Abstract
Airflow obstruction is independent risk factor for cardiovascular events in the general population. The affected vascular bed and contribution of emphysema to cardiovascular risk are unclear. We examined if an obstructive pattern of spirometry and quantitatively defined emphysema were associated with subclinical atherosclerosis in the carotid, peripheral and coronary circulations.
The Multi-Ethnic Study of Atherosclerosis recruited participants age 45–84 years without clinical cardiovascular disease. Spirometry, carotid intima-media thickness, ankle-brachial index and coronary artery calcium were measured using standard protocols. Percent of emphysema-like lung was measured in the lung windows of cardiac computed tomography scans among 3,642 participants. Multiple linear regression was used to adjust for cardiac risk factors including C-reactive protein.
Decrements in the FEV1 and FEV1/FVC were associated with greater internal carotid intima-media thickness among smokers (P=0.03 and P<0.001, respectively) whereas percent emphysema was associated with reduced ankle-brachial index regardless of smoking history (P=0.004). Coronary artery calcium was associated with neither lung function (prevalence ratio for severe airflow obstruction: 0.99; 95% CI, 0.91 to 1.07) nor percent emphysema.
An obstructive pattern of spirometry and emphysema are associated distinctly and independently with subclinical atherosclerosis in the carotid arteries and peripheral circulation, respectively, and were not independently related to coronary artery calcium.
INTRODUCTION
Coronary heart disease, chronic lower respiratory disease, and stroke are the first, third and fourth leading causes of death and jointly accounted for almost 1 million (40%) of deaths in the United States in 2005 (1–2).
An obstructive pattern of spirometry is an independent risk factor for myocardial infarction, cardiovascular mortality (3–10) and stroke (11–14) in general population samples regardless of smoking history. However, the specific systemic vascular beds underlying this strong and consistent relationship are not well defined. Precise and valid measures of subclinical atherosclerosis are available for the carotid, peripheral and epicardial circulations; however, there are few studies of lung function and subclinical atherosclerosis (15–20) and no one study, to date, has compared lung function to measures of subclinical atherosclerosis in all three circulations.
Emphysema is defined as a permanent enlargement of airspaces distal to the terminal bronchioles with destruction of walls (21) and can be measured quantitatively on computed tomography (CT) scan. Percentage of emphysema-like lung (hereafter referred to as percent emphysema) is associated with impaired left ventricular filling (22); however, its association with subclinical atherosclerosis has not been reported. We therefore examined if lung function and percent emphysema were associated with subclinical atherosclerosis in the carotid, peripheral and coronary circulations in a general population sample.
We hypothesized that an obstructive pattern of spirometry, percent emphysema and upper-lobe emphysema would be associated with greater carotid intima medial thickness (IMT), reduced ankle-brachial index (ABI) and increased coronary artery calcium (CAC) on cardiac computed tomography (CT).
METHODS
Study Sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without clinical cardiovascular disease (23). MESA recruited 6,814 men and women ages 45–84 years old in 2000–2002 from six U.S. communities. MESA participants are white, African-American, Hispanic, or Asian (mostly of Chinese origin). Exclusion criteria included clinical cardiovascular disease, weight > 300 lbs, pregnancy and impediment to long-term participation. The protocols of MESA and all studies described herein were approved by the Institutional Review Boards of all collaborating institutions and the National Heart Lung Blood Institute.
The MESA Lung Study enrolled 3,965 MESA participants of 4,484 selected who were sampled randomly among those who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during the MESA-Lung recruitment period in 2004–2006 (99%, 89%, and 91% of the MESA cohort, respectively). Chinese-Americans were over-sampled to improve the precision of estimates in this group.
For comparability with other spirometry studies, the main analyses for spirometry included all participants except for 129 participants lacking valid spirometry measures (due to no technician [n=13], contraindication [n=20], declined [n=29] and no acceptable maneuvers [n=67]). Secondary analyses for obstructive spirometric pattern and the main analyses for emphysema excluded 322 participants with a restrictive pattern of spirometry, defined as a forced vital capacity (FVC) less than the lower limit of normal (LLN) (24) with a FEV1/FVC ratio >LLN, since the primary hypotheses related to obstructive lung disease and emphysema. Participants missing measures of percent emphysema (n=1) and upper-lobe emphysema (n=8) were also excluded from the respective analyses.
Measures
All measures were obtained at the MESA baseline examination, except for spirometry, which was performed during the MESA-Lung recruitment period. All imaging measures were obtained at central reading centers without access to participant information. Random 10% or 100% replicate measures were performed for quality control purposes; results of reproducibility testing are in the Supplementary Material.
Subclinical Atherosclerosis
Carotid Intima-Media Thickness
Trained technicians performed B-mode ultrasonography of the right and left near and far walls of the internal carotid and common carotid arteries (25) using the Logiq 700 ultrasound device (General Electric Medical Systems, Waukesha, WI). Maximal IMT of the internal and common carotid was measured as the mean of the maximum IMT of the near and far walls of the right and left sides.
Ankle-Brachial Index
Measurements for calculation of ABI were obtained using a hand-held Doppler instrument with a 5-mHz probe (Nicolet Vascular, Golden, Colorado). Systolic blood pressure measurements were obtained from bilateral brachial, dorsalis pedis, and posterior tibial arteries (26).
Coronary Artery Calcium
Cardiac CT scans were performed on multi-detector and electron-beam CT scanners following a standardized protocol (27–28). Certified technologists scanned all participants twice on separate breath-holds at full inspiration. A phantom of known physical calcium concentration was included in the field of view; the average phantom-adjusted Agatston score of the two scans was used for analyses (29–30).
Spirometry
Spirometry was conducted in accordance with American Thoracic Society/European Respiratory Society guidelines (31) on a dry-rolling-sealed spirometer with automated quality checks (Occupational Marketing, Inc., Houston, TX) and over-reading by one investigator (32).
For secondary analyses, airflow obstruction was defined as pre-bronchodilator FEV1/FVC ratio < LLN and severity was graded as FEV1 percent predicted: >70%, mild; 50–70%, moderate; <50%, severe (33). Predicted values were calculated using reference equations from the National Health and Nutrition Examination Survey III (24, 31) with a 0.88 correction for Asians (32).
Percent of Emphysema-like Lung
Quantitative measures of emphysema were performed on the lung fields of the cardiac CT scans, which imaged approximately 70% of the lung volume from the carina to the lung bases. Image attenuation was assessed using modified Pulmonary Analysis Software Suite (34). Percent emphysema was defined as the percentage of the voxels in the lung below −910 Hounsfield Units (HU). This threshold was chosen based upon pathology comparisons (35) and the generally mild degree of emphysema in the sample. Upper-lobe emphysema was computed as the percent emphysema in the cranial 1/8th divided by that in the caudal 1/3rd of the imaged lung.
The scan with the higher air volume was used for analyses except in cases of discordant scan quality, in which case the higher quality scan was used (36). Attenuation of air outside the body was measured for each scan and emphysema measures were recalculated after the attenuation of each pixel was corrected to have the value: (−1000 × measured pixel attenuation)/mean air attenuation.
Percent emphysema measures from the carina to lung base are highly correlated (r=0.99) with full-lung measures on the same full-lung scans in smokers, and emphysema measures from cardiac scans correlate with those from full-lung scans from the same MESA participants (e.g., r=0.93 for percent emphysema and r=0.76 for upper-lobe emphysema on multidetector scanners) (36).
Clinical Covariates
Age, gender, race/ethnicity, educational attainment, medical history, medication use and alcohol intake were self-reported. Smoking history was assessed using standard questionnaire items (37) and urinary cotinine levels (Immulite 2000 Nicotine Metabolite Assay, Diagnostic Products Corp., Los Angeles, CA) from the day of CT examination. Hypertension, diabetes, C-reactive protein and cholesterol were assessed using standardized methods (see Supplementary Material).
Statistical Analysis
The cohort was stratified by quintiles of percent emphysema for descriptive purposes. Tests of trend across categories of emphysema and airflow obstruction were performed with Spearman and Cochran-Armitage tests, as appropriate. Generalized additive models with loess smoothing functions were used to test the linearity of relationships of independent variables and covariates with the −2 log likelihood test and to generate multivariate plots. Relationships were generally linear; hence generalized linear models were used, except as described. A Gaussian distribution with identity link was used for all analyses except for that analysis of the presence of CAC, for which a binomial distribution with log link and robust standard errors was used in order to estimate prevalence ratios (38). Initial multivariate analyses regressed subclinical atherosclerosis measures on lung function or quintiles of emphysema after adjustment for age and gender. Full multivariate models also included the variables listed in the footnotes to the tables.
The primary hypothesis tests and 95% confidence intervals (CI) for continuous measures were estimated from linear or smoothed functions (39). Statistical significance was defined as two-tailed P-value <0.05. No adjustment was made for multiple comparisons but all major comparisons are reported, as recommended (40). Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.6 (R Foundation, Vienna, Austria).
RESULTS
The study sample had a mean age of 61 years, 49% were men, and the race/ethnic distribution was 35% white, 26% African-American, 22% Hispanic and 16% Chinese-American. Thirteen percent currently smoked cigarettes, 39% had smoked in the past, and 47% never smoked.
Cardiac Risk Factors
Table 1 shows the characteristics of the 3,642 participants by quintiles of percent emphysema. Participants with greater percent emphysema were more likely to be older, male, white, less obese and former smokers compared to those with lower values of percent emphysema. Participants with greater percent emphysema had a lower FEV1/FVC ratio.
Table 1.
Baseline characteristics of participants stratified by severity of percent emphysema.
| Percent Emphysema
|
|||||
|---|---|---|---|---|---|
| Quintile 1 (n=728) | Quintile 2 (n=729) | Quintile 3 (n=728) | Quintile 4 (n=729) | Quintile 5 (n=728) | |
| Age, mean (SD), years | 60 (10) | 61 (10) | 61 (10) | 62 (10) | 63 (10) |
| Male gender, No. (%) | 164 (23) | 263 (36) | 350 (48) | 454 (62) | 554 (76) |
| Race/ethnicity | |||||
| White, No. (%) | 132 (18) | 213 (29) | 242 (33) | 301 (41) | 391 (54) |
| African American, No. (%) | 217 (30) | 181 (25) | 189 (26) | 193 (26) | 185 (25) |
| Hispanic, No. (%) | 234 (32) | 199 (27) | 154 (21) | 136 (19) | 84 (12) |
| Asian, No. (%) | 145 (20) | 136 (19) | 143 (20) | 99 (14) | 68 (9) |
| Educational attainment | |||||
| No high school degree, No. (%) | 200 (27) | 141 (19) | 122 (17) | 81 (11) | 62 (9) |
| High school degree, No. (%) | 162 (22) | 146 (20) | 123 (17) | 118 (16) | 109 (15) |
| Some college, No. (%) | 206 (28) | 210 (29) | 206 (28) | 207 (28) | 184 (25) |
| College degree, No. (%) | 88 (12) | 119 (16) | 129 (18) | 164 (22) | 167 (23) |
| ≥bachelor degree, No. (%) | 71 (10) | 113 (15) | 148 (20) | 159 (22) | 203 (28) |
| Body mass index, mean (SD), kg/m2 | 29.3 (5.8) | 28.5 (5.7) | 27.8 (5.0) | 27.5 (4.7) | 26.4 (4.1) |
| Cigarette smoking status | |||||
| Never, No. (%) | 405 (56) | 363 (50) | 351 (48) | 335 (46) | 263 (36) |
| Former, No. (%) | 191 (26) | 242 (33) | 282 (39) | 308 (42) | 389 (53) |
| Current, No. (%) | 132 (18) | 124 (17) | 95 (13) | 86 (12) | 76 (10) |
| Pack-years of smoking, median (IQR)* | 17 (5, 33) | 19 (7, 38) | 15 (6, 33) | 20 (7, 38) | 17 (6, 35) |
| Hypertension, No. (%) | 324 (45) | 323 (44) | 320 (44) | 282 (39) | 287 (39) |
| Systolic blood pressure, mean (SD), mmHg | 125 (21) | 125 (20) | 125 (20) | 124 (19) | 123 (18) |
| Diastolic blood pressure, mean (SD), mmHg | 70 (10) | 71 (10) | 72 (10) | 72 (10) | 73 (10) |
| Diabetes mellitus, No. (%) | 108 (15) | 85 (12) | 74 (10) | 69 (9) | 46 (6) |
| Fasting plasma glucose, median (IQR), mg/dL | 98 (90, 108) | 96 (89, 105) | 96 (90, 105) | 96 (90, 105) | 95 (90, 102) |
| LDL, mean (SD), mg/dL | 118 (30) | 116 (31) | 117 (30) | 119 (32) | 117 (30) |
| HDL, mean (SD), mg/dL | 51 (14) | 52 (15) | 51 (15) | 51 (15) | 50 (14) |
| Use of statin, No. (%) | 89 (12) | 111 (15) | 96 (13) | 104 (14) | 116 (16) |
| C-reactive protein, median (IQR), mg/L | 2.3 (1.1, 5.7) | 1.9 (0.8, 4.3) | 1.7 (0.9, 3.7) | 1.7 (0.8, 3.6) | 1.2 (0.6, 2.8) |
| Self-reported asthma < age 45 years, No. (%) | 50 (7) | 54 (7) | 51 (7) | 64 (8) | 67 (9) |
| Self-reported asthma ≥ age 45 years, No. (%) | 11 (1) | 8 (1) | 12 (2) | 12 (2) | 19 (3) |
| FEV1 percent of predicted, mean (SD) | 96 (15) | 96 (16) | 96 (17) | 96 (18) | 95 (20) |
| FVC percent of predicted, mean (SD) | 94 (14) | 96 (14) | 97 (15) | 99 (15) | 102 (16) |
| FEV1/FVC ratio, mean (SD), % | 78 (7) | 76 (7) | 75 (8) | 73 (9) | 70 (10) |
| Percent emphysema, median (IQR), | 3.4 (1.8, 4.8) | 8.9 (7.6, 10.3) | 14.7 (13.2, 16.5) | 22.1 (20.0, 24.8) | 34.3 (30.3, 40.8) |
| Upper-lobe emphysema (ratio), median (IQR) | 1.08 (0.57, 1.93) | 1.09 (0.76, 1.67) | 1.11 (0.85, 1.46) | 1.04 (0.81, 1.33) | 1.02 (0.88, 1.18) |
Packyears of smoking among ever smokers
SD = Standard deviation; IQR = Interquartile range; MRC = Medical Research Council; FEV1 = Forced expiratory volume in one second; FVC = Forced vital capacity.
Lung Function and Subclinical Atherosclerosis
Associations of the FEV1 with IMT and ABI were modified by packyears of smoking (P-interaction<0.05), therefore analyses for lung function were stratified by smoking.
Table 2 shows associations of lung function and measures of subclinical atherosclerosis stratified by smoking history. Among participants with a history of smoking, a lower FEV1 and FEV1/FVC ratio was associated with increased IMT of the internal carotid artery and increased IMT of the common carotid artery. In contrast, there was no evidence for an association of FVC with either IMT measure. Both the FEV1 and FVC were associated with ABI, as was the FEV1/FVC ratio. Findings for IMT and ABI were generally similar among never smokers although the associations were of smaller magnitude and of more borderline statistical significance (Table 2).
Table 2.
Differences in measures of subclinical atherosclerosis in the carotid, peripheral and coronary vascular beds per unit change in lung function among smokers and never smokers.
| Smokers (n=2,024) | FEV1 Difference per 1 L increase (95% CI) |
P-value | FVC Difference per 1 L increase (95% CI) |
P-value | FEV1/FVC ratio Difference per 1 % increase (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| Intima Medial Thickness, Internal Carotid (mm) | ||||||
| Multivariate mean difference | −0.060 (−0.115, −0.005) | 0.03 | −0.000 (−0.051, 0.050) | 0.99 | −0.006 (−0.009, 0.003) | <0.001 |
| Intima Medial Thickness, Common Carotid (mm) | ||||||
| Multivariate mean difference | −0.028 (−0.045, −0.012) | <0.001 | −0.013 (−0.028, 0.022) | 0.09 | −0.001 (−0.002, −0.000) | 0.007 |
| Ankle-Brachial Index | ||||||
| Multivariate mean difference | 0.012 (0.002, 0.023) | 0.02 | 0.004 (−0.005, 0.014) | 0.39 | 0.0008 (0.0002, 0.0014) | 0.006 |
| Presence of Coronary Artery Calcium | ||||||
| Multivariate prevalence ratio | 1.01 (0.97, 1.04) | 0.76 | 1.00 (0.97, 1.03) | 0.96 | 1.001 (0.997, 1.002) | 0.58 |
| Log Agatston Score* | ||||||
| Multivariate mean difference | 0.099 (−0.137, 0.334) | 0.41 | 0.14 (−0.074, 0.360) | 0.20 | −0.004 (−0.016, 0.008) | 0.55 |
|
| ||||||
|
Never Smokers (n=1,812)
| ||||||
| Intima Medial Thickness, Internal Carotid (mm) | ||||||
| Multivariate mean difference | −0.053 (−0.105, −0.001) | 0.047 | −0.008 (−0.053, 0.036) | 0.71 | −0.004 (−0.007, −0.002) | 0.002 |
| Intima Medial Thickness, Common Carotid (mm) | ||||||
| Multivariate mean difference | −0.028 (−0.044, −0.013) | <0.001 | −0.014 | 0.04 | −0.001 | 0.01 |
| Ankle-Brachial Index | ||||||
| Multivariate mean difference | 0.013 (0.003, 0.023) | 0.01 | 0.0066 (−0.002, 0.015) | 0.13 | 0.0006 (0.0000, 0.0011) | 0.045 |
| Presence of Coronary Artery Calcium | ||||||
| Multivariate prevalence ratio | 1.00 (0.97, 1.03) | 0.92 | 0.99 (0.97, 1.02) | 0.65 | 1.001 (0.999, 1.003) | 0.40 |
| Log Agatston Score* | ||||||
| Multivariate mean difference | 0.020 (−0.197, 0.237) | 0.86 | 0.020 (−0.163, 0.203) | 0.82 | −0.001 (−0.012, 0.010) | 0.83 |
Among participants with detectable coronary artery calcium
Multivariate models adjusted for age, gender, race/ethnicity, smoking status, pack-years, urine cotinine, educational attainment, diabetes mellitus, fasting plasma glucose, height, body mass index, hypertension, systolic and diastolic blood pressure, LDL, HDL, statin medication, alcohol use and C-reactive protein.
In contrast, there was no evidence for an association of any measure of lung function with the presence or extent of CAC in smokers or never smokers (Table 2) or in the whole cohort (e.g., prevalence ratio for the presence of CAC in severe airflow obstruction, 0.99, 95% CI, 0.91 to 1.07).
Findings were similar for categories of airflow obstruction. Severity of airflow obstruction was associated with internal carotid IMT, common carotid IMT and, of borderline statistical significance, ABI among smokers but not among never smokers (Online Supplement Table 1).
Percent Emphysema and Subclinical Atherosclerosis
In contrast to findings for lung function, there was no evidence for a consistent relationship of percent emphysema to IMT in fully adjusted models (Table 3). Percent emphysema was associated inversely with ABI and the magnitude of this association was not modified by smoking history (P-interaction=0.56). Indeed, percent emphysema was significantly related to reduced ABI among participants with less than 10 packyears of smoking (P=0.004).
Table 3.
Differences in measures of subclinical atherosclerosis in the carotid, peripheral and coronary vascular beds according to quintiles of CT percent emphysema.
| CT Percent Emphysema
|
Difference per 10 % Increase in Percent Emphysema [95% CI] | P-value | |||||
|---|---|---|---|---|---|---|---|
| quintile 1 (n=728) | quintile 2 (n=729) | quintile 3 (n=728) | quintile 4 (n=729) | quintile 5 (n=728) | |||
| Intima-media Thickness, Internal Carotid (mm) | |||||||
| Median value | 0.778 | 0.807 | 0.844 | 0.840 | 0.897 | <0.001 | |
| Age-sex-adjusted mean difference | 0 | 0.0132 | 0.0270 | −0.0045 | −0.0385 | −0.009 [−0.025, 0.007] | 0.29 |
| Multivariate mean difference | 0 | 0.0182 | 0.0417 | 0.0252 | 0.0068 | 0.005 [−0.012, 0.022] | 0.59 |
| Intima-media Thickness, Common Carotid (mm) | |||||||
| Median value | 0.815 | 0.829 | 0.835 | 0.848 | 0.843 | 0.002 | |
| Age-sex-adjusted mean difference | 0 | −0.0035 | −0.0073 | −0.0051 | −0.0362 | −0.010 [−0.014, −0.005] | <0.001 |
| Multivariate mean difference | 0 | 0.0025 | 0.0014 | 0.0089 | −0.0128 | −0.003 [−0.008, 0.002] | 0.30 |
| Ankle-Brachial Index | |||||||
| Median value | 1.11 | 1.12 | 1.13 | 1.13 | 1.13 | 0.03 | |
| Age-sex-adjusted mean difference | 0 | 0.0061 | 0.0080 | 0.0070 | −0.0048 | −0.002 [−0.006, 0.001] | 0.13 |
| Multivariate mean difference | 0 | 0.0021 | 0.0026 | −0.0005 | −0.0133 | −0.005 [−0.008, −0.002] | 0.004 |
| Presence of Coronary Artery Calcium | |||||||
| Prevalence (%) | 39 | 43 | 46 | 48 | 56 | <.001 | |
| Age-sex-adjusted prevalence ratio | 1 | 1.01 | 0.984 | 0.938 | 0.976 | 0.987 [0.963, 1.01] | 0.33 |
| Multivariate prevalence ratio | 1 | 1.01 | 0.992 | 0.952 | 0.976 | 0.986 [0.960, 1.01] | 0.29 |
| Mean Agatston Score* | |||||||
| Median value | 48.9 | 60.0 | 82.6 | 83.4 | 108 | <0.001 | |
| Age-sex-adjusted mean difference | 0 | 2.07 | 6.27 | 8.80 | 5.49 | 1.035 [−0.999, 3.068] | 0.36 |
| Multivariate mean difference | 0 | 0.57 | 5.35 | 6.26 | 7.21 | 1.045 [−0.995, 3.085] | 0.27 |
Among participants with detectable coronary artery calcium
Multivariate models adjusted for age, gender, race/ethnicity, smoking status, pack-years, urine cotinine, educational attainment, diabetes mellitus, fasting plasma glucose, height, body mass index, hypertension, systolic and diastolic blood pressure, LDL, HDL, statin medication, alcohol use, C-reactive protein, scanner type and mAs.
There was no evidence for an association between percent emphysema and either the presence or the extent of CAC (Table 3).
Upper-Lobe Emphysema and Subclinical Atherosclerosis
Figure 1 shows the associations of upper-lobe emphysema to internal carotid IMT, ABI, and prevalence of CAC. There was evidence for non-linearity in the association of upper-lobe emphysema tointernal carotid IMT (P=0.003) but not to ABI (P=0.12) or CAC (P=0.11).
Figure 1.
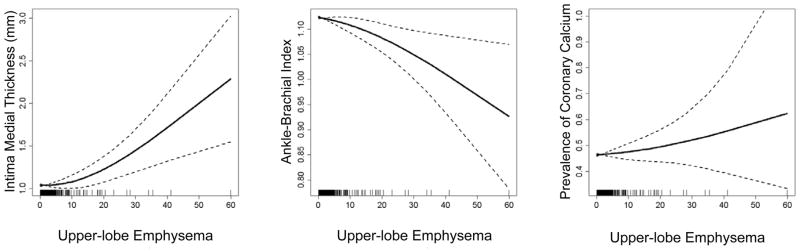
Multivariate relationships of upper-lobe emphysema to measures of subclinical atherosclerosis in the carotid, peripheral and coronary vascular beds.
Multivariate relationships of upper-lobe emphysema to the intima-media thickness of the internal carotid artery (P=0.003), ankle-brachial index (P=0.004), prevalence of coronary artery calcium (P=0.29). Multivariate models adjusted for age, gender, race/ethnicity, smoking status, pack-years, urine cotinine, educational attainment, diabetes mellitus, fasting plasma glucose, height, body mass index, alcohol use, hypertension, systolic and diastolic blood pressure, C-reactive protein, LDL, HDL, statin medication, alcohol use, scanner type and mAs. The relationship of upper-lobe emphysema to the intima-media thickness of the internal carotid artery was non-linear (P=0.002), although those ankle-brachial index and prevalence of coronary artery calcium were not (P=0.12, P=0.45, respectively).
Thick lines = smoothed regression lines, which allows for non-linear relationships; thin lines = 95% confidence intervals.
Upper-lobe emphysema was significantly related to IMT of the internal carotid artery (P=0.003; Figure 3); however, the significance of the association was highly dependent on a few extreme values and no consistent relationship was observed across quintiles of upper-lobe emphysema (Table 4).
Table 4.
Differences in measures of subclinical atherosclerosis in the carotid, peripheral and coronary vascular beds according to quintiles of upper-lobe emphysema.
| Upper to Lower Lobe Ratio of Percent Emphysema
|
Difference per 10 Unit Increase in Upper-lobe Emphysema [95% CI] | P-value | |||||
|---|---|---|---|---|---|---|---|
| quintile 1 (n=727) | quintile 2 (n=727) | quintile 3 (n=727) | quintile 4 (n=727) | quintile 5 (n=727) | |||
| Intima-media Thickness, Internal Carotid (mm) | |||||||
| Median value | 0.860 | 0.863 | 0.826 | 0.829 | 0.786 | 0.056 | |
| Age-sex-adjusted mean difference | 0 | −0.0533 | −0.0909 | −0.0750 | −0.1067 | * | 0.048 |
| Multivariate mean difference | 0 | −0.0199 | −0.0377 | −0.0076 | −0.0371 | * | 0.002 |
| Intima-media Thickness, Common Carotid (mm) | |||||||
| Median value | 0.838 | 0.856 | 0.843 | 0.823 | 0.820 | 0.07 | |
| Age-sex-adjusted mean difference | 0 | −0.0168 | −0.0251 | −0.0337 | −0.0384 | * | 0.20 |
| Multivariate mean difference | 0 | −0.0003 | −0.0038 | −0.0060 | −0.0131 | * | 0.84 |
| Ankle-Brachial Index | |||||||
| Median value | 1.13 | 1.14 | 1.13 | 1.12 | 1.12 | <0.001 | |
| Age-sex-adjusted mean difference | 0 | −0.0012 | −0.0075 | −0.0074 | −0.0103 | −0.027 [−0.043, −0.011] | 0.001 |
| Multivariate mean difference | 0 | −0.0047 | −0.0083 | −0.0075 | −0.0056 | −0.023 [−0.039, −0.007] | 0.004 |
| Presence of Coronary Artery Calcium | |||||||
| Prevalence (%) | 44 | 48 | 46 | 46 | 48 | 0.46 | |
| Age-sex-adjusted prevalence ratio | 1 | 0.942 | 0.915 | 0.916 | 0.972 | 1.034 [0.905, 1.18] | 0.63 |
| Multivariate prevalence ratio | 1 | 0.937 | 0.925 | 0.951 | 1.01 | 1.062 [0.934, 1.21] | 0.40 |
| Mean Agatston Score† | |||||||
| Median value | 63.1 | 75.7 | 88.3 | 80.6 | 70.3 | 0.84 | |
| Age-sex-adjusted mean difference | 0 | −12.2 | −4.6 | −2.79 | −13.8 | 0.858 [−1.47, 3.19] | 0.37 |
| Multivariate mean difference | 0 | −9.19 | 1.17 | 4.17 | −8.07 | 0.992 [−1.00, 2.99] | 0.96 |
Relationship was non-linear therefore linear term not reported. See Figure 3 for graph of association.
Among participants with detectable coronary artery calcium
Multivariate models adjusted for age, gender, race/ethnicity, smoking status, pack-years, urine cotinine, educational attainment, diabetes mellitus, fasting plasma glucose, height, body mass index, alcohol use, hypertension, systolic and diastolic blood pressure, LDL, HDL, statin medication, alcohol use, C-reactive protein, scanner type and mAs.
Upper-lobe emphysema was significantly and inversely associated with the ABI (Figure 1 and Table 4), and this association was not modified by packyears (P-interaction=0.58). There was no evidence for an association of upper-lobe emphysema with either the presence or the extent of CAC.
Lung Phenotypes and Subclinical Atherosclerosis
In multivariate models that simultaneously adjusted for the FEV1/FVC ratio, percent emphysema and upper-lobe emphysema, the significance of the association of obstructive spirometric pattern and upper-lobe emphysema with IMT of the internal carotid artery increased (e.g., mean difference, −0.35mm per 1% increase in the FEV1/FVC ratio, 95% CI, −0.59 to −0.12, P=0.003) whereas the association for CT percent emphysema was further attenuated (P=0.87).
For ABI, the associations with CT percent emphysema and upper-lobe emphysema remained significant (e.g., −0.005 per 10% increase in percent emphysema, 95% CI, −0.09 to −0.0001, P=0.011, respectively) and that of lung function was attenuated (P=0.97).
Additional Analyses
There was no evidence that self-reported physician-diagnosed asthma (at any age) was associated with increased IMT of the internal carotid artery (0.012 mm; 95% CI, −0.032 to 0.057), reduced ABI (−0.002; 95% CI, −0.011 to 0.008) or presence of CAC (RR 1.00, 95% CI, 0.97 to 1.04). Results for self-reported asthma before age 45 years and for symptoms of chronic bronchitis were similarly negative. The main results were generally similar with the inclusion or exclusion of participants with a restrictive pattern of spirometry and were not modified by gender or race/ethnicity.
DISCUSSION
An obstructive pattern of spirometry and upper-lobe emphysema were independently associated with increased IMT of the internal carotid in this population-based study whereas percent emphysema and upper-lobe emphysema were associated with decrements in ABI. In contrast, neither lung function nor emphysema measures were associated with CAC. These findings suggest that cardiovascular risk from smoking-related obstructive ventilatory defects are preferentially related to atherosclerosis of the internal carotid artery whereas emphysema is related to atherosclerosis of the peripheral arteries among smokers and non-smokers alike.
Our results for lung function and IMT are consistent and expand upon those from the Atherosclerosis Risk In Communities (ARIC) Study. In ARIC, lower lung function was associated with increased mean (average of the common and internal carotid arteries and their bifurcation) IMT among smokers but not among never smokers (16). The British Regional Heart Study found IMT of the carotid bifurcation to be inversely associated with FEV1 (15) and a small study of Japanese smokers found increased IMT among participants with an obstructive pattern of spirometry compared to controls (18). None of these studies reported IMT of the internal carotid artery, for which we observed the strongest associations, or ascertained percent emphysema.
Low lung function was also associated with reduced ABI in ARIC and the ‘Men Born in 1918’ cohort (16–17). Ascertainment of percent emphysema in the current study, however, revealed that the association of lung function with ABI was entirely explained by percent emphysema. This finding, along with the lack of association of asthma with subclinical measures of atherosclerosis, might suggest that cardiovascular risk due to low lung function among never smokers may result not from airflow obstruction itself but from subclinical emphysema – panlobular emphysema being not uncommon in never smokers (41).
A retrospective study of 4,905 Korean men undergoing clinical screening for CAC found that CAC was increased in the lowest quartiles of the FEV1 and the FVC but not the FEV1/FVC ratio (20). In contrast, we found no association of CAC with the lung function in MESA. The Korean study may have had better power to detect an association since it was larger, was restricted to one race and gender, and excluded “severe” cardiac disease (whereas MESA excluded clinical cardiovascular disease). On the other hand, results in the Korean study were adjusted only for age, smoking status and BMI. Given the apparent lack of adjustment for other important determinants of lung function and CAC (e.g., packyears), it is unclear if a fully adjusted model in that study would have yielded results similar to those in MESA. In either case, both studies show a consistent and null association of CAC with the FEV1/FVC ratio.
We speculate that the subclinical atherosclerosis occurring in early obstructive lung disease and emphysema may be due to endothelial dysfunction, microvascular disease and oxidative stress rather than the traditional lipid-driven atherosclerosis of the coronary epicardial arteries. Endothelial dysfunction and microvascular disease are prominent features of peripheral vascular disease (42), and flow-mediated dilation of the brachial artery, a measure of nitric oxide-dependent endothelial function, is significantly impaired in peripheral vascular disease (43). Endothelial dysfunction has also been implicated in the pathogenesis of emphysema (44–45) and flow-mediated dilation of the brachial artery is reduced with an obstructive pattern of spirometry and percent emphysema, the association with the former explained by the latter (46) – similar to the current findings for ABI. Oxidative stress is implicated in atherosclerosis and particularly atherosclerosis of the internal carotid artery (47–48), for which we found the strongest associations. In contrast, IMT of the common carotid artery is related more to hypertension and shear stress (48–49). Of note, IMT of the internal carotid artery is a better predictor of myocardial infarction than IMT of the common carotid artery (50).
A novel component of this study was the measurement of percent emphysema, which allowed for the first time the assessment of subclinical atherosclerosis and objectively defined emphysema in a population-based study. Percent emphysema was measured on partial-lung scans, which has been previously validated against full-lung scans in this cohort (36). Nonetheless, the correlation between partial and full-lung scans was lower for upper-lobe emphysema than for percent emphysema. Reassuringly, visual inspection of CT scans with high values for upper-lobe emphysema demonstrated clinical emphysema.
Lung function was assessed approximately four years after the other measures; however, the expected mean change in the FEV1/FVC ratio over four years in an epidemiologic cohort such as MESA is small (approximately 1% (51)) relative to the standard deviation of the FEV1/FVC ratio (9%).
Post-bronchodilator measures of spirometry were not performed in this large cardiovascular cohort. All published epidemiologic studies on lung function and cardiovascular risk are, however, based on pre-bronchodilator measures.
The lack of post-bronchodilator measures limited our ability to distinguish the cause of the obstructive pattern of spirometry. However, associations for lung function were of significantly greater magnitude in smokers than non-smokers, there were consistent associations for upper-lobe emphysema, and there was no evidence for an association of self-reported asthma with subclinical atherosclerosis. Hence, our findings likely apply to smoking-related obstructive lung disease.
Participants with clinical cardiovascular disease were excluded and only 52 participants had severe airflow obstruction. On the one hand, this is a limitation since these results may not fully generalize to patients with severe lung disease. On the other hand, it is a strength, since analyses were not confounded appreciably by medication use. Confounding by unmeasured or imprecisely measured confounders is of concern in any observational study. However, standard and novel cardiac risk factors were measured precisely and current smoking status was confirmed with cotinine levels.
Finally, an alternative explanation for circulation-specific findings for lung function and lung density might be differential measurement error. However, of the measures of subclinical atherosclerosis, CAC was the most precise and the best predictor of cardiovascular events in this cohort (28, 52). Of the measures of lung disease, lung function was more accurate and precise than percent emphysema; however, measurement error in percent emphysema was small and was not differentially related to IMT, ABI and CAC. We therefore doubt the negative findings for CAC or the circulation-specific findings are due to measurement error.
In conclusion, an obstructive pattern of spirometry was independently associated with atherosclerosis in the carotid arteries among smokers whereas quantitatively defined emphysema was associated with reduced ABI regardless of smoking history, and neither measure was associated with CAC. Cardiovascular risk related to chronic lower respiratory disease differs by phenotype and appears unrelated to coronary artery calcium, a measure of epicardial atherosclerosis.
Supplementary Material
Acknowledgments
The authors thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org. This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments have been incorporated prior to submission for publication.
FUNDING SOURCES
The MESA and MESA-Lung Studies are supported by the NIH/NHLBI contracts N01-HC-95159 through N01-HC-95169 and grants R01 HL-077612, HL-093081 and HL-075476.
Footnotes
FINANCIAL DISCLOSURES
Drs. Ahmed, Carr, Jiang and Watson reported no relevant conflict of interest. Dr Barr reported receiving an unrestricted gift from Cenestra Health for supplies for an NIH-funded pilot study. Dr. Hoffman has patents and an interest in Vida Diagnostics. Dr. Kawut received significant grant funding from Pfizer for an investigator-initiated clinical trial in COPD.
References
- 1.Miniño AM, Xu J, Kochanek KD. National vital statistics reports: Deaths: Preliminary data for 2008. 2010 [cited 2010 December 16th]. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. [PubMed]
- 2.Kung HCHD, Xu JQ, Murphy SL. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics; 2008. Deaths: Final data for 2005; p. 56. [PubMed] [Google Scholar]
- 3.Persson C, Bengtsson C, Lapidus L, Rybo E, Thiringer G, Wedel H. Peak expiratory flow and risk of cardiovascular disease and death: A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Am J Epidemiol. 1986;124:942–948. doi: 10.1093/oxfordjournals.aje.a114483. [DOI] [PubMed] [Google Scholar]
- 4.Ebi-Kryston KL. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall Study. J Clin Epidemiol. 1988;41:251–260. doi: 10.1016/0895-4356(88)90129-1. [DOI] [PubMed] [Google Scholar]
- 5.Cook DG, Shaper AG. Breathlessness, lung function and heart attack. Eur Heart J. 1988;9:1215–1222. doi: 10.1093/oxfordjournals.eurheartj.a062432. [DOI] [PubMed] [Google Scholar]
- 6.Tockman MCG. Respiratory risk factors and mortality: Longitudinal studies in Washington County, Maryland. Am Rev Respir Dis. 1989;140:S56–63. doi: 10.1164/ajrccm/140.3_Pt_2.S56. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie PD, Kannel WB, O’Connor G. Mortality associated with respiratory function and symptoms in advanced age. The Framingham Study. Am Rev Respir Dis. 1989;140:379–384. doi: 10.1164/ajrccm/140.2.379. [DOI] [PubMed] [Google Scholar]
- 8.Hole DJ, Watt GCM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: Findings from the Renfrew and Paisley Prospective Population Study. BMJ. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schunemann HJ, Dorn J, Grant BJB, Winkelstein W, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: The Atherosclerosis Risk In Communities Study. Am J Epidemiol. 2003;158:1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Shaper AG, Ebrahim S. Respiratory function and risk of stroke. Stroke. 1995;26:2004–2010. doi: 10.1161/01.str.26.11.2004. [DOI] [PubMed] [Google Scholar]
- 12.Strachan DP. Ventilatory function as a predictor of fatal stroke. BMJ. 1991;302:84–87. doi: 10.1136/bmj.302.6768.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 14.Hozawa A, Billings JL, Shahar E, Ohira T, Rosamond WD, Folsom AR. Lung function and ischemic stroke incidence. Chest. 2006;130:1642–1649. doi: 10.1378/chest.130.6.1642. [DOI] [PubMed] [Google Scholar]
- 15.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GDO. Carotid plaque, intima media thickness, ardiovascular risk factors, and prevalent cardiovascular disease in men and women: The British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis: The ARIC Study. Atherosclerosis. 2005;180:367–373. doi: 10.1016/j.atherosclerosis.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Engstrom G, Hedblad B, Valind S, Janzon L. Asymptomatic leg and carotid atherosclerosis in smokers is related to degree of ventilatory capacity: Longitudinal and cross-sectional results from ‘Men born in 1914’, Sweden. Atherosclerosis. 2001;155:237–243. doi: 10.1016/s0021-9150(00)00557-8. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC. [DOI] [PubMed] [Google Scholar]
- 19.Zureik M, Kauffmann F, Touboul P-J, Courbon D, Ducimetiere P. Association between peak expiratory flow and the development of carotid atherosclerotic plaques. Arch Intern Med. 2001;161:1669–1676. doi: 10.1001/archinte.161.13.1669. [DOI] [PubMed] [Google Scholar]
- 20.Park HY, Lim SY, Hwang JH, Choi J-H, Koh W-J, Sung J, Suh GY, Chung MP, Kim H, Choe YH, Woo S, Jung Kwon O. Lung function, coronary artery calcification, and metabolic syndrome in 4905 korean males. Respiratory Medicine. 2010;104:1326–1335. doi: 10.1016/j.rmed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Celli BR, MacNee W. Ats/ers task force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 22.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal R, Lima J, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction and impaired left ventricular filling. New Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson J, Odencrantz J, Fedan K. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.O’Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. Journal of Vascular Surgery. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 27.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DRJ, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development In young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 28.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 29.Yaghoubi S, Tang W, Wang S. Offline assessment of atherosclerotic coronary calcium from electron beam tomograms. Am J Cardiac Imag. 1995;9:231–236. [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte MJ, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino RGV, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky K, Barr RG. Performance of american thoracic society-recommended spirometry reference values in a multiethnic sample of adults: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson JL, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 34.Guo J, Reinhardt JM, Kitaoka H, Zhang L, Sonka M, McLennan G, Hoffman EA. Integrated system for ct-based assessment of parenchymal lung disease. IEEE International Symposium on Biomedical Imaging; 2002. pp. 871–874. [Google Scholar]
- 35.Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, Staples CA, Pare PD, Hogg JC. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Applied Physiol. 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman EA, Jiang R, Baumhauer HA, Brooks M, Carr JJ, Detrano R, Hinckley K, Reinhardt J, Rodriguez J, Wong N, Barr RG. Reproducibility and validation of lung density measures from cardiac ct scans in the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiology. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferris BG. Epidemiology standardization project. Am Rev Respir Dis. 1978;118 (Supp 2):1–120. [PubMed] [Google Scholar]
- 38.McCullagh P, Nelder JA. Generalized linear models. New York: Chapman & Hall; 1989. [Google Scholar]
- 39.Greenland S, Michels KB, Robins JM, Poole C, Willett WC. Presenting statistical uncertainty in trends and dose-response relations. Am J Epidemiol. 1999;149:1077–1086. doi: 10.1093/oxfordjournals.aje.a009761. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 41.Anderson AE, Jr, Hernandez JA, Eckert P, Foraker AG. Emphysema in lung macrosections correlated with smoking habits. Science. 1964;144:1025–1026. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 42.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J. Atherosclerotic vascular disease conference: Writing group iii: Pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 43.Komai H, Higami Y, Tanaka H, Honda K, Juri M, Okamura Y. Impaired flow-mediated endothelium-dependent and endothelium-independent vasodilation of the brachial artery in patients with atherosclerotic peripheral vascular disease. Angiology. 2008;59:52–56. doi: 10.1177/0003319707303442. [DOI] [PubMed] [Google Scholar]
- 44.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 45.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tudor RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature. 2005;11:491–472. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barr RG, Mesia Vela S, Austin JHM, Basner RC, Keller B, Reeves A, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function & emphysema in ex-smokers. Am J Respir Crit Care Med. 2007;175:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegele RA. The pathogenesis of atherosclerosis. Clin Chem Acta. 1996;246:21–38. doi: 10.1016/0009-8981(96)06224-9. [DOI] [PubMed] [Google Scholar]
- 48.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Current Cardiology Reports. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 49.Tabara Y, Kohara K, Nakura J, Miki T. Risk factor-gene interaction in carotid atherosclerosis: Effect of gene polymorphisms of renin-angiotensin system. J Hum Genet. 2001;46:278–284. doi: 10.1007/s100380170079. [DOI] [PubMed] [Google Scholar]
- 50.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study. New England Journal of Medicine. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 51.Jiang R, Burke GL, Enright PL, Newman AB, Margolis HG, Cushman M, Tracy RP, Wang Y, Kronmal RA, Barr RG. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168:602–10. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


