Abstract
Fine-needle aspiration (FNA) of the thyroid gland is a widely accepted and accurate method for triaging patients with thyroid nodules. Thyroid FNA suffers from a reporting confusion due to multiplicity of category terminologies. To address this, The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was recently introduced for unifying the terminology and morphologic criteria along with the corresponding risk of malignancy.
Objective
The aim of this study was to report the diagnostic utility of TBSRTC at our institution and report the malignancy risk for FNA of thyroid lesions among Saudi patients using this system at KAUH (King Abdulaziz University Hospital), Jeddah, Saudi Arabia.
Materials and methods
A retrospective study identifying 250 thyroid FNAs performed among Saudi patients between Jan 2005–Dec 2010 was undertaken. Cytology specimen data was collected through a computerized search of our cytopathology archives.
Results
Among the 250 thyroid FNAs, 84 were followed by surgical resection. The overall surgical yield of malignancy was 23.8%. The malignancy rate for the 6 categories was as follows: non diagnostic: 20%, benign: 3.1%, atypia of undetermined significance: 50%, suspicious for follicular neoplasm: 20%, suspicious for malignancy: 80%, malignant: 100%.
Conclusion
Retrospective classification of FNAs of thyroid lesions among Saudi patients using TBSRTC at KAAUH, Jeddah, Saudi Arabia, validates the diagnostic reproducibility of this system and yields similar results for risk of malignancy as reported by others. However the associated rates found for non diagnostic (20%) raise the possibility of malignancy risk in this category and validate the past observations that sample inadequacy is a common cause of false negative thyroid FNAs.
Introduction
Fine-needle aspiration (FNA) of the thyroid gland has proven to be an important and widely accepted, cost-effective, simple, safe, and accurate method for triaging patients with thyroid nodules. (1) By using FNA of the thyroid, 70–80% of thyroid lesions can be classified as benign or malignant with 92% negative predictive value for a benign diagnosis and 100% positive predictive value for malignancy. (2) It has an essential role in the evaluation of euthyroid patients with a thyroid nodule as it reduces the rate of unnecessary thyroid surgery for patients with benign nodules and appropriately triages patients with thyroid cancer to appropriate surgery. (3)
Literature is deficient on the exact frequency of thyroid nodules and the associated rate of malignancy in Saudi population. Nonetheless, nodular thyroid diseases are common especially in mountainous areas of the western region of Saudi Arabia (4) and thyroid cancer is the second most common cancer among females accounting for 424 (10.2%) among 4156 total cancers recorded in Saudi females during 2006. (5) In the United States, 4 to 7 percent of the adult population has a palpable thyroid nodule. (6) However, only 1 of 20 clinically identified nodules are malignant. (6) This corresponds to approximately 2 to 4 per 100,000 people per year, constituting only 1 percent of all cancers and 0.5 percent of all cancer deaths. (6) It is estimated that up to 30 million patients in the United States have thyroid nodules larger than 1 cm. In comparison with the high prevalence of thyroid nodules, 30,000 patients are diagnosed with thyroid malignances each year. (6, 7)
Given the high prevalence of nodules combined with the impracticality of surgically excising all nodules, FNA plays a vital role as a screening test. (2) Every patient with a palpable or incidental thyroid nodule is a candidate for FNA. A nodule that appears either iso- or hypo-functioning on radionuclide scan should be considered for FNA based on the US (ultrasound) findings. (6, 8) Incidental lesions detected by US have a cancer risk of approximately 10–15% and should undergo dedicated thyroid sonographic evaluation. (9) A nodule of any size with sonographically suspicious features should also be considered for FNA. (10, 11)
However, despite its widespread use, thyroid FNA currently suffers from a reporting confusion: multiplicity of category names, descriptive reports without categories and variable surgical pathology terminology. (10) Lack of consistency in reporting thyroid FNA has led to wide variances in sensitivity and specificity calculations depending on what one considers to be true and false positives/negatives and resulted in confusion among clinicians on how to manage patients who do not have a clear-cut negative or positive thyroid FNA result. (12)
This confusion in diagnostic terminology and clinician perception of its inconsistency was addressed in 2007 by the National Cancer Institute (NCI) Thyroid FNA of the Science Conference wherein the terminology and morphologic criteria for reporting thyroid FNA were concluded thus forming the framework for The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). (6,13,14) The System improves the clarity of communication among cytopathologists and other health care providers, predicts the cancer risk and reduces unnecessary surgery for patients with benign nodules and appropriately triages patients with malignant nodules for timely clinical intervention. (14, 15) It allows easy and reliable sharing of data from different laboratories for national and international collaboration and comparison by establishing a common language. (14, 15)
There have been few studies validating the utility and diagnostic accuracy of FNA thyroid across different regions of Saudi Arabia, (5,16–19) to the knowledge of authors there are none reporting feedback on the application of the recently established system of TBSRTC within the context of the risk of thyroid malignancies among Saudi population. However several international studies and reviews have been published in this regard. (6, 13, 15, 20–27) This study was performed to study the diagnostic utility of TBSRTC for reporting the rate of thyroid malignancies.
Objective
To study retrospectively the diagnostic utility of TBSRTC at our institution and report the malignancy risk for FNA of thyroid lesions among Saudi patients and compare it with previous studies.
Materials and Methods
A retrospective study including all FNAs of the thyroid lesions between Jan 2005–Dec 2010 was performed. The study identified 1050 FNA procedures performed at KAUH of which 562 were thyroid FNAs. 250 belonged to Saudi patients. All cytology specimen data of these cases was collected through a computerized search of the Cytopathology archives at KAUH (King Abdulaziz University Hospital), Jeddah, KSA. The data was filtered using appropriate SNOWMED (Systematized Nomenclature of Human Medicine) morphology codes indicating the following parameters: Date of FNA, Personal Identity (Medical Record number, Age, Sex, Nationality etc.), Clinical and radiological diagnosis, Morphology, and Topography.
The data was rechecked manually to delete duplications. Computerized search was then exported to Microsoft Excel format and used for analysis. Specific target group was identified. Interpretations were recorded by three cytopathologists as per the existing international standards. Manual review of cytology reports was then completed twice independently and discrepancy, if any, was resolved through a repeat review. Most of the thyroid FNAs performed at KAAUH use the conventional Papanicolaou and Diff Quick stained smears. Occasionally Thin prep slides are used. Thyroid lesions were classified according to the TBSRTC 6-tier diagnostic categories as in Table 1(6, 13) also published in form of an atlas (14) into;
Table (1).
The Bethesda System for Reporting Cytopathology: Recommended Diagnostic Categories (14)
| Diagnostic Category | Risk of malignancy | Usual Management |
|---|---|---|
| I. Nondiagnostic or unsatisfactory | - | Repeat FNA with ultrasound |
| Cyst fluid only | ||
| Virtually acellular specimen | ||
| Other (obscuring blood, clotting artifact, etc) | ||
| II. Benign | 0–3 | Clinical follow-up |
| Consistent with a benign follicular nodule (includes adenomatoid nodule, colloid nodule, etc) | ||
| Consistent with lymphocytic (Hashimoto) thyroiditis in the proper clinical context | ||
| Consistent with granulomatous(subacute) thyroiditis | ||
| III. Atypia of undetermined significance/follicular lesion of undetermined significance | 5–15 | Repeat FNA |
| IV. Follicular neoplasm/"suspicious" for follicular neoplasm Specify if Hürthle cell type | 15–30 | Surgical lobectomy |
| V. Suspicious for malignancy | 60–75 | Near-total thyroidectomy or surgical lobectomy |
| Suspicious for papillary carcinoma | ||
| Suspicious for medullary carcinoma | ||
| Suspicious for metastatic carcinoma | ||
| Suspicious for lymphoma | ||
| VI. Malignant | 97–99 | Near-total thyroidectomy |
| Papillary thyroid carcinoma | ||
| Poorly differentiated carcinoma | ||
| Medullary thyroid carcinoma | ||
| Undifferentiated (anaplastic) carcinoma | ||
| Squamous cell carcinoma | ||
| Carcinoma with mixed features | ||
| Metastatic |
FNA, fine-needle aspiration.
Non diagnostic ND/unsatisfactory UNS; Smears were considered as non-diagnostic when a thyroid FNA sample failed to fulfill the recommended criteria for adequacy which are presence of a minimum of six groups of well-visualized (i.e., well-stained, undistorted, and unobstructed) follicular cells, with at least ten cells per group, preferably on a single slide, absence of colloid or only blood. (28) Aspirates diagnosed as cystic fluid were recorded as such but considered as non-diagnostic.
Benign BN; Lesions were classified into this category if they were diagnosed or reported as colloid nodule, multinodular goiter, lymphocytic or granulomatous thyroiditis as well as if the aspirate showed benign follicular cells only.
Atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS); Lesions were classified into this category if they were diagnosed or reported as adequate with ‘atypical cells/atypical follicular cells’ accompanied by a comment stating that neoplasm could not be excluded and that a repeat FNA of the lesion was recommended.
Follicular neoplasm/Suspicious for follicular neoplasm SFN; Lesions were classified into this category if they were diagnosed or reported as having high follicular cellularity with predominant microfollicle formations, scant colloid. Lesions exhibiting Hurthle cells predominantly and diagnosed as Suspicious for Hurthle cell neoplasm were also included.
Suspicious for malignancy SM; Lesions were classified into this category if they were diagnosed or reported as being suspicious for papillary, medullary or metastatic carcinoma or lymphoma. Smears in this category were mainly cellular with crowded cell groups exhibiting nuclear and cytoplasmic pleomorphism with some occasional single atypical cells. In the context of suspicious papillary carcinoma rare presence of nuclear enlargement, grooves, overlapping and/or pseudoinclusions along with thick colloid were considered suspicious.
Malignant MGT; Lesions were classified into this category if they were diagnosed as frankly malignant with type specification.
We searched for follow-up cytology and histology in the anatomic pathology database. Histological correlation was available in 84 cases.
Calculation of malignancy rate was done as follow:
ND/UNS: No. of malignant cases on surgical resection/no. of cytohistological correlations in this category x100
BN: No. of malignant cases on surgical resection/total BN FNA x100
AUS: No. of malignant cases on surgical resection/no. of cytohistological correlation in this category x100
SFN: No. of malignant cases on surgical resection/no. of cytohistological correlation in this category x100
SM: No. of malignant cases on surgical resection/no. of cytohistological correlation in this category x100
MGT: No. of malignant cases on surgical resection/no. of cytohistological correlation in this category x100
In calculating the malignancy follow-up rate for the benign category, the total number of benign FNA diagnoses was used as the denominator, as similarly performed in other studies. (15, 20, 29) For all other diagnostic categories, malignancy follow-up rates were calculated by using the number of cases with follow-up histology results. If a patient had multiple FNA samples in the same procedure yielding two different diagnoses, only the diagnosis with higher malignant potential was used for calculating malignancy follow-up rates (for example if a single FNA had a diagnosis of "hyperplastic nodule" and "suspicious for medullary carcinoma" on two separate passes, the case was included in the calculation for the SM follow-up rate and not for the benign group).
Results
Among 1050 FNA procedures performed at KAUH between 2005 and 2010 there were 562 thyroid FNA diagnoses of which 250 belonged to Saudi patients. The overall distribution of diagnoses of thyroid FNAs in Saudi patients using TBSRTC are presented in Table 2.
Table (2).
Frequency of Cytological Diagnoses on FNA thyroid of Saudi patients at King Abdulaziz University Hospital, Jeddah, Saudi Arabia using TBSRTC. (N = 250)
| Diagnostic Category | No. (%) of Cases |
|---|---|
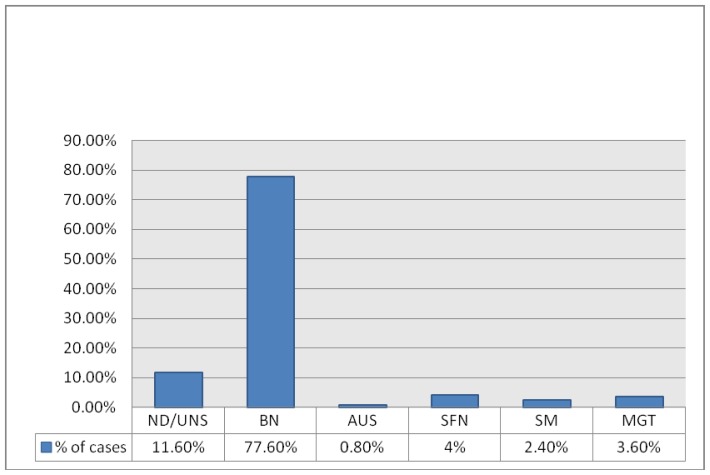
| ND/UNS | 29 (11.6%) |
| BN | 194 (77.6%) |
| AUS | 2 (0.8%) |
| SFN | 10 (4%) |
| SM | 6 (2.4%) |
| MGT | 9 (3.6%) |
FNA: fine needle aspiration, ND/UNS: non diagnostic/unsatisfactory, BN: benign, AUS: atypia of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant.
Frequency of Cytological Diagnoses on FNA thyroid of Saudi patients at King Abdulaziz University Hospital, Jeddah, Saudi Arabia using TBSRTC. (N = 250)
*FNA: fine needle aspiration, ND/UNS: non diagnostic/unsatisfactory, BN: benign, AUS: atypia of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant.
Among the 250 thyroid FNAs in Saudis, 84 were followed by surgical resection (partial or total thyroidectomy). There were 20 cases of malignancy, 63 benign and 1 remained as AUS on resection, giving an overall surgical yield of malignancy of 23.8%. Table 3 presents the total number and the overall distribution of benign and malignant cases on surgical resection. Table 4 presents the rate of malignancy on surgical resection for FNA thyroid diagnostic categories using TBSRTC.
Table (3).
Summary of Diagnoses and Distribution of Malignancies on Surgical Resections of thyroid at King Abdulaziz University Hospital, Jeddah, Saudi Arabia using TBSRTC (N =84)
| Diagnosis on Resection | Total No. (%) of Cases |
|---|---|
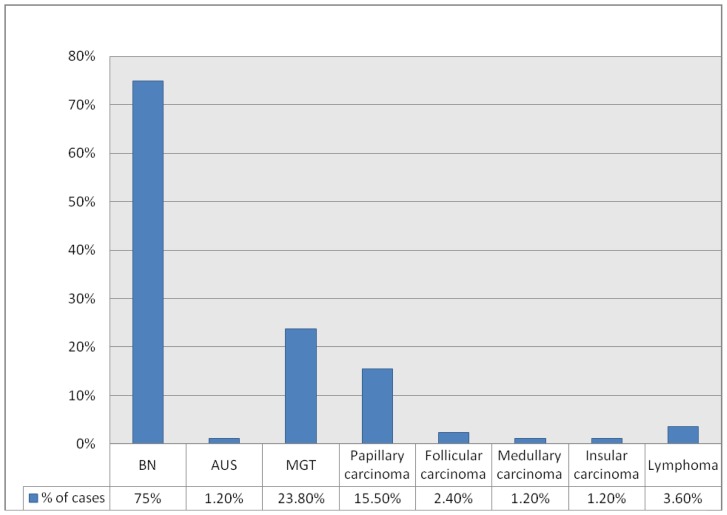
| BN | 63 (75%) |
| AUS | 1 (1.2%) |
| MGT | 20 (23.8%) |
| Papillary carcinoma | 13(15.5%) |
| Follicular carcinoma | 2(2.4%) |
| Medullary carcinoma | 1(1.2%) |
| Insular carcinoma | 1(1.2%) |
| Lymphoma | 3 (3.6%) |
BN: benign, AUS: atypia of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant
Table (4).
Rate of Malignancy on Surgical Resection for FNA Diagnostic Categories using TBSRTC at King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
| Diagnostic Category | Malignancy Rate No. of Cases(% ) |
Reported Rate (%)[atlas] |
|---|---|---|
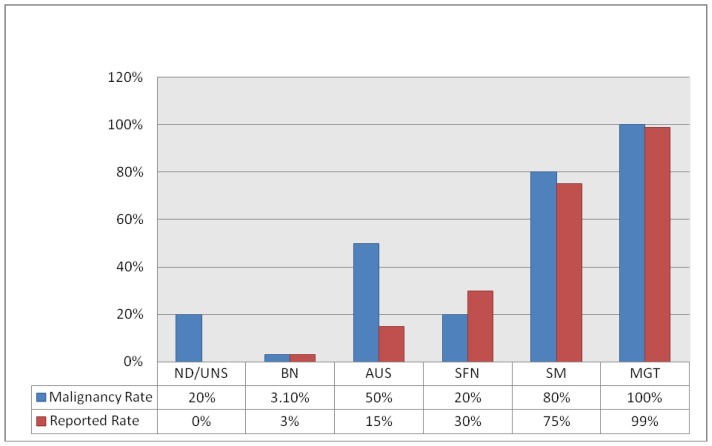
| ND/UNS (n = 5) | 1 (20%) | — |
| BN (n = 194) | 6 (3.1%) | 0–3 |
| AUS (n = 2) | 1 (50%) | 5–15 |
| SFN (n =5) | 1 (20%) | 15–30 |
| SM (n = 5) | 4 (80%) | 60–75 |
| MGT (n = 7) | 7 (100%) | 97–99 |
Diagnoses and Distribution of Malignancies on Surgical Resections of thyroid at King Abdulaziz University Hospital, Jeddah, Saudi Arabia using TBSRTC (N = 84)
*BN: benign, AUS: atypia of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant
Rate of Malignancy on Surgical Resection for FNA Diagnostic Categories using TBSRTC at King Abdulaziz University Hospital, Jeddah, Saudi Arabia
* FNA: fine needle aspiration, ND/UNS: non diagnostic/unsatisfactory, BN: benign, AUS: atypia of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant
Among the 250 thyroid FNAs in Saudis, 29 were ND/UNS. Of these there were 6 repeat FNAs for cases which persisted to be clinically suspicious yielding results as follows: 2 remained ND/UNS (33.3%, with no further clinical suspicion), 2 benign (33.3%), 1 AUS (16.6%) and 1 malignant (16.6%). Subsequent histological correlations among those with single and repeat FNAs together were available for 5 cases and were as follows: 1 Hashimoto's thyroiditis, 1 multinodular goiter, 1 nodular hyperplasia, 1AUS and 1 papillary thyroid carcinoma. The case which remained AUS on follow up histology was mainly composed of groups of atypical follicular cells with considerable degree of nuclear atypia but
Among the 250 thyroid FNAs in Saudis, 194 were categorized as benign, 131 cases of which were diagnosed as benign follicular cells failing to fulfill criteria for any specific category, 63 were diagnosed as benign follicular lesions (15 colloid nodules, 9 nodular hyperplasia, 22 multinodular goiter, 12 Hashimotos thyroditis and 5 lymphocytic thyroiditis). Follow-up histology was available for 61 of the benign cases, yielding the following diagnoses: 43 benign follicular nodules (9 colloid nodules, 6 nodular hyperplasia, 28 multinodular goiter), 8 Hashimoto's thyroiditis; 3 follicular adenoma; 1 Hürthle cell adenoma; 1 minimally invasive follicular carcinoma, 3 papillary carcinoma, including follicular variants; 2 lymphomas (1 Diffuse large B cell lymphoma, 1 MALTOMA). Overall, there were 6 cases of malignancy on resection of 194 benign FNAs, leading to a malignancy risk of 3.1% on follow-up histology.
Among the 250 thyroid FNAs in Saudis, 2 were diagnosed as AUS on FNA. The first case, as mentioned above was diagnosed on surgical follow up of the repeat FNA of a case initially diagnosed as insufficient. The second case was diagnosed as AUS on FNA and on subsequent histology it turned out to be medullary carcinoma leading to an overall malignancy risk of 50%.
Among the 250 thyroid FNAs in Saudis, 10 were classified as SFN. Of these cases, 5 had follow-up histology. The following diagnoses failing to fulfill other morphological criteria for malignancy. Overall, 1 malignant diagnoses was made on resection, yielding a malignancy risk of 20% on follow-up histology. were made: 1 multinodular goiter; 1 Hashimoto's thyroiditis, 1 follicular adenoma, 1 hurthle cell adenoma and 1 follicular carcinoma. There was 1 malignant case leading to an overall malignancy risk of 20% on follow-up histology.
Among the 250 thyroid FNAs in Saudis, 6 were SM of which 5 had follow-up histology. Of these 5 cases, 4 (80%) were malignant, with the following diagnoses: 4 papillary carcinoma and 1 benign with a diagnosis of multinodular goiter. There were 4 malignancies leading to an overall malignancy risk of 80% on follow-up histology.
Nine (9) thyroid FNA samples were classified as malignant. Cytological categories for these were as follows: 7 papillary carcinoma, 1 malignancy unspecified and 1 medullary carcinoma. Of these cases 7 had follow-up histology and all 7 (100%) were confirmed as malignant. The diagnoses of malignancy on resection were as follows: 5 papillary carcinoma, 1 diffuse large B-cell lymphoma and 1 insular carcinoma. There were 7 malignancies leading to an overall malignancy risk of 100% on follow-up histology
In Tables 5 and 6 we present the analytical comparison of the percentage distribution of FNA thyroid diagnostic categories and follow up malignancy rate of our study using TBSRTC with 3 other published studies.
Table (5).
Comparison of Percentages of Distribution of FNA Diagnoses Using TBSRTC Among Published Studies
| Diagnostic Category | Present Study % | Her-Juing Wu H et a l[20] 2011 % no space |
Jo VY et al [15] 2010 % |
Lee K et al [21] 2010 % |
|---|---|---|---|---|
| ND/UNS | 11.6 | 20.1 | 18.6 | 10 |
| BN | 77.6 | 39 | 59.0 | 67.7 |
| AUS/AFLUS | 0.4 | 27.2 | 3.4 | 3.1 |
| SFN | 4 | 8.4 | 9.7 | 1.1 |
| SM | 2.4 | 2.6 | 2.3 | 5.1 |
| MGT | 3.6 | 2.7 | 7.0 | 13 |
FNA: Fine Needle Aspiration, ND/UNS: non diagnostic/unsatisfactory, BN: benign, AUS: atypia of undetermined significance, AFLUS: Atypical follicular lesion of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant
Table (6).
Comparison of Percentages of Follow-up Malignancy Rates among Published Studies
| Diagnostic Category | Present Study % (n=84) | Her-Juing Wu H et al [20] 2011 % (n=221) |
Jo VY et al [15] 2010 % (n=892) |
Lee K et al[21] 2010 % (n=905) |
|---|---|---|---|---|
| ND/UNS | 20 | - | 8.9 | 4.2 |
| BN | 3.1 | 3 | 1.1 | 0 |
| AUS/AFLUS | 50 | 6 | 17 | 41.2 |
| SFN | 20 | 22 | 25.4 | 46.4 |
| SM | 80 | 56 | 70 | 48.4 |
| MGT | 100 | 100 | 98.1 | 78.4 |
ND/UNS: non diagnostic//unsatisfactory, BN: benign, AUS: atypia of undetermined significance AFLUS: Atypical follicular lesion of undetermined significance, SFN: suspicious for follicular neoplasm, SM: suspicious for malignancy, MGT: malignant
Discussion
We found that classification of thyroid lesions according to TBSRTC standardized nomenclature yields similar results for risk of malignancy as reported by others using the same system. Lee K et al (21) performed a similar retrospective study using TBSRTC on 4966 thyroid aspirates and reported malignancy rates as follows: ND, 4.2%; BN, 0%; AUS/AFLUS, 41.2%; SFN, 46.4%; SM, 48.4%; and MGT, 78.4%. Jo VY et al [15] reported the following malignancy rates using TBSRTC on 3080 thyroid aspirates: ND, 8.9; BN, 1.1%; AUS/AFLUS, 17%; SFN, 25.4%; SM, 70%; and MGT, 98.1%. Her-Juing Wu H et al [20] reported their institutional experience using TBSRTC on 1382 thyroid aspirates with a malignancy rates using the same calculations as follows: BN, 3%; AUS/AFLUS, 6%; SFN, 22%; SM, 56%; and MGT, 100%.
The rate of malignancy in the present study was comparable in most of the categories to that mentioned in TBSRTC (14) and other published studies, (15,20,21) except the ND/UNS and AUS. The ND/UNS category in our study yielded a malignancy rate of 20% with 1 case turning out to be malignant among 5 follow up resections. A thyroid FNA sample is considered adequate for evaluation if it contains a minimum of six groups of well-visualized (i.e., well-stained, undistorted, and unobstructed) follicular cells, with at least ten cells per group, preferably on a single slide. (28) The use of these well-defined criteria for adequacy is helpful because they improve the diagnostic efficiency of thyroid FNA and avoid unnecessary surgery for benign non neoplastic thyroid lesions. (30) The most common pitfalls for false-negative diagnoses in FNA thyroid aspirates reported by Bakhos et al consisted of suboptimal material and under diagnosis of papillary carcinoma due to cystic degeneration with a false-negative rate of 4%. (31, 32) Raab et al (33) have also emphasized that suboptimal specimens can be a significant source of false negatives in thyroid cytology. Cystic lesions with a non-diagnostic aspirate should undergo repeat FNA. (34) In our study the case which turned out to be malignant in ND/UNS category was papillary carcinoma with solid and insular pattern. Lesional characteristics other than cystic features which cause sampling error are thyroid glands with multiple nodules, calcification and fibrosis. (35) Sample adequacy of FNA thyroid may be affected by the accuracy of lesion and needle localization ‘geographic miss of the needle’, the method of guidance, the number of aspirated samples, the needle gauge, the aspiration technique, and the presence or absence of on-site facilities for immediate cytologic examination. (36) ND/UNS cytologic findings may also result from poor fixation, preparation, or staining or from excessive blood, necrotic material, or debris obscuring cellular details, misinterpretations. (29, 37) It is, however, important to keep in mind that ND/UNS specimen does not mean negative specimen.
In the AUS category the malignancy rate in the present study is 50% as opposed to 5–15% mentioned in TBSRTC (28) and upto 41.2% (21) as in other studies (Table 6). The less number of cases diagnosed as AUS in the present study could be explained by the strict adherence to diagnostic criteria and the cytopathologist’s efforts in our practice setting to avoid ambiguity and keep the use of AUS to a minimum. The frequency of AUS interpretations should be in the range of approximately 7% of all thyroid FNA interpretations. This figure may be further refined as more laboratories report their experiences using the AUS designation within the context of these criteria. AUS is a category of last resort and should not be used indiscriminately. (38) The case diagnosed as AUS on cytology was sparsely cellular and showed monomorphic population of small non cohesive cells (resembling follicular) with high nuclear cytoplasmic ratio (no colloid) which probably explains why it turned out to be medullary carcinoma on histology. AUS is a heterogeneous category, which reflects the difficulty in the cytological diagnosis of the follicular lesions of thyroid. (10) It includes cases in which the cytomorphological findings are not representative of a benign lesion yet the degree of cellular or architectural atypia is not sufficient to render an interpretation of follicular neoplasm/suspicious for a follicular neoplasm or suspicious for malignancy. (10) This diagnosis may also be used in thyroid FNA specimens that are less than optimal due to limited cellularity, poor fixation and obscuring blood. This group can benefit from repeat FNA and correlation with clinical and radiological findings. (10) However, because the category is heterogeneous and somewhat subjective, there exist and will likely remain differences between observers in using this diagnosis. (39) The use of this category by different pathologists will vary, and AUS can be expected to have, at best, only fair reproducibility. (38)
In the hands of an experienced cytopathologist, accurate localization of the lesion either by palpation or by ultrasound, performing between 1–5 aspirates for thyroid nodules of 1–2 cms size with 20–27 gauge needles, using standard sampling technique, supported by immediate on-site cytological analysis can ensure specimen adequacy. (36,37,40) Nodules with an initial ND/UNS result should be re-aspirated, but no sooner than 3 months later; the 3-month interval is recommended to prevent false positive interpretations due to reactive/reparative changes. (11) Circumstances that require repeat FNA include sample inadequacy, nodule enlargement, cyst recurrence, or clinical or imaging findings that arouse suspicion about the presence of a malignancy even when cytological findings in the biopsy specimen indicate benignity. (36, 41) Ultrasound guidance with immediate, on-site adequacy evaluation is preferred for repeat aspiration after an initial ND/UNS specimen, especially for solid nodules. Repeating the FNA results in a diagnostic interpretation in up to 60% of cases, (42) in several studies US guided FNAs have shown to reduce the rates of non-diagnostic (i.e., insufficient cells and/or colloid) and false-negative aspirates. (43) Given that the diagnostic category of AUS is associated with low specificity and positive predictive value, the appropriate follow-up is controversial. Some authorities recommend repeat FNA, repeat ultrasound scans, or radio-nucleotide uptake studies. (36) Reports have even suggested the use of liquid-based cytology and immunocytochemistry to improve diagnostic accuracy. Radiological correlation may be helpful in improving the positive predictive value of the “AUS” category. Besides increasing size, ultrasonographic features such as hyperechogenicity, irregular nodule borders, calcifications and abnormalities of vasculature all favour a malignancy. (39)
FNA of the thyroid can be used as either a screening test for follicular carcinoma, or as a diagnostic test for other thyroid carcinomas including papillary carcinoma, medullary carcinoma, undifferentiated carcinoma, and lymphoma. (40) Diagnostic limitations inherent to thyroid FNAs underscores the importance of clinical correlation in the management of patients with thyroid nodules Jo VY et al. (15) However, the main purpose of the FNA is to determine the correct surgical procedure when surgery is needed. (44) This allows appropriate triage to FNA versus surgery based on the significant difference in the risk of subsequent malignancy in all the categories. Implementation of TBSRTC appears to improve the quality of reporting by lowering the number of ambiguous and implicit diagnoses and decreases the overall surgery rates, particularly for benign lesions. (44)
Our results were reproduced from retrospective classification, which is a limitation to our study. We did not review all the FNA cases; apart from cases in the gray zone. We used the "most matching criteria” while classifying the lesions. This process relied on the original interpretation (given by 3 cytopathologists at the KAAU Hospital, Jeddah, Saudi Arabia in the period studied) and our review of the original diagnosis report. Most cytopathologists are aware, there can be wide range of interobserver variability in diagnostic thresholds and varied terminologies used by pathologists within the same department (45) which added to the limitations of the study.
In conclusion; at the KAAUH of Jeddah, Saudi Arabia, retrospective classification of FNAs of thyroid lesions among Saudi patients using TBSRTC yields similar results for rate of malignancy as reported by others. However the malignancy rates found for ND/UNS (20%) raise the possibility of malignancy risk in this category and validate the past observations that sample inadequacy is a common cause of false negative thyroid FNAs with a reported false negative rate of up to 25% in literature. (33) It is pertinent to mention here that no risk is reported for this category in the TBSRTC. Our malignancy rate for AUS (50%), underscores the importance of using this terminology carefully and sparingly and that if applied strictly its subsequent malignancy rate may not be always low as reported in TBSRTC. (38) Prospective studies using the TBSRTC will lend further insight into our results and the usefulness of TBSRTC.
Acknowledgements
We would like to thank Razan Asindi and Zahwa Rizwan for their assistance in technical aspects of our research.
Footnotes
Declaration: The authors declare that this study or manuscript has no conflict of interest.
References
- 1.Sakorafas GH. Thyroid nodules; interpretation and importance of fine-needle aspiration (FNA) for the clinician - practical considerations. Surg Oncol. 2010;19(4):e130–9. doi: 10.1016/j.suronc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Yoder BJ, Redman R, Massoll NA. Validation of a five-tier cytodiagnostic system for thyroid fine needle aspiration biopsies using cytohistologic correlation. Thyroid. 2006;16(8):781–6. doi: 10.1089/thy.2006.16.781. [DOI] [PubMed] [Google Scholar]
- 3.Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132(5):658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah L, Thomas J, Fadaq R. Fine needle aspiration in the management of thyroid nodules: experience at King Khalid National Guard Hospital, Jeddah. Ann Saudi Med. 2003;23(6):408–9. doi: 10.5144/0256-4947.2003.408. [DOI] [PubMed] [Google Scholar]
- 5.Al-Eid Haya S. National Cancer Registry. Ministry of Health; Kingdom Of Saudi Arabia: 2006. Cancer incidence report in Saudi Arabia. Last updated 31 august 2010. [Google Scholar]
- 6.Hegedüs L. The Thyroid Nodule. N Eng J Med. 2004;351(17):1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 7.Wong CK, Wheeler MH. Thyroid nodules: rational management. World J Surg. 2000;24(8):934–941. doi: 10.1007/s002680010175. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind A, Sikora AG, Komisar A, Slavit D, Fried K. Rates of malignancy in incidentally discovered thyroid nodules evaluated with sonography and fine-needle aspiration. J Ultrasound Med. 2005;24(5):629–34. doi: 10.7863/jum.2005.24.5.629. [DOI] [PubMed] [Google Scholar]
- 10.Layfield LJ, Cibas ES, Baloch Z. Thyroid fine needle aspiration cytology: a review of the National Cancer Institute state of the science symposium. Cytopathology. 2010;21(2):75–85. doi: 10.1111/j.1365-2303.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 11.Cibas ES, Alexander EK, Benson CB, de Agustín PP, Doherty GM, Faquin WC, Middleton WD, Miller T, Raab SS, White ML, Mandel SJ. Indications for thyroid FNA and pre-FNA requirements: a synopsis of the National Cancer Institute. Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36(6):390–9. doi: 10.1002/dc.20827. [DOI] [PubMed] [Google Scholar]
- 12.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117(3):195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- 13.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19(11):1159–65. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 14.Baloch ZW, Alexander EK, Gharib H, Raab SS. Chapter 1; Overview of Diagnostic Terminology and Reporting. In: Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology. New York, NY: Springer; 2010. pp. 1–4. [Google Scholar]
- 15.Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010;134(3):450–6. doi: 10.1309/AJCP5N4MTHPAFXFB. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shraim MM, Hussein MR, Musalam AO, Al-Ghandi T, Al-Zahramit H, Mahrouz AA, Abu-Eshy SA. Hurthle cell neoplasms of thyroid in South-Western region of Saudi Arabia. West Afr J Med. 2010;29(6):398–402. doi: 10.4314/wajm.v29i6.68275. [DOI] [PubMed] [Google Scholar]
- 17.Fatma Althoubaity Preoperative fine needle aspiration cytology versus frozen section in thyroid surgery. Egyptian Journal of Surgery. 2006;25(1):20–28. [Google Scholar]
- 18.El Hag IA, Kollur SM, Chiedozi LC. The role of FNA in the initial management of thyroid lesions: 7-year experience in a district general hospital. Cytopathology. 2003;14(3):126–30. doi: 10.1046/j.1365-2303.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rikabi AC, Al-Omran M, Cheema M, El-Khwsky F, Al-Nuaim A. Pattern of thyroid lesions and role of fine needle aspiration cytology (FNA) in the management of thyroid enlargement: a retrospective study from a teaching hospital in Riyadh. APMIS. 1998;106(11):1069–74. doi: 10.1111/j.1699-0463.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 20.Her-Juing Wu H, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2011 Jun 16; doi: 10.1002/dc.21754.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Jung CK, Lee KY, Bae JS, Lim DJ, Jung SL. Application of Bethesda System for Reporting Thyroid Aspiration Cytology. The Korean Journal of Pathology. 2010;44(5):521–7. [Google Scholar]
- 22.Ozluk Y, Pehlivan E, Gulluoglu MG, Poyanli A, Salmaslioglu A, Colak N, Kapran Y, Yilmazbayhan D. The Use of the Bethesda Terminology in Thyroid Fine-Needle Aspiration Results in a Lower Rate of Surgery for Nonmalignant Nodules: A Report from a Reference Center in Turkey. Int J Surg Pathol. 2011 Jul 26; doi: 10.1177/1066896911415667. [uid] [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Schinstine M. A brief description of the Bethesda System for reporting thyroid fine needle aspirates. Hawaii Med J. 2010;69(7):176–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19(11):1215–23. doi: 10.1089/thy.2009.0155. [DOI] [PubMed] [Google Scholar]
- 25.Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: Past, present, and future. Cancer Cytopathol. 2011 doi: 10.1002/cncy.20178.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Cross PA, Poller D. The Bethesda thyroid terminology and progress towards international agreement on thyroid FNA cytology reporting. Cytopathology. 2010;21(2):71–4. doi: 10.1111/j.1365-2303.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 27.Dusková J. The new system for reporting fine needle aspiration biopsies of the thyroid gland: Bethesda 2010] Cesk Patol. 2011;47(1):8–14. [PubMed] [Google Scholar]
- 28.Crothers BA, Henry MR, Firat P, Hamper UM. Chapter 2; Non Diagnostic/Unsatisfactory. In: Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology. New York, NY: Springer; 2010. pp. 5–7. [Google Scholar]
- 29.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111(5):306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 30.Haider AS, Rakha EA, Dunkley C, Zaitoun AM. The impact of using defined criteria for adequacy of fine needle aspiration cytology of the thyroid in routine practice. Diagn Cytopathol. 2011;39(2):81–6. doi: 10.1002/dc.21324.. [DOI] [PubMed] [Google Scholar]
- 31.Bakhos R, Selvaggi SM, DeJong S, Gordon DL, Pitale SU, Herrmann M, Wojcik EM. Fine-needle aspiration of the thyroid: rate and causes of cytohistopathologic discordance. Diagn Cytopathol. 2000;23(4):233–7. doi: 10.1002/1097-0339(200010)23:4<233::aid-dc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Sudilovsky D. Interpretation of the paucicellular thyroid fine needle aspiration biopsy specimen. Pathol Case Rev. 2005;10(2):68–73. [Google Scholar]
- 33.Raab S, Vrbin CM, Grzybicki DM, Sudilovsky D, Balassanian R, Zarbo RJ, Meier FA. Errors in thyroid gland fine-needle aspiration. Am J Clin Pathol. 2006;125(6):873–882. doi: 10.1309/7RQE-37K6-439T-4PB4. [DOI] [PubMed] [Google Scholar]
- 34.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941–6. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 35.Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001;30(2):361–400. doi: 10.1016/s0889-8529(05)70191-2. [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Kim EK, Park SI, Kim BM, Kwak JY, Kim SJ, Youk JH, Park SH. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics. 2008;28(7):1869–87. doi: 10.1148/rg.287085033. [DOI] [PubMed] [Google Scholar]
- 37.Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol. 1996;15(1):84–9. doi: 10.1002/(SICI)1097-0339(199607)15:1<84::AID-DC18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Krane JF, Nayar R, Renshaw AA. Chapter 4; Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance. In: Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology. New York, NY: Springer; 2010. pp. 37–45. [Google Scholar]
- 39.Layfield LJ, Morton MJ, Cramer HM, Hirschowitz S. Implications of the proposed thyroid fine-needle aspiration category of "follicular lesion of undetermined significance": a five-year multi-institutional analysis. Diagn Cytopathol. 2009;37(10):710–714. doi: 10.1002/dc.21093. [DOI] [PubMed] [Google Scholar]
- 40.Faquin WC. Diagnosis and Reporting of Follicular-Patterned Thyroid Lesions by Fine Needle Aspiration. Head and Neck Pathol. 2009;3:82–85. doi: 10.1007/s12105-009-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, Steward DL, Zeiger MA, Westra WH, Wang Y, Khanafshar E, Fellegara G, Rosai J, Livolsi V, Lanman RB. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21(3):243–51. doi: 10.1089/thy.2010.0243. Epub 2010 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orija IB, Pineyro M, Biscotti C, Reddy SS, Hamrahian AH. Value of repeating a nondiagnostic thyroid fine-needle aspiration biopsy. Endocr Pract. 2007;13(7):735–742. doi: 10.4158/EP.13.7.735. [DOI] [PubMed] [Google Scholar]
- 43.Lee YH, Kim BH, Suh SI, Seo HS, Seo BK, Cho KR, Lee JH, Kim NH, Seo JA. Comparison of cytological results obtained by repeated US-guided fine-needle aspiration biopsies of thyroid nodules: focus on the rate of malignancy and diagnostic concordance. Diagn Cytopathol. 2009;37(7):492–7. doi: 10.1002/dc.21043. [DOI] [PubMed] [Google Scholar]
- 44.Baloch Z, Layfield LJ. Quest for a uniform cytodiagnostic approach to thyroid aspirates: a consensus proposal. Diagn Cytopathol. 2006;34(2):85–6. doi: 10.1002/dc.20454. [DOI] [PubMed] [Google Scholar]
- 45.Gerhard R, da Cunha Santos G. Inter- and intraobserver reproducibility of thyroid fine needle aspiration cytology: an analysis of discrepant cases. Cytopathology. 2007;18(2):105–11. doi: 10.1111/j.1365-2303.2006.00430.x. [DOI] [PubMed] [Google Scholar]