Abstract
The field of tissue engineering has been growing in the recent years as more products have made it to the market and as new uses for the engineered tissues have emerged, motivating many researchers to engage in this multidisciplinary field of research. Engineered tissues are now not only considered as end products for regenerative medicine, but also have emerged as enabling technologies for other fields of research ranging from drug discovery to biorobotics. This widespread use necessitates a variety of methodologies for production of tissue engineered constructs. In this review, these methods together with their non-clinical applications will be described. First, we will focus on novel materials used in tissue engineering scaffolds; such as recombinant proteins and synthetic, self assembling polypeptides. The recent advances in the modular tissue engineering area will be discussed. Then scaffold-free production methods, based on either cell sheets or cell aggregates will be described. Cell sources used in tissue engineering and new methods that provide improved control over cell behavior such as pathway engineering and biomimetic microenvironments for directing cell differentiation will be discussed. Finally, we will summarize the emerging uses of engineered constructs such as model tissues for drug discovery, cancer research and biorobotics applications.
Keywords: Biorobotics, Cell aggregates, modular tissue engineering, organ models, organ-on-a-chip, pre-vascularization synthetic polypeptides
I.Introduction
Over the last 30 years tissue engineering has resulted in important breakthroughs in the understanding of cell-material interactions and the host response to biodegradable materials and their in vivo integration. There is now a deeper appreciation of the effect of physical properties on cellular behavior such as material stiffness, surface roughness and porosity [1, 2]. From its early stages as single cell type/porous biomaterial constructs to more multi-functional, multi-cellular biomimetic systems, tissue engineering has also provided important insights on how the effects of biomaterials on cellular activities can be harnessed for clinical aims [3].
The initial aim of tissue engineering was to develop tissue or organ substitutes, which are, limited resources in an aging society with prevalent chronic diseases. Driven by the lack of donor tissues and the inability of some tissues such as heart and parts of nervous system to heal themselves, tissue engineering methods for replacement tissues and organs have become a venue to overcome such problems. Despite limited success in some complex organs, the promise of substitute tissues has been fulfilled for some targets. The clinical successes in skin [4], cartilage [5] and more recently in bladder [6] and trachea [7] have already shown that tissue engineering can fill a gap in the biomedical field. In addition, developments due to trials in other target organs, such as cardiac tissue, have resulted in systems that might not be suitable as implantable systems but can satisfy the ever growing needs of biomedical field for complex organ and tissue models. Moreover, novel approaches constantly arise to improve the current tissue engineering efforts by bringing in the developments in other areas of biotechnology and nanotechnology such as pathway engineering to control cell differentiation, nanoscale bioactive agent patterning or noninvasive imaging techniques. Modular approaches, rapid prototyping methods and advances in stem cell research have also contributed to the increasing versatility of tissue engineered constructs. The interactions of different cell types with their surrounding extracellular matrix (ECM) have been recognized as an important determinant of cell behavior. Individual components of ECM have been widely used as scaffold materials in tissue engineering with considerable success. However, the specific composition of ECM in each organ has proven to be essential for better outcomes. Together with the discovery of the importance of cellular microenvironment on stem cell differentiation, obtaining biomimetic environments has become an important goal. Development of artificial ECM structures either based on ECM components or synthetic materials is another area where tissue engineering provides methods for development of cellular microenvironments. It also benefits from advances in protein engineering and synthesis. This review aims to cover new developments in these areas and the outlook of tissue engineering, as an expanding interdisciplinary field.
II. Enhancing Tissue engineering scaffolds
Biodegradable synthetic polymers have been commonly used in tissue engineering applications; however, the most commonly used polymers such as poly-L-lactic acid (PLLA), poly L-lactic-co-glycolic acid (PLGA), poly-caprolactone (PCL), generally lack the necessary signals for cells to reorganize them to generate functioning tissues [8]. Slow remodeling of the scaffolds and prolonged immune response are general problems with such scaffolds [9-11]. Moreover, constructs made of a single synthetic polymer often have isotropic structures which are in contrast to the hierarchical multicomponent organization of tissues. Recently, novel synthetic materials such as engineered synthetic polypeptides have been developed to advance structural complexity of the scaffolds. More conventional, yet active, areas of natural scaffold production are the development of structures based on natural polymers by using various combinations of ECM molecules or the use of decellularized tissues.
A. Enhancing Base Materials
Natural polymer based structures
ECM components such as collagen, fibronectin, laminin have been commonly used as components of natural scaffolds. Through these molecules, cellular activities such as proliferation, differentiation, migration and secretion can be modulated. Moreover, the multi-component nature of ECM environment has prominent effects on cell behavior [12]. For example, the composition of ECM affects how cells interact with it, such as the extent of integrin mediated cell adhesion [13]. These interactions can be imitated by the development of artificial ECM from a mixture of natural ECM components. In order to achieve this, commonly used natural polymers such as hyaluronic acid, collagen and gelatin have been further modified to have additional functionalities such as photocrosslinkable sites [14, 15]. These modifications enable controllable crosslinking between different ECM components which can be used to regulate cellular behavior better than single component scaffolds [16]. Aside from proteins and polysaccharides of mammalian ECM, other natural polymers from different sources such as alginate, chitosan and silk fibroin have also been used for obtaining scaffolds. These base materials have been used in the development of novel natural polymers for tissue engineering such as recombinant chimeric proteins [17]. For example, silk fibroin has been functionalized for antimicrobial activity, conjugated or have been genetically modified to have additional mechanical properties (such as silk-elastin like protein polymers) [18-20]. The versatility offered by recombinant proteins enables more biomimetic scaffolds [21].
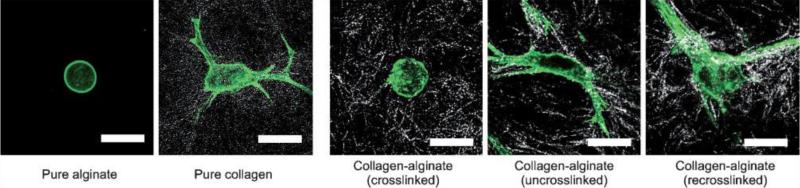
One of the advantages of having artificial ECM system is to obtain highly controllable and preferably reversible 3D systems that would provide an environment very similar to that of natural ECM. For example, recently a mixture of collagen and alginate was developed where the reversible gelation and dissolution of alginate can be controlled by the addition of ions to regulate the dynamics of cellular spreading and migration [22] (Figure 1). Designed artificial ECMs are advantageous as they allow for precise control over composition of the ECM the cells will encounter. For mechanical reinforcement of such ECM-like structures, which are mostly hydrogels, additional materials can be added such as carbon nanotubes [23]. Another possible approach is to control the alignment of fibrillar ECM molecules such as collagen by using magnetic fields or electrochemical processes. These structures better mimic the tissues with highly oriented ECM structures [24, 25].
Figure 1.
Control of cell spreading by reversibly crosslinkable ECM components, by selectively crosslinking, uncrosslinking and re-crosslinking the alginate component within the collagen hydrogel, cell shape can be controlled in 3D. Cells were labeled for actin fibers with AlexiFluor488-Phalloidin (Adapted from [22]).
Synthetic polymer based structures
Synthetic biodegradable polymers can be developed with a high level of control as many of their properties, such as molecular weight, viscosity and degradability can be easily controlled by changing synthesis parameters. This versatility has resulted in widespread use of several classes of biodegradable synthetic polymers such as aliphatic polyesters, polycarbonates and polyphosphoesters in biomedical fields [26]. However, as mentioned before, the interactions of synthetic polymers with cells are generally indirect and limited. To improve cell response, several approaches are available such as blending with natural polymers [27], addition of cell responsive segments to the polymer backbone [28] and chemical functionalization of the polymer chains with bioactive agents [29].
The addition of biological signals to the synthetic materials is a useful tool to control cellular behaviour [30]. By such modifications, existing biomaterials can be rendered remodellable by cells. Poly(ethylene glycol) (PEG) has been widely used for such systems [31]. For example, protease sensitive crosslinkers have been used to generate PEG hydrogels, which improved cell proliferation and spreading due to the enzymatic degradation of the hydrogels by secreted enzymes [32]. Also, the addition of RGD sequences and VEGF directly into PEG hydrogels has promoted cell attachment and formation of tubules by endothelial cells [33]. Angiogenic potential of growth factor modified PEG hydrogels has also been shown in vivo [34].
Another important consideration for engineered scaffolds is mechanical properties. To have more precise control over scaffolds’ mechanical compatibility with tissues, development of new synthetic biomaterials with better defined mechanical, surface and biodegradation properties is still important. One recent addition to such biocompatible and biodegradable materials is poly(glycerol sebacate) (PGS). This elastomer, with its highly tailorable mechanical properties, is especially suitable for soft tissue engineering applications, such as cardiac tissue and cartilage [35, 36]. Its mechanical properties have been used to direct the differentiation of bone mononuclear cells to smooth muscle cells rather than osteochondral cells by producing a suitable biomechanical microenvironment [37]. Also an improved secretion of elastin has been observed in porous PGS scaffolds compared to PLGA scaffolds [38]. Moreover, it can be manufactured in a variety of structures such as electrospun networks [39] or accordion-like scaffolds based on honeycomb architecture [40], which can precisely mimic the anisotropic mechanical properties of soft tissues.
Synthetic polymers can also be produced from nature's building blocks, such as amino acids. The fragile nature of natural polymers generally limits the possible techniques that can be used for their processing. Synthetic polypeptides with functional sequences can overcome this problem. By designing a polypeptide structure, structural or functional drawbacks of the original ECM molecule such as high temperature sensitivity, high immunogenicity or high enzymatic degradability can be avoided.
As the function and structure of crucial protein fragments are better understood, designed polypeptides have become a feasible approach to obtain complex tissue engineering scaffold base materials. The sequencing and the synthesis of polypeptides became easier and some of the polypeptides are already commercially available such as Puramatrix™(RAD16I) [41]. Synthetic polypeptide based scaffolds can be formed from designed self-assembling sequences based on naturally occurring proteins or novel sequences with multiple functionalities [41]. The discovery of Arg-Gly-Asp (RGD), as well as other sequences such as repeat sequences of elastin has shown that full proteins are not always necessary for functional outcomes. Today, many synthetic polypeptides such as antimicrobial sequences within some widespread proteins like ubiquitin [42] are commercially produced. Some of these polypeptides can self-assemble into various forms. They can also be rendered degradable by cellular activities at the same time. A functional peptide sequence can be as small as three or seven amino acids. Smaller building blocks provide precise control of architecture in the new generation of scaffolds. Even with such small segments it is possible to obtain self-assembled hydrogels [43]. Different amino acid sequences with an inherent ability to self-assemble can be used to form a wide variety of structures such as vesicles, ribbons or fibers. Amphiphilicity is a strong tool in nature, as observed in the case of phospholipids that can form vesicles, membrane or micelles in aqueous solutions. Peptides designed similarly to phospholipids with a hydrophobic head and a hydrophilic tail can also form nanoscale vesicles or tubes which can then assemble into networks. This provides nanoscale control over the final scaffold properties. For a hexamer it is possible to define many peptide sequences that can self-assemble up to millimeter scale fibers, each of them conferring different properties (Figure 2) [44].
Figure 2.
Hexamer polypeptides with a hydrophilic head and a hydrophobic tail can self- assemble into millimeter scale fibers. A) Schematic model of the hexamer polypeptide sequences (LIVAGD and AIVAGD) that assemble into fibers via α-helical pairing. B) Fiber mats and single fibers obtained from the self-assembling polypeptides, these fibers can support several cell types such as mesenchymal stem cells and epithelial cells (Adapted from [44]).
The self-assembly process either happens via alpha-helices, beta-hairpin forming structures or beta-sheets. For example, by using ionic complementary properties of peptide sequences beta-sheet structures with distinct hydrophobic and hydrophilic surfaces can be manufactured [41]. Self-assembled peptide scaffolds, especially in the form of nanofibers, have been used in in vitro and in vivo studies, such as repair of brain damage in hamsters after injection of peptide scaffolds [45]. By using peptide moieties, delivery and growth factor retention properties can be given to such scaffolds and they have been used in a wide array of applications such as nucleus pulposus regeneration, stimulation of angiogenesis or as composites with hydroxyapatite for induction of osteogenesis [46-49]. The self-assembly properties can be used in the modeling of diseased states. It is possible to obtain amyloid fiber-like structures with self-assembling synthetic proteins, which is observed in several diseases like Alzheimer or Creutzfeld-Jacobs. However, these scaffolds are still single component scaffolds which cannot imitate the multi-component nature of ECM. As each part of ECM has different ways to direct cell behavior, for whole functionality of the native ECM multi-component scaffolds are necessary. But, obtaining a structure that has all functionalities of ECM by modulating each component can be difficult. One way to achieve such complexity is by using the original tissues or whole organs as scaffolds through a decellularization process. Also by using modular assembly methods microscale control over scaffold architecture is possible.
B. Enhancing Architecture
Biomimicry has been one of the foci of tissue engineering research and it is recognized as an important factor for the functionality of an engineered tissue. Many tissues in the body have modular architectures [50]. Therefore, tissue engineers have been seeking ways to create modular structures, which can be brought together through random-assembly, self-assembly or directed-assembly. By this way, a macroscopic construct that possesses microscopic features can be built. Other than being biomimetic, a modular approach can be used to propagate the engineered tissue construct in a predetermined manner, making the modular approach a high throughput and modular fabrication method.
Random-Assembly
Modularity in the context of tissue engineering can be at different levels. As covered in the synthetic polypeptide section, the materials that are used for fabricating the scaffolds can be modular as well as the scaffold itself. The motivation for both approaches is the same: mimicking the native tissue structure, which is modular in both macromolecular (i.e. ECM) and cellular (i.e. liver lobules) level. For example, Davis et al. used genetically engineered protein-based polymers which have regular repeats of lysine and glutamine that can be used for enzymatic crosslinking and hydrogel formation [51]. The controllable nature of the repeating units (i.e. the modularity of its monomers) provided tailorable mechanical and physical properties to the polymer and eventually to the gel produced. In a more complex manner, multiple hyaluronic acid derivatives were used as “puzzle pieces” to produce tunable composite hydrogels through exploiting the modularity of the polymers comprising it [52]. The material commercialized as Extracel™, which consists of 4 basic components to fabricate various gels with different properties by changing the ratio of these building blocks. A similar approach for fabricating composite hydrogels starting from modular polymers was done using PEG-based micron-sized hydrogels [53]. In this study, PEG derivatives with different properties were crosslinked around living HepG2 liver cells to control mechanical properties, degradation and bioactive agent delivery. Same modular PEG-based system has also been used for encapsulation of HL-1 cardiomyocytes, which resulted in long-term viability, growth and expression of functional cardiac markers as well as activation peak synchronization upon electrical stimulation. Combination of PEG-based polymers and peptide sequences was also used to produce modular macromolecules to fabricate synthetic ECM-like structures [54]. These were used for introducing bioactive agents such as VEGF and RGD sequences and the gel structure was able to accommodate HUVECs in a concentration dependent manner.
Modular tissues have also been proposed as a potential way of providing vasculature to the engineered tissues. In one study, McGuigan et al. fabricated tissue parts using liver cell-encapsulated collagen gels seeded with HUVECs on their exterior surface. The constructs were cultured for 2-3 days until the endothelial cells became confluent and then allowed to self-assemble inside a tube to form a network of interconnected channels (Figure 3) [55]. A similar approach was used to fabricate constructs of umbilical cord vein smooth muscle cells (UVSMCs) encapsulated in hydrogels prepared using a lactoyl poloxamine derivative mixed with collagen. These constructs were then seeded with HUVECs to create the vasculature-like interstitial spaces. Presence of UVSMCs in the hydrogels increased the HUVEC attachment and proliferation on the surface compared to constructs containing HepG2 cells [56]. Moreover, the presence of UVSMCs significantly affected the HUVEC phenotype as determined by their nitric oxide production [57]. It was possible to perfuse fluids through the interstitial spaces between the constructs confirming the interconnectivity of the pores [58]. When implanted, these modular constructs with endothelial cell lining were able to form a stable, perfusable chimeric vascular bed [59]. The modular approach is a strong tool for the development of vascularized tissues and it was also applied to cardiac tissue engineering. In this example, cardiomyocytes were encapsulated in collagen type I-Matrigel mixture and the fabricated constructs were seeded with endothelial cells to create a vascularized tissue [60]. Later on, these tissue constructs were assembled into functional, macroporous structures with adequate contractile properties and gene expression. Presence of endothelial cells resulted in nonthrombogenic properties and enabled continuous whole blood perfusion without clogging [61]. Although interconnected, the flow path was torturous due to the random assembly. This tortuous nature of the flow path was shown to adversely affect the endothelial cell functionality , depending on the flow rate, due to promotion of disturbed flow causing activated endothelial cells [62]. Therefore, using a random assembly approach might not be the ideal way of achieving vasculature within modular engineered tissues.
Figure 3.
A) Schematics of the modular construct design and fabrication. HepG2 cell encapsulated cylindrical collagen gels were seeded with human umbilical-vein endothelial cells (HUVECs). After HUVECs completely covered the gel surface, which takes usually 2 to 3 days, the HUVEC-seeded cylinders can be assembled into a larger structure to form the modular construct that can be used to perfuse supply nutrients to the cells through formed network of interconnected channels. B) Light microscopy images of collagen–HepG2 module prior to HUVEC seeding. C) On Day 7 of the culture period, HUVECs on the surface of the modules was stained for VE-cadherin and imaged using confocal microscopy to show the confluent layer of HUVECs on the surface of the modules. D) A flow circuit was used to perfuse PBS or media the modular construct. E) After media perfusion of 7 days, collagen–HepG2–HUVEC modules were retrieved and imaged using confocal microscopy (Adapted from [58]).
Controlled-Assembly
Researchers have developed approaches that can give more control over the architecture of the final construct compared to random assembly. Controlled assembly of the tissue constructs is important for creating structures with higher biomimicry that comprise defined architectures and can be used to engineer tissues in a bottom-up manner. Towards this end, tissue constructs fabricated using photocrosslinkable polymers were assembled by the aid of hydrophobic-hydrophilic interactions [63-67]. Depending on the initial geometry of the constructs, various secondary structures were fabricated [66]. For example, cell-laden PEG-based hydrogels were produced in arrays of micron-sized predefined geometries and assembled in a directed manner to form macron-sized engineered tissues [64]. To include a microvasculature, more intricate than the mere use of spaces between randomly assembled constructs, microchannel networks were incorporated in constructs by using photolithography. Encapsulated cells were viable after the fabrication process and media perfusion through the microvasculature was possible. More control over the assembly process can be achieved by choosing the construct geometries so that it would allow them to be assembled in a directed way through a naturally occurring lock and key mechanism that fit the right constructs together [63, 66-68]. Tissue engineering constructs fabricated using controlled-assembly approaches have been shown to accommodate co-culture of different cell types with high cell viability.
As an alternative approach for controlling assembly, Yamaguchi et al exploited molecular recognition to achieve photoregulation of the assembly process [69]. In this study polyacrylamide was modified with an azobenzene moiety, which can interact differently with cyclodextrin groups (again added on polyacrylamide) upon photoisomerization. Through exploiting the different affinities of trans-azobenzene and cisazobenzene to cyclodextrin, adhesion and dissociation of the constructs could be controlled via photoirradiation. In another study, either cyclodextrin (host gels) or small hydrocarbon-groups (guest gels) were used to modify acrylamide-based gels and used for directing their assembly through their mutual molecular recognition [70]. It was also possible to create, sort and selectively assemble subpopulations by modifying the size and shape of the gels (Figure 4).
Figure 4.
Macroscale assembly of gel modules through selective molecular recognition of acrylamide gels modified with cyclodextrin or hydrocarbon groups. A) Selective assembly of α-CD-gel (blue) and n-Bu-gel (yellow) upon shaking in water in the presence of t-Bu-gel (dark green). B) Selective assembly of β-CD-gel (red) and t-Bu-gel (dark green) upon shaking in water in the presence of n-Bu-gel (yellow). C) Selective assembly of α-CD-gel/n-Bu-gel and β-CD-gel/t-Bu-gel upon shaking in water in the presence of all gel types (Adapted from [70]).
In addition to tissue constructs of cell-laden or cell-seeded materials, constructs that comprise of only cells have also been created and assembled towards modular tissue engineering. One approach for directed assembly of such structures that comprise only cells is to use complementary DNA sequences [71]. In order to modify cell surfaces, azide sugars (i.e. N-azidoacetylmannosamine) were added to cell culture media, which did not show any detectable adverse affects in cellular metabolism, yet could be incorporated to extracellular glycocalyx [72]. It was possible to assemble cells, which are covalently modified using complementary oligonucleotides, in a controllable manner through the ratio of cells with the different oligonucleotides [71]. For example, with a ratio of 1 to 50 it was possible to assemble cell aggregates within rosette geometry; cells with the less common oligonucleotide were in the middle of more abundant cells. Similar DNA oligonucleotide modification of the cell surfaces was also achieved through modification of the cell surface using phosphoramidites in a protein and glycan independent manner [73]. In another study, modification of cell surfaces was achieved through liposome fusion and selective delivery of ketone and oxyamine groups to desired subpopulation of cells [74]. Assembly was achieved through oxime ligation and was shown to be effective in controlling the formation of cell aggregates and cell layers to form 3D multilayered structures.
As an alternative approach to chemical modification of cell or cell aggregate surfaces, Brat-Leal et al used physical means for the assembly of cell aggregates by incorporating magnetic microparticles into them [75]. By this way, it was possible to immobilize, translocate and assemble the aggregates and control these processes temporally.
Decellularized tissues as scaffolds
As long as it can be rendered non-immunogenic an allogenic or xenogenic tissue contains all the necessary ECM components in the right architecture. Although decellularized tissues have been in use in the field for a long time, the recent mild decellularization processes [76] that preserve the ECM architecture have improved their effectiveness. Since, the intricate structure of the native tissue can be preserved, the differentiation and orientation of the seeded cells can be directed. When applied to whole organs, this also ensures the presence of well-defined vascular networks that can be filled faster by endothelial cells in vivo. Some common sources of the decellularized matrices are adipose tissue, porcine small intestine submucosa and skin, pericardium, trachea and heart valves [77-79].
Decellularization is generally achieved by a combination of physical and chemical techniques to induce minimal damage to the ECM structures. This can be achieved either by hyper/hypotonic solutions, treatment with acids and bases or detergent treatments. Physical methods including freeze-thawing and pressure application have been also used to minimize chemical use. Decellularized tissues have already achieved success in whole organ replacements in esophagus and trachea [7,80]. However, decellularization is often lengthy and also a slightly deteriorating protocol for the ECM. Moreover, incomplete decellularization can lead to immunogenicity and adverse side effects [81]. In addition, the initial degradation of ECM-based structures is fast and results in rapid loss of mechanical properties [82].
Alternatively, there have been efforts to use cell culture techniques to obtain newly synthesized ECM with a higher level of control over its structure [83]. With the help of bioreactors and biochemical control of cell behavior, a completely autologous tissue with properties close to that of the native tissue can be developed. This and similar methods will be covered in the following section.
III. Scaffold Free Approaches
On the opposite end of the spectrum, cell-based engineered tissues, without any scaffolding material have been under investigation for their potential use in regenerative medicine. Cells are physically not robust enough when they are isolated from their ECM and other neighboring cells. Thus many attempts using cell suspension injections to the site of a deficient tissue (i.e. infarcted myocardium) have resulted in poor viability, low retention and limited integration with the host tissue [84]. To avoid these drawbacks, sheets or aggregates of cells that were cultured in vitro for some time prior to their in vivo application have been tried. Aggregation and sheet formation can increase the physical stability of the cells through cell-cell interactions as well as accumulation of secreted ECM molecules by the cells.
To generate cell aggregates many different substrates have been used including agarose [85], PEG [86, 87], polydimethylsiloxane (PDMS) [88] and poly(N-isopropylacrylamide) (PNIPAAm) [89]. Often, these substrates are prepared using soft lithographical approaches [90], through which the material of choice is cast into a template with desired patterns (i.e. sphere, toroid, cube), and then solidified upon crosslinking or polymerization. Aggregation of the seeded cells on these substrates occurs after a certain incubation time, which is specific for different cell types.
The properties of the substrate used should enable the retrieval of the cellular constructs. Temperature responsive polymers such as PNIPAAm are very suitable for this process as PNIPAAm becomes hydrophilic and swells when the temperature is changed from 37°C to room temperature. By using this reversible property, aggregates can be retrieved with a high yield from substrate (Figure 5) [89]. PNIPAAm can be either used as a mold or as a coating material to modify molds that were made out of other polymers (i.e. PDMS). For example, PDMS molds possessing microgrooves have been coated with PNIPAAm through chemical crosslinking and used to form tissue fibers [91].
Figure 5.
Fabrication of temperature responsive substrates for generating cell aggregates. A-B) PNIPAAm microwells were fabricated by soft lithography, then cells were seeded and retrieved after aggregate formation. Phase contrast and fluorescent microscopy images of the aggregates released from C) PNIPAAm compared to D) PEG microwells (Adapted from [89]).
Usually substrates that have been used for cell aggregation have a flat and smooth structure. This can result in additional diffusion constraints in a system that has already limitations due to bulkiness of cell aggregates. To avoid this, electrospinning techniques have been combined with micromolding approaches to achieve PCL substrates with a permeable bottom made of a nanofibrous sheet [92]. Using this system, aggregates of HepG2 cells, embryonic stem cells (ESCs), pancreatic cells and cardiomyocytes have been produced. As an alternative approach, toroid-shaped cell aggregates have been proposed as a possible remedy for diffusion and/or vascularization limitations. In this approach, rat hepatocytes were seeded into agarose microwells to form toroid shaped aggregates after 2 days of incubation [93]. These aggregates spontaneously combined with each other to form tubular or double-lumen structures.
Cell aggregates have also been used for studying cellular phenotype in a 3D environment [94, 95], and for controlling the differentiation of ESCs [86, 87, 96]. For example, primary hepatocyte aggregates produced in a size controlled manner using PDMS concave microwell arrays have shown higher albumin secretion and higher enzymatic activity when they were co-aggregated with hepatic stellate cells compared to single cell aggregates [94]. In another study, it was shown that primary ventricular cardiac cell aggregates demonstrated phenotypical differences specifically in expression of tissue maturation markers and under hormonal stimuli compared to 2D culture [95].
One of the most exploited aggregate types is ESC aggregates. Since in their natural state, ESCs are in an aggregated configuration, many researchers have tried to mimic this embryo-like structure in vitro, which is named as embryoid body (EB). These structures have been utilized to achieve effective differentiation of the ESCs to the desired cell types [97]. Although there are other means of creating ESC aggregates such as hanging drop or suspension culture method, using microwells for aggregation purposes is beneficial since this method enables production of more uniform and size-controllable aggregates [97, 98]. Especially the size of the EBs is important since it has been shown that the aggregate size can influence their differentiation [87]. A number of materials including PEG [86] and hyaluronic acid [99] have been used to fabricate microwells to produce ESC aggregates or EBs. Moeller et al. have examined the effect of various PEG types on aggregation and attachment of ESCs, and for their EB production capacity [100]. Soon after, other high throughput and facile approaches for EB formation were developed using PDMS [101] and polyesters [102]. Also, recently, it was shown that the incorporation of biomaterials to cell aggregates could manipulate stem cell behavior [103].
Another widely used and successful approach for tissue engineering in the last decade is the use of cell sheets. Cell sheets provide more control over the architecture of the formed tissue compared to cell aggregates since it is possible to align the cells in a particular manner using molds with predetermined topological cues [104]. For cell sheet production, again PNIPAAm has been widely used. It is possible to produce cell sheets, pattern the constituent cells of the sheet, and also stack the sheets to form thicker tissues [105]. For example, cell sheets were used to create tissue engineered blood vessel with adequate and biomimetic mechanical properties [106]. Towards this end, circumferential orientation of ECM in the native vessel was replicated using pNIPAAM coated micropatterned PDMS molds (with grooves and ridges patterns) as templates for production of aligned cell sheets. By this method, it was possible to obtain cell sheets of several layers with aligned cells and ECM, which affected the mechanical properties of the engineered tissue rendering it anisotropic as in the natural tissue (i.e. stronger in the direction of the alignment).
In other studies, cardiac tissue engineering was attempted using this cell sheet-based approach [107, 108]. For example, cardiomyocyte cell sheets were stacked together to form 3D tissue structures, which could improve the myocardial function upon implantation following a myocardial infarction [109]. Although cell sheets gave better in vivo results compared to single cell injection, the cell source used to fabricate the cell sheets was neonatal rat cardiomyocytes, which is not a sustainable cell source for clinical applications. In order to find a clinically more relevant cell source, ESC-derived cardiomyocytes have been used [110]. In this process, the ESCs were cultured in suspension culture in the presence of growth factors and then enriched for cardiomyocytes. Although ESC-derived cardiomyocytes did not form sheets, co-culturing with cardiac fibroblasts resulted in cell sheets, which were able to beat spontaneously, synchronize and express Connexin 43. In another study, ESCs were used for producing cell sheets without prior selection for cardiomyocytes [111]. Cell sheet formation was shown to enhance cardiomyogenic differentiation. The individual cell sheets were used for fabricating 3D scaffolds by sandwiching these cell sheets in between porous scaffolds.
Cell sheet-based approaches were also tried for liver tissue engineering and characterized both in vivo and in vitro for their survival, engraftment with the host tissue and functionality [112, 113]. In an attempt to preserve functionality of the hepatocytes for longer periods, hepatocyte sheets were stacked with endothelial sheets [113]. Cell sheet co-cultures resulted in bile canaliculi network development and higher liver specific gene expression compared to hepatocyte sheets. Co-culture of endothelial and hepatocyte cell sheets preserved albumin secretion for 28 days while the single culture of hepatocyte cell sheets showed a decrease in albumin secretion.
Cell sheets of mesenchymal stem cells (MSCs) from different sources such as periodontal ligament, bone marrow and umbilical cord have also been produced [114, 115]. In vivo studies using a swine model showed that periodontal ligament stem cell sheets were more effective in regeneration of periodontal defects compared to cell suspension application [114]. Cell sheet formation can be further improved by the addition of exogenous factors. For example, it has been shown that ascorbic acid (Vitamin C) enhanced the cell sheet formation by increasing ECM production [114]. Also, incorporation of gelatin microspheres loaded with transforming growth factor β1 (TGF-β1) facilitated chondrogenic differentiation and cell sheet formation.
As an alternative to thermo-responsive polymer based substrates, polyelectrolyte multilayers have been used to produce cell sheets [116]. In this study, myoblast cell sheets were created on poly-l-(lysine)/ hyaluronic acid multilayers with a fibronectin top layer. Cell sheets were collected after dissolution of the polyelectrolyte multilayers by addition of divalent ions to the culture media.
IV. Cell Sources and Controlling Cell Behavior
A. Stem Cells and Induced Pluripotent Cells
One of the most important aspects of an engineered tissue construct is the cell source. As, over time it has been proven that the primary cells cannot be a sufficient cell source for some target tissues, other cell sources such as ESCs have been proposed. ESCs can be expanded in culture indefinitely under proper conditions [117, 118]. ESCs have the potential to differentiate into all the cell types in the body. ESCs have been successfully differentiated into many cell types such as cardiomyocytes [119-121], endothelial cells [122], hepatocytes [123-125], pancreatic beta cells [126], osteoblasts [127, 128], and neural cells [129-133]. Despite these properties, ESCs have been the focus of many ethical questions and concerns as well as scientific concerns regarding directing their differentiation in a controlled manner and without tumour formation [134].
A relatively recent development in the area of genetic manipulation of adult cells resulted in the development of induced pluripotent stem cells (iPSCs). This cell source has most of the advantageous properties of the ESCs [135], while being patient-specific and less controversial [136-138]. It has been shown that these cells can be differentiated to many cell types including hepatocytes [139] and cardiomyocytes [140]. Also, there are few studies that use iPSCs for tissue engineering purposes in the literature. For example, there is a recent study where the cell source used for cardiac tissue engineering was iPSC-derived cardiomyocytes [141].
Despite initial promising results with iPSCs, there are concerns on issues such as the efficacy of their differentiation [142] and the additional risk of introduction of somatic coding mutations [143]. For these cells to be routinely used in tissue engineering and regenerative medicine applications, these problems should be addressed [144]. To use these cell sources more efficiently, controlled differentiation of stem cells using biomimetic environments and pathway engineering are promising approaches.
B. Controlled Differentiation
Cell preparation and differentiation are multi-step and time-consuming procedures, while the yield of the target cell type is usually low. This makes off-the-shelf tissue engineering products hard to achieve; especially for multicellular tissues. Therefore, controlled differentiation of stem cells using biomaterials has become an actively researched topic in the tissue engineering field [145].
Differentiation of stem cells using biomaterials has the advantage of providing a biomimetic environment, which can potentially increase the efficacy of the differentiation. Other advantages include reducing the processing steps in tissue fabrication by combining cell differentiation and incorporation as well as enabling simultaneous differentiation of multiple cell types in a single platform for engineering complex tissues. Towards this end, a number of studies have been conducted to control the stem cell differentiation using physical [146] and chemical [147] properties of the biomaterials.
As the substrate physical properties (i.e. elastic modulus) can affect the lineage commitment of the stem cells [1], many researchers have investigated ways to exploit this phenomena for potential tissue engineering applications. For example, MSCs were encapsulated in an injectable hyaluronic acid-tyramine (HA-Tyr) hydrogel system with tunable mechanical properties for potential use in cartilage tissue engineering [148]. Hydrogels with lower crosslinking degrees yielded more chondrogenic differentiation, with enhanced ECM deposition. On the other hand, higher crosslinking degrees resulted in generation of cells with fibrous phenotypes that generated fibrocartilage and fibrous tissue. In another study, collagen and fibronectin functionalized polyacrylamide substrates with mechanical properties mimicking tendon and bone tissue were used to modulate bone marrow MSC differentiation towards these tissues [149]. It was shown that differentiation could be modulated using the substrate mechanical properties, but it was interdependent to biochemical cues as well. Differentiation of MSCs towards endothelial cells was also studied in 3D using fibrinogen modified with PEG derivatives [150]. MSCs encapsulated in various PEGylated fibrinogen hydrogels showed alterations in their differentiation towards endothelial cells in gels with different mechanical properties. Similarly, when nerve progenitor cells (NPCs) were cultured in hyaluronic acid with tunable mechanical properties ranging from that of neonatal brain to adult brain, it was possible to change the NPC phenotype from mature neuronal to astroglial [151].
Another recent way to control stem cell differentiation is to incorporate proteins or peptide sequences to the scaffold, preferably in specific patterns to achieve biomimetic structures. For example, insulin-like growth factor binding protein 4 (IGFBP4) was immobilized on polystyrene substrates using elastin-like polypeptides in order to direct the differentiation of ESCs towards cardiogenic lineage [152]. In another study, differentiation factors sonic hedgehog (SHH) and ciliary neurotrophic factor (CNTF) were patterned in 3D through modification of hydrogel chemistry using two-photon laser scanning technology (Figure 6) [153]. Barnase-barstar and streptavidin-biotin chemistries were used for simultaneous patterning of two different growth factors. Patterning of growth factors with high precision in 3D can be a promising approach for controlling stem cell differentiation.
Figure 6.
A) Schematics of the simultaneous protein immobilization using 2 photon laser scanning method. A femtosecond laser was used to immobilize maleimide-barnase, represented with the black circle, followed by a wash step to remove unbound maleimide-barnase. Then, maleimide-streptavidin was immobilized, represented by orange square, and again followed by a wash step. After this step, a protein of interest which has been fused to barstar and biotin was introduced. They will specifically bind to barnase and streptavidin, respectively. B and C) Confocal microscopy images of simultaneous patterning of biotin–CNTF (red) and barstar–SHH (green) (Scale bar: 100 μm) (Adapted from [153]).
C. Pathway engineering
Pathway engineering is the science of understanding cells’ major pathways, especially the metabolic ones, and manipulating them through genetic modifications [154]. Pathway engineering has been exploited on yeast [155], bacteria [156] and plants [157] for overproduction of desired metabolites for nearly two decades. This generally involves the enhancement of the expression of a key enzyme in a certain pathway usually by the aid of transcription factors [158]. More elaborate approaches have emerged such as knocking other pathways simultaneously while enhancing one or altering more than one enzyme in a given pathway to have exponential effects. Moreover, with the manipulation of correct pathways, cells could be reprogrammed to a desired phenotype [159].
As a result of the growing knowledge on signaling and metabolic pathways in mammalian cells, pathway engineering approaches have recently been extended to mammalian cells [160-162]. It has also been considered for tissue engineering and regenerative medicine applications [163]. Currently, the studies in mammalian pathway engineering are for utilization of mammalian cells for production of therapeutic proteins [164]. However, reprogramming a cell through pathway engineering can also be used for controlled and sustainable differentiation of stem cells with high yields as well as for directing other cellular behaviors such as expression and/or secretion of a specific protein. By this way cells can be manipulated utilizing the knowledge on the signaling pathways for chondrogenic differentiation of MSCs [165], skin aging [166], fracture healing of the bone [167], liver regeneration [168] or cellular apoptosis [169], in order to provide solutions for engineering complex tissues. For example, human pluripotent stem cells were reprogrammed into multipotent neural crest cells through activation of the canonical Wnt signaling and suppression of the Activin A/Nodal pathway simultaneously [170]. It was also possible to control cell migration and homing through engineering the pathways for surface glycans, which could have profound effects in wound healing and tissue regeneration [171]. Although they are few in number, such promising studies suggest that pathway engineering and cellular reprogramming can potentially be used for solving the existing problems in tissue engineering and regenerative medicine.
V. Emerging Tissue engineering Applications
Aside from the demand for organ substitutes, there is also a growing need for relevant tissue models of diseases for basic science purposes or pharmaceutical trials. The most prominent of these efforts based on tissue engineering methods are drug models and cancer models.
A. Tissue Models for Drug Testing
Despite the developments in other areas, drug discovery and development still constitute the biggest portion of the biomedical field. However, as the regulations tighten and the cost of drug discovery constantly increases, the drug developers are looking for more intelligent ways to test drug candidates. There is a great need for biomimetic testing systems and the current developments in tissue engineering provide a means to help drug discovery and toxin screening via microtissue based diagnostics and testing [172].
Although, high throughput drug testing systems based only on enzymes have been used for testing cytotoxicity and drug conversion, they cannot demonstrate the whole metabolic effect of a drug on liver [173]. The incorporation of cells in microfluidic systems has improved such drug platforms. However, 3D effects of drug distribution cannot be evaluated by 2D culture systems. 3D models developed based on cell encapsulated hydrogels would be a better model of drug distribution [174]. For example, an alginate-based hydrogel system has been developed for measuring the toxicity of drugs on encapsulated liver cells with results comparable to LD50 values in vivo [175]. This system was further modified with the addition of a cancer cell line, thus simultaneously the toxicity and the anticancer drug efficacy could be tested. The comparison of several widely used drugs’ LD50 values in mice and CT50 values in culture has shown that 3D structures provide a better estimate (R2=0.97) than 2D cultures (R2=0.85) [175]. This implies that tissue-like 3D models were a better method for assessing drug effects. Such systems are also important for assessment of toxicity for growing number of new materials and chemicals in biomedical engineering field, which cannot be tested effectively and economically with the currently available tests. Another advantage of miniaturized 3D models is the packing of cells in a smaller volume compared to a 2D surface. This is a better mimic of cell densities in tissues in vivo. Moreover such artificial constructs would also be an answer to the prevailing questions surrounding animal use in experiments, particularly for drug and cosmetic tests.
Another aspect of host reaction to drugs is the immune response, which can be unpredictable with standard in vitro and animal models. Utilization of humanized mice has improved the test outcomes [176], but still cheaper and animal test-free systems would be desirable. For this end, microorganoids resembling the lymph nodes have been developed which can be kept alive and exposed to drugs for several weeks [177]. Analysis of cellular immunity by cytokine release can be done simultaneously with monitoring of other cellular events, such as proliferation and apoptosis, in this system. Another attempt to provide alternatives to animal testing via tissue engineering was the development of multicell type artificial cornea mimics. Such structures could replace Draize test which utilizes rabbit eyes to test the allergic reactions to new chemicals [178].
The complex, multicellular structure of some organs is hard to imitate. Especially the problems related to the vascularization of engineered construct above volumes of several mm3 limit organ production at macro scale. However, with the developments in microfluidics-based systems, miniaturized organs can be produced. With the help of microfabrication techniques these miniaturized organs can be produced as valuable drug development tools.
These tissue-on-a-chip or organ on-a-chip structures can be viewed as miniaturized organs where all the physiological activities pertinent to the metabolization of a drug or a toxin can be closely mimicked. Microscale tissues are advantageous for monitoring due to decreased amount of reagent use, smaller sample size and the possibility of high throughput analysis. Moreover, via miniaturization, it is possible to mimic physiological shear stress conditions better as well as to provide relevant physiological fluid composition spatially and temporally. Analysis of drug candidates with microtissues can both increase the reliability of the tests and enable detection of candidates that can be eliminated in animal tests due to false negative results.
Even though microengineered tissue based tests would be an important improvement, it cannot answer the questions related to another important aspect of drug testing and monitoring which are the short and long term systemic effects of the drugs. For example, a single toxicity test based on an artificial liver system would not account for the possible side effects of the metabolic by-products of the hepatocyte activity on the drug on other tissues. A possible solution proposed for this problem is the body-on a chip systems. By connecting several organs, it is possible to obtain a body-on-a-chip system where the effect of one drug or therapy on multiple organs can be elaborated. Already, there are several such systems available, such as gastrointestinal tract models, kidney models and lung air sac models [179, 180]. In such systems, reciprocal interactions of the cells can be monitored, such as increase in hepatic glucose synthesis by hepatocytes in the presence of endothelial cells [181].
A body-on-a-chip will offer multiple advantages: i) metabolization of the drugs and their possible systemic effects can be monitored and the sequence of the events can be elucidated, ii) small scale effects that can be missed in animal tests which can result in negative outcomes in later clinical trials can be detected, iii) testing of drug-drug interactions can be done in a cheaper and more efficient way for several organs at the same time [182]. They will also allow elaboration of the mechanisms of the drug toxicity and side effects without animal studies and in a more complex manner than cell culture studies.
However, having many cell types in a small system creates some practical problems, such as the composition of the culture medium [183]. Some solutions have already been available such as development of a blood surrogate which would be suitable for all cell types. Other options are the separation of the feeding of each microorgan so that they can be in contact with their own medium where metabolite exchange between microorgans is achieved by separate connections or addition of controlled delivery functions into the chip for specific growth factor delivery to each compartment [181, 184, 185].
Another important issue in the development of such systems is the utilization of suitable methodologies for their monitoring. Since the aim is to observe long-term effects and also temporal changes in responses, noninvasive methods for visualization of tissue integration and cellular activity at high resolutions are necessary. The microscopy methods such as Lens-free microscopy [186], Coherent Anti-Stokes Raman Microscopy (CARS) and Secondary Harmonic Generation (SHG) [187, 188] can provide real-time information about the model ECM and cells (Figure 7).
Figure 7.
Non-linear microscopy techniques SHG and CARS allow simultaneous, real-time monitoring of cell-fibrous scaffold (bacterial cellulose) interactions. A) Interactions of vascular smooth muscle cells with nanofibrillar bacterial cellulose was monitored at specific time points to quantify cell proliferation, migration and ECM secretion B) Secreted collagen fibers can be distinguished from the cellulose fibers due to the substantial difference in their average fiber size (A collagen fiber is indicated with an arrow around vascular smooth muscle cells) (Adapted from [206]).
As for monitoring of metabolic activity, since in such small volumes collection of analytes is problematic, general choice of analysis is electrical or optical methods [189], such as cellular impedance measurements via surface plasmon resonance. Also, in addition to microscopy methods such as time lapse microscopy, by using fluorogenic molecules specific interactions of cells with drugs or metabolites can be monitored. For example, autofluorescence of doxorubicin allows monitoring of its uptake [190]. Correlation of cell's physical properties with their activities is a strong indicator of their health that can be incorporated into long term monitoring of cellular activities.
Other possible outcomes of tissue engineering would be disease models, such as robust in vitro 3D models which can closely mimic the in vivo condition. These models can be used for basic science research and also for therapeutic drug testing [191]. Especially with the strong indications of non-applicability of animal test results for human trials, 3D artificial, diseased human tissues might be a reliable model for drug testing.
B. Cancer Models
The ability to develop healthy and diseased tissue at the same time, possibly from the same cell source is a strong tool to understand the onset and propagation of diseases such as cancer. The large variation of cancer cells from patient to patient such as differences in their metastasis behavior, requires models that can reflect these discrepancies with high fidelity.
In vitro 2D culture of cancer cells cannot provide information about their movement in 3D. A tissue engineering-based cancer model can reflect the invasive properties of the carcinomas. It can also be used to monitor the feedback mechanisms between tumor and stromal cells and also the interactions of cancer cells with different cytokines.
For modeling metastasis, the 3D cell migration process needs to be imitated. There are new techniques available for achieving control over the cell migration and spreading in 3D structures. Tumorogenesis can be accurately imitated in systems which contain all relevant cell types together with a biomimetic ECM structure. For example, for breast cancer models, 2D culturing of epithelial cells will give an incomplete picture as epithelial-stroma interaction is an important part of tumor progression. The addition of ECM components via a natural scaffold would also be necessary to reinforce the robustness of the model. In the absence of ECM, 3D cancer models have been produced with spheroid cultures, which can be prepared from epithelial tumors (homotypic aggregation) and can be rendered also multi-cellular (via heterotypic aggregation) to model the interactions of stroma and the cancer cells [192]. Such structures not only can be used to model tumor architecture but also provide all the necessary autocrine and paracrine effects to better mimic the tumor microenvironment. Mesenchymal induction of basement membrane deposition by epithelial cells [193] has been shown to be an important determinant in development of epithelium based cancer models. For example, laminin rich ECM was previously used to distinguish the differences between normal and malignant breast cells. The healthy cells formed polarized colonies and the cancerous cells grow into proliferative colonies with no definite organization. This model can be used to elaborate the differences between healthy and diseased structures which will give a more complete understanding of how the disease progresses than 2D culture models [194].
Another important factor during embryonic development and cancer metastasis is the epithelial to mesenchyme transition. The interaction of mesenchyme and the epithelium defines their function and structure in a reciprocal way during development. Understanding the underlying principles of this transition via co-cultures in scaffold systems would help in understanding the complex interactions between these cell types. For example, it has been shown that epithelialmesenchyme transition resulted in cells that demonstrate stem cell-like properties [195]. Imitation of the microenvironment that leads to this event would be beneficial both for cancer research and standard tissue engineering applications [196].
These developments in artificial disease models would also help in optimization of surgical protocols. Cryosurgery is a growing field in the cancer resection area as it is a minimally invasive technique with high efficiency. But the microenvironment induced by the application of extremely low temperatures, which is below the thresholds for thermodamage, can cause damage to the area surrounding the target. The optimal application conditions and their long term effects will only be available after years of data gathering with conventional methods. However tissue engineered constructs can be exposed to the same conditions of the surgery and the effect of the treatment can be visualized in real–time. This way, in-depth measurements of the physical conditions within the tissue can be made and the surgery conditions can be rectified accordingly [197]. Moreover, these kinds of models can be further used for the tissue preservation and organ preservation studies and the complex effect of freezing on the tissues.
C. Biorobotics
Biorobotics is a field that aims to design robots that can mimic animal or human movements and to analyze natural control systems to use them in new robot designs. For example, each muscle in animals can act as a multiple actuator with the ability to respond to multitudes of inputs. This provides a substantial advantage to the single actuator/ single input systems in conventional robots. Bio-inspired “cellular actuators” based on piezoelectric materials have been developed to mimic this property [198]. Aside from physical biomimicry, recently research focus also turned on using cell based systems as actuators. Cell types that can act as actuators such as vorticella [199], cardiomyocytes, myoblasts can be used to develop MEMS systems that can also function in aqueous conditions. For a feasible actuator high density of cells is necessary. For this end, there have been trials with extracted tissues such as dorsal vessel obtained from insects [200] or in vitro assembled systems based on fibroblasts and myoblasts that can actuate microdevices [201]. But better actuators with more control over cell localization can be obtained by tissue engineering methods. For example, myoblasts labeled with magnetite cationic liposomes have been used to form an artificial muscle construct under magnetic force to act as a bioactuator [202]. Also cardiomyocytes within ECM-based gels has been developed as microdevice actuators [203]. There are also ongoing trials to use neuron cell cultures as biological processors that can control robotic systems [204], which can benefit from tissue engineering methodologies. Tissue engineered actuators would provide more sophisticated structures with more control over mode of actuation. They can also be used to form the interface between robotic systems and tissues. Although, most of the tissue engineering efforts are limited to mammalian cells for regenerative medicine purposes, another possibility in biorobotic/tissue engineering interface is the utilization of cold-blooded species’ cells such as insects and zebrafish, which have less stringent requirements for culture.
VI. future perspectives
The ties and contribution of tissue engineering methodologies to biotechnology have been growing strongly. With recent clinical successes, combined with the better understanding of host/biomaterial interactions tissue engineering still has room to grow. The novel scaffolds are smart in many ways, but they generally lack the temporal control over the modeling process in vivo. The next generation would also incorporate structures that would actively measure the health of the implant. Such lab on a chip applications in vivo, could improve the quality of monitoring available for implant systems [205]. Also developments in disease models and body-on-a-chip applications and demands in biorobotics will create new requirements for the next generation of tissue engineered constructs which will necessitate more widespread use of new methodologies such as pathway engineering or use of alternative cell sources.
Acknowledgments
This paper was supported by the Institute for Soldier Nanotechnology, National Institutes of Health (HL092836, EB02597, AR057837, HL099073), the National Science Foundation (DMR0847287), and the Office of Naval Research Young Investigator award
Contributor Information
Pinar Zorlutuna, Center for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, MA, USA.; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Nihal Engin Vrana, Center for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, MA, USA.; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Ali Khademhosseini, Center for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, MA, USA.; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, USA. Wyss Institute for Biologically Inspired Engineering, Harvard Medical School, Boston, MA, USA.
References
- 1.Engler AJ, et al. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006 Aug;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien FJ, et al. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005 Feb;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 3.Khademhosseini A, et al. Progress in Tissue Engineering. Scientific American. 2009 May;300:64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 4.Lazic T, Falanga V. Bioengineered Skin Constructs and Their Use in Wound Healing. Plastic and Reconstructive Surgery. 2011 Jan;127:75S–90S. doi: 10.1097/PRS.0b013e3182009d9f. [DOI] [PubMed] [Google Scholar]
- 5.Roberts SJ, et al. Clinical applications of musculoskeletal tissue engineering. British Medical Bulletin. 2008 Jun;86:7–22. doi: 10.1093/bmb/ldn016. [DOI] [PubMed] [Google Scholar]
- 6.Atala A. Engineering organs. Current Opinion in Biotechnology. 2009 Oct;20:575–592. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Macchiarini P, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008 Dec;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 8.Gomes S, et al. Natural and genetically engineered proteins for tissue engineering. Progress in Polymer Science. 2012 Jan;37:1–17. doi: 10.1016/j.progpolymsci.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAllister TN, et al. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regenerative Medicine. 2008 Nov;3:925–937. doi: 10.2217/17460751.3.6.925. [DOI] [PubMed] [Google Scholar]
- 10.Peck M, et al. Tissue engineering by self-assembly. Materials Today. 2011 May;14:218–224. [Google Scholar]
- 11.Fujihara Y, et al. Immunological response to tissue-engineered cartilage derived from auricular chondrocytes and a PLLA scaffold in transgenic mice. Biomaterials. 2010 Feb;31:1227–1234. doi: 10.1016/j.biomaterials.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Huang NF, Li S. Regulation of the Matrix Microenvironment for Stem Cell Engineering and Regenerative Medicine. Annals of Biomedical Engineering. 2011 Apr;39:1201–1214. doi: 10.1007/s10439-011-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. Journal of Cell Science. 2001 Oct;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 14.Ifkovits JL, Burdick JA. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Engineering. 2007 Oct;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 15.Nichol JW, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010 Jul;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartgerink JD, et al. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proceedings of the National Academy of Sciences. 2002 Apr;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracalello A, et al. Design and Production of a Chimeric Resilin-, Elastin-, and Collagen-Like Engineered Polypeptide. Biomacromolecules. 2011 Aug;12:2957–2965. doi: 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- 18.Gomes SC, et al. Antimicrobial functionalized genetically engineered spider silk. Biomaterials. 2011 Jun;32:4255–4266. doi: 10.1016/j.biomaterials.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numata K, et al. Silk-Based Nanocomplexes with Tumor-Homing Peptides for Tumor-Specific Gene Delivery. Macromolecular Bioscience. 2012 Jan;12:75–82. doi: 10.1002/mabi.201100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia XX, et al. Tunable Self-Assembly of Genetically Engineered Silk-Elastin-like Protein Polymers. Biomacromolecules. 2011 Nov;12:3844–3850. doi: 10.1021/bm201165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitajima T, et al. Recombinant Human Gelatin Substitute With Photoreactive Properties for Cell Culture and Tissue Engineering. Biotechnology and Bioengineering. 2011 Oct;108:2468–2476. doi: 10.1002/bit.23192. [DOI] [PubMed] [Google Scholar]
- 22.Gillette BM, et al. Dynamic Hydrogels: Switching of 3D Microenvironments Using Two-Component Naturally Derived Extracellular Matrices. Advanced Materials. 2010 Feb;22:686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 23.Shin SR, et al. Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation. Acs Nano. 2012 Jan;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng XG, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008 Aug;29:3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Builles N, et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials. 2010 Nov;31:8313–8322. doi: 10.1016/j.biomaterials.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 26.Tian H, et al. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress in Polymer Science. 2012 Feb;37:237–280. [Google Scholar]
- 27.Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Progress in Polymer Science. 2011 Sep;36:1254–1276. [Google Scholar]
- 28.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010 Oct;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 29.Edlund U, et al. A Strategy for the Covalent Functionalization of Resorbable Polymers with Heparin and Osteoinductive Growth Factor. Biomacromolecules. 2008 Mar;9:901–905. doi: 10.1021/bm701267u. [DOI] [PubMed] [Google Scholar]
- 30.Mahony O, et al. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Advanced Functional Materials. 2010 Nov;20:3835–3845. [Google Scholar]
- 31.Moon JJ, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010 May;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials. 2011 Feb;32:1301–1310. doi: 10.1016/j.biomaterials.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Leslie-Barbick JE, et al. Micron-Scale Spatially Patterned, Covalently Immobilized Vascular Endothelial Growth Factor on Hydrogels Accelerates Endothelial Tubulogenesis and Increases Cellular Angiogenic Responses. Tissue Engineering Part A. 2011 Jan;17:221–229. doi: 10.1089/ten.tea.2010.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saik JE, et al. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomaterialia. 2011 Jan;7:133–143. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. Journal of Biomedical Materials Research Part A. 2010 Jul;94A:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 36.Kenar H, et al. A 3D aligned microfibrous myocardial tissue construct cultured under transient perfusion. Biomaterials. 2011 Aug;32:5320–5329. doi: 10.1016/j.biomaterials.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W, et al. Artificial Niche Combining Elastomeric Substrate and Platelets Guides Vascular Differentiation of Bone Marrow Mononuclear Cells. Tissue Engineering Part A. 2011 Aug;17:1979–1992. doi: 10.1089/ten.tea.2010.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crapo PM, Wang Y. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 2010 Mar;31:1626–1635. doi: 10.1016/j.biomaterials.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sant S, et al. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. Journal of Tissue Engineering and Regenerative Medicine. 2011 Apr;5:283–291. doi: 10.1002/term.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelmayr GC, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Materials. 2008 Dec;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu EC, et al. Self-Assembling Peptides as Cell-Interactive Scaffolds. Advanced Functional Materials. 2012 Feb;22:456–468. [Google Scholar]
- 42.Kieffer AE, et al. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. Faseb Journal. 2003 Feb;17:776–778. doi: 10.1096/fj.02-0699fje. [DOI] [PubMed] [Google Scholar]
- 43.Loo Y, et al. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnology Advances. 2012 May-Jun;30:593–603. doi: 10.1016/j.biotechadv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Mishra A, et al. Ultrasmall natural peptides self-assemble to strong temperature-resistant helical fibers in scaffolds suitable for tissue engineering. Nano Today. 2011 Jun;6:232–239. [Google Scholar]
- 45.Zhang SG. Fabrication of novel biomaterials through molecular self-assembly. Nature Biotechnology. 2003 Oct;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 46.Cho H, et al. Regulation of endothelial cell activation and angiogenesis by injectable peptide nanofibers. Acta Biomaterialia. 2012 Jan;8:154–164. doi: 10.1016/j.actbio.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galler KM, et al. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Engineering Part A. 2012 Jan;18:176–184. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang BC, et al. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. Journal of Biomedical Materials Research Part A. 2012 Mar;100A:646–653. doi: 10.1002/jbm.a.33300. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JM, et al. Biphasic Peptide Amphiphile Nanomatrix Embedded with Hydroxyapatite Nanoparticles for Stimulated Osteoinductive Response. Acs Nano. 2011 Dec;5:9463–9479. doi: 10.1021/nn203247m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenas P, et al. Modularity in developmental biology and artificial organs: A missing concept in tissue engineering. Artificial Organs. 2011 Jun;35:656–662. doi: 10.1111/j.1525-1594.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 51.Davis NE, et al. Modular enzymatically crosslinked protein polymer hydrogels for in situ gelation. Biomaterials. 2010 Oct;31:7288–7297. doi: 10.1016/j.biomaterials.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serban MA, Prestwich GD. Modular extracellular matrices: Solutions for the puzzle. Methods. 2008 May;45:93–98. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott EA, et al. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a noncytotoxic porogen. Acta Biomaterialia. 2010 Jan;6:29–38. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrbar M, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007 Sep;28:3856–3866. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 55.McGuigan AP, et al. Fabrication of cell-containing gel modules to assemble modular tissue-engineered constructs. Nature protocols. 2006 Mar;1:2963–2969. doi: 10.1038/nprot.2006.443. [DOI] [PubMed] [Google Scholar]
- 56.Sosnik A, et al. Lactoyl-poloxamine/collagen matrix for cell-containing tissue engineering modules. Journal of Biomedical Materials Research - Part A. 2008 Aug;86:339–353. doi: 10.1002/jbm.a.31594. [DOI] [PubMed] [Google Scholar]
- 57.Leung BM, Sefton MV. A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Annals of Biomedical Engineering. 2007 Dec;35:2039–2049. doi: 10.1007/s10439-007-9380-0. [DOI] [PubMed] [Google Scholar]
- 58.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences of the United States of America. 2006 Aug;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamberlain MD, et al. Chimeric vessel tissue engineering driven by endothelialized modules in immunosuppressed sprague-dawley rats. Tissue Engineering - Part A. 2011 Jan;17:151–160. doi: 10.1089/ten.tea.2010.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leung BM, Sefton MV. A modular approach to cardiac tissue engineering. Tissue Engineering - Part A. 2010 Oct;16:3207–3218. doi: 10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGuigan AP, Sefton MV. The thrombogenicity of human umbilical vein endothelial cell seeded collagen modules. Biomaterials. 2008 Jun;29:2453–2463. doi: 10.1016/j.biomaterials.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan OF, Sefton MV. Endothelial cell behaviour within a microfluidic mimic of the flow channels of a modular tissue engineered construct. Biomedical Microdevices. 2011 Jan;13:69–87. doi: 10.1007/s10544-010-9472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du Y, et al. Surface-directed assembly of cell-laden microgels. Biotechnology and Bioengineering. 2010 Feb;105:655–62. doi: 10.1002/bit.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Y, et al. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnology and Bioengineering. 2011 Jul;108:1693–1703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du Y, et al. Convection-driven generation of long-range material gradients. Biomaterials. 2010 Mar;31:2686–94. doi: 10.1016/j.biomaterials.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du Y, et al. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jul;105:9522–7. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du Y, et al. Method of Bottom-Up Directed Assembly of Cell-Laden Microgels. Cellular and Molecular Bioengineering. 2008 Feb;1:157–162. doi: 10.1007/s12195-008-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamanian B, et al. Interface-directed self-assembly of cell-laden microgels. Small. 2010 Apr;6:937–44. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi H, et al. Photoswitchable gel assembly based on molecular recognition. Nature Communications. 2012 Jan;3 doi: 10.1038/ncomms1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harada A, et al. Macroscopic self-assembly through molecular recognition. Nature Chemistry. 2011;3:34–37. doi: 10.1038/nchem.893. [DOI] [PubMed] [Google Scholar]
- 71.Gartner ZJ, Bertozzi CR. Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009 Mar;106:4606–4610. doi: 10.1073/pnas.0900717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chemical Society Reviews. 2010 Jan;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selden NS, et al. Chemically programmed cell adhesion with membrane-anchored oligonucleotides. Journal of the American Chemical Society. 2012;134:765–768. doi: 10.1021/ja2080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dutta D, et al. Synthetic chemoselective rewiring of cell surfaces: Generation of three-dimensional tissue structures. Journal of the American Chemical Society. 2011;133:8704–8713. doi: 10.1021/ja2022569. [DOI] [PubMed] [Google Scholar]
- 75.Bratt-Leal AM, et al. Magnetic manipulation and spatial patterning of multi-cellular stem cell aggregates. Integrative Biology. 2011 Mar;3:1224–1232. doi: 10.1039/c1ib00064k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiao T, et al. Measurements of the Effects of Decellularization on Viscoelastic Properties of Tissues in Ovine, Baboon, and Human Heart Valves. Tissue Engineering Part A. 2012 Feb;18:423–431. doi: 10.1089/ten.tea.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Remlinger NT, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010 May;31:3520–3526. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 78.Vorotnikova E, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biology. 2010 Oct;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Crapo PM, et al. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011 Apr;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badylak SF, et al. Esophageal Preservation in Five Male Patients After Endoscopic Inner-Layer Circumferential Resection in the Setting of Superficial Cancer: A Regenerative Medicine Approach with a Biologic Scaffold. Tissue Engineering Part A. 2011 Jun;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keane TJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012 Feb;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Gilbert TW, et al. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007 Jan;28:147–50. doi: 10.1016/j.biomaterials.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 83.L'Heureux N, et al. A completely biological tissue-engineered human blood vessel. Faseb Journal. 1998 Jan;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 84.Haraguchi Y, et al. Concise Review: Cell Therapy and Tissue Engineering for Cardiovascular Disease. Stem Cells Translational Medicine. 2012 Feb;1:136–141. doi: 10.5966/sctm.2012-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Napolitano AP, et al. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 2007 Oct;43:496–500. doi: 10.2144/000112591. [DOI] [PubMed] [Google Scholar]
- 86.Karp JM, et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007 Jun;7:786–94. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 87.Hwang YS, et al. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009 Oct;106:16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi YS, et al. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 2012 Mar;33:2482–2491. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tekin H, et al. Stimuli-responsive microwells for formation and retrieval of cell aggregates. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2010 Oct;10:2411–2418. doi: 10.1039/c004732e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007 Dec;28:5087–92. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Tekin H, et al. Responsive microgrooves for the formation of harvestable tissue constructs. Langmuir. 2011 May;27:5671–9. doi: 10.1021/la200183x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallego-Perez D, et al. High throughput assembly of spatially controlled 3D cell clusters on a micro/nanoplatform. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2010 Mar;10:775–782. doi: 10.1039/b919475d. [DOI] [PubMed] [Google Scholar]
- 93.Livoti CM, Morgan JR. Self-Assembly and Tissue Fusion of Toroid-Shaped Minimal Building Units. Tissue Engineering Part A. 2010 Jun;16:2051–2061. doi: 10.1089/ten.tea.2009.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong SF, et al. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials. 2011 Nov;32:8087–8096. doi: 10.1016/j.biomaterials.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 95.Akins RE, et al. Three-dimensional culture alters primary cardiac cell phenotype. Tissue Engineering - Part A. 2010 Feb;16:629–641. doi: 10.1089/ten.tea.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi H, et al. Patterned differentiation of individual embryoid bodies in spatially organized 3D hybrid microgels. Advanced Materials. 2010 Dec;22:5276–5281. doi: 10.1002/adma.201002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spelke DP, et al. Methods for embryoid body formation: the microwell approach. Methods in molecular biology (Clifton, N.J.) 2011;690:151–162. doi: 10.1007/978-1-60761-962-8_10. [DOI] [PubMed] [Google Scholar]
- 98.Bratt-Leal AM, et al. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnology Progress. 2009 Jan-Feb;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerecht S, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moeller H-C, et al. A microwell array system for stem cell culture. Biomaterials. 2008 Feb;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi YY, et al. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010 May;31:4296–4303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- 102.Selimovic S, et al. Microfabricated polyester conical microwells for cell culture applications. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2011 Nov;11:2325–2332. doi: 10.1039/c1lc20213h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bratt-Leal AM, et al. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011 Jan;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin JB, et al. Thermo-responsive poly(N-isopropylacrylamide) grafted onto microtextured poly(dimethylsiloxane) for aligned cell sheet engineering. Colloids and Surfaces B: Biointerfaces. 2012 Nov;99:108–115. doi: 10.1016/j.colsurfb.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 105.Elloumi-Hannachi I, et al. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. Journal of Internal Medicine. 2010 Jan;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 106.Isenberg BC, et al. Micropatterned cell sheets with defined cell and extracellular matrix orientation exhibit anisotropic mechanical properties. Journal of Biomechanics. 2012 Mar;45:756–761. doi: 10.1016/j.jbiomech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 107.Sekine H, et al. Myocardial tissue engineering: toward a bioartificial pump. Cell and Tissue Research. 2012 Mar;347:775–782. doi: 10.1007/s00441-011-1267-6. [DOI] [PubMed] [Google Scholar]
- 108.Haraguchi Y, et al. Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiology Research and Practice. 2011;1 doi: 10.4061/2011/845170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sekine H, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Engineering - Part A. 2011 Nov;17:2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 110.Matsuura K, et al. Creation of mouse embryonic stem cell-derived cardiac cell sheets. Biomaterials. 2011 Oct;32:7355–7362. doi: 10.1016/j.biomaterials.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 111.Huang C-C, et al. A strategy for fabrication of a three-dimensional tissue construct containing uniformly distributed embryoid body-derived cells as a cardiac patch. Biomaterials. 2010 Aug;31:6218–6227. doi: 10.1016/j.biomaterials.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 112.Ohashi K, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nature Medicine. 2007 Jun;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 113.Kim K, et al. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials. 2012;33:1406–1413. doi: 10.1016/j.biomaterials.2011.10.084. [DOI] [PubMed] [Google Scholar]
- 114.Wei FL, et al. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. Journal of Cellular Physiology. 2011 Sep;227:3216–3224. doi: 10.1002/jcp.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solorio LD, et al. Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-β1. Journal of Controlled Release. 2012 Mar;158:224–232. doi: 10.1016/j.jconrel.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahn R, et al. Ion-induced cell sheet detachment from standard cell culture surfaces coated with polyelectrolytes. Biomaterials. 2012 Apr;33:3421–3427. doi: 10.1016/j.biomaterials.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 117.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 118.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000 Feb;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 119.Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003 Jun;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 120.Kehat I, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. Journal of Clinical Investigation. 2001 Aug;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kehat I, et al. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circulation Research. 2002 Oct;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 122.Levenberg S, et al. Endothelial cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002 Apr;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamazaki T, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Letters. 2001 May;497:15–9. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 124.Ishii T, et al. In vitro differentiation and maturation of mouse embryonic stem cells into hepatocytes. Experimental Cell Research. 2005 Jul; doi: 10.1016/j.yexcr.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 125.Lavon N, et al. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004 Jun;72:230–8. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 126.Moritoh Y, et al. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003 May;52:1163–8. doi: 10.2337/diabetes.52.5.1163. [DOI] [PubMed] [Google Scholar]
- 127.Bielby RC, et al. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Engineering. 2004 Sep-Oct;10:1518–25. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 128.Karp JM, et al. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006 Apr;24:835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 129.Kim JH, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002 Jul;418:50–6. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 130.Bjorklund LM, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proceedings of the National Academy of Sciences of the United States of America. 2002 Feb;99:2344–9. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang SC, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnology. 2001 Dec;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 132.Reubinoff BE, et al. Neural progenitors from human embryonic stem cells. Nature Biotechnology. 2001 Dec;19:1134–40. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 133.Schuldiner M, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Research. 2001 Sep;913:201–5. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 134.Manzar N, et al. The Ethical Dilemma of Embryonic Stem Cell Research. Science and Engineering Ethics. 2011:1–10. doi: 10.1007/s11948-011-9326-7. [DOI] [PubMed] [Google Scholar]
- 135.Bilic J, Izpisua Belmonte JC. Concise review: Induced pluripotent stem cells versus embryonic stem cells: Close enough or yet too far apart? Stem Cells. 2012 Jan;30:33–41. doi: 10.1002/stem.700. [DOI] [PubMed] [Google Scholar]
- 136.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009 Dec;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 137.Fluri DA, et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nature Methods. 2012 Mar; doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seki T, et al. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nature Protocols. 2012 Mar;7:718–728. doi: 10.1038/nprot.2012.015. [DOI] [PubMed] [Google Scholar]
- 139.Nagamoto Y, et al. The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3T3 cell sheets. Biomaterials. 2012 Jun;33:4526–4534. doi: 10.1016/j.biomaterials.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 140.Josowitz R, et al. Induced pluripotent stem cell-derived cardiomyocytes as models for genetic cardiovascular disorders. Current Opinion in Cardiology. 2011 May;26:223–229. doi: 10.1097/HCO.0b013e32834598ad. [DOI] [PubMed] [Google Scholar]
- 141.Tulloch NL, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 2011 May;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hussein SM, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011 Mar;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 143.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011 Mar;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011 Mar;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 145.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Engineering - Part A. 2009 Feb;15:205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dado D, et al. Mechanical control of stem cell differentiation. Regenerative Medicine. 2012 Jan;7:101–116. doi: 10.2217/rme.11.99. [DOI] [PubMed] [Google Scholar]
- 147.Hudalla GA, Murphy WL. Biomaterials that regulate growth factor activity via bioinspired interactions. Advanced Functional Materials. 2011 May;21:1754–1768. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toh WS, et al. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012 May;33:3835–3845. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 149.Sharma RI, Snedeker JG. Paracrine interactions between mesenchymal stem cells affect substrate driven differentiation toward tendon and bone phenotypes. PLoS ONE. 2012 Feb;7 doi: 10.1371/journal.pone.0031504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang G, et al. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomaterialia. 2010 Sep;6:3395–3403. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 151.Seidlits SK, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010 May;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 152.Minato A, et al. Cardiac differentiation of embryonic stem cells by substrate immobilization of insulin-like growth factor binding protein 4 with elastin-like polypeptides. Biomaterials. 2012 Jan;33:515–523. doi: 10.1016/j.biomaterials.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 153.Wylie RG, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nature Materials. 2011 Oct;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 154.Koffas M, et al. Metabolic engineering. Annual Review of Biomedical Engineering. 1999 Aug;1:535–557. doi: 10.1146/annurev.bioeng.1.1.535. [DOI] [PubMed] [Google Scholar]
- 155.Graf A, et al. Yeast systems biotechnology for the production of heterologous proteins. Fems Yeast Research. 2009 May;9:335–348. doi: 10.1111/j.1567-1364.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 156.Ruffing A, Chen RR. Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microbial Cell Factories. 2006 Jul;5 doi: 10.1186/1475-2859-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iwase A, et al. Manipulation of plant metabolic pathways by transcription factors. Plant Biotechnology. 2009 Mar;26:29–38. [Google Scholar]
- 158.Bailey JE, et al. Inverse metabolic engineering: A strategy for directed genetic engineering of useful phenotypes. Biotechnology and Bioengineering. 1996 Oct;52:109–121. doi: 10.1002/(SICI)1097-0290(19961005)52:1<109::AID-BIT11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 159.Tyo KE, et al. Expanding the metabolic engineering toolbox: more options to engineer cells. Trends in Biotechnology. 2007 Mar;25:132–137. doi: 10.1016/j.tibtech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 160.Godia F, Cairo JJ. Metabolic engineering of animal cells. Bioprocess and Biosystems Engineering. 2002 Jan;24:289–298. [Google Scholar]
- 161.Baik JY, et al. Metabolic engineering of Chinese hamster ovary cells: Towards a bioengineered heparin. Metabolic engineering. 2012 Mar;14:81–90. doi: 10.1016/j.ymben.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pouilly S, et al. Metabolic glycoengineering through the mammalian GalNAc salvage pathway. Febs Journal. 2012 Feb;279:586–598. doi: 10.1111/j.1742-4658.2011.08448.x. [DOI] [PubMed] [Google Scholar]
- 163.Du J, Yarema KJ. Carbohydrate engineered cells for regenerative medicine. Adv Drug Delivery Reviews. 2010 Jun;62:671–682. doi: 10.1016/j.addr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seth G, et al. Hu WSST, editor. Engineering cells for cell culture bioprocessing - Physiological fundamentals. Cell Culture Engineering. 2006;101:119–164. doi: 10.1007/10_017. [DOI] [PubMed] [Google Scholar]
- 165.Shanmugarajan TS, et al. Growth Factors and Signaling Pathways in the Chondrogenic Differentiation of Mesenchymal Stem Cells. Tissue Engineering and Regenerative Medicine. 2011 Jun;8:292–299. [Google Scholar]
- 166.Osborne R, et al. Understanding Metabolic Pathways for Skin Anti-Aging. Journal of Drugs in Dermatology. 2009 Jul;8:S4–S7. [PubMed] [Google Scholar]
- 167.Hofmann A, et al. Osteoblasts - Cellular and molecular regulatory mechanisms in fracture healing. Orthopade. 2009 Nov;38:1009–1019. doi: 10.1007/s00132-009-1488-5. [DOI] [PubMed] [Google Scholar]
- 168.Kurinna S, Barton MC. Cascades of transcription regulation during liver regeneration. International Journal of Biochemistry & Cell Biology. 2011 Feb;43:189–197. doi: 10.1016/j.biocel.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Majors BS, et al. Links between metabolism and apoptosis in mammalian cells: Applications for anti-apoptosis engineering. Metabolic Engineering. 2007 Jul;9:317–326. doi: 10.1016/j.ymben.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 170.Menendez L, et al. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proceedings of the National Academy of Sciences of the United States of America. 2011 Nov;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sackstein R. Glycoengineering of HCELL, the Human Bone Marrow Homing Receptor: Sweetly Programming Cell Migration. Annals of Biomedical Engineering. 2012 Apr;40:766–76. doi: 10.1007/s10439-011-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Derda R, et al. Multizone Paper Platform for 3D Cell Cultures. Plos One. 2011 May;6 doi: 10.1371/journal.pone.0018940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fox S, et al. High-Throughput Screening: Update on Practices and Success. Journal of Biomolecular Screening. 2006 Oct;11:864–869. doi: 10.1177/1087057106292473. [DOI] [PubMed] [Google Scholar]
- 174.Xu Y, et al. A Microfluidic Hydrogel Capable of Cell Preservation without Perfusion Culture under Cell-Based Assay Conditions. Advanced Materials. 2010 Jul;22:3017–3021. doi: 10.1002/adma.201000006. [DOI] [PubMed] [Google Scholar]
- 175.Lan SF, Starly B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicology and Applied Pharmacology. 2011 Oct;256:62–72. doi: 10.1016/j.taap.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 176.Shultz LD, et al. Humanized mice in translational biomedical research. Nature Reviews Immunology. 2007 Feb;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 177.Giese C, et al. Immunological substance testing on human lymphatic micro-organoids in vitro. Journal of Biotechnology. 2010 Jul;148:38–45. doi: 10.1016/j.jbiotec.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 178.Vrana NE, et al. Development of a Reconstructed Cornea from Collagen–Chondroitin Sulfate Foams and Human Cell Cultures. Investigative Ophthalmology & Visual Science. 2008 Dec;49:5325–5331. doi: 10.1167/iovs.07-1599. [DOI] [PubMed] [Google Scholar]
- 179.Sung JH, et al. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab on a Chip. 2011 Nov;11:389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 180.Huh D, et al. Reconstituting Organ-Level Lung Functions on a Chip. Science. 2010 Jun;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vozzi F, et al. Connected Culture of Murine Hepatocytes and HUVEC in a Multicompartmental Bioreactor. Tissue Engineering Part A. 2009 Jun;15:1291–1299. doi: 10.1089/ten.tea.2008.0066. [DOI] [PubMed] [Google Scholar]
- 182.Ingber D. ‘Organ-on-a-chip’ technology: on trial. Chemistry & Industry. 2011 Jul;:18–20. [Google Scholar]
- 183.Esch MB, et al. The Role of Body-on-a-Chip Devices in Drug and Toxicity Studies. In: Yarmush ML, et al., editors. Annual Review of Biomedical Engineering, Vol 13. Vol. 13. Annual Reviews; Palo Alto: 2011. pp. 55–72. [DOI] [PubMed] [Google Scholar]
- 184.Zhang C, et al. Towards a human-on-chip: Culturing multiple cell types on a chip with compartmentalized microenvironments. Lab on a Chip. 2009 Sep;9:3185–3192. doi: 10.1039/b915147h. [DOI] [PubMed] [Google Scholar]
- 185.Chia SM, et al. TGF-beta 1 regulation in hepatocyte-NIH3T3 co-culture is important for the enhanced hepatocyte function in 3D microenvironment. Biotechnology and Bioengineering. 2005 Mar;89:565–573. doi: 10.1002/bit.20372. [DOI] [PubMed] [Google Scholar]
- 186.Kim SB, et al. A cell-based biosensor for real-time detection of cardiotoxicity using lensfree imaging. Lab on a Chip. 2011 Nov;11:1801–1807. doi: 10.1039/c1lc20098d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Enejder A, et al. Periasamy A, et al., editors. CARS and SHG microscopy of artificial, bioengineered tissues. Multiphoton Microscopy in the Biomedical Sciences X. 2010;7569 [Google Scholar]
- 188.Brackmann C, et al. Visualization of the Cellulose Biosynthesis and Cell Integration into Cellulose Scaffolds. Biomacromolecules. 2010 Mar;11:542–548. doi: 10.1021/bm901153t. [DOI] [PubMed] [Google Scholar]
- 189.Choi JR, et al. Investigation of portable in situ fluorescence optical detection for microfluidic 3D cell culture assays. Optics Letters. 2010 May;35:1374–1376. doi: 10.1364/OL.35.001374. [DOI] [PubMed] [Google Scholar]
- 190.Tatosian DA, Shuler ML. A Novel System for Evaluation of Drug Mixtures for Potential Efficacy in Treating Multidrug Resistant Cancers. Biotechnology and Bioengineering. 2009 May;103:187–198. doi: 10.1002/bit.22219. [DOI] [PubMed] [Google Scholar]
- 191.Götz C, et al. Xenobiotic Metabolism Capacities Of Human Skin In Comparison to a 3D-epidermis model and keratinocyte-based cell culture as In vitro alternatives for chemical testing : phase ii enzymes. Experimental Dermatology. 2012 May;:358–363. doi: 10.1111/j.1600-0625.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 192.Jong Bin K. Three-dimensional tissue culture models in cancer biology. Seminars in Cancer Biology. 2005 Oct;15:365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 193.Ingber DE, et al. Tissue engineering and developmental biology: Going biomimetic. Tissue Engineering. 2006 Dec;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 194.Lee GY, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature Methods. 2007 Mar;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008 May;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sewell-Loftin MK, et al. EMT-Inducing Biomaterials for Heart Valve Engineering: Taking Cues from Developmental Biology. Journal of Cardiovascular Translational Research. 2011 Oct;4:658–671. doi: 10.1007/s12265-011-9300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Han B, et al. A cryoinjury model using engineered tissue equivalents for cryosurgical applications. Annals of Biomedical Engineering. 2005 Jul;33:972–982. doi: 10.1007/s10439-005-3478-z. [DOI] [PubMed] [Google Scholar]
- 198.Ueda J, et al. Large Effective-Strain Piezoelectric Actuators Using Nested Cellular Architecture With Exponential Strain Amplification Mechanisms. Ieee-Asme Transactions on Mechatronics. 2010 Oct;15:770–782. [Google Scholar]
- 199.Nagai M, et al. Chemical control of Vorticella bioactuator using microfluidics. Lab on a Chip. 2010 Oct;10:1574–1578. doi: 10.1039/c003427d. [DOI] [PubMed] [Google Scholar]
- 200.Akiyama Y, et al. Long-term and room temperature operable bioactuator powered by insect dorsal vessel tissue. Lab on a Chip. 2009 Aug;9:140–144. doi: 10.1039/b809299k. [DOI] [PubMed] [Google Scholar]
- 201.Xi J, et al. Self-assembled microdevices driven by muscle. Nature Materials. 2005 Jan;4:180–184. doi: 10.1038/nmat1308. [DOI] [PubMed] [Google Scholar]
- 202.Yamamoto Y, et al. Functional Evaluation of Artificial Skeletal Muscle Tissue Constructs Fabricated by a Magnetic Force-Based Tissue Engineering Technique. Tissue Engineering Part A. 2011 Jan;17:107–114. doi: 10.1089/ten.TEA.2010.0312. [DOI] [PubMed] [Google Scholar]
- 203.Horiguchi H, et al. Fabrication and Evaluation of Reconstructed Cardiac Tissue and Its Application to Bio-actuated Microdevices. Ieee Transactions on Nanobioscience. 2009 Dec;8:349–355. doi: 10.1109/TNB.2009.2035282. [DOI] [PubMed] [Google Scholar]
- 204.Ferrandez JM, et al. Human Neuroblastoma Cultures for Biorobotics. 2011 Sep;:6672–6675. doi: 10.1109/IEMBS.2011.6091645. [DOI] [PubMed] [Google Scholar]
- 205.Kubon M, et al. A Microsensor System to Probe Physiological Environments and Tissue Response. 2010 Ieee Sensors. 2010:2607–2611. [Google Scholar]
- 206.Brackmann C, et al. Non-linear microscopy of smooth muscle cells in artificial extracellular matrices made of cellulose. Journal of Biophotonics. 2012 May;5:404–414. doi: 10.1002/jbio.201100141. [DOI] [PubMed] [Google Scholar]