Abstract
More than one-fifth of the world’s population live in extreme poverty, where a lack of safe water and adequate sanitation enables high rates of enteric infections and diarrhoea to continue unabated. Although oral rehydration therapy has greatly reduced diarrhoea-associated mortality, enteric infections still persist, disrupting intestinal absorptive and barrier functions and resulting in up to 43% of stunted growth, affecting one-fifth of children worldwide and one-third of children in developing countries. Diarrhoea in children from impoverished areas during their first 2 years might cause, on average, an 8 cm growth shortfall and 10 IQ point decrement by the time they are 7–9 years old. A child’s height at their second birthday is therefore the best predictor of cognitive development or ‘human capital’. To this ‘double burden’ of diarrhoea and malnutrition, data now suggest that children with stunted growth and repeated gut infections are also at increased risk of developing obesity and its associated comorbidities, resulting in a ‘triple burden’ of the impoverished gut. Here, we Review the growing evidence for this triple burden and potential mechanisms and interventions that must be understood and applied to prevent the loss of human potential and unaffordable societal costs caused by these vicious cycles of poverty.
Introduction
The fact that children who live in poverty have disproportionately high levels of hunger and disease is an unaccept able reality. The World Bank estimates that 1.3 billion people (>20% of the world’s population) live in extreme poverty, most of whom are women and children who survive on less than US$1.25 per day.1,2 The gut, as the single largest interface of humans with their external environ ment, is unique among organ systems in its role in, and responses to, the challenges of diseases caused by poverty, undernutrition and their combinations. Highlighting this importance, the application of basic discoveries in intestinal sodium–glucose cotransport to oral rehydration therapy (ORT) has prevented millions of deaths from diarrhoea in the past four decades. However, the persistence of poor sanitation and crowded living conditions in developing countries continues to contribute to high rates of enteric infections, particularly among young children.3
When enteric infections lead to overt diarrhoea, they can cause a high mortality rate. Indeed, this rate exceeded 13.6 per 1,000 children <5 years (>23 per 1,000 children <1 year of age) in studies reviewed from 1955 to 1979, although it has improved to <5 per 1,000 children <5 years (<8 per 1,000 <1 year of age) since 1990, largely as a result of ORT.3 However, high morbidity rates of diarrhoeal illnesses continue unabated,3 and children living in developing areas continue to experience ongoing enteric infections, which contribute to long-term effects of stunted growth and impaired cognitive development. This interaction between infections and malnutrition has been recognized as a vicious cycle since classic work conducted by Scrimshaw et al.4 and Mata5 during the 1960s and 1970s. These studies showed that repeated diarrhoeal illnesses as well as other common childhood infections progressively altered the normal growth trajectories of children. Ultimately, poor growth and impaired cognitive development have been linked to societal effects on both productivity and ‘human capital’—a term used by Victora et al.6 to reflect long-term morbidity from impaired cognition and reduced productivity. Although the term ‘double burden’ has been applied to the two problems of malnutrition and obesity occurring in developing areas, we suggest that these problems are both related to early childhood enteric infections. Hence, we propose that the link between enteric infections and child growth and development is a double burden of enteric infections and malnutrition, and the potential link of both of these factors to obesity in later life is an interrelated ‘triple burden’ (Figure 1).7
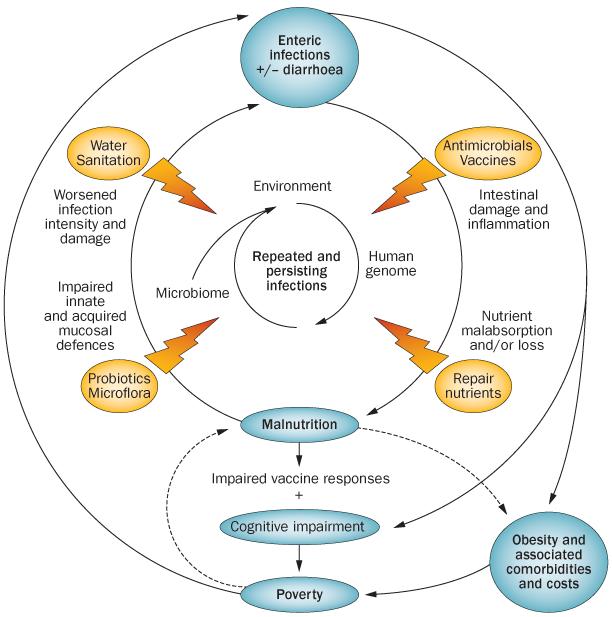
Figure 1.
The vicious cycles of diseases of poverty. Enteric infections, especially in the first 2–3 years of life, with or without overt diarrhoea, can predispose an individual to malnutrition and stunted growth through multiple mechanisms. Stunting by 2 years of age, in turn, is associated with impaired cognitive development that extends into later childhood and even adulthood and adult productivity. In addition, malnourished children experience both greater frequency and duration of diarrhoeal illnesses, and, documented in animal models, heavier infections. The latter is documented with Cryptosporidium and with enteroaggregative E. coli. Finally, enteric infections or stunting can predispose to obesity and its comorbidities of diabetes, hypertension, cardiovascular disease, metabolic syndrome and burgeoning health-care expenditures, contributing to individual and societal poverty in vicious cycles.
Mounting evidence now indicates that further links exist between enteric infections and poverty. Indeed, infections and stunting in early childhood might predispose to greater risk of obesity, type 2 diabetes, metabolic syndrome or cardio vascular disease (CVD) later in life, which are usually considered as major noncommunicable diseases. Therefore, potential, as yet not well defined, ‘thrift’ genes, which have a role in promoting fat storage to protect against starvation and signalling pathways responsible for catch-up growth—a term used to describe accelerated child growth after resolution of infections or under nutrition providing that diarrhoea burdens do not continue—(Figure 2) might increase an individual’s, and potentially their children’s risk, of obesity and associated comorbidities.8,9
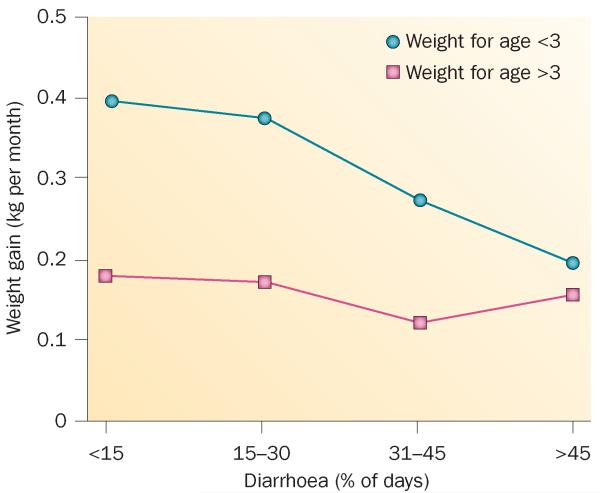
Figure 2.
Catch-up growth in malnourished children and its eradication by recurring diarrhoea. Malnourished children (that is, with weight-for-age <3 z-scores, less than three standard deviations below normal weight for age) tend to catch up with a doubling of weight gains, if they do not experience heavy diarrhoeal burdens (that is, <15% of their days are spent with diarrhoea in this observation period in the first 2 years of life). However, heavy diarrhoeal burdens are associated with a progressive ablation of this crucial catch-up growth. Permission obtained from Elsevier © Schorling, J. B. & Guerrant, R. L. Lancet 335, 599–600 (1990).
Here, we Review the epidemiology, intestinal pathophysiology and interventions for the double burden of infection and malnutrition, in which enteric infections and undernutrition follow each other in a vicious cycle to result in adverse acute and chronic health and developmental outcomes in children. We highlight emerging evidence for the triple burden of disease in survivors of the vicious enteric infection–malnutrition cycle.
The double burden
The concept of an impoverished gut provides compelling targets for potential interventions to break the infection–malnutrition cycle. Evidence for enteropathy (such as blunted small intestinal villi with lamina propria inflammation), functional impairment with increased intestinal permeability leading to bacterial or lipopolysaccharide translocation from the gut to the blood, as well as chronic systemic immune activation, has arisen from clinical and animal model studies of undernutrition and enteric infections. Lindenbaum and colleagues10-12 described malabsorption, weight loss and jejunitis in Peace Corps volunteers under going intestinal biopsies in the 1960s. This phenotype has come to be known as ‘environmental enteropathy’ or the ‘impoverished gut’ because of clear relationships between the setting itself (that is, tropical, developing areas with endemic enteric infections), the histological findings and the effects on gut function.
A review of work by Lunn and co-workers13-16 in Gambian children showed that mucosal enteropathy, as assessed by the lactulose:mannitol urinary excretion ratio—an indicator of intestinal permeability per available surface area—explained up to 43% of observed growth faltering.15,17 Furthermore, this increased intestinal permeability was a chronic condition, far exceeding the 7.3% of days over their first 2 years of life that these children spent with diarrhoea. Indeed, their lactulose:mannitol excretion ratios were associated with growth suppression on 76% of days during this period. In a follow-up study, total IgG antibody and anti-endotoxin core antibody (EndoCAb) were assessed as a marker of intestinal bacterial endotoxin translocation across a disrupted intestinal barrier.18 The weight-for-age z-score (WAZ) and height-for-age z-score (HAZ) anthropometry, lactulose:mannitol ratio and plasma EndoCAb levels were all similar in Gambian and UK infants at 2 months of age.18 By 15 months of age, however, the Gambian childrens’ HAZ and WAZ had fallen from mean values of −0.6 to −1.8, and −0.4 to −2.4, respectively; their lactulose:mannitol ratio almost tripled (by contrast, this ratio declined in UK children); and mean IgG and EndoCAb concentrations were twofold and fivefold higher in Gambian children than UK children. Lactulose:mannitol ratios, total IgG and EndoCAb concentrations were all correlated with each other and were negatively correlated with linear and ponderal growth, accounting for 51–56% of linear growth shortfalls.17,18
The causal relationships between infection and mal nutrition have been confirmed in mouse models in which enteric infections with Cryptosporidium or enteroaggregative E. coli species caused enteropathy and growth impairment.19-21 In addition, as infected mice showed heavy pathogen burdens and worsened intestinal damage and weight loss when malnourished (that is, milk deprived or protein deprived), these findings support causal relationships in the vicious cycle of enteric infection and malnutrition (Figure 1).19-21 Furthermore, weanling undernutrition itself perturbed small intestinal morphology and barrier function in a mouse model.22 Thus, although overt diarrhoea could account for ~25% of stunted growth,23 this vicious cycle of enteric infection and malnutrition often ‘smoulders’ as enteropathy for extended periods of time without overt diarrhoea in young children exposed to multiple enteric pathogens when adequate water and sanitation are lacking. Enteric infections in these children could therefore account for around half of all stunting, as well as the lasting effects on development.
This double burden stunts not only a child’s growth, but also cognitive development and full human potential, as well as the economic productivity and progress of the community. Christopher Eppig24 suggests that recognized improvements in national IQ seen with development of nations (the so-called Flynn effect) are a result of reductions in the burden of infectious diseases even when controlling for gross domestic product per capita and for malnutrition. Clearly a link exists between common, potentially preventable, infectious diseases (especially in early childhood), undernutrition and impaired cognitive development (Figure 1). Whether early childhood enteric infections have a direct effect on cognitive development that is independent of the effects through malnutrition (most notably HAZ at 2 years, HAZ-2) remains unclear. In either case, the importance of interventions that interrupt the vicious diarrhoea–malnutrition cycle and its double burden remains paramount.
Vicious cycles of poverty
Malnutrition, which can occur following famines and food shortages, illustrates the potential relevance of infections of poverty as the impoverished gut becomes impaired in its absorptive capacity by multiple and repeated enteric infections from contaminated water and inadequate sanitation. Indeed, Eppig24 suggests that infections themselves blunt human development. We suggest that the disrupted intestinal barrier and blunted absorptive function and common mucosal or even systemic inflammation that are seen with repeated enteric infections are pivotal points in the increasingly appreciated vicious cycles of poverty. In addition to mortality from acute enteric infections, early childhood infections are also linked with more than half of the 7.6 million deaths in children <5 years of age, caused in part by malnutrition and the life-long consequences of the moderate–severe stunting that occurs in 178 million children worldwide (20% of children worldwide; 32% of children in developing countries)25-32 (Box 1). From early childhood, diarrhoea accounts for substantial amounts of stunting observed worldwide. Indeed, a 20-year multicountry analysis revealed that five or more diarrhoeal infections in the first 2 years of life accounted for 25% of all stunting observed; moreover, every five diarrhoeal episodes increased stunting risk by 13%.23 These data indicate that diarrhoea and stunting combine to dramatically increase the global mortality, often seen with diseases such as pneumonia and malaria, as well as hinder human capital.6 The potential added burden of obesity, type 2 diabetes, and CVD (the triple burden) further compounds individual and societal costs (Figure 3).
Box 1. The global burden of infections and malnutrition.
-
■
Disability Adjusted Life Years incorporate both mortality (years of potential life lost) and morbidity (years lost to disability)
-
■
Global mortality of children <5 years is most commonly caused by respiratory diseases, diarrhoea, malaria and other illnesses, but over half of all deaths in young children are also associated with malnutrition
-
■
Although diarrhoea morbidity has declined over the past four decades, morbidity has not declined
-
■
The full, potential lifelong effect of enteric infections (that is, diarrhoea and stunting) on human development, productivity and chronic diseases is not adequately appreciated
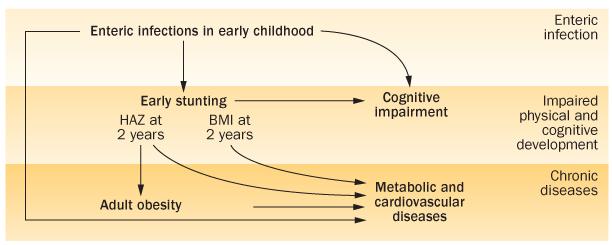
Figure 3.
Chronic consequences of early childhood enteric infections and stunting. The triple burden of enteric infections, impaired physical development (including low HAZ-2, or stunting and BMI-2) and cognitive development, and later life risk of obesity and its comorbidities are shown. Abbreviation: HAZ-2, height-for-age z-score at age 2 years.
Stunting and cognitive impairment
Evidence for role of enteric infections
The effects of enteric infections on stunting and cognitive impairment have been extensively reviewed elsewhere.6,7 Below, we summarize the key points of evidence in support of these relationships.
Stunting
Evidence for the effect of diarrhoea or enteric infections on growth failure stems from the classic studies in Guatemala, which showed that impoverished children often start off on a fairly good growth trajectory, similar to healthy children, only to be reduced after repeated diarrhoeal episodes and other infections (including respiratory as well as enteric infections) during the first 2 years of life.4,5,33,34 This pattern has also been observed in multiple studies over several decades in populations throughout Asia, Africa and Latin America.35,36 Early childhood diarrhoea was shown to have a specific effect on subsequent growth impairment in prospective studies in Northeast Brazil, Peru, Bangladesh, Ginea-Bissau and Ghana.23,37-41 Indeed, it is the crucial catch-up growth that is linearly ablated by progressively heavier diarrhoeal burdens (Figure 2) and malnourished children are at greater risk of both increased diarrhoea frequency and duration than better nourished children.5,8,37,42-44 As noted above, findings from mouse models have further confirmed the vicious infection–malnutrition cycle with specific pathogens.20,21,45
Stunting and cognition
The cognitive impairment associated with stunting at 2 years of age is clear from studies in the Philippines, Brazil, Peru, Jamaica, Thailand, Bangladesh and Guatemala.46-59 Not only does stunting delay schooling (with progressive delays of age at starting school by 1 year to ≥3 years in children with mild-severe stunting), but the cognitive (IQ) benefit of schooling is also reduced >25% by 11 years by stunting in early life (that is, HAZ-2 <2).52 Furthermore, a follow-up study was conducted in 1,448 individuals 25–35 years after feeding studies were carried out in four villages in Guatemala. The original feeding studies were conducted from 1969 to 1977 when the children were 0–7 years; children were randomly assigned to receive a calorie-protein supplement (91 kcal and 6.4 g protein per 100 ml) versus 33 kcal and no protein supplement in age-matched controls. Supplementation improved IQ (10%), wages (46%) and reading and schooling (8–20%) when compared with controls when assessed by Raven testing and follow-up visits, but only if the supplement was given in the first 2–3 years of life.60,61
Cognition
Whether enteric infections have an independent effect on cognitive development, through such mechanisms as chronic inflammation,46,53,54,62-66 in addition to their unambiguous indirect effects through growth impairment remains somewhat controversial. Fischer and colleagues have reviewed this evidence from careful studies done over time in Brazil, Philippines, Guatemala and Peru.67 Similar to Eppig’s suggestion that infections associate with impaired IQ independently of gross domestic product and education as well as malnutrition,24 we find that associations of early childhood diarrhoea with later cognitive impairment might include both stunting-dependent as well as stunting-independent correlations (R. L. Guerrant, A. A. M. Lima and R. C. Pinkerton, personal communication). However, many of the cognitive outcomes in studies of early childhood illness reflect the multifactorial origin of developmental delay that includes such factors as birthweight, household stimulation, and maternal behaviour. More studies are needed to clarify potential direct, as well as indirect effects, and mechanisms by which early childhood enteric infections might impair cognitive development.
The triple burden
Chronic, noncommunicable diseases, such as CVD and type 2 diabetes, have increased in incidence in the developing and the developed world, resulting in a growing need for resources.68,69 An emerging line of research links poor growth in foetal and early life to an increased risk of adult chronic disease. This research—often referred to as the developmental origins of health and disease69—had its beginnings in the findings of David Barker, who demonstrated that children born small for gestational age had increased risk of cardiovascular mortality, as well as multiple risk factors for CVD and type 2 diabetes.71-76 Nutrient deprivation is believed to have a role in these processes, as supported by data from the Dutch Hunger Winter of 1944–1945, when individuals, including pregnant women, had to survive on minimal food rations imposed by the government owing to food scarcity.77 Children born from these pregnancies were more likely than those conceived after the famine to become obese and hypertensive as adults.78 Although the mechanism behind these findings is under continued investigation, multiple researchers have hypothesized that nutrient deprivation in particular, as well as other potential insults including maternal stress78 and inflammation,79 during gestation result in epigenetic changes such as DNA methylation and histone acetylation, modifying expression of genes related to metabolism80 and growth, particularly insulin growth factor-2 (IGF-2)74 to prepare the individual for potential future caloric deficiencies. In contrast to the thrifty genotype, in which individuals inherit specific alleles contributing to early life metabolic advantage, the alteration in gene expression as a result of these epigenetic changes are hypothesized to produce a thrifty phenotype.9
Adult chronic disease risk Stunting
Research over the past 10 years has expanded the concept of the developmental origins of health and disease to investigate whether caloric deprivation, protein or micronutrient deficiencies, infection or other challenges in young children might affect long-term risk of future adult disease. Currently, the majority of published data on this topic relate to poor weight gain in early childhood and are centred on data from large retrospective evaluations of childhood weight patterns among individuals diagnosed in adulthood with prediabetes (glucose intolerance) or CVD. In these studies, individuals who developed these diseases had on average poor weight gain in early childhood (up to 2–3 years old), followed by rapid weight gain in later childhood starting around age 6 years.81-83 These studies followed long-term associations of low BMI in early life, without assessment of the aetiology behind the poor weight gain.83 One of the studies was performed in New Delhi, India, which, during the 1960s (when the study was conducted), had a high prevalence of enteric infections; whether enteric disease contributed to the children’s poor weight gain, however, remains unclear.82 Overall, these data provide inferential evidence of a causal link between poor early weight gain and later disease, although genetic factors could also have influenced both poor early childhood weight gain and later adult disease.
Further links between poor early weight gain and later disease are suggested by findings from cross-sectional studies, which demonstrate that stunting (as assessed by HAZ <2 for children or adults from regions with high rates of enteric infection) is associated with central obesity, high body fat, insulin resistance, hypertension, and low HDL cholesterol in adults.84-87 Changes related to stunting in blood pressure levels in both sexes88 and body habitus in girls89 were already noted in later childhood, whereas, among adults, most of the findings (with the exception of central obesity)87 were only noted among women84,85 and not men,86 suggesting that stunting-related alterations can be apparent early in life and might be affected by gender. The studies cited here were all performed in developing areas with a high prevalence of enteric infections, raising the potential that poor early weight gain was related to underlying enteropathy. Nevertheless, these studies are based on the evaluation of growth alone (and not enteric disease) and their cross-sectional nature has considerable drawbacks. There might have been confounding issues, such as differences in family environments, which influence both early growth and adult metabolic disease. Other studies performed during childhood—both using longitudinal and cross-sectional approaches—have not noted links between childhood stunting and later obesity90,91 and insulin resistance92 during childhood, emphasizing that before manifestation many of these effects might require additional metabolic challenge such as that seen during puberty and with lifestyle changes in early adulthood.
Perhaps the most notable evidence for the link between childhood growth and adult disease risk comes from long-term studies in developing countries evaluating future risk factors among children who exhibited poor growth in childhood. One such study from New Delhi, India, found negative correlations between BMI at age 2 years and glucose intolerance (a strong risk factor for future diabetes) at age 30 years.93 After adjustment for adult BMI, low BMI at age 2 years was strongly linked with insulin resistance, high triglyceride levels, hypertension, glucose intolerance and metabolic syndrome (a cluster of risk factors for CVD that frequently precede type 2 diabetes).93 Similarly, following adjustment for adult BMI, a multicountry analysis of long-term cohorts from five developing countries in four continents revealed links between low BMI at 2 years and high levels of fasting glucose and blood pressure at a mean age of 23 years of follow-up.6
Overall, the evidence of poor early weight gain among individuals who go on to develop CVD and glucose intolerance, associations between stunting and risk factors for CVD, and the association of low BMI at 2 years with CVD risk factors provides a basis for relationships between stunting or low BMI in childhood and risk of adult obesity and CVD (Figure 3). However, the causative mechanisms explaining these relationships remain uncertain, although the potential exists that nutrient deprivation in early life is associated with epigenetic changes in a manner similar to what has been postulated to link low birth weight and future CVD risk. Although this nutrient deprivation contributing to poor childhood growth could theoretically be a result of food scarcity among affected children, the strong link between childhood enteric disease and stunting discussed above is also likely to contribute.
Early infections
Additional data move beyond evaluating the risk of adult chronic diseases based on poor gains in weight or height in childhood to instead investigate potential links of childhood disease with the increase in adult risk factors. Follow-up of children in the Nutrition Institute of Central America and Panama (INCAP) revealed links between early childhood diarrhoea and low HDL cholesterol levels, elevated fasting glucose levels, and abdominal obesity in adulthood (aged 25–37 years).94,95 Febrile illness in early childhood was also associated with an increased risk of low HDL cholesterol levels, high levels of tri glycerides and metabolic syndrome in adulthood at ages 25–42 years.95 The association of diarrhoea frequency with adult CVD risk factors provides early evidence that childhood enteric infections have a direct link with adult CVD risk. As such, the previously mentioned links between adult CVD risk factors and low BMI at age 2 years could be related to upstream events such as childhood enteric disease (Figure 3). The association with febrile illnesses raises the possibility that inflammatory cytokines have a role in epigenetic changes, as has been demonstrated in the regulation of blood pressure.79 Nevertheless, further research is clearly needed to solidify these associations and clarify potential mechanisms, including whether aetiologic roots, including calorie deprivation, deficiencies in protein or micronutrients, inflammation or some other aetiology, are related to long-term risk of CVD.
Controlling vicious cycles
Biomarkers of the impoverished gut
Effective biomarkers of the impoverished gut are essential for our understanding of the causes, pathogenesis and patient responses to interventions. These bio markers need to be applicable in resource-limited community settings in which the impoverished gut develops, and where any effective interventions must ultimately apply. Simple noninvasive markers are therefore key, be they in faecal, urine, or blood specimens96,97 or in the medical histories or measurements of childrens’ clinical course and growth.98 Although several reports noted below and in Table 1 suggest that biomarkers hold promise,13-18 considerably further study is required to critically assess how specific infections and interventions alter selected biomarkers, in contrast to those altered by growth or particular nutrient deficiencies (Table 1).
Table 1.
Known and potential biomarkers of the impoverished gut or environmental enteropathy
| Assessing | Urine | Stool | Blood | Study |
|---|---|---|---|---|
| Damage to intestinal barrier and absorptive function |
Lactulose: mannitol ratio* |
α-1-antitrypsin | EndoCAb*, lipopolysaccharide or soluble CD14 |
Goto;13 Camilleri;99 Lima;100 Campbell;18 Lima;39 Petri;66 Barbosa;101 Rahaman;102 Brenchley;96 Sandler105 |
| ND | ND | Bacterial 16S rRNA | Jiang97 | |
| Creatinine | Zonulin | Zonulin | Fasano;112 Tripathi113 | |
| I-FABP | I-FABP | I-FABP | Sandler105 | |
| Intestinal inflammation | ND | Methylene blue stain detects faecal leukocytes using microscopy |
ND | Steiner;103 Masoodi;108 Langhorst;110 Campbell109 |
| ND | (Hemoccult) | ND | ||
| ND | Lactoferrin (nonbreastfed infants)* |
ND | ||
| ND | Myeloperoxidase | ND | ||
| Nitric oxide | IL-8 and other proinflammatory cytokines IL-8 mRNA |
ND | ||
| ND | Calprotectin Calprotectin mRNA |
ND | ||
| ND | Neopterin | ND | ||
| ND | SAA3 | ND | Reigstad111 | |
| Intestinal barrier repair | Citrulline | ND | Citrulline | Papadia116 |
| ND | Regl | ND | Peterson104 | |
| Small bowel overgrowth | ND | Lactulose breath test | ND | George;114 Esposito115 |
| Tissue biopsy to assess intestinal barrier disruption and inflammation |
ND | + Quantitative culture | ND | Lindenbaum;11 Gerson10 |
| Metabonome | ND | ND | Swan;106 Saric107 | |
| Field history | ND | Any prolonged or persistent diarrhoeal illness (>7 days)* |
ND | Lima;39 Moore98 |
Recognized biomarkers in published reports.
Abbreviations: EndoCAb, anti-endotoxin core antibody; I-FABP, fatty-acid binding protein, intestinal; ND, not determined; SAA3, serum amyloid A3 protein.
Established and experimental laboratory studies have shown the potential biomarkers in urine, stool, blood and potentially saliva to assess intestinal barrier function or damage, impaired absorptive function, intestinal or systemic inflammation or intestinal injury repair (Table 1). Thus far, the best established biomarkers from human clinical studies are urine lactulose:mannitol ratios,13,99,100 serum EndoCAb, faecal lactoferrin (if the child has not been breastfed), α-1-antitrypsin (A1AT) and Reg1.18,39,66,101-104 In addition, plasma bacterial DNA (16S rRNA gene) levels and/or plasma soluble CD14 or fatty-acid binding protein, intestinal (I-FABP) levels96,97,105 and urine metabonomics106,107 could provide alternative (or additional) markers for microbial translocation as a consequence of increased gut permeability.96,97 Other markers of inflammation or immune activation include faecal myeloperoxidase, neopterin, calprotectin or serum C-reactive protein or serum amyloid A protein;108-111 zonulin is being explored as a potential marker of inflammation.112,113 A lactulose breath test has also been used to assess small bowel bacterial overgrowth.114,115
Finally, plasma citrulline might be a quantitative biomarker of small bowel mass integrity that correlates with crypt depth and xylose absorption in HIV-associated villous atrophy in a tropical entero pathy population in Zambia.116 An example of the clinical utility of these biomarkers is provided by the substantial improvement in urinary lactulose excretion after 10 days of alanyl-glutamine therapy in undernourished children whose weight recovery improved up to 4 months after therapy.117 In addition, the simple assessment of any child experiencing a diarrhoeal illness extending beyond 7 or 14 days in duration (termed prolonged or persistent diarrhoea, respectively), who is at risk of heavy diarrhoeal burdens and growth shortfalls, thus warrants special attention. Provision of nutritional supplementation and potential targeted antimicrobial therapy should be considered, depending on the local predominant pathogens or specific test results, perhaps targeting protozoa or predominant bacterial pathogens, analogous to single dose albendazole therapy for the geohelminths.64,118 Zinc supplementation is currently recommended by the WHO for all episodes of childhood diarrhoea in developing countries.119 Some clinicians recommend a trial of nitazoxanide for persistent diarrhoea. Yogurt-based or amino-acid-based diets could also accelerate recovery from persistent diarrhoea in children120
Advances in interventions
Discovery of biomarker signatures that capture complex interactions of host factors, including nutritional status, intestinal barrier function, microbiome and inflammation in coordination with specific enteropathogens, will lead to novel understanding of microbial pathogenesis. We and others have described the effects of undernutrition, alone or in combination with enteric infections, on growth intestinal mucosal architecture, barrier function and tight junctions in mouse models.19-22,45,121,122 These models should be also examined for susceptibility to chronic diseases such as metabolic syndrome. Multiplex PCR assays123-125 have shown that children acquire an increasing array of enteropathogens in the first years of life; however, the presence of these pathogens is not always clearly associated with overt diarrhoea or growth failure, emphasizing a fundamental question: when is a gut microorganism a gut pathogen? We propose that a systems biology approach incorporating information about host and microbial genetics, nutrition and growth, epithelial homeostasis, inflammation and metabolomics is needed to untangle this web and point the way to novel interventions.
Novel interventions
Prevention
Preventive and therapeutic interventions were the focus of a 2009 WHO Report “Diarrhoea: Why are children still dying and what can be done”.119 These interventions included several measures that had a clear evidence base: prompt and adequate ORT; promotion of breast feeding; use of rotavirus vaccine and potentially other vaccines such as cholera vaccine; zinc and other nutritional therapies; and improved water and sanitation. Long-term investments in sanitation and hygiene represent the largest challenges, along with strengthening nutrition programmes, education and primary care in low-income and middle-income settings around the world. Thus, we should consider the importance of water, sanitation, micronutrients and vaccines as preventing not just diarrhoea, but also malnutrition, its developmental consequences and perhaps obesity in later life.
Therapy
Stopgap measures include improving the availability and efficacy of ORT, defining the optimal dosing and timing of micronutrient supplementation (such as zinc and vitamin A) and repair nutrient supplementation (such as glutamine or alanylglutamine) that have been shown to improve intestinal barrier function or weight gain in undernourished children.100,117 Probiotics deserve further study, although data from developing countries are limited. Extensive reviews of 16 randomized controlled trials in the Cochrane database showed a mean reduction in duration of diarrhoea by 29 h, approximate 4-day reductions in persistent diarrhoea and 13–14% reductions in diarrhoea incidence, as well as variably improved growth and vaccine-induced antibody production with probiotic treatment.126-128 In addition, an updated meta-analysis of 34 studies including >4,000 patients suggests nearly a 50% reduction in antibiotic-associated diarrhoea after probiotics.129 Certain infections involve anorexia and malabsorption compounded with faecal protein loss and febrile caloric consumption making its effect on malnutrition even worse. Helicobacter pylori has been variably associated with increased risk of diarrhoea, including shigellosis, perhaps via hypochlorhydria,130,131 but this association has been debated.132 Carefully targeted single-dose antimicrobial therapy (analogous to or including single-dose albendazole) might also warrant further study.64,118 Interventions to improve the efficacy of oral rotavirus and other enteric vaccines in low-income countries are needed.133 These potential approaches include novel adjuvants or mucosal repair nutrients that might improve intestinal mucosal function and help to optimize vaccine immunogenicity and protection.
Conclusions
Multiple preventive and therapeutic measures, including improved water and sanitation, ORT and micronutrient delivery, existing and new vaccines, hygiene education and innovative therapies such as probiotics, prebiotics, key nutrients and carefully targeted single-dose antimicrobial therapy will be needed to break the vicious cycles of poverty. The continued lack of adequate water and sanitation can now be seen to have increasingly costly consequences for the health of an individual and for societal budgets: a triple burden that compounds the costs of poverty through enteric infections, malnutrition and noncommunicable diseases. These costs thus become increasingly unaffordable, not to mention unconscionable. Indeed, we predict that multiple synergistic approaches to interrupt the vicious cycles of enteric infections, malnutrition and noncommunicable diseases will be required to reduce the human and societal costs.
Key points.
-
■
High diarrhoea rates continue unabated in developing countries, despite benefits from oral rehydration therapy in reducing mortality
-
■
One-fifth (178 million) children worldwide have stunted growth; early childhood enteric infections, with or without overt diarrhoea, are predicted to account for 25–43% of this burden
-
■
Malnutrition severe enough to cause stunting contributes to more than half of global mortality in children >5 years old, as well as to impaired cognitive development
-
■
Enteric infections and undernutrition each increase the risk of the other in a vicious cycle
-
■
Increasing data show that early childhood infections and stunting are associated with obesity and its comorbidities in later life, forming a triple burden of poverty
-
■
Enteric infections, malnutrition and noncommunicable diseases form vicious cycles with poverty that are best reduced using multiple approaches including improved water purity and availability, sanitation, vaccines and supplementary nutrients
Review criteria.
A search for original articles published between 1960 and 2012 and focusing on the effect of diarrhoea or enteric infections on long-term growth, cognition and chronic diseases was performed using the MEDLINE and PubMed databases. The search terms used were “diarrhoea”, “enteric disease”, “environmental enteropathy”, “tropical enteropathy”, “stunting”, “wasting”, “development”, “cognitive function”, “early childhood”, “obesity”, “metabolic syndrome” and “cardiovascular disease”, alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Acknowledgements
The authors’ collaborative work in this area is supported in part by NIH (ICIDR-UO1AI026512, FIC GIDRT D43TW006578) and the MAL-ED study, funded by an award from the Bill and Melinda Gates Foundation to the Foundation for the National Institutes of Health (FNIH). S. Moore is supported by an Independent Scientist in Global Health Award K02-TW008767 from the Fogarty International Center at NIH.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed equally to all aspects of producing this article.
References
- 1.Ravallioin M, Chen S, Sangraula P. Policy Research Working Paper No. 4620. Washington DC, USA: 2008. Dollar a day revisited. [Google Scholar]
- 2.The World Bank . The World Bank Poverty Overview. 2012. [online], http://www.worldbank.org/en/topic/poverty. [Google Scholar]
- 3.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monogr. Ser. World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- 5.Mata Leonardo J. The children of Santa Maria Cauque: a prospective field study of health and growth. MIT Press; Cambridge, USA: 1978. [Google Scholar]
- 6.Victora CG, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr. Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schorling JB, Guerrant RL. Diarrhoea and catch-up growth. Lancet. 1990;335:599–600. doi: 10.1016/0140-6736(90)90378-i. [DOI] [PubMed] [Google Scholar]
- 9.Stoger R. The thrifty epigenotype: an acquired and heritable predisposition for obesity and diabetes? Bioessays. 2008;30:156–166. doi: 10.1002/bies.20700. [DOI] [PubMed] [Google Scholar]
- 10.Gerson CD, Kent TH, Saha JR, Siddiqi N, Lindenbaum J. Recovery of small-intestinal structure and function after residence in the tropics. II. Studies in Indians and Pakistanis living in New York City. Ann. Intern. Med. 1971;75:41–48. doi: 10.7326/0003-4819-75-1-41. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Kent TH, Sprinz H. Malabsorption and jejunitis in American Peace Corps volunteers in Pakistan. Ann. Intern. Med. 1966;65:1201–1209. doi: 10.7326/0003-4819-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 12.Lindenbaum J, Gerson CD, Kent TH. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann. Intern. Med. 1971;74:218–222. doi: 10.7326/0003-4819-74-2-218. [DOI] [PubMed] [Google Scholar]
- 13.Goto R, Mascie-Taylor CG, Lunn PG. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br. J. Nutr. 2009;101:1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- 14.Goto R, Mascie-Taylor CG, Lunn PG. Impact of anti-Giardia and anthelminthic treatment on infant growth and intestinal permeability in rural Bangladesh: a randomised double-blind controlled study. Trans. R. Soc. Trop. Med. Hyg. 2009;103:520–529. doi: 10.1016/j.trstmh.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 16.Lunn PG. Growth retardation and stunting of children in developing countries. Br. J. Nutr. 2002;88:109–110. doi: 10.1079/BJNBJN2002652. [DOI] [PubMed] [Google Scholar]
- 17.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J. Nutr. 2003;133:1332–1338. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 19.Costa LB, et al. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of cryptosporidium infection and malnutrition. J. Infect. Dis. 2012;205:1464–1471. doi: 10.1093/infdis/jis216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coutinho BP, et al. Cryptosporidium infection causes undernutrition and, conversely, weanling undernutrition intensifies infection. J. Parasitol. 2008;94:1225–1232. doi: 10.1645/GE-1411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J. Infect. Dis. 2010;202:506–514. doi: 10.1086/654894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno PM, et al. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G612–G622. doi: 10.1152/ajpgi.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Checkley W, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int. J. Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppig C, Fincher CL, Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc. Biol. Sci. 2010;277:3801–3808. doi: 10.1098/rspb.2010.0973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Bhutta ZA, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 26.Black RE, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 27.Black RE, et al. Global, regional, and national causes of child mortality in 2008, a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 28.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 29.Guerrant RL, Kosek M, Lima AA, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002;18:191–193. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 30.Kosek M, et al. Directing diarrhoeal disease research towards disease-burden reduction. J. Health Popul. Nutr. 2009;27:319–331. doi: 10.3329/jhpn.v27i3.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 32.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 33.Mata LJ, Kronmal RA, Urrutia JJ, Garcia B. Antenatal events and postnatal growth and survival of children in a rural Guatemalan village. Ann. Hum. Biol. 1976;3:303–315. doi: 10.1080/03014467600001531. [DOI] [PubMed] [Google Scholar]
- 34.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr. Opin. Infect. Dis. 2011;24:496–502. doi: 10.1097/QCO.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrimpton R, Victora CG, de OM, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001;107:E75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- 36.Victora CG, de OM, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 37.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 38.Guerrant RL, et al. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J. Infect. Dis. 1983;148:986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- 39.Lima AA, et al. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J. Infect. Dis. 2000;181:1643–1651. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- 40.Moore SR, et al. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int. J. Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 41.Moore SR, Lima AA, Guerrant RL. Infection: Preventing 5 million child deaths from diarrhea in the next 5 years. Nat. Rev. Gastroenterol. Hepatol. 2011;8:363–364. doi: 10.1038/nrgastro.2011.103. [DOI] [PubMed] [Google Scholar]
- 42.Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am. J. Trop. Med. Hyg. 1992;47:28–35. doi: 10.4269/ajtmh.1992.47.28. [DOI] [PubMed] [Google Scholar]
- 43.Mata L. Diarrheal disease as a cause of malnutrition. Am. J. Trop. Med. Hyg. 1992;47:16–27. doi: 10.4269/ajtmh.1992.47.16. [DOI] [PubMed] [Google Scholar]
- 44.Schorling JB, McAuliffe JF, de Souza MA, Guerrant RL. Malnutrition is associated with increased diarrhoea incidence and duration among children in an urban Brazilian slum. Int. J. Epidemiol. 1990;19:728–735. doi: 10.1093/ije/19.3.728. [DOI] [PubMed] [Google Scholar]
- 45.Costa LB, et al. Cryptosporidium-malnutrition interactions: mucosal disruption, cytokines, and TLR signaling in a weaned murine model. J. Parasitol. 2011;97:1113–1120. doi: 10.1645/GE-2848.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer Walker CL, et al. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PLoS ONE. 2012;7:e7908. doi: 10.1371/journal.pone.0047908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adair LS, et al. Cohort profile: the Cebu longitudinal health and nutrition survey. Int. J. Epidemiol. 2011;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 49.Chang SM, Walker SP, Grantham-McGregor S, Powell CA. Early childhood stunting and later behaviour and school achievement. J. Child. Psychol. Psychiatry. 2002;43:775–783. doi: 10.1111/1469-7610.00088. [DOI] [PubMed] [Google Scholar]
- 50.Cusick SE, Georgieff MK. Nutrient supplementation and neurodevelopment: timing is the key. Arch. Pediatr. Adolesc. Med. 2012;166:481–482. doi: 10.1001/archpediatrics.2012.199. [DOI] [PubMed] [Google Scholar]
- 51.Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969-77) and its follow-up (1988–1989) J. Nutr. 1995;125(Suppl.):1027S–1041S. doi: 10.1093/jn/125.suppl_4.1027S. [DOI] [PubMed] [Google Scholar]
- 52.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J. Nutr. 1999;129:1555–1562. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 53.Niehaus MD, et al. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am. J. Trop. Med. Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 54.Patrick PD, et al. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child. Neuropsychol. 2005;11:233–244. doi: 10.1080/092970490911252. [DOI] [PubMed] [Google Scholar]
- 55.Pollitt E, Gorman KS, Engle PL, Martorell R, Rivera J. Early supplementary feeding and cognition: effects over two decades. Monogr. Soc. Res. Child. Dev. 1993;58:1–99. [PubMed] [Google Scholar]
- 56.Pongcharoen T, et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch. Pediatr. Adolesc. Med. 2012;166:411–416. doi: 10.1001/archpediatrics.2011.1413. [DOI] [PubMed] [Google Scholar]
- 57.Popkin BM, et al. Breast-feeding and diarrheal morbidity. Pediatrics. 1990;86:874–882. [PubMed] [Google Scholar]
- 58.Tarleton JL, et al. Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 2006;74:475–481. [PubMed] [Google Scholar]
- 59.Walker SP, Grantham-McGregor SM, Powell CA, Chang SM. Effects of growth restriction in early childhood on growth, IQ, and cognition at age 11 to 12 years and the benefits of nutritional supplementation and psychosocial stimulation. J. Pediatr. 2000;137:36–41. doi: 10.1067/mpd.2000.106227. [DOI] [PubMed] [Google Scholar]
- 60.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371:411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 61.Stein AD, et al. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Arch. Pediatr. Adolesc. Med. 2008;162:612–618. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ajjampur SS, et al. Effect of cryptosporidial and giardial diarrhoea on social maturity, intelligence and physical growth in children in a semi-urban slum in south India. Ann. Trop. Paediatr. 2011;31:205–212. doi: 10.1179/1465328111Y.0000000003. [DOI] [PubMed] [Google Scholar]
- 63.Guerrant DI, et al. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 64.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. Parasitic helminth infection and cognitive function in school children. Proc. Biol. Sci. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- 65.Partovi F, Khalili G, Kariminia A, Mahmoudzadeh-Niknam H. Effect of Giardia lamblia infection on the cognitive function of school children. Iranian J. Publ. Health. 2007;36:73–78. [Google Scholar]
- 66.Petri WA, Jr, et al. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer Walker CL, et al. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PLoS ONE. 2012;7:e47908. doi: 10.1371/journal.pone.0047908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008;93(Suppl. 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 69.Yach D, Hawkes C, Gould CL, Hofman KJ. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. 2004;291:2616–2622. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- 70.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol. Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 72.Barker DJ, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 73.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J. Clin. Endocrinol. Metab. 2002;87:4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 76.Williams S, St. George IM, Silva PA. Intrauterine growth retardation and blood pressure at age seven and eighteen. J. Clin. Epidemiol. 1992;45:1257–1263. doi: 10.1016/0895-4356(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 77.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 78.Roseboom TJ, et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol. Cell Endocrinol. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 79.Nilsson PM. Elevated blood pressure predicts type 2 diabetes, but why? J. Hypertens. 2008;26:1740–1741. doi: 10.1097/HJH.0b013e32830c6939. [DOI] [PubMed] [Google Scholar]
- 80.Tobi EW, et al. DNA methylation differences after exposure to prenatal famine are common and timing-and sex-specific. Hum. Mol. Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 82.Bhargava SK, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deboer MD, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr. Rev. 2012;70:642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferreira HS, et al. Short stature of mothers from an area endemic for undernutrition is associated with obesity, hypertension and stunted children: a population-based study in the semi-arid region of Alagoas, Northeast Brazil. Br. J. Nutr. 2009;101:1239–1245. doi: 10.1017/S0007114508059357. [DOI] [PubMed] [Google Scholar]
- 85.Florencio TT, Ferreira HS, Cavalcante JC, Stux GR, Sawaya AL. Short stature, abdominal obesity, insulin resistance and alterations in lipid profile in very low-income women living in Maceio, north-eastern Brazil. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14:346–348. doi: 10.1097/hjr.0b013e328010f24d. [DOI] [PubMed] [Google Scholar]
- 86.Florencio TT, Ferreira HS, Cavalcante JC, Sawaya AL. Short stature, obesity and arterial hypertension in a very low income population in North-eastern Brazil. Nutr. Metab. Cardiovasc. Dis. 2004;14:26–33. doi: 10.1016/s0939-4753(04)80044-9. [DOI] [PubMed] [Google Scholar]
- 87.Schroeder DG, Martorell R, Flores R. Infant and child growth and fatness and fat distribution in Guatemalan adults. Am. J. Epidemiol. 1999;149:177–185. doi: 10.1093/oxfordjournals.aje.a009784. [DOI] [PubMed] [Google Scholar]
- 88.Sesso R, Barreto GP, Neves J, Sawaya AL. Malnutrition is associated with increased blood pressure in childhood. Nephron Clin. Pract. 2004;97:c61–c66. doi: 10.1159/000078402. [DOI] [PubMed] [Google Scholar]
- 89.Grillol LP, et al. Lower resting metabolic rate and higher velocity of weight gain in a prospective study of stunted vs nonstunted girls living in the shantytowns of Sao Paulo, Brazil. Eur. J. Clin. Nutr. 2005;59:835–842. doi: 10.1038/sj.ejcn.1602150. [DOI] [PubMed] [Google Scholar]
- 90.Walker SP, Chang SM, Powell CA. The association between early childhood stunting and weight status in late adolescence. Int. J. Obes. 2007;31:347–352. doi: 10.1038/sj.ijo.0803383. [DOI] [PubMed] [Google Scholar]
- 91.Timaeus IM. Stunting and obesity in childhood: a reassessment using longitudinal data from South Africa. Int. J. Epidemiol. 2012;41:764–772. doi: 10.1093/ije/dys026. [DOI] [PubMed] [Google Scholar]
- 92.Martins VJ, Martins PA, Neves J, Sawaya AL. Children recovered from malnutrition exhibit normal insulin production and sensitivity. Br. J. Nutr. 2008;99:297–302. doi: 10.1017/S0007114507793959. [DOI] [PubMed] [Google Scholar]
- 93.Fall CH, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care. 2008;31:2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Margolis R. Childhood Morbidity and Health in Early Adulthood: life course linkages in a high morbidity context. Adv. Life Course Res. 2010;15:132–146. doi: 10.1016/j.alcr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Margolis R. University of Pennsylvania ScholarlyCommons: Repository. 2008. The effects of early childhood diseases on young adult health in Guatemala. [online], http://repository.upenn.edu/cgi/viewcontent.cgi?article=1019&context=parc_working_papers. [Google Scholar]
- 96.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu. Rev. Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore SR, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camilleri M, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol. Motil. 2010;22:e15–e26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lima AA, et al. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr. 2005;40:28–35. doi: 10.1097/00005176-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 101.Barboza Junior MS, Silva TM, Guerrant RL, Lima AA. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz. J. Med. Biol. Res. 1999;32:1499–1504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 102.Rahaman MM, Wahed MA. In: Diarrhea and Malnutrition: Interactions, Mechanisms and Interventions. Chen LC, Sears CL, editors. Plenum Press; New York and London: 1983. pp. 155–160. [Google Scholar]
- 103.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 104.Peterson KM, et al. The expression of REG 1A and REG 1B is increased during acute amebic colitis. Parasitol. Int. 2011;60:296–300. doi: 10.1016/j.parint.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swann JR, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl Acad. Sci. USA. 2011;108(Suppl. 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saric J, et al. Integrated cytokine and metabolic analysis of pathological responses to parasite exposure in rodents. J. Proteome Res. 2010;9:2255–2264. doi: 10.1021/pr901019z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masoodi I, et al. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J. Gastroenterol. Hepatol. 2009;24:1768–1774. doi: 10.1111/j.1440-1746.2009.06048.x. [DOI] [PubMed] [Google Scholar]
- 109.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J. Pediatr. Gastroenterol. Nutr. 2004;39:153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Langhorst J, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm. Bowel Dis. 2005;11:1085–1091. doi: 10.1097/01.mib.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 111.Reigstad CS, Lunden GO, Felin J, Backhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS ONE. 2009;4:e5842. doi: 10.1371/journal.pone.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012;10:1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tripathi A, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl Acad. Sci. USA. 2009;106:16799–16804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig. Dis. Sci. doi: 10.1007/s10620-012-2426-7. http://doi:10.1007/s10620-012-2426-7. [DOI] [PubMed] [Google Scholar]
- 115.Esposito I, et al. Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: an observation on non-absorbable antibiotics. World J. Gastroenterol. 2007;13:6016–6021. doi: 10.3748/wjg.v13.45.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Papadia C, et al. Plasma citrulline as a quantitative biomarker of HIV-associated villous atrophy in a tropical enteropathy population. Clin. Nutr. 2010;29:795–800. doi: 10.1016/j.clnu.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 117.Lima NL, et al. Wasting and intestinal barrier function in children taking alanyl-glutamine-supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr. 2007;44:365–374. doi: 10.1097/MPG.0b013e31802eecdd. [DOI] [PubMed] [Google Scholar]
- 118.Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Brigham H. Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. Am. J. Trop. Med. Hyg. 1989;41:78–87. [PubMed] [Google Scholar]
- 119.Wardlaw T, Salama P, Brocklehurst C, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;13:870–872. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- 120.Moore SR. Update on prolonged and persistent diarrhea in children. Curr. Opin. Gastroenterol. 2011;27:19–23. doi: 10.1097/MOG.0b013e32833f215d. [DOI] [PubMed] [Google Scholar]
- 121.Ladd FV, et al. Zinc and glutamine improve brain development in suckling mice subjected to early postnatal malnutrition. Nutrition. 2010;26:662–670. doi: 10.1016/j.nut.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitter SS, et al. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics (Sao Paulo) 2012;67:11–18. doi: 10.6061/clinics/2012(01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taniuchi M, et al. Development of a multiplex polymerase chain reaction assay for diarrheagenic Escherichia coli and Shigella spp. and its evaluation on colonies, culture broths, and stool. Diagn. Microbiol. Infect. Dis. 2012;73:121–128. doi: 10.1016/j.diagmicrobio.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Platts-Mills JA, Operario DJ, Houpt ER. Molecular diagnosis of diarrhea: current status and future potential. Curr. Infect. Dis. Rep. 2012;14:41–46. doi: 10.1007/s11908-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stroup S, et al. Dual probe DNA capture for sensitive real-time PCR detection of Cryptosporidium and Giardia. Mol. Cell Probes. 2012;26:104–106. doi: 10.1016/j.mcp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oberhelman RA, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J. Pediatr. 1999;134:15–20. doi: 10.1016/s0022-3476(99)70366-5. [DOI] [PubMed] [Google Scholar]
- 127.Preidis GA, et al. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology. 2011;140:8–14. doi: 10.1053/j.gastro.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guandalini S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011;45(Suppl.):S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 129.Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2012;35:1355–1369. doi: 10.1111/j.1365-2036.2012.05104.x. [DOI] [PubMed] [Google Scholar]
- 130.Passaro DJ, et al. Acute Helicobacter pylori infection is followed by an increase in diarrheal disease among Peruvian children. Pediatrics. 2001;108:E87. doi: 10.1542/peds.108.5.e87. [DOI] [PubMed] [Google Scholar]
- 131.Shmuely H, et al. Association of Helicobacter pylori infection with Shigella gastroenteritis in young children. Am. J. Gastroenterol. 2004;99:2041–2045. doi: 10.1111/j.1572-0241.2004.40120.x. [DOI] [PubMed] [Google Scholar]
- 132.Cohen D, Shoham O, Orr N, Muhsen K. An inverse and independent association between Helicobacter pylori infection and the incidence of shigellosis and other diarrheal diseases. Clin. Infect. Dis. 2012;54:e35–e42. doi: 10.1093/cid/cir916. [DOI] [PubMed] [Google Scholar]
- 133.Serazin AC, Shackelton LA, Wilson C, Bhan MK. Improving the performance of enteric vaccines in the developing world. Nat. Immunol. 2010;11:769–773. doi: 10.1038/ni0910-769. [DOI] [PubMed] [Google Scholar]