Abstract
Highly proliferative cells, including cancer cells, require a constant supply of molecular building blocks to support their growth. To acquire substrates such as glucose and amino acids from the extracellular space, dividing cells rely on transporter proteins in the plasma membrane. Numerous studies link transcriptional and post-translational control of nutrient transporter expression with proliferation, highlighting the importance of nutrient transporters in both physiologic and pathologic growth. Here we review recent work that spotlights the crucial role of nutrient transporters in cell growth and proliferation, discuss post-translational mechanisms for coordinating expression of different transporters, and consider the therapeutic potential of targeting these proteins in cancer and other diseases characterized by inappropriate cell division.
Keywords: nutrient transporters, proliferation, cancer, growth, glucose, amino acids
Proliferation creates nutrient demand that is met by elevated nutrient transporter expression
Rapidly dividing cells must not only replicate their genomes, but also accumulate the biomass necessary to make daughter cells. To support biosynthesis, proliferating cells import nutrients that both fuel ATP generation and allow the synthesis of building blocks for new cells. Lipids, nucleic acids, and non-essential amino acids are both acquired from the extracellular space and generated from metabolic intermediates. Although anabolic metabolism is considered a hallmark of cancer [1], many of these metabolic changes also occur in normal proliferating cells [2]. First described by Otto Warburg in the 1920s, the increased conversion of pyruvate to lactate under normoxic conditions in cancer cells was initially attributed to defects in mitochondrial respiration [3, 4]. Now, however, aerobic glycolysis is recognized as an adaptive strategy that provides the cell with biosynthetic precursors. As early as the 1950s, scientists identified increased glutamine consumption as another metabolic change characteristic of rapidly proliferating cells [5]. Somewhat surprisingly, the demand for glutamine in growing cells has little to do with increasing the amino acid pool for protein synthesis, as demonstrated by the excretion of ~50% of glutamine nitrogens from glioma cells [6]. Instead, rapidly proliferating normal lymphocytes, enterocytes, fibroblasts, in addition to many cancers, use glutamine for other important tasks, such as synthesizing the anti-oxidant glutathione, maintaining cellular NADPH pools, and fueling anaplerotic reactions to replenish TCA cycle intermediates [6, 7]. To summarize, “cancer metabolism” is in most cases the same as “proliferative metabolism.”
In multicellular organisms, amino acids and carbohydrates are plentiful in the extracellular milieu, but these polar molecules cannot cross the cell membrane without transporter proteins [3]. Accordingly, extrinsic growth signals are tightly linked to increased nutrient transporter expression. Growth factor signals transduced through the phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin (PI3K/Akt/mTOR) pathway up-regulate nutrient transporters (such as the glucose transporter GLUT1 and the amino acid transporter dimerization partner 4F2hc) at the transcriptional and post-transcriptional levels, permitting the influx of nutrients required for factor-dependent cell growth [8]. At the same time, pathways that regulate nutrient transporter expression also respond to nutrient availability; nutrient starvation triggers the adaptive up-regulation of transporters for the limiting nutrients, as demonstrated by the up-regulation of leucine transporters in prostate cancer lines or increased expression of cystine transporters in cultured cell lines that are cysteine-limited in vitro [9–11]. This adaptive response is likely conserved from unicellular organisms, which are regularly exposed to large fluctuations in extracellular nutrient levels. The dual regulation of nutrient transporter expression by growth signals and nutrients may make it difficult to distinguish between cause and effect when transporters are elevated during neoplastic growth. Up-regulation of nutrient transporters through increased pro-growth signaling may promote or enable the switch to anabolic metabolism, but inadequate perfusion of tumors may contribute to the observed increases in transporter expression. In this review we will discuss recent studies that demonstrate the important role that regulated nutrient transporter expression plays in driving proliferation.
Recent advances in our understanding of how metabolic changes support cancer initiation and progression have led to a push to develop drugs that target the specific anabolic pathways activated in various cancer classes [12]. This approach to therapy is likely to be selective, as most normal cells are more metabolically quiescent than cancer cells and better able to adapt to reductions in nutrient import. Cancer cells express constitutively-active anabolic oncogenes that lock them into a pro-growth metabolic profile and sensitize them to starvation. Cancer cells are also often autophagy deficient, further sensitizing them to nutrient limitation. While anabolic strategies can differ even within a tumor class, ability to genotype and phenotype individual tumors will increase the chance that therapies targeted to specific biosynthetic pathways will be successful. However, we propose that targeting nutrient transporter proteins, particularly the simultaneous targeting of multiple transporters, could be a more globally effective approach as all biosynthetic pathways depend on imported extracellular nutrients. Given that glucose and glutamine are critical carbon sources in cancer cells [2, 3, 7, 13], we will highlight current therapeutic strategies to block the activity of glucose and amino acid transporters as a means of limiting neoplastic cell growth. The challenges associated with this approach will also be discussed.
Amino acid and glucose transporters: necessary but not sufficient to drive proliferation
The role of glucose transporters in proliferation
Many rapidly proliferating cells depend heavily on glucose. Glucose and other hexose molecules cross the plasma membrane through either facilitated diffusion via a glucose transporter (GLUT) or by active transport through a sodium-glucose transporter (SGLT). The characteristics of selected glucose transporters known to have a role in promoting cell growth (Figure 1) are summarized in Table 1; additional details are available in recent and thorough reviews [14–18]. As the proximal step at which glucose metabolism can be regulated, glucose import appears to limit the growth rate of at least some cells. Consistent with this, glucose transporter expression levels are elevated in proliferating cells and in a wide variety of tumor types [14, 16, 19]. In fact, measuring the rate of glucose uptake via 18FDG-PET imaging allows for the detection and staging of tumors in patients, emphasizing the connection between glucose uptake and rapid cell growth [16].
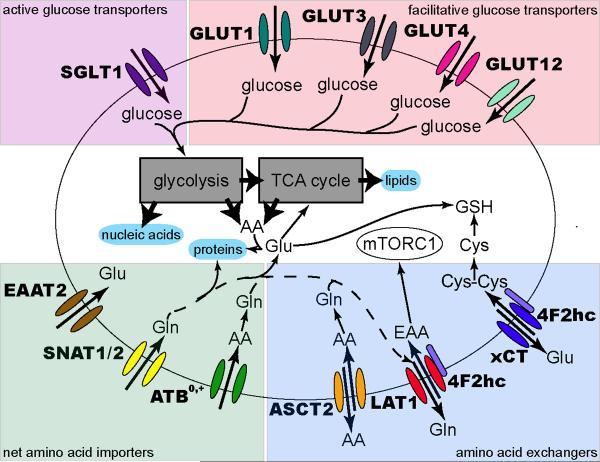
Figure 1. Nutrient transporters involved in proliferation.
Transporters clearly linked to cell growth are shown. Glucose imported through SGLTs or GLUTs feeds into glycolysis to promote biosynthesis and generate ATP. Net amino acid import through transporters including SNAT1, SNAT2, and ATB0,+ supplies glutamine that enters the TCA cycle and is used for glutathione synthesis. Additionally, these transporters supply glutamine and other amino acids that serve as exchange substrates for transporters such as ASCT2, 4F2hc/LAT1, and 4F2hc/xCT. EAA import through LAT1 activates pro-growth pathways through mTORC1, while cystine transported through xCT helps protect against oxidative stress by supporting glutathione (GSH) production. While glutamine is the indicated LAT1 exchange substrate, other amino acids may take its place. See Table 1 for all preferred transporter substrates. In all cases, co-transported ions have been omitted for simplicity.
Table 1.
Nutrient transporters implicated in cell growth Common names and SLC designations are both provided and amino acid substrates are given in single letter code. Signaling pathways known to affect the function of the transporter are designated as transcriptional (t) or post-transcriptional (p).
| Common name | SLC designation | Substrates | Normal distribution | Known pathways affecting function | Cancers over-expressing transporter | References |
|---|---|---|---|---|---|---|
| AMINO ACID EXCHANGERS | ||||||
| ASCT2 | SLC1A5 | A, S, C, T, Q, V, N | kidney, lung, skeletal muscle, large intestine, adipocytes | c-Myct | hepatoma, glioma | [38, 55, 64, 78] |
| xCT/4F2hc | SLC7A11/SLC3A2 | E, cystine | pancreas, brain | Nrf2t | pancreatic, gastrointestinal, brain, hepatocellular, leukemia, lymphoma | [10, 46, 48, 79] |
| LAT1/4F2hc | SLC7A5/SLC3A2 | L, I, F, M, Y, H, W, V (low affinity for Q,N) | brain, spleen, placenta, bone marrow | ATF4t, HIF2α | brain, colon, lung, liver, skin, prostate, stomach, larynx | [9, 38, 53, 79, 80] |
| LAT3 | SLC43A1 | L, I, M, F, V | androgen receptort | prostate | [9, 38, 81] | |
| CAT-1 | SLC7A1 | K, R, Ha | ubiquitous (except adult liver) | PKCp | glioma | [8, 38, 82] |
| NET AMINO ACID IMPORTERS | ||||||
| ATB0,+ | SLC6A14 | all neutral AAs | estrogent | colorectal, cervical, ER-positive breast | [83] | |
| SNAT1 | SLC38A1 | G, A, N, C, Q, H, M, S, Q | brain, retina, heart, placenta, adrenal gland | T-cell activationt | [38, 56, 84] | |
| SNAT2 | SLC38A2 | G, P, A, S, C, Q, N, H, M | neurons, placenta, adrenal glands, testes, thymus, muscle, liver, intestine, kidney, lung, adipose, spleen, skin | T-cell activationt,p | [38, 56, 84] | |
| SNAT4 | SLC38A4 | G, A, S, C, Q, N, M, T | liver, skeletal muscle, kidney, pancreas | [38, 84] | ||
| SNAT5 | SLC38A5 | G, N, H, A, S, Q | stomach, brain, liver, lung, small intestine, spleen, colon, kidney | c-Myct | [38, 55, 56, 84] | |
| EAAT2 | SLC1A2 | D, E | brain | gastric | [38, 50] | |
| PAT1/LYAAT-1 | SLC36A1 | G, P, A | ubiquitous | [85] | ||
| cystinosin | C, cystine | [38] | ||||
| GLUCOSE TRANSPORTERS | ||||||
| GLUT1 | SLC2A1 | glucose, 2-DG, galactose, mannose, glucosamine | ubiquitous | Rast, Srct, Fujinami sarcoma virust, HIF-1t, c-Myct, Aktt, estrogent, PKA-cAMPt, PI3K/Aktp, mTORt ,p53t | lymphoma, colorectal, hepatocellular, head and neck, gastric, prostate, thyroid, renal, lung, pancreatic, sarcoma, laryngeal, esophageal, brain, breast | [14–16, 24, 26, 27, 34, 63, 79] |
| GLUT3 | SLC2A3 | glucose, galactose, mannose, maltose, xylose | neurons, placenta, testes, white blood cells, sperm, pre-implantation embryo | p53t, HIF-1t, CAV1t, cAMPt, miR-195-5pp | breast, choriocarcinoma, ovarian, colorectal, retinoblastoma, rhabdomyosarcoma, lung, stomach, glioma, cervical, gallbladder, oral squamous cell, bladder | [14–16, 31, 32, 79, 86, 87] |
| GLUT4 | SLC2A4 | glucose, glucosamine | heart, adipose tissue, skeletal muscle | Insulin/PI3K/Aktp, p53t, AMPKP, PPARyt | astrocytic, lung, gastric, rhabdomyosarcoma, thyroid, multiple myeloma | [14, 15, 34, 35] |
| GLUT12 | SLC2A12 | glucose, fructose, galactose, 2-DG | heart, small intestine, skeletal muscle, prostate, kidney | mTORCp | prostate, breast | [14–16, 62] |
| SGLT1 | SLC5A1 | glucose, galactose | intestine, trachea, kidney, heart, brain, testes, prostate | cAMP-PKAt, p, PKCp, EGFRp | colorectal, head and neck, prostate, lung, pancreatic | [17, 79] |
Histidine is a good substrate for CAT1 only at low pH (~5.5) [82]
A consequence of the switch to pro-proliferative metabolic programs including glycolysis is the increased production of lactate. To combat decreased intracellular pH, cells up-regulate monocarboxylate transporters (MCTs) to pump out excess lactate; for example, MCT1 is up-regulated following the glycolytic switch associated with T cell activation and pharmacologically blocking this transporter prevents proliferation [20]. Lactate and other monocarboxylates may also be used for fuel in oxidative tissues, including the brain and skeletal muscle. While there is evidence to suggest that coordinated expression of MCT proteins contribute to a cell-cell lactate shuttle in which oxygenated cancer cells import and metabolize lactate released by their hypoxic neighbors [21], there are conflicting data regarding the expression of these proteins in cancer cells [22]. The contribution of MCT substrates to building biomass remains unclear and remains an important area for future investigation; readers are referred to recent reviews on MCTs and their role in cancer [20–22].
Glucose transporter 1 (GLUT1)
GLUT1 (SLC2A1) is considered the transporter responsible for basal glucose uptake in most tissues. In keeping with this, GLUT1 activity is necessary for the growth of many cell types and deleting GLUT1 results in embryonic lethality in mice [15, 23–25]. Studies in T cells and mouse mammary cancer lines indicate that GLUT1 expression may be limiting, as increasing GLUT1 expression increases growth [24, 26, 27]. In a mouse mammary tumor model, implantation of GLUT1 knock-down cells led to smaller tumors with a reduced proliferation index, while the injection of cells over-expressing GLUT1 led to increased tumor volume secondary to decreased apoptosis and increased proliferation [24]. However, as expected, GLUT1 over-expression is not sufficient to drive proliferation in the absence of exogenous growth signals [27]. Interestingly, single nucleotide polymorphisms (SNPs) within the promoter and introns of GLUT1 have been associated with the progression of clear-cell renal carcinoma and increased 18FDG uptake and higher rates of proliferation in breast cancer, suggesting that inborn variation in glucose transporter expression may predispose an individual to certain cancers [28, 29].
Other glucose transporters
Given that GLUT1 is ubiquitously expressed, its up-regulation in many transformed cells is not surprising. However, cancers also over-express other GLUT proteins, reactivating embryonic transporters or expressing proteins characteristic of other differentiated cell types [16]. For example, the high-affinity GLUT3 (SLC2A3) is over-expressed in many cancers (including colorectal, breast, and bladder cancers), even though it is normally expressed primarily in neurons [18, 30–32]. Reducing GLUT3 expression limits proliferation in a bladder cancer cell line [32] and GLUT3 knock-down reduces colorectal cancer cell growth [30]. Although colony formation by p53−/− murine embryonic fibroblasts is not affected by GLUT3 over-expression alone, GLUT3 over-expression further enhanced colony formation by these cells in the context of Ha-RasV12 expression [33]. The insulin-responsive GLUT4, which catalyzes the rate-limiting step of glucose uptake in insulin-sensitive tissues, may also play a role in cancer [15, 18]. While best known for its role in insulin resistance, obesity, and type II diabetes, GLUT4 (SLC2A4) is de-repressed in p53-driven cancers, leading to increased glucose uptake [34]. GLUT4 is also up-regulated and constitutively localized to the plasma membrane in multiple myeloma, whereas knock-down leads to cytostasis and cytotoxicity [35]. GLUT8 (SLC2A8) and GLUT11 (SLC2A11) are also up-regulated in myelomas, though the impact on glucose transport is not clear [35]. The role of these and other class II and class III glucose transporters needs to be better characterized.
Finally, SGLT1 (SLC5A1) may also increase glucose transport in some cancers. The epidermal growth factor receptor (EGFR) was recently shown to stabilize SGLT1 through a kinase-independent mechanism, the details of which remain to be elucidated [36]. Consistent with this finding, HDAC inhibitors that reduce EGFR expression produce a parallel decrease in SGLT1 levels and cellular glucose content [37]. The effect of SGLT1 over-expression in the absence of parallel EGFR over-expression has not been assessed. In summary, these studies demonstrate that glucose transporters are up-regulated in rapidly proliferating cells and are necessary, and in several cases limiting, for cell growth.
Amino acid transporters implicated in cell growth
In contrast to the two SLC families responsible for hexose import, there are 11 SLC families dedicated to the transport of amino acids. The biochemistry and disease association of many members of these families has recently been reviewed by Bröer and Palacin [38]; we will focus on the more limited array of amino acid transporters that have been implicated in cell growth (Table 1 and Figure 1).
The nutrient transporter chaperone 4F2hc
Expression of the heterodimeric amino acid transporters that are linked to the 4F2 heavy chain (4F2hc, CD98, or SLC3A2) has been tied to proliferation in many studies. 4F2hc is not itself a nutrient transporter. Rather, it is a type II membrane protein that dimerizes with a number of different light chains, including LAT1 (SLC7A5) and xCT (SLC7A11), and acts as a chaperone for the trafficking of these multipass transporter proteins to the plasma membrane [38]. 4F2hc has recently been reported to interact with and stabilize GLUT1 and it will be interesting to follow up on this finding [39]. 4F2hc is up-regulated in T cells upon activation and by cytokine stimulation (reviewed in [8]). While there is much data suggesting that 4F2hc promotes cell growth through effects on nutrient transport, it is important to consider that 4F2hc also binds to integrin β chains to regulate processes including cell spreading, proliferation, and growth [40]. Based on rescue experiments with a 4F2hc-CD69 chimera that associates with integrins but does not fully support isoleucine uptake, several studies have suggested that the integrin-binding function of 4F2hc is of primary importance during T and B cell proliferation [41, 42]. However, it is not clear that this chimera fully disrupts the ability of 4F2hc to promote import of all amino acids. In an in vitro T cell activation assay where integrin binding is unlikely to play a key role (plate-bound CD3 and CD28 antibodies plus IL-2), 4F2hc−/− T cells still exhibit severe proliferation defects [43]. Additionally, conditional 4F2hc knockout mice have been used to show that dextran sodium sulfate-induced colitis and tumorigenesis are less severe in the absence of 4F2hc [44]. While the effect of 4F2hc loss on amino acid uptake in this disease model was not considered, it was also not excluded. Similar to results with the conditional T cell knockout mouse, an antibody to 4F2hc surface domains blocked the development of type 1 diabetes in a mouse model; however, as T cells require 4F2hc for both the nutrient influx that supports proliferative metabolism and for integrin-mediated cell-cell contact and migration, the loss of both of these activities may contribute to the protection from experimental diabetes [43, 45]. In sum, as 4F2hc has a dual role in regulating amino acid transporter and β integrin activity, it is important to consider both effects when interpreting experimental results.
The xCT transporter
Another 4F2hc-associated amino acid transporter frequently up-regulated in cancers and activated T cells is xCT [46, 47]. Knocking down xCT inhibits cancer cell growth not by limiting amino acids for protein synthesis, but by compromising glutathione production by limiting cysteine availability [48]. xCT is over-expressed in a wide variety of cell lines in vitro; however, as standard cell culture conditions select for cells that can take up cystine, its expression pattern in vivo is likely more restricted [11]. While xCT−/− mice are relatively normal, xCT−/− fibroblasts require the reducing agent β-mercaptoethanol to proliferate in cell culture. Because xCT exchanges glutamate for cystine, gliomas over-expressing xCT may secrete sufficient glutamate to trigger excitotoxic death in the surrounding neurons [46]. The xCT transporter is also important in the interaction of chronic lymphocytic leukemia cells with their environment; bone marrow stromal cells convert cystine imported through xCT/4F2hc into cysteine, which is then released into the media and taken up by neighboring leukemia cells that express this transporter only at low levels [49]. Interestingly, a splice variant of CD44 found in some cancer cell lines interacts with and stabilizes xCT, increasing its surface expression and affording cells more protection from oxidative stress both in vitro and in vivo [10]. Another association between CD44 and amino acid transporter up-regulation that has recently emerged is a translocation discovered in gastric cancer that fuses CD44 with the high affinity glutamate transporter EAAT2 (SLC1A2); only 17 amino acids of the transporter are lost and the fusion protein appears to retain transport activity [50]. CD44-EAAT2 expression increased cellular glutamate levels and promoted both proliferation and colony formation by transformed cells.
Other plasma membrane amino acid transporters
Over-expression of other amino acid transporters has also been detected in many proliferating cells. The LAT3 transporter is elevated in hormone-dependent stages of prostate cancer, while the related 4F2 light chain LAT1 is up-regulated in metastatic and castration-resistant disease [9]. In the same study, over-expression of LAT1 or LAT3 increased the clonogenicity of LNCaP cells, suggesting that access to amino acids can limit cell growth. Like other 4F2hc heterodimeric amino acid transporters, the 4F2hc/LAT1 complex is an exchanger. Preferred LAT1 substrates include essential amino acids (EAAs) that enter the cell in exchange for the non-essential amino acids glutamine and alanine [51]. Although incapable of net amino acid import, up-regulation of 4F2hc/LAT1 in proliferating cells could increase uptake of EAAs. These amino acids are potent activators of mTORC1, a key regulator of growth and proliferation [52]. In fact, it has recently been demonstrated that pro-proliferative effects of HIF2α in lung and renal carcinoma cells result from mTORC1 activation subsequent to LAT1 up-regulation [53].
The Na+-dependent transporter ASCT2 (SLC1A5) works in tandem with 4F2hc/LAT1 by providing glutamine as an exchange substrate for EAA import [54]. Cancer cells also require glutamine to feed biosynthetic pathways and maintain redox balance through glutathione synthesis [7]. Accordingly, oncogenic transformation frequently up-regulates the expression of transporters like ASCT2 and of enzymes that capture glutamine for use in the TCA cycle [54, 55]. In activated T cells, the glutamine transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) are also up-regulated [56]. Another transporter that can increase net amino acid transport and appears to have an important role in cell growth and oncogenesis is ATB0,+ (SLC6A14); ATB0,+ expression is transcriptionally and translationally regulated by estrogen in breast cancer cells [57]. Given the broad substrate specificity of ATB0,+ and the ability of ATB0,+, SNAT1, and SNAT2 to increase net amino acid influx, it will be important to evaluate whether these transporters play a larger role in cancer than currently recognized. In summary, multiple amino acid transporters facilitate rapid cell growth and are up-regulated in cancer.
Lysosomal amino acid transporters
In addition to serving as biosynthetic precursors, amino acids also promote growth and proliferation by feeding into signaling pathways. As mentioned above, EAAs, especially leucine, are critical for the activation of mTORC1. Interestingly, it appears that lysosomal amino acids also play a key role in mTORC1 regulation [58, 59]. However, lysosomal amino acid transporters remain poorly characterized. To date, proton-coupled amino acid transporter 1 (PAT1 or SLC36A1) and cystinosin are the only human lysosomal amino acid transporters that have been defined [38], although PQLC2 has recently been identified as a lysosomal transporter of lysine and arginine based on homology to the C. elegans transporter LAAT-1 [60]. These transporters, along with others that have yet to be discovered, are likely to play important roles in promoting cell growth.
Nutrient transporter expression is coordinately regulated at the post-translational level
Numerous microarray studies have demonstrated that amino acid and glucose transporters are transcriptionally up-regulated in proliferating cells. However, the PI3K pathway coordinates uptake of glucose and amino acids in a growing cell by affecting the translation and trafficking of nutrient transporters as well. As reviewed elsewhere, GLUT1 expression and trafficking are tightly regulated by cytokines via PI3K during lymphocyte proliferation [8]; the PI3K pathway also post-transcriptionally regulates other glucose transporters, including GLUT12 and the insulin-regulated GLUT4 [8, 61, 62]. Downstream effectors in this signaling pathway including mTOR, the serine/threonine protein kinase glycogen synthase kinase 3 (GSK3), and tuberous sclerosis protein 2 (TSC2) also play a role in GLUT1 regulation in different cell types [8, 63]. Through effects on mTOR, the PI3K pathway also promotes the expression of the amino acid transporters 4F2hc, LAT1, and ASCT2 [8, 64]. The roles of other signaling pathways in nutrient transporter regulation are not as well characterized, although activation of PKA by cAMP up-regulates surface GLUT1 levels in proliferating murine stem cells and PKC activation down-regulates the cationic amino acid transporter CAT1 in gliomas [8, 26].
Sphingolipids provide a mechanism for coordinated, post-translational regulation of multiple nutrient transporters in both yeast and mammalian cells [25, 65–68]. The sphingolipid ceramide, produced in response to a number of stresses that trigger growth arrest in mammalian cells, inhibits proliferation in part by disrupting the trafficking of glucose transporters, including GLUT1 and GLUT4, and the amino acid transporters SNAT2, CAT1, and 4F2hc [25, 65]. While the molecular mechanism behind transporter down-regulation is not completely clear, the internalization of several of these proteins, including 4F2hc, LAT1, GLUT1, and basigin (CD147, a protein responsible for membrane localization of lactate transporters), occurs through a clathrin-independent endocytic pathway [69]. Thus, clustering of multiple nutrient transporters in the same membrane domains may allow for their co-regulation. This idea is further supported by the finding that ASCT2 and LAT1 associate with lactate transporters and cell proliferation factors such as epithelial cell adhesion molecule (EpCAM) in a CD147-4F2hc complex [38]. The fact that a screen for proteins trafficked through this clathrin-independent pathway did not identify other known ceramide targets could be due to low expression of these transporters in the HeLa system used by Eyster et al. or could indicate the presence of multiple ceramide-sensitive trafficking mechanisms.
More recently, the Donaldson lab has shown that upon leaving the cell surface, 4F2hc trafficking becomes ubiquitin-dependent [70]. A proteome-wide search for ubiquitylation sites identified multiple residues within nutrient transporters, including GLUT1, 4F2hc, LAT1, and xCT [71]. Similarly, phosphorylation sites have been identified for GLUT1, LAT1, and 4F2hc in a global analysis of cell-cycle dependent phosphorylation sites; in general, metabolic proteins were found to be heavily phosphorylated during M phase but not during S phase, suggesting that activity of nutrient transporters and other metabolic enzymes may be limited to periods of growth [72]. Nutrient transporter localization may also be modulated by glycosylation, as the PI3K pathway increases glucose uptake in T cells via glycosylation-mediated alterations in GLUT1 trafficking [27]. These findings suggest important goals for future studies, including identification of the kinases and ubiquitin ligases involved, the stimuli regulating these post-translational modifications, and the effect of these modifications on transporter activity and localization.
miRNA silencing may be yet another means to post-transcriptionally co-regulate nutrient transporters. miRNAs are known to regulate the levels of individual nutrient transporters; for example, GLUT3 and LAT1 are targeted by miR-195 and miR-126, respectively [32, 73]. The promiscuous pairing of miRNAs with their targets means that they can silence multiple mRNAs in the same pathway to elicit a coordinated response; a recent analysis of biochemical Gene Ontology and KEGG information for human miRNA targets demonstrated specificity to certain biochemical pathways [74]. Metabolic pathways were the most highly enriched for miRNA target sets, supporting the hypothesis that miRNAs are likely to coordinate nutrient transporter levels. Unfortunately, studies addressing the functional significance of post-translational regulation through phosphorylation, ubiquitylation, glycosylation, or miRNA silencing are in many cases limited by the lack of specific antibodies that can be used to follow trafficking or surface levels of the endogenous proteins. Given the increasing interest in the relationship between metabolism and neoplastic growth, it is likely that additional reagents will soon become commercially available.
Targeting nutrient transporters to limit pathological cell growth
Given the strong link between nutrient transporters and proliferation and the apical position of these proteins in metabolic pathways, nutrient transporters are intriguing, if challenging, pharmacologic targets in cancer and autoimmune disorders. The strategy of starving cancer cells of required amino acids has been proven effective based on the success of recombinant bacterial L-asparaginase in the treatment of acute lymphoblastic leukemia [75]. More recently, efforts have been directed at blocking nutrient import rather than availability. Several compounds that inhibit nutrient transport also prevent proliferation and induce cell death (Table 2). However, as nutrients are present at high concentrations in the blood, competitive inhibitors of these transporters requiring millimolar concentrations to block import in vitro are unlikely to be therapeutically useful. Furthermore, the full extent of off-target effects and broad activities expected of these compounds requires further study. Several compounds with low micromolar activity have been identified; additional information regarding the specificity and mechanism of action of these compounds is likely to be forthcoming. Finally, the recent publication of models for GLUT1-4 based on the structure of the XylE bacterial xylose transporter, which resembles the human glucose transporters, may aid future rational drug design, leading to novel inhibitors or improved efficacy of known compounds [76].
Table 2.
Chemical inhibitors of selected nutrient transporters
| Inhibitor | Transporter | Mechanism | Cell line | Ref |
|---|---|---|---|---|
| Phloretin | GLUT1-4, GLUT10, GLUT13 | inhibition | liver cancer cells and xenografts | [15, 88] |
| STF-31 | GLUT1 | inhibition | VHL negative RCC xenografts | [89] |
| WZB117 | GLUT1 | inhibition | lung cancer xenografts | [90, 91] |
| anti-GLUT1 mAb | GLUT1 | inhibition | breast cancer, lung cancer | [23] |
| HDACi | SGLT1 | destabilization and down-regulation through EGFR | colorectal cancer | [37] |
| Ceramide | 4F2hc, CAT-1,GLUT1, SNAT2 | down-regulation | murine hematopoietic, prostate cancer, cervical cancer (4F2hc, CAT-1, GLUT1) muscle (SNAT2) | [25, 65] |
| FTY720/AAL-149 | 4F2hc, GLUT1, CAT-1 | down-regulation | murine hematopoietic, prostate cancer, cervical cancer, mouse leukemia model | [77] |
| KYT-0353 (JPH203) | LAT1 | inhibition | colon adenocarcinoma cells and xenografts | [80] |
| anti-Lat1 mAb (SOL22) | LAT1/4F2hc | down-regulation | cervical cancer | [92] |
| anti-4F2hc mAb | 4F2hc | T cells | [45] | |
| BCH | LAT1, LAT2 | inhibition | prostate cancer, oral epidermoid carcinoma, osteogenic sarcoma, rat glioma | [9, 93] |
| Sulfasalazine | 4F2hc/xCT | inhibition | glioma, leukemia, lymphoma, prostate cancer, breast, colorectal cancer xenografts | [10, 79, 94, 95] |
| 4-carboxyphenylglycine | 4F2hc/xCT | glioma | [79] | |
| L-γ-glutamyl-p-nitroanilide (GPNA) | ASCT2 | inhibition | cervical cancer | [52] |
| α-Methyltryptophan | ATB0, + | inhibition | ER+ breast cancer cell | [57] |
It is important to recognize that any drug targeting a single transporter or transporter family is likely to promote the emergence of resistant cancers that have switched to an alternate fuel. Like successful combination therapies that target resistance pathways at the outset, simultaneously inhibiting both glucose and amino acid transporters would be a superior approach to limiting neoplastic growth. This is a key advantage of sphingolipid drugs modeled after the immunosuppressant Fingolimod (FTY720) and its analog AAL-149. While these compounds have other cellular effects that likely contribute to their efficacy, they simultaneously down-regulate GLUT1, CAT1, and 4F2hc to selectively kill cancer cells through a mechanism that closely parallels nutrient limitation [77]. These effects on nutrient transporter proteins are a key facet of their anti-cancer actions based on the protective effect of cell-permeant nutrients and the resistance of low nutrient-adapted cells to the drugs. As mentioned above, targeting transporters should be cancer-selective because normal cells are able to adapt to reduced nutrient influx. Compounds that inhibit nutrient transporters might also be combined with drugs that work through complementary mechanisms. For example, AAL-149 exhibits synergy with the autophagy blocker chloroquine; the combination kills even multi-drug resistant relapsed pre-B cell leukemias that are not sensitive to either compound alone [77]. Similarly, combining cisplatin and the xCT inhibitor sulfasalazine improves activity in colon cancer xenograft models, most likely because sulfasalazine sensitizes the cell to cisplatin-generated reactive oxygen species by reducing glutathione production [10]. Given their apical position in all biosynthetic pathways, nutrient transporter proteins could represent the Achilles' heel of cancer; drugs that target these proteins have the potential to be highly effective and broadly active, particularly in combination with existing therapies and it is therefore worth the effort to overcome the challenges associated with their identification and use.
Concluding remarks
While microarray data provide information about the transcriptional regulation of nutrient transporters, study of their translational and post-translational control is limited in part by the lack of antibodies that detect surface epitopes and recognize the endogenous protein in intact, fixed cells. Now that the genes behind the different transport activities have been defined, an important next step will be to develop and make available antibodies that can be used to study how and when these proteins are post-translationally modified, and the effect of phosphorylation, ubiquitylation, and glycosylation on transporter trafficking. Given that nutrient transporters are essential enablers of cancerous growth, it will also be important to further investigate the roles of less well-characterized nutrient transporters in growth control, particularly the concentrative amino acid transporters and glucose transporters beyond GLUT1 and GLUT4. A better understanding of how nutrient transporter trafficking is regulated should lead to the development of new strategies and additional compounds that can limit inappropriate cell growth in conditions such as autoimmunity and cancer (see Outstanding Questions Box). Given the ability of calorie restriction to extend health span, determining the effect of such compounds on aging would also be of interest.
Outstanding Questions Box
What are the relative contributions of the amino acid transport and integrin functions of 4F2hc in proliferating cells?
What is the role of concentrative amino acid transporters (SNATs, ATB0,+, EAAT2) that can increase net amino acid uptake in normal and pathological cell proliferation?
How do ubiquitylation, phosphorylation, and glycosylation regulate nutrient transporter trafficking? Which enzymes are responsible for these post-translational modifications?
Does the regulation of metabolism by miRNAs extend to the coordinated regulation of nutrient transporters?
How are amino acids transported out of the lysosome?
Acknowledgements
A.N.M. is supported by Grant Number T32CA009054 from the National Cancer Institute (NCI). A.L.E. is supported by grants from the NIH (R01 GM089919), the American Cancer Society (RSG-11-111-01-CDD), the Department of Defense (W81XWH-11-1-0535), and University of California, Irvine Council on Research, Computing, and Libraries (MIIG-2011-12-24).
GLOSSARY
- 4F2 cell-surface antigen heavy chain (4F2hc)
also called cluster of differentiation 98 heavy chain (CD98hc) / solute carrier family 3 member 2 (SLC3A2), is a ubiquitous cell surface transmembrane protein that associates with both amino acid transporters and integrins.
- System ASC amino acid transporter 2 (ASCT2)
also called ATB0 / SLC1A5, is a neutral amino acid exchanger frequently up-regulated in cancers.
- Sodium- and chloride-dependent neutral and basic amino acid transporter (ATB0,+)
also known as SLC6A14, is a transporter with specificity for all essential amino acids. Co-transport with H+, Na+, and Cl− allows net amino acid import. Expression is low in normal tissues but increased in some cancers.
- Basigin
or CD147 / extracellular matrix metalloproteinase inducer (EMMPRIN), is an ancillary protein required for proper targeting of monocarboxylate transporters 1, 3, and 4.
- High affinity cationic amino acid transporter 1 (CAT1)
or SLC7A1, is a ubiquitously expressed (with the exception of liver) cationic amino acid transporter.
- Cystinosin
A lysosomal transmembrane protein that transports cystine and cysteine.
- Excitatory amino acid transporter 2 (EAAT2)
or SLC1A2, is a protein that transports glutamate and aspartate across the plasma membrane in an Na+, H+, and K+-dependent manner. Its primary function is in neurotransmitter re-uptake, but it is expressed in some cancers as a fusion protein.
- Glucose transporter 1 (GLUT1)
also called solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is the first protein that facilitates the transport of glucose across the plasma membrane to be characterized. It is ubiquitously expressed and highly conserved.
- Glucose transporter 3 (GLUT3)
or SLC2A3, is a high-affinity glucose transporter that is highly expressed in cell types with elevated glucose demand, including neurons, testes, and tumor cell lines.
- Glucose transporter 4 (GLUT4)
or SLC2A4, is an insulin-sensitive glucose transporter expressed in cardiac and skeletal muscle and adipose tissue.
- Large neutral amino acid transporter small subunit 1 (LAT1)
also called SLC7A5, is a transporter that associates with 4F2hc to exchange bulky and aromatic amino acids across the plasma membrane. Over-expression of the LAT1/4F2hc dimer is linked to proliferation in a number of cancers.
- Monocarboxylate transporters 1–4 (MCT1-4)
belong to the SLC16 family of proteins that transport monocarboxylates such as lactate, pyruvate, and ketone bodies bi-directionally across the membrane in a proton-dependent manner. MCT1, 3, and 4 required the chaperone basignin for proper localization.
- Proton-coupled amino acid transporter 1 (PAT1)
also known as lysosomal amino acid transporter 1 (LYAAT1) / SLC36A1,,is a broadly expressed, low-affinity, pH-dependent transporter of glycine, proline, and alanine, localized to the lysosomal membrane.
- Sodium/glucose co-transporter 1 (SGLT1)
also called SLC5A1, is the first active transporter of glucose to be identified. Its primary function is glucose absorption in the intestine.
- Sodium-coupled neutral amino acid transporter 1 and 2 (SNAT1/2)
also known as SLC38A1 and SLC28A2 are transporter proteins that use the Na+ gradient to concentrate neutral amino acids within the cell. SNAT2 expression is nearly ubiquitous, while SNAT1 expression is more limited.
- Cystine/glutamate transporter (xCT)
also called SLC7A11, is a transporter that forms the amino acid transport system xc− when coupled with 4F2hc. It exchanges cystine for glutamate in the pancreas and brain, but is frequently up-regulated in cancer and cultured cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, et al. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O. The Metabolism of Carcinoma Cells. The Journal of Cancer Research. 1925;9:148–163. [Google Scholar]
- 5.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 6.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edinger AL. Controlling cell growth and survival through regulated nutrient transporter expression. Biochem J. 2007;406:1–12. doi: 10.1042/BJ20070490. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71:7525–7536. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 10.Ishimoto T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Lo M, et al. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 12.Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Valencia I, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adekola K, et al. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012 doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augustin R. The protein family of glucose transport facilitators: It's not only about glucose after all. IUBMB Life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 16.Yeluri S, et al. Cancer's craving for sugar: an opportunity for clinical exploitation. J Cancer Res Clin Oncol. 2009;135:867–877. doi: 10.1007/s00432-009-0590-8. [DOI] [PubMed] [Google Scholar]
- 17.Wright EM, et al. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 18.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buller CL, et al. GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am J Physiol Renal Physiol. 2011;301:F588–596. doi: 10.1152/ajprenal.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halestrap AP, Wilson MC. The monocarboxylate transporter family--role and regulation. IUBMB Life. 2012;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 21.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–732. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro C, et al. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–139. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi S, et al. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007;257:244–251. doi: 10.1016/j.canlet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Young CD, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenther GG, et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MO, et al. PKA and cAMP stimulate proliferation of mouse embryonic stem cells by elevating GLUT1 expression mediated by the NF-kappaB and CREB/CBP signaling pathways. Biochim Biophys Acta. 2012;1820:1636–1646. doi: 10.1016/j.bbagen.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page T, et al. Glucose transporter polymorphisms are associated with clear-cell renal carcinoma. Cancer Genet Cytogenet. 2005;163:151–155. doi: 10.1016/j.cancergencyto.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Grabellus F, et al. The XbaI G>T polymorphism of the glucose transporter 1 gene modulates 18F-FDG uptake and tumor aggressiveness in breast cancer. J Nucl Med. 2010;51:1191–1197. doi: 10.2967/jnumed.110.075721. [DOI] [PubMed] [Google Scholar]
- 30.Ha TK, et al. Caveolin-1 Increases Aerobic Glycolysis in Colorectal Cancers by Stimulating HMGA1-Mediated GLUT3 Transcription. Cancer Res. 2012;72:4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- 31.Meneses AM, et al. Regulation of GLUT3 and glucose uptake by the cAMP signalling pathway in the breast cancer cell line ZR-75. J Cell Physiol. 2008;214:110–116. doi: 10.1002/jcp.21166. [DOI] [PubMed] [Google Scholar]
- 32.Fei X, et al. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586:392–397. doi: 10.1016/j.febslet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Kawauchi K, et al. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 34.Calvo MB, et al. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int J Endocrinol. 2010;2010 doi: 10.1155/2010/205357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBrayer SK, et al. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012;119:4686–4697. doi: 10.1182/blood-2011-09-377846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weihua Z, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou CW, et al. HDAC inhibition decreases the expression of EGFR in colorectal cancer cells. PloS one. 2011;6:e18087. doi: 10.1371/journal.pone.0018087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broer S, Palacin M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 2011;436:193–211. doi: 10.1042/BJ20101912. [DOI] [PubMed] [Google Scholar]
- 39.Ohno H, et al. 4F2hc stabilizes GLUT1 protein and increases glucose transport activity. Am J Physiol Cell Physiol. 2011;300:C1047–1054. doi: 10.1152/ajpcell.00416.2010. [DOI] [PubMed] [Google Scholar]
- 40.Cantor JM, et al. Integrin-associated proteins as potential therapeutic targets. Immunol Rev. 2008;223:236–251. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 41.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantor J, et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nature immunology. 2009;10:412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantor J, et al. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J Immunol. 2011;187:851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen HT, et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lian G, et al. Manipulation of CD98 resolves type 1 diabetes in nonobese diabetic mice. J Immunol. 2012;188:2227–2234. doi: 10.4049/jimmunol.1102586. [DOI] [PubMed] [Google Scholar]
- 46.Lo M, et al. The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008;99:464–472. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levring TB, et al. Activated human CD4 T cells express transporters for both cysteine and cystine. Sci Rep. 2012;2:266. doi: 10.1038/srep00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W, et al. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer letters. 2011;312:55–61. doi: 10.1016/j.canlet.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao J, et al. CD44-SLC1A2 gene fusions in gastric cancer. Sci Transl Med. 2011;3:77–30. doi: 10.1126/scitranslmed.3001423. [DOI] [PubMed] [Google Scholar]
- 51.Broer S. Adaptation of plasma membrane amino acid transport mechanisms to physiological demands. Pflugers Arch. 2002;444:457–466. doi: 10.1007/s00424-002-0840-y. [DOI] [PubMed] [Google Scholar]
- 52.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elorza A, et al. HIF2alpha Acts as an mTORC1 Activator through the Amino Acid Carrier SLC7A5. Mol Cell. 2012;48:681–691. doi: 10.1016/j.molcel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karunakaran S, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogmundsdottir MH, et al. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One. 2012;7:e36616. doi: 10.1371/journal.pone.0036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoncu R, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B, et al. LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science. 2012;337:351–354. doi: 10.1126/science.1220281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasaki-Suzuki N, et al. Growth hormone inhibition of glucose uptake in adipocytes occurs without affecting GLUT4 translocation through an insulin receptor substrate-2-phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2009;284:6061–6070. doi: 10.1074/jbc.M808282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson-O'Brien AL, et al. Mitogen-stimulated and rapamycin-sensitive glucose transporter 12 targeting and functional glucose transport in renal epithelial cells. Endocrinology. 2008;149:917–924. doi: 10.1210/en.2007-0985. [DOI] [PubMed] [Google Scholar]
- 63.Buller CL, et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836–843. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs BC, et al. ASCT2 silencing regulates mammalian target-of-rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol Cell Physiol. 2007;293:C55–63. doi: 10.1152/ajpcell.00330.2006. [DOI] [PubMed] [Google Scholar]
- 65.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daquinag A, et al. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol. 2007;27:633–650. doi: 10.1128/MCB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowart LA, Obeid LM. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim Biophys Acta. 2007;1771:421–431. doi: 10.1016/j.bbalip.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bultynck G, et al. Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol. 2006;26:4729–4745. doi: 10.1128/MCB.01973-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eyster CA, et al. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eyster CA, et al. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol Biol Cell. 2011;22:3218–3230. doi: 10.1091/mbc.E10-11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Guan KL. Amino acid signaling in TOR activation. Annu Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 72.Olsen JV, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 73.Miko E, et al. miR-126 inhibits proliferation of small cell lung cancer cells by targeting SLC7A5. FEBS Lett. 2011;585:1191–1196. doi: 10.1016/j.febslet.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Backes C, et al. A dictionary on microRNAs and their putative target pathways. Nucleic Acids Res. 2010;38:4476–4486. doi: 10.1093/nar/gkq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Berg H. Asparaginase revisited. Leuk Lymphoma. 2011;52:168–178. doi: 10.3109/10428194.2010.537796. [DOI] [PubMed] [Google Scholar]
- 76.Sun L, et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature. 2012;490:361–366. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 77.Romero Rosales K, et al. Sphingolipid-based drugs selectively kill cancer cells by down-regulating nutrient transporter proteins. Biochem J. 2011;439:299–311. doi: 10.1042/BJ20110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 79.Ganapathy V, et al. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Oda K, et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer science. 2010;101:173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukuhara D, et al. Protein characterization of NA+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. Am J Pathol. 2007;170:888–898. doi: 10.2353/ajpath.2007.060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hatzoglou M, et al. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 2004;24:377–399. doi: 10.1146/annurev.nutr.23.011702.073120. [DOI] [PubMed] [Google Scholar]
- 83.Karunakaran S, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 85.Thwaites DT, Anderson CM. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br J Pharmacol. 2011;164:1802–1816. doi: 10.1111/j.1476-5381.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ha TK, et al. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012;72:4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- 87.Simpson IA, et al. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu CH, et al. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210–2219. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- 89.Chan DA, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94–70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, et al. Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 2010;298:176–185. doi: 10.1016/j.canlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Ohno Y, et al. Production and characterization of highly tumor-specific rat monoclonal antibodies recognizing the extracellular domain of human L-type amino-acid transporter 1. Cancer Sci. 2008;99:1000–1007. doi: 10.1111/j.1349-7006.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim CS, et al. BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull. 2008;31:1096–1100. doi: 10.1248/bpb.31.1096. [DOI] [PubMed] [Google Scholar]
- 94.Doxsee DW, et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- 95.Guo W, et al. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett. 2011;312:55–61. doi: 10.1016/j.canlet.2011.07.024. [DOI] [PubMed] [Google Scholar]