Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the treatment of choice for patients with chronic myelogenous leukemia (CML) who have failed or are intolerant to tyrosine kinase inhibitors (TKI). Myeloablative conditioning regimens have been associated with high treatment-related mortality (TRM) rate in such patients, and reduced-intensity conditioning (RIC) regimens are often preferred but have high rates of disease recurrence and graft-versus-host-disease (GVHD). We report our experience with nine CML patients (four chronic phase and five with accelerated phase or blast crisis) who failed TKI and underwent allo-HSCT using an alemtuzumab-based RIC regimen. The conditioning regimen was well tolerated and induced engraftment in all patients, and complete cytogenetic remission (CCyR) in eight of nine. Four patients, all with a history of accelerated phase or blast crisis, died. Four of the five remaining patients had a cytogenetic relapse a median of 10 months after transplantation. Donor lymphocyte infusion (DLI), TKI or both induced a CCyR in all cases. With a median follow-up of 47 months, five patients, including all those transplanted in first or second chronic phase, are alive and in remission. Allo-HSCT with an alemtuzumab-based conditioning regimen induces remission in patients with CML that have failed TKI therapy and has a low incidence of GVHD. Disease recurrence is frequent but responds to DLI. In some cases, restoration of susceptibility to TKI was observed. Outcomes may improve with the routine administration of post-transplant TKI.
Keywords: Chronic myelogenous leukemia, reduced-intensity conditioning, alemtuzumab, tyrosine kinase inhibitors
Introduction
Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) is still considered the only curative therapy in chronic myelogenous leukemia (CML), it is currently mainly used for patients who failed, or are intolerant to imatinib or other tyrosine kinase inhibitors (TKI) [1,2]. Such patients tend to have higher complication rates and decreased survival after myeloablative transplantation [3–5]. Reduced-intensity conditioning (RIC) regimens were developed to allow allo-HSCT in older patients and those with co-morbidities. CML patients treated with RIC have acceptable treatment-related mortality (TRM), and overall survival but a high incidence of graft-versus-host-disease (GVHD) as well as higher relapse rate [6,7]. Alemtuzumab is a humanised monoclonal antibody directed against CD52 antigen, expressed on T and B lymphocytes, as well as other non-target cells [8]. Widely used in chronic lymphocytic leukemia, there is now mounting evidence of its benefits in stem cell transplantation, particularly in the prevention of acute and chronic GVHD [7,9–12]. Here, we report our experience with alemtuzumab-based RIC regimens in nine consecutive patients with CML.
Patients and results
Between March 2002 and December 2007, nine patients with CML underwent allo-HSCT using an alemtuzumab-based conditioning at the University of Chicago Medical Center. One additional patient left the hospital after allo-HSCT against medical advice on Day 18 and explicitly refused follow-up. He is not included in this analysis. All patients were enrolled on treatment protocols approved by the Institutional Review Board and had provided written informed consent.
Interval from diagnosis to transplantation ranged from 10 to 156 months. As shown in Table I, most patients were extensively pretreated and had a high comorbidity score and decreased performance status at the time of transplantation. All had received prior imatinib. Seven of the nine were resistant to imatinib and two (Patients 2 and 8, Table I) were intolerant; Patient 2 developed diarrhea and Patient 8, liver toxicity. They both lost their response upon discontinuation of imatinib. Four patients (Patients 1, 3, 4 and 6, Table I) received dasatinib after failure of imatinib; only Patient 6 achieved a major cytogenetic response to dasatinib.
Table I.
Patients, disease, and transplant.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age at transplantation/sex | 37/F | 63/F | 61/M | 61/F | 54/M | 64/M | 44/F | 55/F | 64/M |
| Performance status | 3 | 0 | 3 | 2 | 1 | 0–1 | 1 | 1 | 1 |
| HCT CI | ≥3 | <3 | ≥3 | ≥3 | <3 | <3 | <3 | <3 | ≥3 |
| Number of prior therapies | 4 | 1–2 | 3 | 3 | 1 | 5 | 1 | 1–2 | 3 |
| Prior TKI | Imatinib; dasatinib | Imatinib | Imatinib; dasatinib | Imatinib; dasatinib | Imatinib | Imatinib; dasatinib | Imatinib | Imatinib | Imatinib |
| Reason for stopping TKI | Resistance | Intolerance | Resistance | Resistance | Resistance | Resistance | Resistance | Intolerance | Resistance |
| Disease status at AlloSCT | MyBC | MyBC | LyBC | AP | 2d CP | 2d CP | CP | CP | CP |
| Cytoreduction before SCT | Cladribine | Cladribine | Cladribine | Cladribine | Cladribine | No | No | No | No |
| Interval from diagnosis | 10 m | 18 m | 10 m | 116 m | 29 m | 156 m | 14 m | 15 m | 36 m |
| Conditioning regimen | Bu/Flu/Camp | Flu/Mel/Camp | Bu/Flu/Camp | Bu/Flu/Camp | Bu/Cy/Camp | Bu/Flu/Camp | Flu/Mel/Camp | Flu/Mel/Camp | Flu/Mel/Camp |
| Donor | Sibling | Sibling | Sibling | Sibling | Sibling | MUD 8/8 | MUD 7/8 | Sibling | Sibling |
| Time to ANC > 500 | D+12 | D+9 | D+15 | D+9 | D+11 | D+15 | D+18 | D+10 | D+9 |
| D 30 cytogenetic response | NoCyR | CCyR | CCyR | CCyR | CCyR | CCyR | CCyR | CCyR | CCyR |
| Chimerism d+30 | NA | FDC | FDC | FDC | FDC | FDC | FDC | FDC | FDC |
| Relapse | NA | No | No | No | 4 m | No | 12 m | 12 m | 11 m |
| GVHD | No | Skin | Skin | Skin | Skin | No | No | Skin+liver | Skin+liver |
| Salvage therapy | Nilotinib | NA | NA | NA | Imatinib+DLI; dasatinib | NA | Imatinib | DLI | DLI |
| Response to salvage | NR | NA | NA | NA | CCyR | NA | CCyR | CCyR | CCyR |
| Last follow-up | Died +4 m | Died +15 m | Died +6 m | Died +9 m | Alive +52 m | Alive +30 m | Alive +49 m | Alive +54 m | Alive +48 m |
| Cause of death | BC CML | Infection | Infeetion | CV event | NA | NA | NA | NA | NA |
| Hematopoietic function | Good | Poor | Poor | Good | Good | Poor | Good | Good | Good |
CML, chronic myelogenous leukemia; M, male; F, female; HCT CI, hematopoietic cell transplantation-specific comorbidity index; TKI, tyrosine kinase inhibitor; CP, first chronic phase; 2dCP, second chronic phase; AC, accelerated phase; MyBC, myeloid blast crisis; LyBC, lymphoid blast crisis; m, months; D, day; MUD, matched unrelated donor; CCyR, complete cytogenetic response; NoCyR, no cytogenetic response; NR, no response; FDC, full donor chimerism; MDC, mixed donor chimerism; GVHD, graft-versus-host disease; DLI, donor lymphocyte infusion; NA, not applicable; CV, cardiovascular.
At the time of initial transplant recommendation, three patients (Patients 1–3, Table I) had blast crisis, three (Patients 4–6, Table I) were in accelerated phase and three (Patients 7–9, Table I) were in first chronic phase. One patient with accelerated phase (Patient 6, Table I) achieved a second chronic phase with dasatinib; the five other patients with blast crisis or accelerated phase received cytoreductive therapy before transplantation with cladribine 15 mg/m2 given intravenously daily for 5 days [13]. This was followed by a course of high-dose cytarabine and mitoxantrone in one patient. Patients 1–4 underwent transplantation during the nadir of chemotherapy-induced cytopenia. Patients 5 and 6 were transplanted in a second chronic phase.
Conditioning and GVHD prophylaxis
All patients received alemtuzumab 20 mg daily for five consecutive days before transplant. In one patient (Patient 5, Table I), this was combined with busulfan 0.8 mg/kg/day intravenously for 4 days and cyclophosphamide 60 mg/kg/day for 2 days. In four patients (Patients 1, 3, 4 and 6, Table I), it was combined with fludarabine 30 mg/m2 daily for 5 days and area under the curve (AUC)-targeted busulfan daily for 4 days [14]. In the remaining four patients (Patients 2, 7, 8 and 9, Table I), it was combined with fludarabine 30 mg/m2 daily for 5 days and melphalan 140 mg/m2 for 1 day [7]. Post-transplant GVHD prophylaxis consisted of tacrolimus in all patients. Transplant supportive care was as previously described [12,15] and included high-dose valacyclovir as CMV prophylaxis for most patients [16], though the earliest patients received high-dose acyclovir instead strategy [17]. All patients received peripheral blood stem cell transplantation; seven had Human leucocyten antigen (HLA)-identical sibling donors and two had unrelated donors. No prophylactic TKI therapy or donor lymphocyte infusion (DLI) was given after engraftment.
Regimen-related toxicity was graded according to the common toxicity criteria (CTC) criteria [18]. White blood cell (WBC) engraftment was defined as the first of three consecutive days with an absolute neutrophil count of at least 0.5×109/L. Acute and chronic GVHD were graded according to standard criteria [19,20]. Post-transplantation donor–recipient chimerism was assessed by means of DNA microsatellite analysis as previously described [21] and/or by fluorescent in situ hybridization (FISH) when a sex mismatch was present. Hematologic response was defined as complete if all peripheral blood counts had normalised. For molecular and cytogenetic response, blood and bone marrow were analysed for BCR-ABL by RT-PCR or t(9;22) using FISH [22]. Most of the patients were managed before mutational analysis of BCR-ABL was routinely done.
Toxicity, treatment-related mortality, engraftment
Life threatening toxicities occurred only in patients with advanced disease and/or a high comorbidity score (HCT-CI). Three patients (Patients 1, 2 and 4, Table I) required intensive care admissions with prolonged hospitalisations and ventilation assistance in two of them, but recovered. No treatment-related deaths occurred in the immediate post-transplant period. WBC engraftment occurred in all patients between Day +9 and Day +18. At the time of the first disease assessment on Day +30, eight of the nine patients showed full engraftment with more than 95% donor chimerism in unfractionated cells. T-cell chimerism on Day +30 was available in three patients and showed more than 95% donor chimerism in two patients and 82% donor chimerism in Patient 4. But Patients 2, 3 and 6 had persistent poor hematopoietic function and continued to require growth factor and transfusion support with blood and platelets, despite persistent donor chimerism. No further stem cell infusion was administered.
Outcomes
On Day 30, Patients 2–9 were in complete cytogenetic response by FISH or conventional karyotype analysis. Patient 1 never achieved a cytogenetic response after transplantation despite salvage therapy with nilotinib and died 4 months later. Among the 8 patients who achieved a complete cytogenetic response after transplant, Patients 2–4, all transplanted in blast crisis, died 15, 6 and 9 months after transplant, without evidence of CML. Patient 2 died from respiratory failure secondary to a septic shock. Patient 3 developed CMV pneumonia with subsequent multiorgan failure, despite high-dose valacyclovir prophylaxis. These two events occurred in the setting of persistent poor hematopoietic function. Patient 4 who had a long history of severe renal and cardiac illness died suddenly at home from a presumed cardiovascular event.
Four of the remaining five other patients relapsed. Three had a durable response to DLI. Their course is briefly described. Patient 5, who was resistant to imatinib prior to transplant and who was transplanted in second chronic phase, relapsed 4 months after transplant with 10% Philadelphia positive cells on cytogenetic analysis and still 94% donor chimerism in the bone marrow. He was started on imatinib 400 mg per day resulting in complete response by FISH within 30 days. Five months later, ~9 months after transplant, 34% of cells were Philadelphia positive and donor chimerism decreased to 70%. Imatinib was increased to 800 mg per day but without further response. Ten months after transplant and while continuing imatinib, he received a DLI containing a dose of 2×107 CD3+/kg. A second DLI containing 8×107 CD3+/kg was given 1 month later. He developed limited GVHD of the skin and dry eyes. Thirteen months after transplant he obtained a durable complete cytogenetic response with more than 95% donor chimerism. BCR-ABL remained however detectable in the blood for 36 months after achieving cytogenetic remission, he was recently switched to dasatinib. Two recent molecular assays have been negative for BCR/ABL.
Patient 7, who was resistant to imatinib before transplant and who was transplanted in chronic phase, relapsed 12 months after transplant with detection of 10% Philadelphia positive cells on cytogenetic analysis and positive BCR/ABL RT-PCR. At that time she still had 92% donor chimerism. She was started on imatinib 400 mg per day and obtained a durable hematological response and complete cytogenetic remission (CCyR) that is currently ongoing 4 years after transplant despite persistent minimal residual disease detectable by BCR/ABL RT-PCR.
Patient 8 who was intolerant to imatinib and transplanted in chronic phase, relapsed 12 months after transplant with detection of 10% Philadelphia positive cells on cytogenetic analysis and 87% donor chimerism. She received a first DLI containing 2×107 CD3+/kg followed 2 months later by a second DLI containing 5×107 CD3+/kg. She achieved a durable complete cytogenetic response but developed skin and liver GVHD after the second infusion. She is currently alive and in complete molecular remission 54 months after the transplant.
Patient 9 who was resistant to imatinib before transplant and transplanted in chronic phase, lost his cytogenetic response almost 1 year after transplant when he presented with 18% Philadelphia positive cells by FISH and 65% donor chimerism. Fifteen months after transplant he received a DLI containing 20×106 CD3+/kg. He developed limited GVHD of skin, liver, gut and lungs but achieved a durable complete cytogenetic response and complete molecular remission.
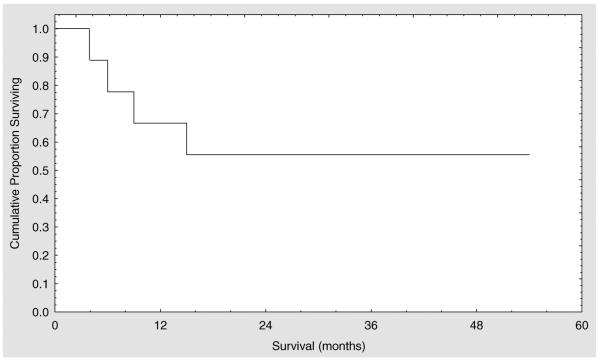
At the time of the last follow-up in May 2008 five of the nine patients were alive, all of them in CCyR or better. All these patients were transplanted in first or second chronic phase (Figure 1).
Figure 1.
Kaplan–Meier survival curve for patients with CML transplanted using an alemtuzumab-based regimen.
GVHD
Grade II acute GVHD mainly involving skin occurred after transplantation in four patients and responded well to treatment with steroids. Two more patients developed GVHD only after DLI infusion. Patient 8 who had no previous history of GVHD, developed skin and liver GVHD after DLI and required treatment with prednisone and tacrolimus for approximately 1 year. Patient 9 had a previous history of GVHD when he received DLI 1 year after transplant. After DLI he developed Grade 2 skin and liver GVHD that was refractory to prednisone, tacrolimus and mycophenolate. He subsequently was treated with extracorporeal photopheresis and finally received one course of rituximab resulting in a slow improvement. At the time of the last follow-up, he was still taking prednisone.
Discussion
Allo-HSCT remains an important salvage therapy for patients with CML who are resistant or intolerant to TKI. Patients with CML in chronic phase who have failed prior TKI have acceptable rates of disease control after myeloablative transplantation, but are prone to toxicities and TRM. Oehler et al. reported that patients who underwent transplantation in chronic phase with a suboptimal response to TKI had a significant worse overall survival when compared with chronic phase patients who were in complete cytogenetic response [3]. RIC are being increasingly used in high-risk patients with co-morbidities as well as older patients, and rely mainly on GvL (graft-versus-leukemia) effects to induce remissions [23]. Studies of RIC in CML show feasibility, but increased relapse rate and GVHD [6,7]. In vivo alemtuzumab, a monoclonal antibody directed against CD52, allows depletion of recipient and donor T cells and significantly reduces the risk for acute and chronic GVHD without much increasing the risk of graft rejection [24,25]. Nevertheless, the T-cell depletion causes a delay in immune reconstitution, and an impairment of GVL effect.
Here we report our experience with alemtuzumab-based GVHD prophylaxis regimen in nine patients with CML with both myeloablative and RIC. Seven had become resistant to TKI and two were intolerant. More than half the patients were in accelerated phase or blast crisis before transplantation and received cytoreductive therapy with cladribine in the weeks leading up to transplant [13]. Several patients had a decreased performance status and three had a high HCT-CI. They represent therefore a very high risk group for TRM and relapse. We hypothesised that the tolerability of alemtuzumab-based conditioning might be to their advantage despite the increase risk of disease recurrence.
Most patients achieved rapid neutrophil engraftment and eight out nine patients achieved a complete cytogenetic response with more than 95% donor chimerism on Day (30. The incidence of severe acute and chronic GVHD was also quite low. Of the four patients transplanted in blast crisis or accelerated phase, one relapsed, one died of unrelated cardiovascular event and two, despite full donor chimerism, had persistent poor hematopoietic function contributing to late, fatal opportunistic infections. The reason for the incomplete hematopoietic reconstitution is unclear, is extremely uncommon after fludarabine alemtuzumab melphalan conditioning in patients with other hematologic malignancies, and may be related to stromal dysfunction perhaps related to exposure to cladribine in the weeks leading up to the transplant.
Four of the five patients transplanted in first or second chronic phase relapsed after a median time of 10 months. These data are consistent with those of Crawley et al. who reported outcomes of RIC in CML patients using different regimens and noticed a higher relapse rate after the use of alemtuzumab than after the use of ATG (anti-thymocyte globulin) [6]. Two other reports about the use of alemtuzumab in myeloablative transplant in CML patients showed a high relapse rate as well [26,27]. More intensive conditioning therefore does not seem to overcome the impairment of GvL effect by alemtuzumab, but interestingly additional therapy with DLI is quite effective in this setting. All our relapsed patients responded to salvage therapy with DLI and/or imatinib and all are currently alive and in ongoing remission. We also observed one transient and one durable response to imatinib in Patients 1 and 3, respectively, both of whom were resistant to imatinib before transplantation.
In summary, for patients with CML who failed imatinib, but are in chronic phase at the time of allo-HSCT, alemtuzumab-based conditioning is well tolerated, leads to excellent engraftment, a high percentage of remissions with a low risk of GVHD and excellent long-term survival. For patients with more advanced disease, the results were disappointing. Because of a very high recurrence rate for patients transplanted in chronic phase, additional prophylactic treatment is necessary. Prophylactic low dose DLI is the most effective approach but puts patients at risk of pancytopenia and GVHD [28]. Maintenance treatment with a TKI appears safer and well tolerated and should therefore be routinely considered [28–31]. Because several non-cross resistant TKIs are available, we propose a systematic maintenance therapy during 1 year with dasatinib or nilotinib in imatinib failures. DLI could then be given in case of molecular or cytogenetic relapse as was suggested by Olavarria et al [32]. It is likely that increasingly patients will be referred who have failed all available TKIs. For such patients our observation of restoration of sensitivity to TKI justifies their reintroduction after transplant. One could also consider combination of DLI with TKI as suggested by Savani et al. [31], combination of TKI to overcome resistance, or the use of aurora kinase inhibitors or other experimental drugs [33–35]. Close disease monitoring usually by monitoring BCR-ABL levels in the peripheral blood is particularly important. Mutation analysis of BCR-ABL should be helpful as well.
Acknowledgements
Xavier Poiré is supported by a grant from Franqui-De Roover Foundation (Salus Sanguinis), Brussels, Belgium. Koen van Besien is supported in part by NCI grant K24CA116471. Koen van Besien declares having received research support from Berlex Pharmaceuticals.
References
- 1.Hehlmann R, Berger U, Pfirrmann M, Heimpel H, Hochhaus A, Hasford J, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–4692. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 2.Zaretsky Y, Rifkind J, Lockwood G, Tsang R, Kiss T, Hasegawa W, et al. Long-term follow-up of allogeneic bone marrow transplantation for patients with chronic phase chronic myeloid leukemia prepared with a regimen consisting of cyclophosphamide, cytarabine and single-dose total body irradiation conditioning. Bone Marrow Transplant. 2007;40:423–430. doi: 10.1038/sj.bmt.1705755. [DOI] [PubMed] [Google Scholar]
- 3.Oehler VG, Gooley T, Snyder DS, Johnston L, Lin A, Cummings CC, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109:1782–1789. doi: 10.1182/blood-2006-06-031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radich JP, Olavarria E, Apperley JF. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Hematol Oncol Clin North Am. 2004;18:685–702. x. doi: 10.1016/j.hoc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Weisser M, Schmid C, Schoch C, Hiddemann W, Kolb HJ. Resistance to pretransplant imatinib therapy may adversely affect the outcome of allogeneic stem cell transplantation in CML. Bone Marrow Transplant. 2005;36:1017–1018. doi: 10.1038/sj.bmt.1705172. [DOI] [PubMed] [Google Scholar]
- 6.Crawley C, Szydlo R, Lalancette M, Bacigalupo A, Lange A, Brune M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the chronic leukemia working party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 7.Kebriaei P, Detry MA, Giralt S, Carrasco-Yalan A, Anagnostopoulos A, Couriel D, et al. Long-term follow-up of allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning for patients with chronic myeloid leukemia. Blood. 2007;110:3456–3462. doi: 10.1182/blood-2007-04-085969. [DOI] [PubMed] [Google Scholar]
- 8.Morris PJ, Russell NK. Alemtuzumab (Campath-1H): a systematic review in organ transplantation. Transplantation. 2006;81:1361–1367. doi: 10.1097/01.tp.0000219235.97036.9c. [DOI] [PubMed] [Google Scholar]
- 9.Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 10.Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 11.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 12.van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 13.Dann EJ, Anastasi J, Larson RA. High-dose cladribine therapy for chronic myelogenous leukemia in the accelerated or blast phase. J Clin Oncol. 1998;16:1498–1504. doi: 10.1200/JCO.1998.16.4.1498. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell P, Artz A, Undevia S, Hart J, van Besien K. Phase I study of dose-escalated busulfan with fludarabine and alemtuzumab as conditioning for allogeneic hematopoietic stem cell transplantation (alloHCT) J Clin Oncol. 2008;26 doi: 10.3109/10428194.2010.520773. May 20 Suppl; abst 7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devine SM, Hoffman R, Verma A, Shah R, Bradlow BA, Stock W, et al. Allogeneic blood cell transplantation following reduced-intensity conditioning is effective therapy for older patients with myelofibrosis with myeloid metaplasia. Blood. 2002;99:2255–2258. doi: 10.1182/blood.v99.6.2255. [DOI] [PubMed] [Google Scholar]
- 16.Kline J, Pollyea DA, Stock W, Artz A, Rich E, Godley L, et al. Pre-transplant ganciclovir and post transplant high-dose valacyclovir reduce CMV infections after alemtuzumab-based conditioning. Bone Marrow Transplant. 2006;37:307–310. doi: 10.1038/sj.bmt.1705249. [DOI] [PubMed] [Google Scholar]
- 17.Verma A, Devine S, Morrow M, Chen YH, Mihalov M, Peace D, et al. Low incidence of CMV viremia and disease after allogeneic peripheral blood stem cell transplantation. Role of pretransplant ganciclovir and post-transplant acyclovir. Bone Marrow Transplant. 2003;31:813–816. doi: 10.1038/sj.bmt.1703916. [DOI] [PubMed] [Google Scholar]
- 18.Sakiyama M, Kami M, Hori A, Imataki O, Hamaki T, Murashige N, et al. Regimen-related toxicity following reduced-intensity stem-cell transplantation (RIST): comparison between Seattle criteria and National Cancer Center Common Toxicity Criteria (NCI-CTC) version 2.0. Bone Marrow Transplant. 2004;34:787–794. doi: 10.1038/sj.bmt.1704673. [DOI] [PubMed] [Google Scholar]
- 19.Akpek G, Lee SJ, Flowers ME, Pavletic SZ, Arora M, Lee S, et al. Performance of a new clinical grading system for chronic graft-versus-host disease: a multicenter study. Blood. 2003;102:802–809. doi: 10.1182/blood-2002-10-3141. [DOI] [PubMed] [Google Scholar]
- 20.Martino R, Romero P, Subira M, Bellido M, Altes A, Sureda A, et al. Comparison of the classic Glucksberg criteria and the IBMTR severity index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24:283–287. doi: 10.1038/sj.bmt.1701899. [DOI] [PubMed] [Google Scholar]
- 21.Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–1060. doi: 10.1038/sj.bmt.1701779. [DOI] [PubMed] [Google Scholar]
- 22.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonising current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valcarcel D, Martino R, Sureda A, Canals C, Altes A, Briones J, et al. Conventional versus reduced-intensity conditioning regimen for allogeneic stem cell transplantation in patients with hematological malignancies. Eur J Haematol. 2005;74:144–151. doi: 10.1111/j.1600-0609.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 24.Lowdell MW, Craston R, Ray N, Koh M, Galatowicz G, Prentice HG. The effect of T cell depletion with Campath-1M on immune reconstitution after chemotherapy and allogeneic bone marrow transplant as treatment for leukaemia. Bone Marrow Transplant. 1998;21:679–686. doi: 10.1038/sj.bmt.1701153. [DOI] [PubMed] [Google Scholar]
- 25.Thursky KA, Worth LJ, Seymour JF, Miles Prince H, Slavin MA. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab*. Br J Haematol. 2006;132:3–12. doi: 10.1111/j.1365-2141.2005.05789.x. [DOI] [PubMed] [Google Scholar]
- 26.Chakrabarti S, MacDonald D, Hale G, Holder K, Turner V, Czarnecka H, et al. T-cell depletion with Campath-1H “in the bag” for matched related allogeneic peripheral blood stem cell transplantation is associated with reduced graft-versus-host disease, rapid immune constitution and improved survival. Br J Haematol. 2003;121:109–118. doi: 10.1046/j.1365-2141.2003.04228.x. [DOI] [PubMed] [Google Scholar]
- 27.von dem Borne PA, Beaumont F, Starrenburg CW, Oudshoorn M, Hale G, Falkenburg JH, et al. Outcomes after myeloablative unrelated donor stem cell transplantation using both in vitro and in vivo T-cell depletion with alemtuzumab. Haematologica. 2006;91:1559–1562. [PubMed] [Google Scholar]
- 28.Weisser M, Tischer J, Schnittger S, Schoch C, Ledderose G, Kolb HJ. A comparison of donor lymphocyte infusions or imatinib mesylate for patients with chronic myelogenous leukemia who have relapsed after allogeneic stem cell transplantation. Haematologica. 2006;91:663–666. [PubMed] [Google Scholar]
- 29.DeAngelo DJ, Hochberg EP, Alyea EP, Longtine J, Lee S, Galinsky I, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res. 2004;10:5065–5071. doi: 10.1158/1078-0432.CCR-03-0580. [DOI] [PubMed] [Google Scholar]
- 30.Olavarria E, Ottmann OG, Deininger M, Clark RE, Bandini G, Byrne J, et al. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia. 2003;17:1707–1712. doi: 10.1038/sj.leu.2403068. [DOI] [PubMed] [Google Scholar]
- 31.Savani BN, Montero A, Kurlander R, Childs R, Hensel N, Barrett AJ. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36:1009–1015. doi: 10.1038/sj.bmt.1705167. [DOI] [PubMed] [Google Scholar]
- 32.Olavarria E, Siddique S, Griffiths MJ, Avery S, Byrne JL, Piper KP, et al. Post-transplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients undergoing reduced-intensity allografts for chronic myeloid leukemia. Blood. 2007;110:4614–4617. doi: 10.1182/blood-2007-04-082990. [DOI] [PubMed] [Google Scholar]
- 33.Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood. 2007;110:2828–2837. doi: 10.1182/blood-2007-04-038943. [DOI] [PubMed] [Google Scholar]
- 34.Kujawski L, Talpaz M. Strategies for overcoming imatinib resistance in chronic myeloid leukemia. Leuk Lymphoma. 2007;48:2310–2322. doi: 10.1080/10428190701665988. [DOI] [PubMed] [Google Scholar]
- 35.Valent P. Emerging stem cell concepts for imatinib-resistant chronic myeloid leukaemia: implications for the biology, management, and therapy of the disease. Br J Haematol. 2008 doi: 10.1111/j.1365-2141.2008.07197.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]