Abstract
Alcohol and dietary fat both play an important role in alcohol-mediated multi-organ pathology, including gut and liver. In the present study we hypothesized that the combination of alcohol and dietary unsaturated fat (USF) would result in intestinal inflammatory stress and mucus layer alterations, thus contributing to disruption of intestinal barrier integrity. C57BL/6N mice were fed Lieber-DeCarli liquid diets containing EtOH and enriched in USF (corn oil/linoleic acid) or SF (medium chain triglycerides: beef tallow) for 8 weeks. Intestinal histology, morphometry, markers of inflammation, as well as levels of mucus protective factors were evaluated. Alcohol and dietary USF triggered an intestinal pro-inflammatory response, characterized by increase in Tnf-α, MCP1, and MPO activity. Further, alcohol and dietary USF, but not SF, resulted in alterations of the intestinal mucus layer, characterized by decreased expression of Muc2 in the ileum. A strong correlation was observed between down-regulation of the antimicrobial factor Cramp and increased Tnf-α mRNA. Therefore, dietary unsaturated fat (corn oil/LA enriched) is a significant contributing factor to EtOH-mediated intestinal inflammatory response and mucus layer alterations in rodents.
Keywords: Unsaturated Fat Diet, Ethanol, Intestinal Inflammation
INTRODUCTION
The concept that alcohol and dietary fat both play an important interactive role in the development of alcoholic liver disease (ALD) is well documented (Nanji, 2004; Nanji and French, 1986). The majority of the evidence from rodent models supports both a protective effect of dietary saturated fatty acids against ALD, and a deleterious effects of dietary unsaturated fatty acids, linoleic acid (LA) in particular, in alcohol-mediated liver damage (Kono et al., 2000; Nanji and French, 1989; Nanji et al., 2001; Nanji et al., 1989; Nanji et al., 1996; Ronis et al., 2004). Moreover, Nanji and French have shown that LA is required for the development of experimental ALD (Nanji and French, 1989). However, the underlying molecular mechanisms are not fully elucidated. Hence, the role of fat composition in the development and progression of ALD remains an area of considerable interest.
The importance of the gut-liver axis in alcohol-mediated liver pathology has received increasing interest (Purohit et al., 2008; Szabo and Bala, 2010; Wang et al., 2010). Clinical and experimental data have demonstrated that gut-derived endotoxin, lipopolysaccharide (LPS), plays an important role in the pathogenesis of ALD (Bode et al., 1987; Fukui et al., 1991; Keshavarzian et al., 2009; Mathurin et al., 2000; Parlesak et al., 2000; Szabo and Bala, 2010; Tang et al., 2009). Multiple mechanisms contribute to alcohol-associated endotoxemia, including alcohol-mediated intestinal bacterial overgrowth (Bode et al., 1993), alterations in gut microbiota (Mutlu et al., 2009; Mutlu et al., 2012; Yan et al., 2011), as well as increased LPS translocation caused by the disruption of intestinal barrier integrity (Banan et al., 1999; Ma et al., 1999; Tang et al., 2008; Zhong et al., 2010).
The most recent studies from our group demonstrated that EtOH disrupts intestinal tight junctions in the ileal mucosa, followed by increased intestinal permeability, elevated blood LPS levels and consequent liver steatosis and injury (Kirpich et al., 2012; Zhong et al., 2010). Understanding the effects of EtOH, dietary fat, and their potential interactions on the intestine is critical in determining the mechanisms of alcohol-mediated multi-organ pathology. The intestine, is an important component of the immune systems, and is an initial organ exposed to alcohol and ingested nutrients; however, little is known about the potential effects of alcohol in combination with different dietary factors on the intestinal inflammatory response and its associations with intestinal barrier integrity.
In the present study, we used a mouse model of chronic EtOH feeding to evaluate the effects of dietary saturated and unsaturated fat on the gut pathology associated with chronic alcohol consumption. We hypothesized that dietary unsaturated fat (corn oil/linoleic acid enriched) is a cofactor in ethanol-mediated intestinal inflammatory stress and mucus layer alterations. Mice were fed liquid Lieber-DeCarli EtOH diet ad libitum for 8 weeks with two different sources of fat. Unsaturated fat (USF) diet was enriched in corn oil/linoleic acid (LA), and saturated fat (SF) was mainly comprised of medium chain triglycerides (MCT). Our findings revealed that alcohol and dietary USF triggered an intestinal pro-inflammatory response characterized by increased Tnf-α, MCP-1, and MPO activity. Further, alcohol and dietary USF, but not SF, resulted in alterations of the intestinal mucus layer and antimicrobial defense, characterized by decreased expression of Muc2 and Cramp in the ileum. Overall, these findings contribute to the understanding of the deleterious effects of dietary unsaturated fatty acids, LA in particular, in EtOH-mediated intestinal pathology.
MATERIALS AND METHODS
Experimental Animal Model
C57BL/6N male mice obtained from Harlan (Indianapolis, IN) were fed a modified Lieber–DeCarli liquid diet enriched in USF (corn oil/LA) or SF (an 18:82 ratio beef tallow:MCT (Ronis et al., 2004)). The diets were purchased from the Research Diet, New Brunswick, NJ. Mice were fed control or EtOH-containing diets ad libitum for 8 weeks. Control mice were pair-fed SF or USF maltose-dextrin diets that were isocaloric with the EtOH diets. In the control group diets, the levels of protein, carbohydrate, and fat were held constant at 17, 43, and 40% of total energy, respectively. In the alcohol diets, EtOH (35% of total calories) was substituted for carbohydrate energy. Detailed nutritional composition and dietary calories of the experimental diets are provided in the Table 1. The fatty acid compositions of corn oil, MCT and beef tallow are shown in the Table 2. At the end of the experiment, the mice were anesthetized; and blood and tissue samples were collected for assays. The detailed experimental protocol has been described previously (Kirpich et al., 2012). The study protocol was approved by the University of Louisville Institutional Animal Care and Use Committee. The study was performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
Table 1.
Nutritional composition of the SF+EtOH and USF+EtOH experimental diets.
| Ingredients | SF+EtOH | USF+EtOH |
|---|---|---|
| (kcal%) | ||
| Protein | ||
| Casein | 166 | 166 |
| DL - Methionine | 1 | 1 |
| L - Cystine | 2 | 2 |
| Carbohydrates | ||
| Maltodextrin | 63 | 63 |
| 1Cellulose | 0 | 0 |
| 2Xantham Gum | 0 | 0 |
| Fat | ||
| Soybean Oil | 40 | 40 |
| Corn Oil | 356 | 0 |
| 4Beef Fat | 0 | 65 |
| MCT Oil | 0 | 292 |
| 3Minerals S10018 | 0 | 0 |
| Vitamins V10036 | 10 | 10 |
| Choline Bitartrate | 0 | 0 |
| Ethanol | 350 | 350 |
The diets were obtained from Research Diet, New Brunswick, NJ
Cellulose – 10gm/1000g;
Xantham Gum – 3gm/1000g;
Minerals 8.75 g/1000g;
Choline Bitartrate – 0.53gm/1000g
18:82 ratio beef tallow:MCT
Table 2.
Fatty acid composition of fat sources in SF and USF experimental diets*.
| Fatty acids | Corn oil | MCT | Beef tallow |
|---|---|---|---|
| g/kg total fatty acids | |||
| <C8:0 | - | 60 | - |
| C8:0 | - | 670 | - |
| C10:0 | - | 230 | 1 |
| >C10:0 | 1000 | 40 | 999 |
| C12:0 | - | - | 1 |
| C14:0 | - | - | 33 |
| C 14:1 | - | - | 2 |
| C 15:0 | - | - | 13 |
| C 15:1 | - | - | 2 |
| C16:0 | - | - | 255 |
| C16:1 | 122 | - | 34 |
| C 17:0 | 1 | - | 15 |
| C 17:1 | - | - | 7 |
| C 18:0 | 22 | - | 216 |
| C 18:1 n9 | 275 | - | 387 |
| C 18:2 n6 | 570 | - | 22 |
| C 18:3 n3 | 9 | - | 6 |
| C 19:0 | - | - | 1 |
| C 20:0 | 1 | - | 1 |
| C24:0 | - | - | 4 |
Blood Alcohol Level Measurement
Blood alcohol levels were measured using NAD-ADH Reagent Multiple Test (Sigma, Saint Louis, MO) according to the manufacturer’s instructions.
Histological Analysis of the Intestinal Mucosa
After sacrifice, segments of the terminal ileum (1 cm proximal of the cecum) were excised and fixed in 10% buffered formalin. After processing, 4 μm sections were cut, stained with hematoxylin and eosin (H&E) and examined using light microscopy. The histological evaluation of the intestinal mucosa was performed by a trained pathologist in a blinded fashion. The ileum was chosen for analysis because the ileum is the region that has the most permeability in response to alcohol feeding (Kirpich et al., 2012). Histological assessment also included semi-quantitative analysis of the goblet cell density by counting the number of goblet cells per villus. Fifteen villi per group (n=3) were examined for that analysis.
Morphometric Analysis of the Intestinal Mucosa
Ten complete villus-crypt structures per group (n=3) were randomly selected in H&E stained sections, and villus height, width, and crypt depth were measured using MetaMorph software (Molecular Devices, LLC, Sunnyvale, CA). Villus length was measured from the tip to the base of villus, villus width was measured at the base of villus, and the crypt length was measured from the bottom of the crypt to the opening of the crypt (Collins et al., 2008).
Myeloperoxidase Activity Measurement
Myeloperoxidase (MPO) activity in the intestine was measured using Myeloperoxidase Activity Colorimetric Assay Kit (BioVision Research Products, Mountain View, CA) according to the manufacturer’s instructions.
RNA Isolation and Real Time Reverse Transcription Polymerase Chain Reaction Assay
Total RNA from the ileum was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Reverse transcription (RT) was performed with qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD) and qRT-PCR with Perfecta SYBR Green FastMix (Quanta Biosciences) using an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). The reverse and forward specific primers were as following: Monocyte chemotactic protein-1 (Mcp-1; F:5’-ggctcagccagatgcagt-3’; R:5’-gagcttggtgacaaaaactacag-3’), Cathelin related antimicrobial peptide (Cramp; F:5’-cagccctttcggttcaagaa-3’; R:5’-cccacctttgcggagaagt-3’), Mucin-2 (Muc2; F:5’-actgcacattcttcagctgc-3’; R:5’-attcatgaggacggtcttgg-3’), Interleukin-1β (IL-1β; F:5’-ttcatctttgaagaagagcccat-3’; R:5’-tcggagcctgtagtgcagtt-3’), Interleukin-6 (IL-6; F:5’-tggaaatgagaaaagagttgtgc-3’; R:5’-ccagtttggtagcatccatca-3’). Primers were designed using Primer3 software (Rozen and Skaletsky, 2000). Tumor necrosis factor-alpha (Tnf-α) primer was purchased from SA Biosciences (Frederick, MD). All primer pairs were validated by demonstrating high amplification efficiency, consistent single peak dissociation patterns and the presence of single products of the expected size on agarose gels. The relative gene expression was normalized with 18s rRNA (SA Biosciences (Frederick, MD) as the internal control, and calculated using the 2−ΔΔCt method.
Intestinal Cytokine Production Measurement
Intestinal segments from the distal ileum were used for the measurement of cytokine production. The specimens were homogenized in 1 ml of normal saline (Ding and Li, 2003). The homogenates were then centrifuged at 4500 g for 15 minutes at 4°C, and supernatants were used for the assay. Intestinal cytokines were determined by multianalyte chemiluminescent detection using MILLIPLEX MAP Mouse Cytokine/Chemokine Panel kit (Millipore, Billerica, MA) on the Luminex100 IS System (Luminex, Austin, TX). Specifically, IL-1β, IL-6, IL-10, MCP-1, and TNF-α were measured. Data were normalized to total protein content.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc., La Jolla, CA). Two-way ANOVA followed by Tukey’s multiple comparison test was used to evaluate significant differences between the 4 compared groups (SF, SF+EtOH, USF, USF+EtOH). The data were expressed as mean ± SEM. A p-value of < 0.05 was considered statistically significant. Pearson’s correlation analysis was used to determine the association between pro-inflammatory cytokine and TJ protein variables.
RESULTS
Effects of Alcohol and Saturated and Unsaturated Fat Diets on Body Weight and Blood Alcohol Levels
The experimental animals were pair-fed liquid diets enriched in saturated fat (SF) or unsaturated fat (USF) with or without EtOH for 8 weeks. Mice fed USF+EtOH diet gained more body weight compared to mice fed SF+EtOH diet during the first 2 weeks of the experiment (15.2+1.7 g vs 6.06+1.6 g, p < 0.05). A noticeable gradual reduction of body weight was observed in both SF+EtOH and USF+EtOH groups starting the second month of the feeding period. There were no differences in final body weight in both EtOH fed groups compared to the initial body weight (25.28+0.4 g vs 25.07+0.4 g in SF+EtOH; and 25.41+0.5 g vs 26.72+0.6 in USF+EtOH).
At the end of the experiment the blood alcohol levels were similar in the SF+EtOH (0.15+0.02%) and USF+EtOH (0.12+0.02%) groups indicating that type of dietary fat did not affect systemic blood alcohol levels. However, portal vein alcohol levels were not determined.
Evaluation of the Intestinal Pathology in Response to Ethanol and Saturated or Unsaturated Fat Diets
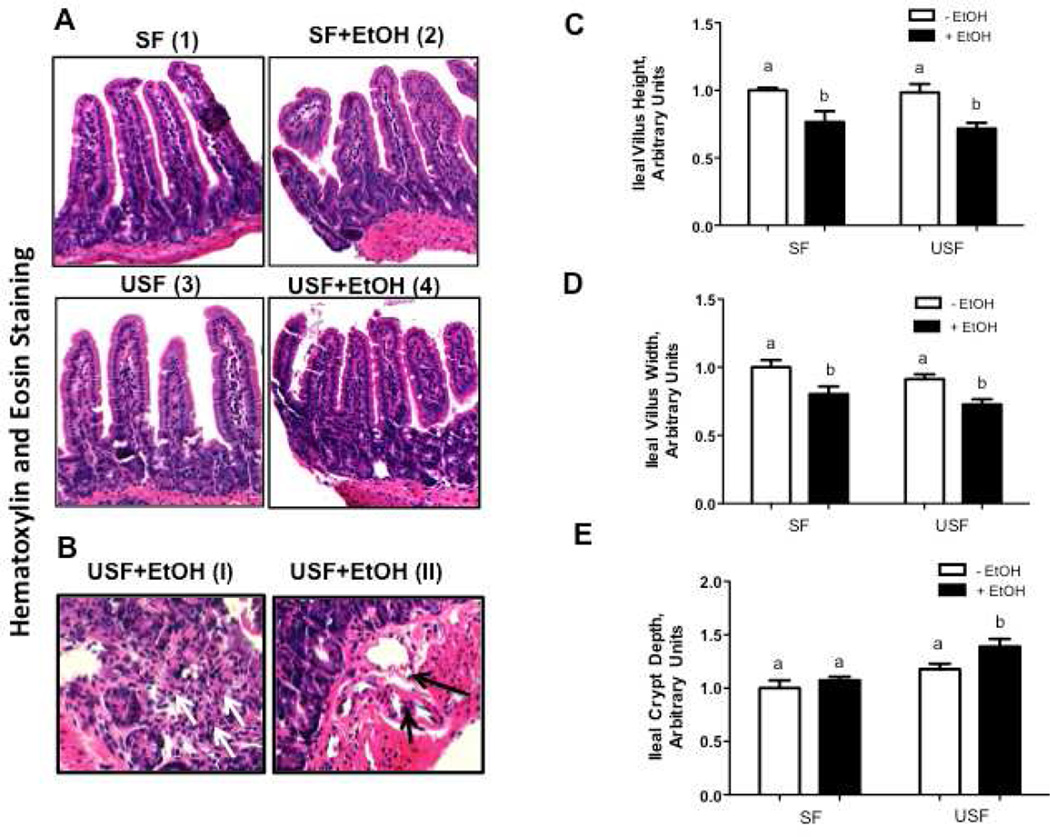
To determine the effects of EtOH and different types of dietary fat on the intestine, we first performed histological evaluation of intestinal tissue sections and quantitative analysis of the intestinal morphometry. Mice receiving SF and USF diet had intact intestinal mucosa, and the villi looked normal. No inflammatory cell infiltration was noted either in the intraepithelial or in the lamina propria compartments (Fig.1A-I, 1A-III). In contrast, EtOH addition to the diets damaged the intestinal epithelium architecture, characterized by blunting of the villi (Fig.1A-II, 1A-IV), accompanied by moderate inflammatory cell infiltrates, predominantly mononuclear lymphocytes with some neutrophils, in the lamina propria; this effect of EtOH was more pronounced in USF+EtOH compared with SF+EtOH group (Fig.1B-I). Thinning of the fibrovascular core in the villi, and dilation of the capillaries in the lamina propria (Fig.1B-II) were also observed, which was also more pronounced in the USF+EtOH group.
Figure 1. Evaluation of the intestinal pathology in response to ethanol and saturated fat (SF) or unsaturated fat (USF) diets.
(A-B) Representative microphotographs of hematoxylin and eosin staining of the intestinal sections. Distal ileum from mice fed Lieber-DeCarli control and EtOH liquid diets for 8 weeks. Sections were analyzed using a light microscope (original magnification for A: x200, for B:x400). The mucosa appear normal in SF and USF groups (A-1, and A-3). Histological injury is noted in mice fed alcohol, characterized by shorten villi (A-2, and A-4). Inflammatory cell infiltrates (B-1) and dilation of blood vessels (B-2) were observed predominantly in the lamina propria of USF+EtOH group. Arrows indicate infiltration of lymphocytes and neutrophils, and dilated capillaries. (C-D) Morphometrical analysis of the ileal mucosa in response to ethanol and dietary fat. Ten complete villus-crypt junctions of the ileal segments of the individual mouse were measured, n=3 animals/per group. Values are mean+SEM. The value for the SF group was set at 1 as a control for comparison purposes. Statistical differences were analyzed by two-way ANOVA followed by the Tukey’s multiple comparison test. Means without a common letter differ at p<0.05.
The morphometric indices (villus length and width, crypt depth) indicate intestinal enterocyte maturity and functional capacity. Importantly, there were no differences in studied parameters between the SF or USF diets in the absence of EtOH. A reduction in villus height (Fig. 1C) and width (Fig. 1D) was noted in response to EtOH, regardless of the type of fats in the diet (SF vs SF+EtOH, p < 0.05; USF vs USF+EtOH, p <0.05). The crypt depth was observed to be significantly greater in the ileum of USF+EtOH fed animals compared with other groups (Fig. 1E). The increase in crypt depth was about 20% in USF+EtOH compared with USF group (p < 0.05), and about 40% in USF+EtOH compared with SF+EtOH group (p < 0.05).
Ethanol and Unsaturated Fat Diet Fed Mice Exhibited Intestinal Inflammatory Stress
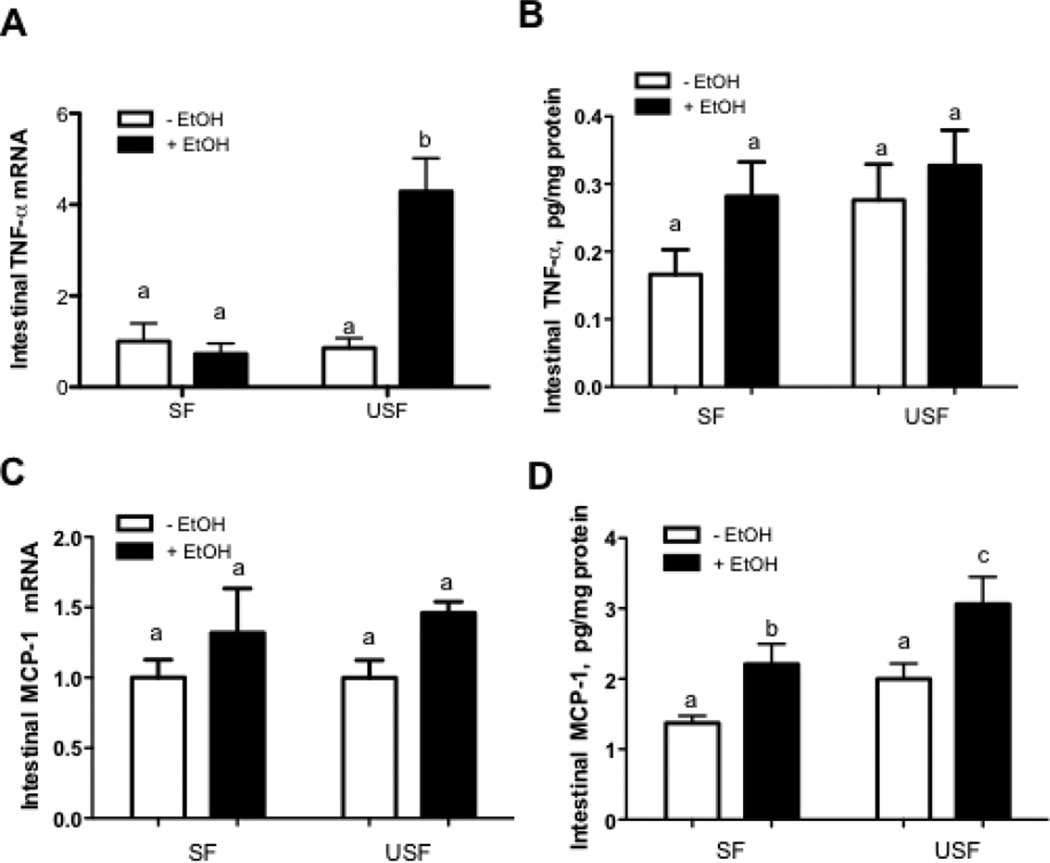
Our recent study demonstrated that USF+EtOH resulted in prominent hepatic inflammation compared with USF as well as with SF+EtOH feeding (Kirpich et al., 2012). To determine whether USF+EtOH can cause similar effects in the intestine, and to evaluate the potential mechanisms by which different types of dietary fat may contribute to the alcohol-mediated intestinal barrier dysfunction, we assessed markers of intestinal inflammatory activity in this animal model. Compared with USF diet alone, the USF+EtOH treatment increased ileal Tnf-α and Mcp-1 mRNA levels, key mediators of inflammation and macrophage activation. We found that USF+EtOH fed mice exhibited 4-fold higher intestinal Tnf-α mRNA levels compared with USF, as well as with SF+EtOH fed animals (Fig.2A, p<0.05). Despite the up-regulation of Tnf-α mRNA in response to USF+EtOH, a correspondent statistically significant increase in the TNF-α protein levels was not observed (Fig.2B); the discrepancy in the TNF protein data could be due to the interfering proteins present in the intestinal homogenates used for the assay. Intestinal Mcp-1 mRNA levels were elevated by EtOH in both USF+EtOH and SF+EtOH groups compared with pair-fed control animals (Fig.2C) with the statistically significant increase at the protein levels (Fig.2D: SF vs SF+EtOH, and USF vs USF+EtOH, p<0.05). Even though MCP-1 was significantly elevated by EtOH regardless the types of fat in the diets, the effect was more pronounced in response to USF+EtOH treatment (Fig.2D: SF+EtOH vs USF+EtOH, p<0.05). The correspondent increase in the Mcp-1 mRNA levels was not statistically significant likely due to the discrepancy in the mRNA and protein stability. We have evaluated several other cytokines, including IL-1β and IL-6. The expression of these pro-inflammatory cytokines was insignificantly elevated in response to EtOH at both mRNA and protein levels (data not shown). We also found that IL-10, a potent anti-inflammatory cytokine, was not altered by EtOH or the type of fat in the diet (data not shown).
Figure 2. Intestinal markers of inflammation in response to alcohol and saturated fat (SF) and unsaturated fat (USF) diets.
(A and B) Tnf-α mRNA and protein levels. (C and D) Mcp1 mRNA and protein levels. The relative mRNA expression was measured by qRT-PCR. Values are mean+SEM, n=6 animals/per group. Cytokine protein levels were determined using MILLIPLEX Cytokine/Chemokine Panel kit (Millipore, Billerica, MA) on the Luminex100 IS System (Austin, TX). Values are mean+SEM, n=6–9 animals/per group. Statistical differences were analyzed by two-way ANOVA followed by the Tukey’s multiple comparison test. Means without a common letter differ at p<0.05.
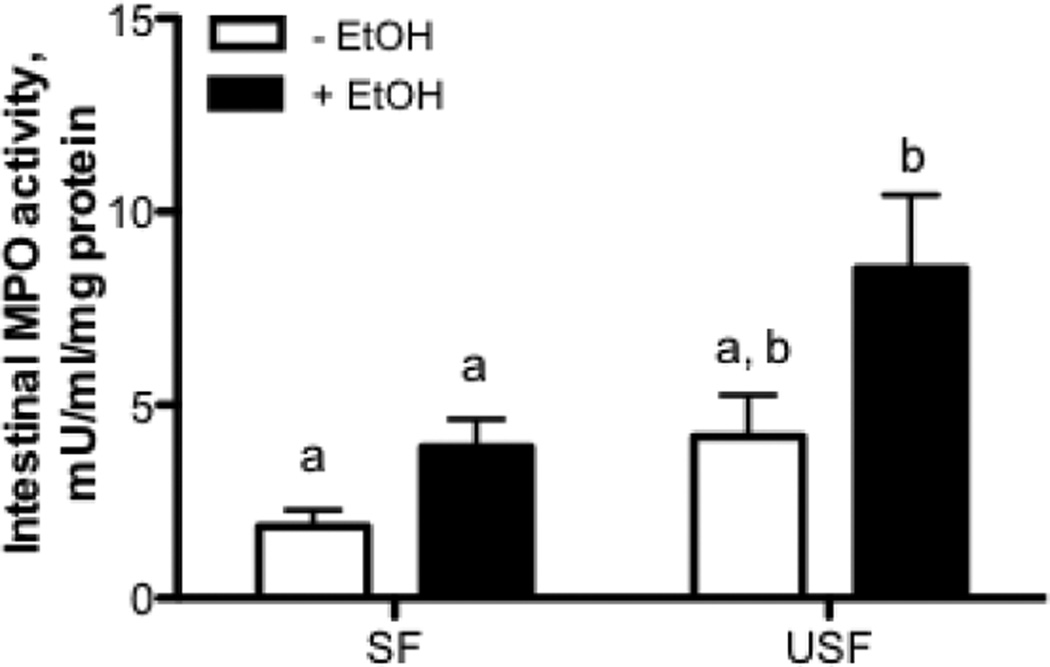
To further evaluate the effects of dietary fat on alcohol mediated intestinal inflammatory response we measured the ileal MPO activity, the downstream event of cytokine activation. USF+EtOH significantly increased ileal MPO activity, a marker of inflammation and neutrophil infiltration, compared with SF+EtOH (Fig.3, p<0.05).
Figure 3. Intestinal MPO activity in response to EtOH and SF and USF dietary fat.
USF+EtOH significantly increased ileal MPO activity, a marker of inflammation and neutrophil infiltration, compared with SF+EtOH. Values are mean+SEM, n=5–6 animals/per group. Statistical differences were analyzed by two-way ANOVA followed by the Tukey’s multiple comparison test. Means without a common letter differ at p<0.05.
Cytokine-mediated alterations of intestinal TJ have recently been demonstrated by several groups (Al-Sadi et al., 2012; Suzuki et al., 2011; Wang et al., 2005). In the present study, we used linear regression analysis to test for potential association between ileal pro-inflammatory markers and down-regulation of TJ proteins and protein-adaptors in response to USF+EtOH (Kirpich et al., 2012). We found that increased secretion of MCP-1 in USF+EtOH group was negatively correlated with ZO-1 expression (Pearson r = −0.81, p = 0.05), occludin (Pearson r = − 0.81, p = 0.05), and the protein adaptor fodrin (Pearson r = − 0.83, p = 0.03).
Alterations of the Intestinal Mucus Layer in Response to Ethanol and Unsaturated Dietary Fat
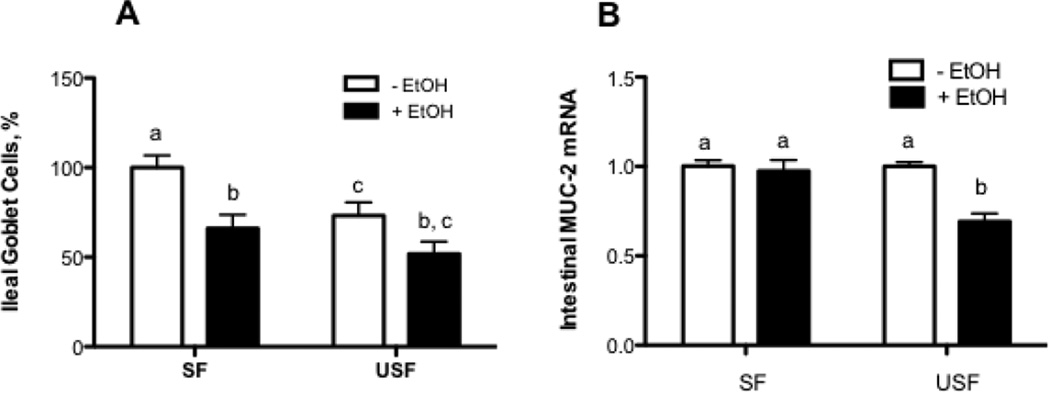
The gut epithelium typically provides a structural barrier to both commensal and pathogenic flora by secreting a protective coat of mucus. In our study, we focused our investigations on MUC2, a major component of intestinal mucus layer. MUC2 is secreted by intestinal goblet cells. Significantly decreased density of goblet cells in the ileum was observed in USF group compared with SF (Fig.4A, p < 0.05), with further decline in response to EtOH regardless of the types of dietary fat. Significantly decreased ileal Muc2 mRNA levels was observed in response to USF+EtOH compared with USF, but not SF+EtOH compared with SF treatments (Fig.4B: USF+EtOH vs USF, p<0.05).
Figure 4. Effects of saturated fat (SF) or unsaturated fat (USF) on the intestinal mucus layer integrity in response to chronic alcohol feeding.
(A) Quantitative analysis of intestinal Goblet cells. (B) Muc2 mRNA levels assessed by qRT-PCR. Values are mean+SEM, n=6 animals/per group. Statistical differences were analyzed by two-way ANOVA followed by the Tukey’s multiple comparison test. Means without a common letter differ at p<0.05.
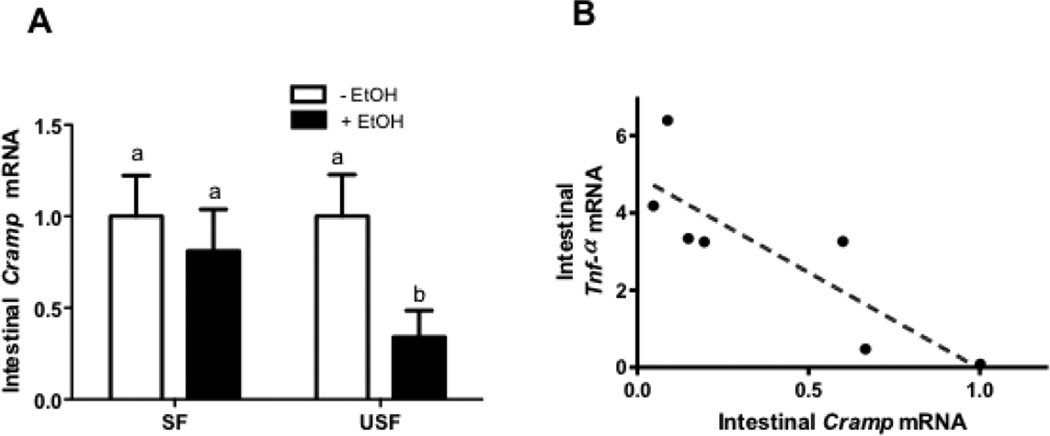
Expression of the Antibacterial Peptide Cramp in Response to Ethanol and Dietary Fat
To further explore the effects of different types of dietary fat in combination with EtOH on the intestinal mucosa, we next studied Cramp, one of the cathelicidin antimicrobial peptides secreted by Paneth cells in the intestinal epithelium. Mice on USF+EtOH diet showed a marked decrease in expression of the ileal Cramp compared with USF diet, whereas there were no significant changes of Cramp levels in the SF+EtOH fed mice compared with SF pair-fed animals (Fig. 5A: USF+EtOH vs USF, p<0.05). It has been shown that cathelicidin peptides exhibit immunomodulatory effects, including modulation of the release of TNF-α (Pinheiro da Silva et al., 2009). A significant inverse correlation was found between Cramp mRNA and levels of intestinal pro-inflammatory marker, Tnf-α mRNA (Pearson r = − 0.88, p = 0.02, Fig.5B).
Figure 5. Down-regulation of the intestinal mucosa antimicrobial peptide, Cramp, in response to ethanol and dietary unsaturated but not saturated fat.
(A) Cramp mRNA levels assessed by qRT-PCR. Values are mean+SEM, n=6 animals/per group. Statistical differences were analyzed by two-way ANOVA followed by the Tukey’s multiple comparison test. Means without a common letter differ at p<0.05. (B) Negative correlation was observed between intestinal antimicrobial factor Cramp and intestinal Tnf-α mRNA levels.
DISCUSSION
There are several known injurious effects of alcohol consumption on the gut, including alcohol induced gut permeability (Parlesak et al., 2000; Purohit et al., 2008; Rao et al., 2004), impairment of the intestinal microcirculation, macro- and micronutrient malabsorption, and altered gut motility (reviewed in (Bode and Bode, 2003; Rajendram and Preedy, 2005). However, little is known about the combined effects of EtOH and dietary fat on the intestinal inflammatory response, as well as on intestinal mucus layer integrity. The major new finding of the present study is that EtOH and dietary USF (corn oil/LA enriched) but not SF (MCT enriched) triggered inflammatory stress, and mucus layer alterations in the ileum, the region that is the most highly permeable in response to chronic alcohol feeding (Kirpich et al., 2012).
We report that USF+EtOH compared with SF+EtOH treatment resulted in intestinal inflammation, characterized by moderate infiltration of inflammatory cells, predominantly lymphocytes with some neutrophils, in the lamina propria, increased expression of Tnf-α and Mcp1 pro-inflammatory cytokines, and elevated MPO activity. Our findings are in alignment with the study by Fleming et al. (Fleming et al., 2001), in which they demonstrated that mice fed EtOH and diet containing USF fat for 2 weeks exhibited increased intestinal levels of TNF-α, IL-1β, IL-6 mRNA. The importance of our results is in demonstrating the higher potential of USF (corn oil/LA enriched) compared with SF (MCT enriched) to induce these pro-inflammatory effects in the intestine in response to EtOH. The strong correlation between increased MCP1 levels and down-regulation of TJ proteins observed in our experimental model suggests the contributory role of USF+EtOH diet induced inflammation in the disruption of intestinal barrier integrity. Indeed, cytokine-mediated enhancement of intestinal permeability via alterations of intestinal TJ has been demonstrated by other groups (Ma et al., 2004; Wang et al., 2005; Ye et al., 2006). Moreover, cytokines increase the flux of large molecules, such as bacterial lipopolysaccharides, across the intestinal TJ (Wang et al., 2005). Therefore, USF+EtOH induced alterations of intestinal TJ and increased intestinal permeability observed in this model might, at least in part, be mediated by the intestinal inflammation.
The ability of EtOH per se to induce a pro-inflammatory response has been shown in Caco-2 cells, an in vitro model of intestinal epithelium (Amin et al., 2009). However, alcohol consumption is always coupled with dietary factors in vivo. Therefore, to evaluate the contribution of a diet to alcohol-mediated effects is essential. The increased intestinal pro-inflammatory response observed in our study in response to USF+EtOH but not SF+EtOH strongly suggests that USF diet (corn oil/LA enriched) modulates the intestinal inflammatory tone in response to EtOH. Dietary USF, LA in particular, may affect the inflammatory response in several different ways, including conversion of LA to arachidonic acid (AA), and its subsequent metabolism to pro-inflammatory leukotoxins. Nanji et al. have demonstrated that EtOH and corn oil feeding was associated with an increase in cyclooxygenase-2, thromboxane B2, and leukotriene B4, important mediators of ALD (Nanji et al., 1993). Of high interest is the recent study by Yang et al. (Yang et al., 2010) demonstrating that chronic alcohol administration increases circulating bioactive peroxidized phospholipids (hydroxyoctadecanoic acids [HODEs], and hydroxyeicosatetraenoic acids [HETEs]), pro-inflammatory derivatives of LA and AA.
There are several potential mechanisms and signaling pathways underlying intestinal pro-inflammatory response. It has been shown that arachidonic acid metabolites may increase IL-8 production by enhancing NF-kB-dependent transcription of IL-8 (Alzoghaibi et al., 2004). The important role of p38α MAPK in TNF-α regulation has been demonstrated in the inflamed colonic mucosa of IBD patients (Waetzig et al., 2002). In addition to p38α, the authors also showed significant activation of colonic mucosa JNK1/2, and ERK1/2 MAPKs in these patients. Costantini at al. have reported increased phosphorylation of the intestinal p38, ERK1/2 MAPKs, and elevated IL-6 in burn-induced intestinal inflammation, which were attenuated by pentoxifylline treatment (Costantini et al., 2009). We are currently investigating potential mechanisms underlying observed alcohol/USF-induced intestinal inflammation.
Another point to consider in the USF+EtOH induced intestinal inflammation is the alterations of gut microbiota, which can occur in response to alcohol (Kirpich et al., 2008; Mutlu et al., 2009; Mutlu et al., 2012; Yan et al., 2011) as well as high fat diet (Cani et al., 2007; Hildebrandt et al., 2009; Turnbaugh et al., 2006). On the other hand, intestinal inflammation caused by EtOH and dietary USF may, in turn, alter microbiota composition creating a vicious cycle in the intestine. This notion is supported by a study showing that chemically or genetically (IL-10 deficiency) induced intestinal inflammation can alter intestinal microbiota (Lupp et al., 2007).
Impairment of the intestinal microbiota can significantly modulate mucosal inflammatory response and alter host immunity (Hooper and Gordon, 2001), therefore maintaining the integrity of the intestinal mucosal barrier is essential in this scenario. One of the mechanisms by which this is accomplished is the production of a thick mucus layer that overlies the entire intestinal epithelium. Ethanol (Andrade et al., 2006) and dietary USF fat (Kono et al., 2003) can affect the mucus layer by diminishing the number of goblet cells (which secrete mucins). Decreased density of goblet cells in response to USF (corn oil/LA enriched) compared to SF (MCT enriched) was observed in our experiment with further decline in response to EtOH regardless of the type of dietary fat. Further, ileal Muc2, a major component of intestinal mucus secreted by goblet cells, was down regulated in mice fed USF+EtOH but not SF+EtOH diet. Importantly, dietary SF (MCT enriched) prevented the decrease in Muc2 levels in response to EtOH, despite not preventing the decrease in goblet cell numbers. MUC2 plays a crucial role in maintaining the intestinal mucosal barrier. It has been shown that MUC2 genetic depletion or mutation resulted in spontaneous intestinal inflammation (Johansson et al., 2008; Van der Sluis et al., 2006), and increased epithelial permeability (Heazlewood et al., 2008). Taking into consideration that disruption of mucus integrity allows invasion of bacteria and other luminal pathogens into intestinal mucosa resulting in increased expression of pro-inflammatory cytokines and chemokines (Goto and Kiyono, 2012; Kagnoff, 2006), Muc2 alterations may contribute to the increased intestinal pro-inflammatory tone observed in our study.
Dietary fat also modified the ethanol-mediated down-regulation of Cramp, another important factor of the intestinal mucosal barrier protection. In our study, expression of Cramp, a peptide with antimicrobial and immunomodulatory effects, was significantly decreased in response to USF+EtOH but not SF+EtOH diets. The strong correlation between low levels of Cramp and increased Tnf-α expression observed in our study suggests the potential role of Cramp in intestinal pro-inflammatory response. This is supported by the fact that human cathelicidin LL-37 can suppress LPS-induced macrophage and dendritic inflammatory responses, including the release of nitric oxide and TNF-α (Mookherjee et al., 2006). However, it has been also reported that LPS induced cytokine activation in macrophages was not inhibited in CRAMP−/− mice (Pinheiro da Silva et al., 2009). Therefore the role of Cramp in the intestinal pro-inflammatory response is controversial and required further study.
One of the limitations of the study is that our experimental diets do not necessarily reflect the human diets. The calories from the fat were predominantly derived from MCT in SF fed groups, and from the corn oil in USF fed animals. However, in the real life, the dietary fat is mixed and comprised of the different proportions of saturated and unsaturated fatty acids. Nevertheless, the obtained data support the hypothesis that USF (corn oil/LA enriched) is a contributing factor to the EtOH-mediated intestinal inflammatory response, and a key player in the intestinal mucus layer alterations.
Acknowledgments
The authors thank Hanan Farghaly, MD for help in the intestinal histological evaluation; David Barker, PhD for the qPCR primer design; and Marion McClain for manuscript proofreading.
Funding
The work presented in this study was supported by NIH grants R21 AA020849-01A1 (IK), P01 AA017103 (CJM), R01 AA0015970 (CJM), R01 AA018016 (CJM, SB), R01 DK071765 (CJM), R37 AA010762 (CJM), R01 AA018869 (CJM), P30 AA019360 (CJM), RC2AA019385 (CJM), and the Department of Veterans Affairs (CJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Al-Sadi R, Guo S, Dokladny K, Smith MA, Ye D, Kaza A, Watterson DM, Ma TY. Mechanism of Interleukin-1beta Induced-Increase in Mouse Intestinal Permeability In Vivo. J. Interferon Cytokine Res. 2012 doi: 10.1089/jir.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoghaibi MA, Walsh SW, Willey A, Yager DR, Fowler AA, 3rd, Graham MF. Linoleic acid induces interleukin-8 production by Crohn's human intestinal smooth muscle cells via arachidonic acid metabolites. Am. J. Physiol. Gastroint. Liver Physiol. 2004;286:G528–G537. doi: 10.1152/ajpgi.00189.2003. [DOI] [PubMed] [Google Scholar]
- Amin PB, Diebel LN, Liberati DM. Dose-dependent effect of ethanol and E.Coli on gut permeability and cytokine production. J. Surg. Res. 2009;157:187–192. doi: 10.1016/j.jss.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Andrade MC, Menezes JS, Cassali GD, Martins-Filho OA, Cara DC, Faria AM. Alcoholinduced gastritis prevents oral tolerance induction in mice. Clin. Exp. Immunol. 2006;146:312–322. doi: 10.1111/j.1365-2249.2006.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. JPharmacol. Exp. Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Practice Res. Clin. Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Bode C, Kolepke R, Schafer K, Bode JC. Breath hydrogen excretion in patients with alcoholic liver disease--evidence of small intestinal bacterial overgrowth. Zeitschrift fur Gastroenterologie. 1993;31:3–7. [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;6:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, Browne RW. Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am. J. Physiol. Gastroint. Liver Physiol. 2008;294:G948–G962. doi: 10.1152/ajpgi.00274.2007. [DOI] [PubMed] [Google Scholar]
- Costantini TW, Peterson CY, Kroll L, Loomis WH, Putnam JG, Wolf P, Eliceiri BP, Baird A, Bansal V, Coimbra R. Burns, inflammation, and intestinal injury: protective effects of an antiinflammatory resuscitation strategy. J. Trauma. 2009;67:1162–1168. doi: 10.1097/TA.0b013e3181ba3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World. J. Gastroenterol. 2003;9:1327–1332. doi: 10.3748/wjg.v9.i6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol. Clin. Exp. Res. 2001;25:579–589. [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J. Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol. Rev. 2012;245:147–163. doi: 10.1111/j.1600-065X.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–e1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff MF. Microbial-epithelial cell crosstalk during inflammation: the host response. Ann.NYAcad. Sci. 2006;1072:313–320. doi: 10.1196/annals.1326.038. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The Type of Dietary Fat Modulates Intestinal Tight Junction Integrity, Gut Permeability, and Hepatic Toll-Like Receptor Expression in a Mouse Model of Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol . 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Enomoto N, Connor HD, Wheeler MD, Bradford BU, Rivera CA, Kadiiska MB, Mason RP, Thurman RG. Medium-chain triglycerides inhibit free radical formation and TNFalpha production in rats given enteral ethanol. Am. J. Physiol. Gastroint. Liver Physiol. 2000;278:G467–G476. doi: 10.1152/ajpgi.2000.278.3.G467. [DOI] [PubMed] [Google Scholar]
- Kono H, Fujii H, Asakawa M, Yamamoto M, Matsuda M, Maki A, Matsumoto Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Annals of surgery. 2003;237:246–255. doi: 10.1097/01.SLA.0000048450.44868.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alphainduced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastroint. Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol. Clin. Exp. Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastroint. Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW. Dietary factors and alcoholic cirrhosis. Alcohol. Clin. Exp. Res. 1986;10:271–273. doi: 10.1111/j.1530-0277.1986.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44:223–227. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J. Pharmacol. Exp. Ther. 2001;299:638–644. [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am. J. Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol. Clin. Exp. Res. 1989;13:15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Yang EK, Fogt F, Sadrzadeh SM, Dannenberg AJ. Medium chain triglycerides and vitamin E reduce the severity of established experimental alcoholic liver disease. J. Pharmacol. Exp. Ther. 1996;277:1694–1700. [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Pinheiro da Silva F, Gallo RL, Nizet V. Differing effects of exogenous or endogenous cathelicidin on macrophage toll-like receptor signaling. Immunol. Cell Biol. 2009;87:496–500. doi: 10.1038/icb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendram R, Preedy VR. Effect of alcohol consumption on the gut. Dig Dis. 2005;23:214–221. doi: 10.1159/000090168. [DOI] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am. J. Physiol. Gastroint. Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J. Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World. J. Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol. Clin. Exp. Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesityassociated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J. Immunol. 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferongamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World. J. Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, Nagy LE, Feldstein AE, McIntyre TM. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J. Biol. Chem. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastroint. Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcoholinduced intestinal barrier dysfunction. Am. J. Physiol. Gastroint. Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]