Abstract
Background
Cotinine, a nicotine metabolite, is a biomarker of tobacco, nicotine and carcinogen exposure. However a given cotinine level may not represent the same tobacco exposure; for example, African Americans have higher cotinine levels than Caucasians after controlling for exposure.
Methods
Cotinine levels are determined by the amount of cotinine formation and the rate of cotinine removal which are both mediated by the enzyme CYP2A6. Since CYP2A6 activity differs by sex (estrogen induces CYP2A6) and genotype, their effect on cotinine formation and removal were measured in non-smoking Caucasians (Study 1, n=181) infused with labeled nicotine and cotinine. The findings were then extended to ad libitum smokers (Study 2, n=163).
Results
Study 1: Reduced CYP2A6 activity altered cotinine formation less than cotinine removal resulting in ratios of formation to removal of 1.31 and 1.12 in CYP2A6 reduced and normal metabolizers (P=0.01), or 1.39 and 1.12 in males and females (P=0.001), suggesting an overestimation of tobacco exposure in slower metabolizers. Study 2: Cotinine again overestimated tobacco and carcinogen exposure by ≥25% in CYP2A6 reduced metabolizers (≈2 fold between some genotypes) and in males.
Conclusions
In people with slower, relative to faster, CYP2A6 activity cotinine accumulates resulting in substantial differences in cotinine levels for a given tobacco exposure.
Impact
Cotinine levels may be misleading when comparing those with differing CYP2A6 genotypes within a race, between races with differing frequencies of CYP2A6 gene variants (i.e. African Americans have higher frequencies of reduced function variants contributing to their higher cotinine levels) or between the sexes.
Keywords: Tobacco, Cotinine, CYP2A6, Polycyclic aromatic hydrocarbons, NNAL
INTRODUCTION
In humans, cotinine is metabolically formed from nicotine in a reaction catalyzed by CYP2A6 and is further metabolized to trans’-3-hydroxycotinine (3HC) by CYP2A6 (1, 2). Cotinine is routinely used as an objective index of tobacco and tobacco-derived carcinogen exposure. The use of cotinine is particularly useful because of wide individual variability in the relationship between self-reported cigarettes smoked per day and systemic exposure to tobacco and tobacco-derived carcinogen (3). Higher plasma cotinine levels have been associated with increased lung cancer risk (4), and variation in cotinine levels have recently been used as evidence that the mechanism mediating the association between genetic variation in CHRNA5-A3-B4 and lung cancer risk is almost entirely through the modulation of tobacco consumption (5).
The systemic intake of nicotine is correlated with exposure to tobacco-derived carcinogen (3). When used as a biomarker of tobacco-derived carcinogen exposure, it is generally assumed that plasma cotinine levels reflect intake of nicotine, and that variability in the relationship between cotinine levels and nicotine intake is random among smokers. However, some research suggests that a particular level of cotinine does not predict the same level of tobacco exposure among different groups of smokers. For example, directional inconsistencies have been observed between plasma cotinine levels and other indicators of tobacco exposure in genetic association studies involving CYP2A6. Caucasian smokers with one or more reduced function CYP2A6 alleles (i.e. CYP2A6 reduced metabolizers, or RM) smoke fewer cigarettes per day compared to the CYP2A6 normal metabolizers (NM), consistent with titration of smoke intake to obtain desired levels of nicotine in the body (6, 7). Yet despite smoking fewer cigarettes per day, smokers with reduced CYP2A6 activity have similar plasma cotinine levels compared to the smokers with faster CYP2A6 activity (8). Another example is found in studies of African American light smokers, where similar levels of cigarettes per day are reported between CYP2A6 genotypes (i.e. CYP2A6 RM have similar cigarettes per day compared to CYP2A6 NM), but the plasma cotinine levels are significantly higher in CYP2A6 RM compared to CYP2A6 NM (9). Furthermore, gene variants at the CYP2A6 loci (i.e. tag SNPs) that are associated with decreased lung cancer risk are paradoxically associated with increased, rather than decreased, plasma cotinine levels (10). These observations suggest that CYP2A6 reduced activity genotype may increase plasma cotinine levels in addition to its role in reducing nicotine inactivation, tobacco consumption and nitrosamine metabolic activation (11).
Variation in plasma cotinine levels is also observed between the sexes. The male participants of the National Health and Nutrition Examination Surveys have significantly higher plasma cotinine levels compared to the female participants even after adjusting for cigarettes per day, machine-determined nicotine delivery of cigarettes, race, age, body mass index, poverty status, and the use of either menthol or regular cigarettes (12). Since CYP2A6 activity differs between the sexes, this systematic variation in cotinine levels between the sexes could be the result of the difference in CYP2A6 activity (13).
Notable variation in cotinine levels is also observed between races. African American smokers generally have higher plasma cotinine levels compared to Caucasian smokers after adjusting for the number of cigarettes smoked per day and the machine-determined nicotine delivery in cigarettes (14), which could be partially due to the slower cotinine clearance in African Americans compared to Caucasians (15). Since the prevalence of CYP2A6 RM is high in African Americans compared to Caucasians, it is possible that the racial variation in cotinine levels originates from the difference in CYP2A6 activities between racial groups.
Steady state plasma cotinine levels are determined by three factors: the daily intake of nicotine, the fraction of nicotine converted to cotinine (fNIC→COT), and the systemic clearance of cotinine. The steady state plasma cotinine level is described by the following equation:
| (1) |
where COTplasma represents the steady state plasma cotinine levels, Dosenic is the daily nicotine intake. fNIC→COT is the fraction of nicotine converted to cotinine (i.e. cotinine formation), and ClCOT represents systemic plasma cotinine clearance (i.e. cotinine removal) (16). Hence, the steady state plasma cotinine levels are proportional to the ratio of fNIC→COT/ClCOT and the Dosenic. Cotinine is both formed and removed by CYP2A6. Thus, both fNIC→COT and cotinine clearance are highly dependent on CYP2A6 activity. We hypothesize that reduced CYP2A6 activity will increase the ratio of fNIC→COT/ClCOT, and alter the quantitative relationship between plasma cotinine and nicotine and tobacco-derived carcinogen exposure in cigarette smokers.
In this study, we use CYP2A6 genotype as the primary indicator of CYP2A6 activity. CYP2A6 genetic variants are known to alter in vivo CYP2A6 activity as well as nicotine and cotinine clearance (17–23). In addition to CYP2A6 genotype, we also use the nicotine metabolite ratio (NMR) as a secondary indicator of CYP2A6 activity (24). For the evaluation of nicotine intake in ad libitum smokers, we use urinary total nicotine equivalents (TNE) (25). For the evaluation of tobacco-derived carcinogen in ad libitum smokers, we used total urinary (methylnitrosamino)-1-(3) pyridyl-1-butanol (NNAL), 1-hydroxyfluorene and 1-hydroxypyrene levels (26, 27).
MATERIALS AND METHODS
Study design
Data for the present analysis were taken from two studies that have been previously published (21, 28).
Study 1
The pharmacokinetics of nicotine and cotinine were characterized in 181 non-smoking Caucasian male and female sets of twins (both monozygotic and dizygotic). Demographic variable comparisons are shown in supplementary table 1. A comprehensive description of the study procedures has been published previously (29, 30). Briefly, participants received a simultaneous 30-minute infusion of deuterium-labeled nicotine and cotinine in the fasting condition. Blood samples were collected for the measurement of labeled nicotine and cotinine for determination of pharmacokinetic parameters and DNA was extracted for CYP2A6 genotyping. The cotinine and 3HC levels were measured in the 480-minute plasma sample for determination of the NMR (21). The pharmacokinetic calculations can be found in the supplementary methods.
Study 2
The relationship between plasma cotinine, nicotine intake and carcinogen exposure was studied in daily ad libitum smokers (Supplementary table 1). In a cross sectional study of 163 Alaska Native smokers, the plasma cotinine levels urinary TNE, total NNAL and PAH metabolites were measured. A comprehensive description of the study procedures has been published elsewhere (28).
Tobacco and tobacco derived carcinogen exposure biomarkers
To evaluate the quantitative relationship between plasma cotinine and tobacco exposure in cigarette smokers, we used urinary total nicotine equivalents (TNE) from Study 2 participants as the reference biomarker of nicotine and tobacco exposure as it is the summation of multiple nicotine metabolic pathways. TNE is the total urinary level of nicotine and 8 of its metabolites (i.e. nicotine, nicotine glucuronide, cotinine, cotinine glucuronide, 3HC, 3HC glucuronide, nicotine-N-oxide, cotinine-N-oxide, and nornicotine). Together, the 9 analytes account for about 90% of nicotine (determined from a transdermal administered nicotine dose (31)), and creatinine adjusted spot urinary TNE correlates with daily tobacco consumption (32). TNE is not influenced by the different rates of nicotine and cotinine metabolism since it measures the nicotine metabolites generated via different metabolic pathways.
To investigate the relationship between plasma cotinine levels and tobacco-derived carcinogen exposure, we used total urinary (methylnitrosamino)-1-(3) pyridyl-1-butanol (NNAL) levels as the biomarker for tobacco specific nitrosamines exposure in study 2 participants (33). NNAL is a reductive metabolite of the highly carcinogenic 4-(methylnitrosamino)-1-(3) pyridyl-1-butanone (NNK); prediagnostic levels are associated with subsequent lung cancer risk (34).
We also investigated the relationship between plasma cotinine levels and exposure to another class of tobacco-related carcinogens which are not nicotine-derived, the polycyclic aromatic hydrocarbons (33). The urinary levels of two hydroxylated polycyclic aromatic hydrocarbons, 1-hydroxypyrene and 1-hydroxyfluorene, were used as biomarkers of polycyclic aromatic hydrocarbons exposure. 1-Hydroxypyrene levels have been directly associated with cancer risk (35), and recent evidence suggests that 1-hydroxyfluorene is more specific to tobacco exposure than 1-hydroxypyrene (27).
CYP2A6 genotyping
Prevalent CYP2A6 alleles with altered function were genotyped by two step-allele specific PCR reactions in the Pharmacogenetics laboratory at CAMH and University of Toronto as previously reported (7, 20, 36, 37). Those individuals with one or two copies of reduced function allele (*2, *4, *7, *9, *10, *12, *17 and *35) were classified as CYP2A6 RM (20, 36, 37).
The measurement of CYP2A6 activity
The in vivo CYP2A6 activity was measured using NMR, which is the ratio between plasma 3HC and cotinine. NMR is widely used as an in vivo indicator of CYP2A6 activity (24, 36, 38, 39). In the context of our data analysis, the use of NMR may be confounded with plasma cotinine since it uses plasma cotinine as the denominator. Thus, we use NMR to extend our findings with CYP2A6 genotype as it is a continuous variable and for comparisons to the literature.
Tobacco and tobacco-derived carcinogen exposure measurements
Plasma nicotine, cotinine and 3HC levels, as well as urinary nicotine and metabolites, total NNAL, and PAH metabolites were quantified by gas/liquid chromatography-tandem mass spectrometry in the Clinical Pharmacology Laboratory at the University of California San Francisco as previously described (24, 40–43). NMR stratification was done by a median split of plasma NMR within each study population; participants who were in the higher NMR stratum were considered the faster CYP2A6 activity group, while those in the lower NMR stratum were considered the slower CYP2A6 activity group.
Statistical analysis
Statistical analyses were performed using R statistical package (version 2.13, R foundation for statistical computing). In study 1 statistical comparisons between CYP2A6 genotypes and sex were performed using mixed-effect linear regressions. All analyses controlled for non-independence of data in twin pairs by modeling twining as a random effect (21). Spearman’s correlation was used to evaluate the relationship between 1) NMR and fNIC→COT/ClCOT; 2) NMR and cotinine to TNE ratio; 3) Plasma cotinine and urinary TNE, NNAL, 1-hydroxypyrene and 1-hydroxyflurene. Fisher’s r to z transformation was used to compare the correlation coefficients. To minimize the influence of potential confounders such as race and smoking status on cotinine pharmacokinetics, the effects of CYP2A6 genotype and sex were examined within non-smoking Caucasians in Study 1.
RESULTS
CYP2A6 Reduced function genetic variants decreased cotinine removal more than cotinine formation (Study 1)
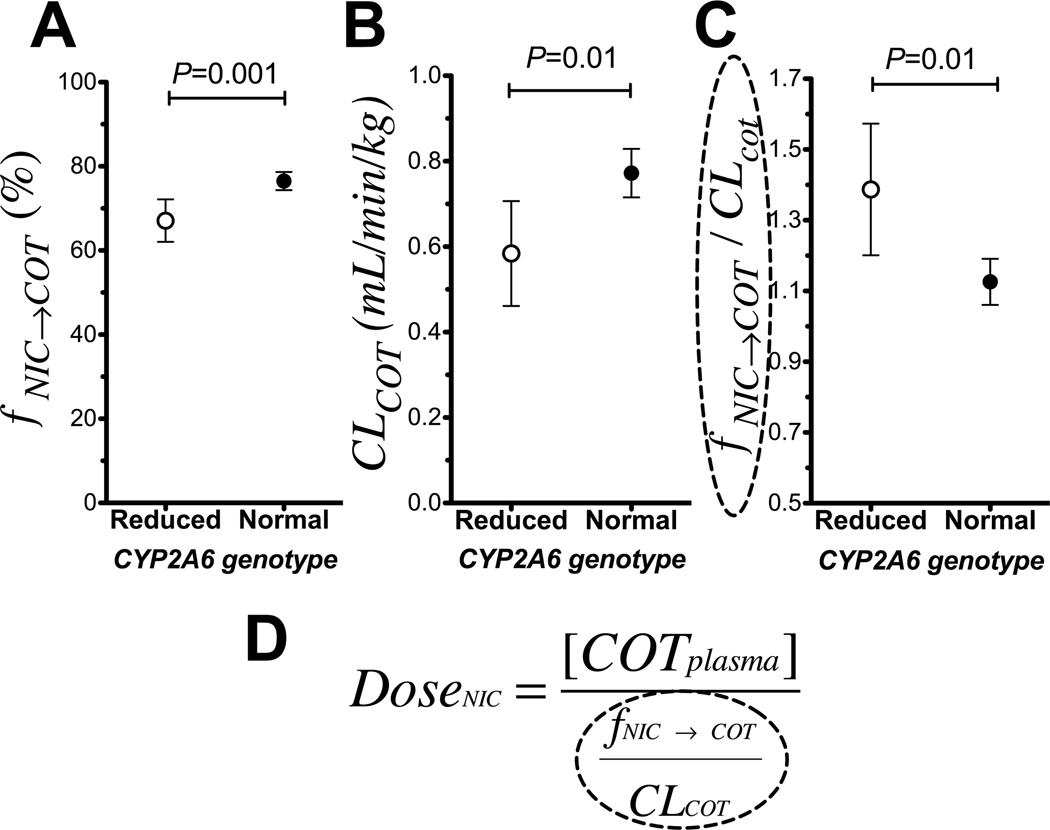
Fig. 1A compares cotinine formation (fNIC→COT) between CYP2A6 genotypes in non-smoking Caucasians. CYP2A6 RM (n=33) had significantly lower fNIC→COT compared to CYP2A6 NM (n=148). In CYP2A6 RM, 67% of the nicotine dose was metabolized to cotinine compared to the 77% in CYP2A6 NM, a 15% relative reduction, P=0.001 (Fig. 1A). CYP2A6 RM also had lower cotinine clearance compared to the CYP2A6 NM (0.58 ml/min/kg in CYP2A6 RM versus 0.77 ml/min/kg in CYP2A6 NM, a 33% reduction, P=0.01, Fig. 1B). The ratio of the fractional conversion of nicotine (reflecting cotinine formation) to cotinine clearance (reflecting cotinine removal), fNIC→COT/ClCOT, was significantly higher in CYP2A6 RM compared to CYP2A6 NM (1.31 in CYP2A6 RM versus. 1.12 in CYP2A6 NM, P=0.01, Fig. 1C). Of note, the resulting fNIC→COT/CLCOT ratio is the conversion factor, or average correction needed in plasma cotinine levels to accurately indicate the same nicotine dose (Fig. 1D). These genetic differences were consistent in both males and females (Supplementary fig. 1A–F). In agreement with the findings between CYP2A6 genotypes, statistically significant differences in fNIC→COT, cotinine clearance and fNIC→COT/ClCOT were observed when comparing the faster versus the slower NMR strata (Supplementary fig. 1G–H). There was a significant inverse correlation between NMR (i.e. a continuous measure of CYP2A6 activity) and fNIC→COT/ClCOT, suggesting the effect size is even larger when comparing the two extreme ends of CYP2A6 activity (Rho=−0.57, P<0.0001).
Figure 1.
Reduced CYP2A6 activity had a greater impact on cotinine removal than cotinine formation (Study 1), which would result in the accumulation of cotinine and higher cotinine levels at a given tobacco exposure. A. CYP2A6 reduced metabolizers metabolized (n=33) a significantly lower amount of nicotine to cotinine compared to CYP2A6 normal metabolizers (n=148). B. CYP2A6 reduced metabolizers (n=33) had lower cotinine clearance (CLCOT) compared to CYP2A6 normal metabolizers (n=148). C. The fNIC→COT/CLCOT ratio was higher in CYP2A6 reduced metabolizers (n=33) compared to CYP2A6 normal metabolizers (n=148). Data presented as mean ± 95% confidence interval. D. The fNIC→COT/CLCOT ratio is the conversion factor between plasma cotinine levels and the actual nicotine dose.
Different relationships between plasma cotinine and tobacco exposure were observed between smokers with different CYP2A6 genotype groups (Study 2)
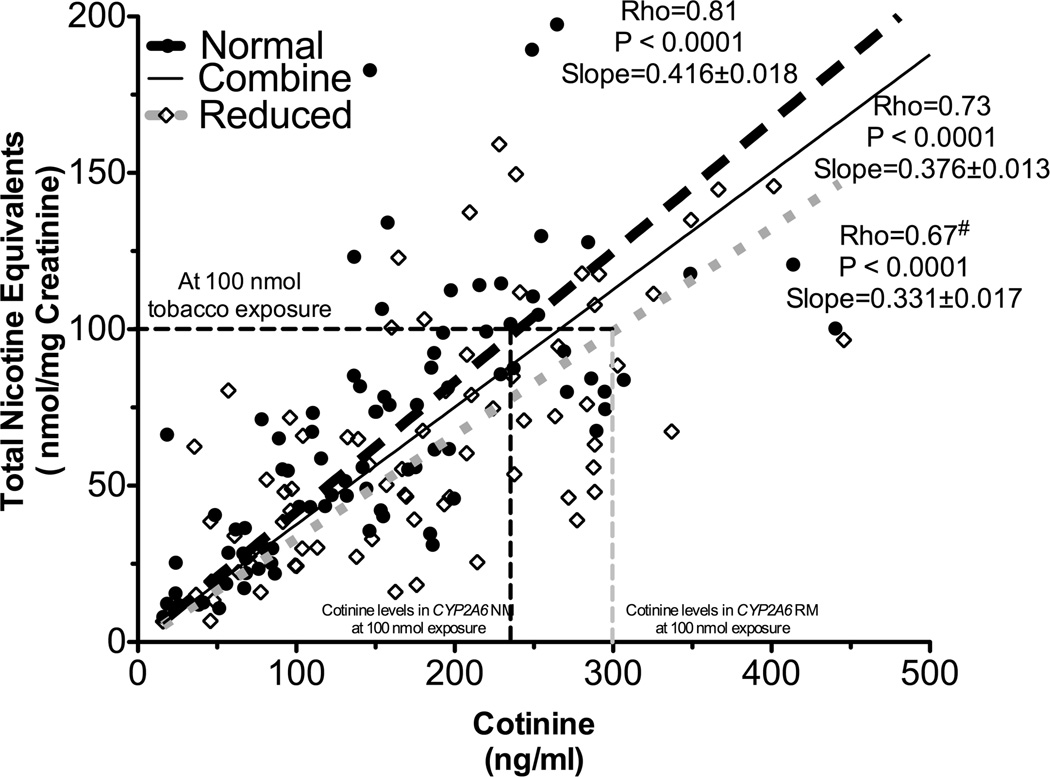
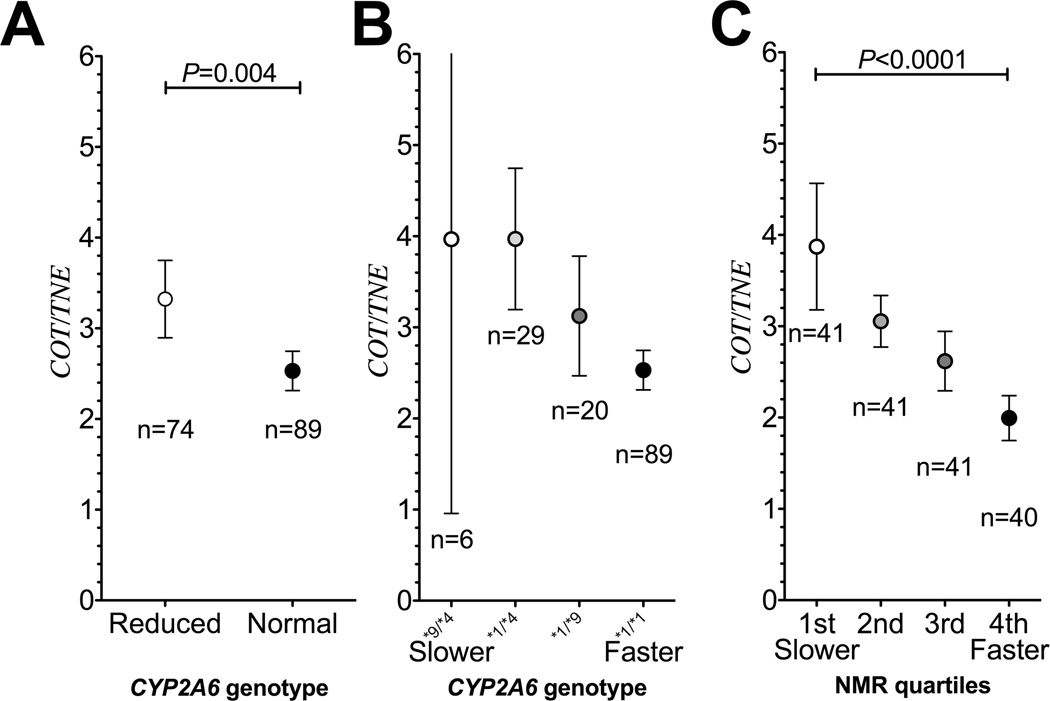
Next, the quantitative relationship between plasma cotinine and nicotine intake in smokers was investigated. Demographic variable comparisons are shown in supplementary table 1. Independent regression lines between urinary TNE and plasma cotinine were constructed in CYP2A6 RM and NM (n=74 and n=89, respectively). As illustrated by fig. 2, the slope of the regression line in CYP2A6 RM was significantly lower compared to the slope in CYP2A6 NM (slope: 0.33 in CYP2A6 RM versus 0.42 in CYP2A6 NM, P=0.001, Fig. 2). Statistical significance is demonstrated by a significant interaction between CYP2A6 genotype and cotinine levels in the linear regression analysis presented in supplementary table 2A. A similar difference in regression line slopes was observed between the NMR strata (Supplementary fig. 2). There was a significant inverse correlation between NMR, a continuous measure of CYP2A6 activity, and the cotinine to TNE ratio (Rho=−0.46, P<0.0001). These observations indicate that cotinine predicts nicotine intake, and therefore tobacco consumption, differently in smokers with reduced compared to normal CYP2A6 activity. As illustrated by fig. 2, a 300 ng/mL (which is equivalent to 1702 nmol/L) cotinine level was indicative of a 100 nmol nicotine equivalents exposure in CYP2A6 NM, whereas the same cotinine level was indicative of roughly 125 nmol exposure in CYP2A6 RM. Alternatively, at 100 nmol tobacco exposure, CYP2A6 RM would have 300 ng/mL plasma cotinine levels, whereas the CYP2A6 NM would have only 240 ng/mL. This is a 25% difference (60 ng/mL or 340 nmol/L) in cotinine levels between CYP2A6 genotype groups. Since reduced metabolizers include those with a range of decreased activity combined together, the 25% reflects an average difference (Fig. 3A). As illustrated by some specific genotypes, or quartiles of NMR, these differences in cotinine estimates of dose can be ≈2 fold when comparing between the extremes of CYP2A6 activity or among different genotypes (fig. 3B and fig. 3C).
Figure 2.
Cotinine’s ability to predict tobacco exposure was different between CYP2A6 genotypes (Study 2). The slope between urinary TNE and plasma cotinine was significantly lower in CYP2A6 reduced metabolizers (n=74) compared to that of CYP2A6 normal metabolizers (n=89, supplementary table 2A), suggesting the quantitative relationship between cotinine and tobacco exposure (i.e. TNE) differed between CYP2A6 genotypes. # indicates statistical significant difference in Spearman’s Rho compared to the CYP2A6 normal metabolizers. The numbers after the slopes are standard error. Of note, the strength of correlations between plasma cotinine and urinary TNE in the CYP2A6 RM was significantly weaker than in CYP2A6 NM (Fisher’s r to Z transformation: P<0.01).
Figure 3.
A. The cotinine to TNE ratio (i.e. cotinine levels per nicotine intake) was significantly lower in CYP2A6 NM compared to the CYP2A6 RM. (Study 2) B. The cotinine to TNE ratio decreased with CYP2A6 genotypes with increasing activity (Study 2). As illustrated using some different CYP2A6 genotypes, containing reduced function (*9) or loss of function (*4) allele compared to the wild type individuals (*1/*1). Of note, individuals who are fully null for CYP2A6 (i.e. these with two copies of gene deletions, CYP2A6*4/*4) had unexpectedly lower COT to TNE ratio compared to the wild-type individuals (*1/*1, data not shown), suggesting the minor remaining cotinine formation pathway (likely CYP2B6 or CYP2A13) is considerably slower than the low affinity cotinine removal pathway (likely UGT2B10 glucuronidation or renal clearance). C. The cotinine to TNE ratio decreased by NMR quartiles (Study 2). Data presented as mean ± 95% confident intervals. Statistical comparisons were performed by Mann Whitney or Kruskal-Wallis tests.
Different relationships between plasma cotinine and tobacco specific nitrosamine exposures were observed in smokers with different CYP2A6 genotype groups (Study 2)
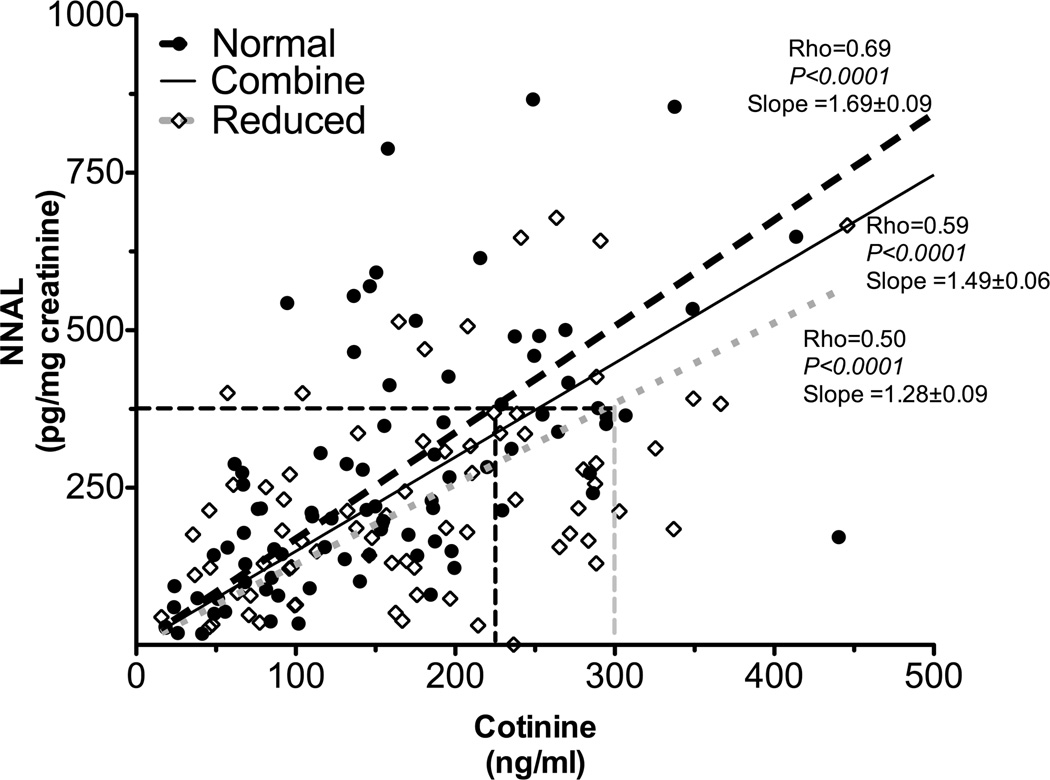
Next, we investigated the quantitative relationship between plasma cotinine and tobacco specific nitrosamines exposure in smokers. As seen with TNE, the slope of the regression line between urinary NNAL and plasma cotinine in CYP2A6 RM was significantly lower compared to the slope in CYP2A6 NM (slope: 1.279 in CYP2A6 RM versus 1.687 in CYP2A6 NM, P=0.003, fig. 4. Statistical significance demonstrated by a significant interaction term in the linear regression analysis presented in supplementary table 2B). These results demonstrated that that cotinine levels predict NNAL levels, and therefore nitrosamine exposure, differently in smokers with reduced compared to normal CYP2A6 activity. As illustrated by fig. 4, a 300 ng/mL (which is equivalent to 1702 nmol/L) cotinine level was indicative of a 510 pg/mg creatinine NNAL exposure in CYP2A6 NM, whereas the same cotinine level was indicative of roughly 375 pg/mg creatinine NNAL exposure in CYP2A6 RM. Alternatively, at 375 pg/mg creatinine NNAL exposure, CYP2A6 RM would have 300 ng/mL plasma cotinine levels, whereas the CYP2A6 NM would have only 225 ng/mL. The magnitude of the CYP2A6 genotype effect (25%) on plasma cotinine levels was similar to that observed with TNE as the exposure marker; as with the TNE biomarker this is an underestimation of effect size for some genotype or phenotype comparisons (see figure 3).
Figure 4.
Cotinine’s ability to predict tobacco specific nitrosamines exposure (as indicated by NNAL levels) was different between CYP2A6 genotypes (Study 2). The slope between urinary NNAL levels and plasma cotinine was significantly lower in CYP2A6 reduced metabolizers (n=74) compared to that of CYP2A6 normal metabolizers (n=89, supplementary table 2B). This suggested the quantitative relationship between cotinine and tobacco specific nitrosamines exposure differed between CYP2A6 genotypes. The numbers after the slopes are standard error.
To determine whether the above observations were unique to plasma cotinine, independent regression lines between urinary NNAL and urinary TNE levels were constructed in CYP2A6 RM and NM. The slope of the regression lines did not differ between CYP2A6 genotypes (Supplementary fig 3, and supplementary table 2C), indicating that as expected TNE’s relationship to tobacco specific nitrosamines exposure in smokers was independent of CYP2A6 genotype. Together this demonstrates that the impact of CYP2A6 genotype on plasma cotinine levels was specific to cotinine as an exposure biomarker.
Different relationships between plasma cotinine and polycyclic aromatic hydrocarbon exposure were observed in smokers with different CYP2A6 genotype groups (Study 2)
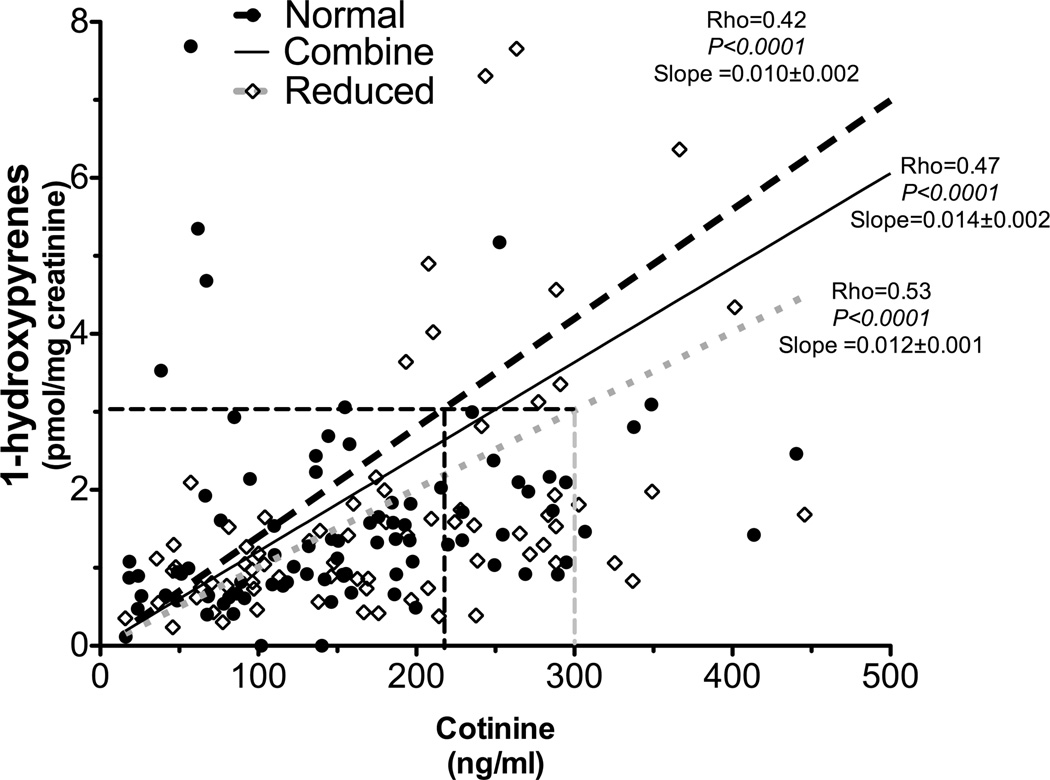
Next, we investigated the quantitative relationship between plasma cotinine and polycyclic aromatic hydrocarbon exposure in smokers. As illustrated by supplementary fig. 4 and fig. 5, the slopes of the regression lines in CYP2A6 RM were lower compared to the slope in CYP2A6 NM (1-hydroxyfluorene slopes: 0.024 in CYP2A6 RM vs. 0.027 in CYP2A6 NM, P=0.013, supplementary fig. 4 and supplementary table 2D; 1-hydroxypyrene slopes: 0.012 in CYP2A6 RM versus 0.010 in CYP2A6 NM, P=0.079, Fig. 5 and supplementary table 2E).
Figure 5.
Cotinine’s ability to predict polycyclic aromatic hydrocarbon exposure (as indicated by 1-hydroxypyrene) was different between CYP2A6 genotypes (Study 2). The slope between 1-hydroxypyrene levels and plasma cotinine was lower in CYP2A6 reduced metabolizers (n=74) compared to that of CYP2A6 normal metabolizers (n=89, supplementary table 2E). The numbers after the slopes are standard error.
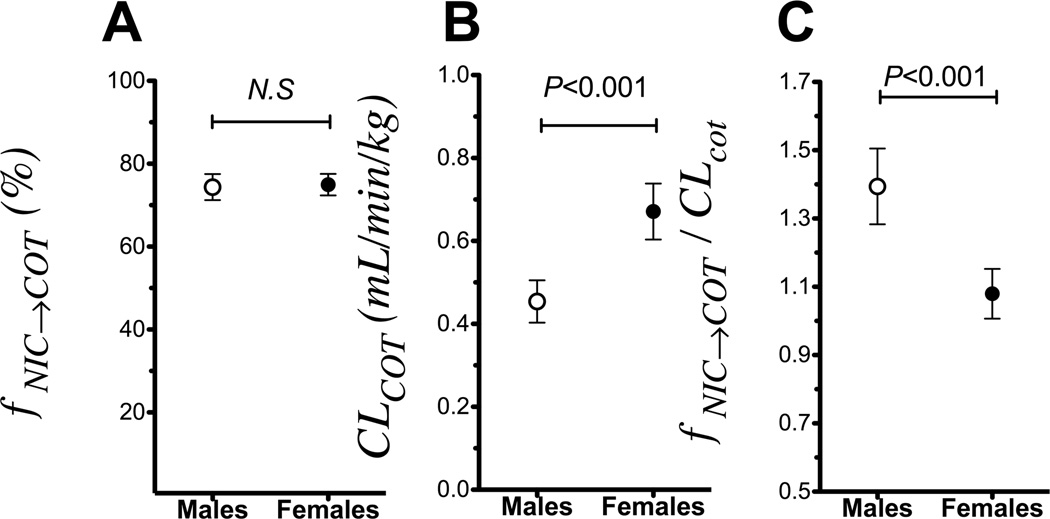
Males had a greater reduction in cotinine clearance than in cotinine formation (Study 1)
Because CYP2A6 activity differs between the sexes, we investigated the impact of sex on cotinine formation and cotinine clearance. The fNIC→COT did not differ between the sexes (74% in males compared to 75% in females, non-significant, Fig. 6A). However males had a significantly lower cotinine clearance compared to females (0.58 versus 0.81 ml/min/kg in males and females, respectively, P<0.001, Fig. 6B). A significantly higher fNIC→COT / CLCOT ratio, which is the conversion factor between plasma cotinine levels and the nicotine dose (Fig. 1D), was observed in males compared to females (1.39 versus 1.12 in males and females, respectively, P=0.001, Fig. 6C). As observed with the entire study population, similar impacts of sex on fNIC→COT, cotinine clearance and fNIC→COT / CLCOT could be observed when examined only within the subgroup of CYP2A6 NM (i.e. excluding the CYP2A6 RM, supplementary fig. 5).
Figure 6.
Males (n=54), who have reduced CYP2A6 activity compared to females (n=127), had a greater ratio of cotinine formation over cotinine removal, which would result in the accumulation of cotinine and higher cotinine levels at a given tobacco exposure in male smokers (Study 1). A. The fNIC→COT did not differ between the sexes. B. Males had significantly lower ClCOT compared to the females. C. Males had a higher fNIC→COT/CLCOT compared to females indicating an over-estimation of nicotine dose for their cotinine levels (see Fig 1D). Data presented as mean ± 95% confidence interval.
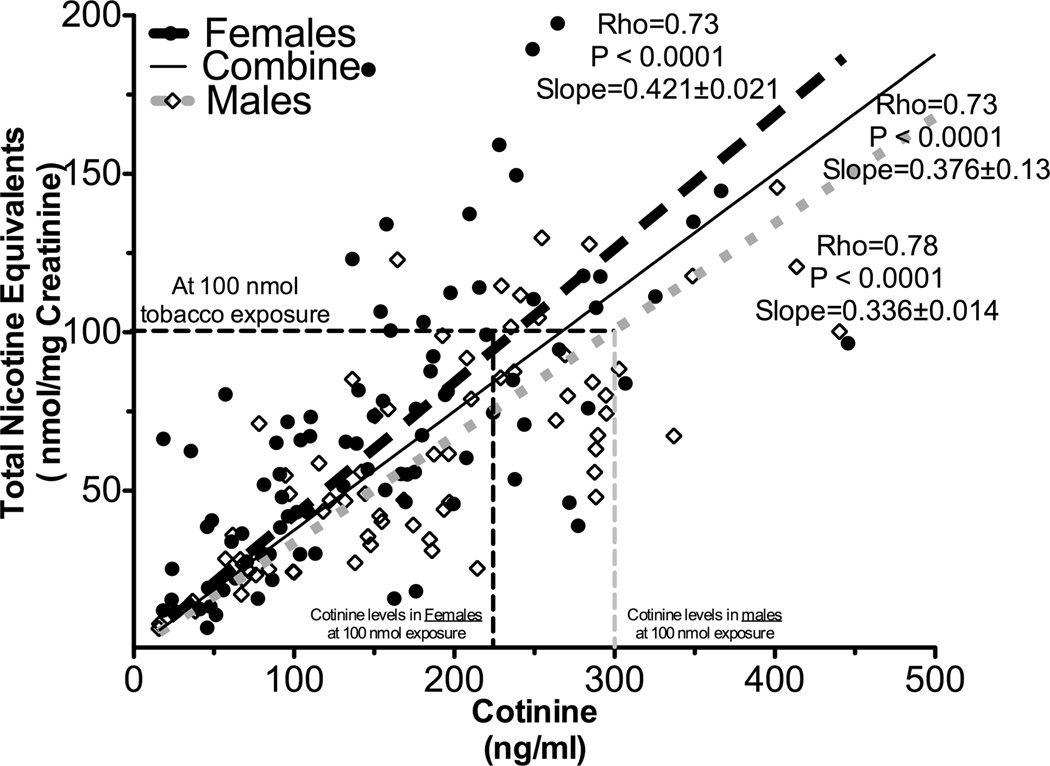
Different relationships between plasma cotinine and tobacco exposure were observed in male smokers compared to female smokers (Study 2)
Independent regression lines between urinary TNE and plasma cotinine were constructed by sex. The slope of the regression line in males was significantly lower compared to the slope in females (0.34 in males versus 0.42 in females, P=0.01, Fig 7 and supplementary table 2F). The magnitude of the sex effect on cotinine levels was similar to the effect of CYP2A6 genotype. At 100 nmol tobacco exposure, there is a 25% difference in plasma cotinine levels (60 ng/mL or 340 nmol/L) between the sexes. As observed with the entire study population, similar impacts of sex could be observed when examined within just the CYP2A6 NM (slopes: 0.36±0.02 in males versus 0.54±0.03 in females, P=0.001)
Figure 7.
Cotinine’s ability to predict tobacco exposure was different between the sexes. The slope between urinary TNE and plasma cotinine was significantly lower in males (n=71) compared to that of females (n=92, supplementary table 2F), suggesting cotinine over-estimates tobacco exposure (i.e. TNE) in males compared to females (Study 2). The numbers after the slopes are standard error.
DISCUSSION
Our analyses demonstrate that variation in CYP2A6 metabolic activity, such as those that exist between CYP2A6 genotypes or the sexes, may alter cotinine removal (i.e. cotinine clearance) to a different extent than cotinine formation (i.e. fNIC→COT). Individuals with lower CYP2A6 activities, such as CYP2A6 RM and males, have higher ratios of cotinine formation to cotinine removal (i.e. fNIC→COT / CLCOT) compared to individuals with faster CYP2A6 activity, such as CYP2A6 NM and females. Thus, the quantitative relationship between plasma cotinine and tobacco and tobacco derived carcinogen exposures in smokers varies between those with different CYP2A6 activity.
The pharmacokinetic mechanism
The greater effect of altered CYP2A6 activity on cotinine clearance compared to formation is likely related to differences in nicotine and cotinine’s hepatic metabolism. Nicotine exhibits a higher hepatic extraction ratio compared to cotinine, which is largely due to differences in their intrinsic clearances. The intrinsic clearance (Vmax/Km) of nicotine is 1.69 µL/min/mg protein in human liver microsomes, which is 10 times faster than the 0.16 µL/min/mg protein of cotinine (1, 2, 44, 45). For drugs like nicotine, with high extraction ratios, clearance is determined in large part by liver blood flow and is less affected by changes in liver enzymatic activity. In contrast for low extraction drugs like cotinine clearance is largely affected by changes in liver enzymatic activity. This differential impact of variation in CYP2A6 activity is consistent with previous observations on the effects of CYP2A6 induction. Estrogen induces CYP2A6 protein expression in humans (46). During pregnancy, when estrogen levels are elevated, the clearance of nicotine increases by 60% whereas the clearance of cotinine increased by 140% (47). Thus, higher CYP2A6 activity during pregnancy has a greater effect on cotinine clearance than nicotine clearance.
CYP2A6, sex and race
Consistent with a higher fNIC→COT / CLCOT ratio predicting higher cotinine levels for the same tobacco and tobacco-derived carcinogen exposure (equation 1), we observed different quantitative relationships between cotinine and tobacco and tobacco-derived carcinogen exposure in smokers with reduced CYP2A6 activities (CYP2A6 RM and males). These observations provide a mechanistic explanation for the systematic variation in plasma cotinine levels previously observed between CYP2A6 genotypes, the sexes and possibly races (9, 10, 12).
Our observations explain the directional inconsistencies between cotinine and other indicators of tobacco consumption in genetic association studies involving CYP2A6 (9, 10). Due to the higher fNIC→COT/CLCOT ratio, CYP2A6 RM would have higher plasma cotinine levels upon the same tobacco exposure compared to the CYP2A6 NM. This masks, or reduces, the true size of the effect of CYP2A6 genotype on tobacco consumption based on plasma cotinine (i.e. CYP2A6 RM have higher cotinine levels with lower tobacco consumption). These results could also explain why the same CYP2A6 genetic variants have been associated with lower lung cancer risk while having paradoxically higher plasma cotinine levels (10).
Similarly, during transdermal nicotine patch treatment (i.e. one week after starting treatment) steady state plasma cotinine levels in biochemically confirmed abstinent individuals (38) were inversely related to the pre-treatment CYP2A6 activity (supplementary fig. 6). The 25% (50 ng/mL) average differences in cotinine levels between participants with faster and slower CYP2A6 activity (NMR) was in agreement with the effect size observed in smokers in study 2. The difference double to 50% (100 ng/mL) when comparing those in the first versus fourth quartile of CYP2A6 activity. Thus for the same systemic nicotine exposure (delivered by 21mg patch for all participants), the individuals with slower CYP2A6 activity had higher steady state cotinine levels than the individuals with faster CYP2A6 activity (supplementary fig. 6).
Our results could explain the systematic variation in plasma cotinine levels between the sexes (48). Due to the lower average estrogen levels, males have lower CYP2A6 protein expression and activity compared to females (49). Thus, males have higher fNIC→COT/CLCOT ratios, which suggest that males would have higher plasma cotinine levels compared to females with similar tobacco exposure.
Finally, our observation could also explain the differences in plasma cotinine levels between Caucasians and African Americans even when controlling for tobacco exposure. About 50% of African Americans are CYP2A6 RM compared to the 20% in Caucasians (50). Therefore, African Americans have, on average, higher fNIC→COT/CLCOT ratios compared to Caucasians, resulting in higher plasma cotinine levels for similar tobacco exposure.
Implications for cancer research
Plasma cotinine is often used as an objective, non-self-report, indicator of tobacco exposure in smoking-related cancer research. However, there could be a 25% or greater variation in plasma cotinine levels following the same tobacco and tobacco-derived carcinogen exposure depending on CYP2A6 genotype or in the presence of other inducers/inhibitors of CYP2A6 activity. To put this difference in perspective, Munafò et al. reported that each risk allele in rs16969968, a single nucleotide polymorphism in the α5 nicotinic acetylcholine receptor, is associated with a 138.4 nmol/L (or 24 ng/mL) increase in plasma cotinine levels, which in turn was predicted to increase lung cancer risk by 1.3 times (5). Our data suggest that the difference between CYP2A6 genotypes (or sex) would be approximately 340 nmol/L (or 60 ng/mL) at 100 nmol tobacco exposure (or roughly 375 pg/mg Cre NNAL). Hence, cotinine levels alone, without considering CYP2A6 activity, may not provide the most accurate information regarding tobacco and tobacco-derived carcinogen exposure in smokers, particularly when comparing groups with different frequencies of CYP2A6 genotypes (or race) or sex. Biomarkers of nicotine intake such as TNE that are less dependent on individual differences in nicotine metabolism or biomarkers of toxicants that are more directly involved in the carcinogenic process, will enhance our understanding of the contribution of variable levels of tobacco consumption on lung cancer risk.
In conclusion, the quantitative relationship between plasma cotinine level and tobacco and tobacco-derived carcinogen exposure differs among groups with different levels of CYP2A6 activity. These comparison groups include the different CYP2A6 genotype groups within a race (e.g. CYP2A6 RM versus CYP2A6 NM among Caucasians) and different races with distinct prevalence of CYP2A6 RM (e.g. Caucasians with 20% CYP2A6 RM versus African Americans with 50% CYP2A6 RM). Furthermore, groups with different estrogen levels may also have variation in cotinine levels unrelated to differences in tobacco exposure. Those include different sexes, or those with varying pregnancy and menopause statuses. Therefore cotinine levels, as a quantitative marker of tobacco and tobacco-derived carcinogen exposure, are not directly comparable between CYP2A6 genotypes, sexes, and races.
Supplementary Material
ACKNOWLEDGEMENTS
This work was co-supported by the Indian Health Service, the National Institute for Drug Abuse at the National Institutes of Health and the National Cancer Institute at the National Institutes of Health (DA02277, DA012353, DA020830, DA012353, DA11170, NARCH III U26IHS300012, HHSN261200700462P, UL1 RR024131 and CA114609); the Endowed Chair in Addiction for the Department of Psychiatry (RFT); Canadian Institutes of Health Research (MOP86471 and TMH109787); Ontario Graduate Scholarship (for AZZ); CAMH and the CAMH foundation; the Canada Foundation for Innovation (#20289 and #16014)and the Ontario Ministry of Research and Innovation. We thank Clifford Watson and Connie Sosnoff for performing the analytical chemistry.
Footnotes
Conflict of Interest
NLB has been a paid consultant to pharmaceutical companies that market medications for smoking cessation treatment, and has served as a paid expert witness in litigation against tobacco companies. RFT has participated in one day advisory meetings for Novartis and McNeil. DKH has received grant funding from Nabi Biopharmaceuticals to conduct a clinical trial. GES participated in a one-day advisory meeting for Pfizer in 2008.
REFERENCES
- 1.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24:1212–1217. [PubMed] [Google Scholar]
- 2.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3'-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277:1010–1015. [PubMed] [Google Scholar]
- 3.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1184–1188. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 5.Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst. 2012;104:740–748. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship Between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 Variation and Smoking Behaviors and Lung Cancer Risk. Journal of the National Cancer Institute. 2011;103:1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho MK, Faseru B, Choi WS, Nollen NL, Mayo MS, Thomas JL, et al. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:3426–3434. doi: 10.1158/1055-9965.EPI-09-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timofeeva MN, McKay JD, Smith GD, Johansson M, Byrnes GB, Chabrier A, et al. Genetic polymorphisms in 15q25 and 19q13 loci, cotinine levels, and risk of lung cancer in EPIC. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:2250–2261. doi: 10.1158/1055-9965.EPI-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AZ, Binnington MJ, Renner CC, Lanier AP, Hatsukami DK, Stepanov I, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco specific nitrosamine exposure and lower tobacco specific nitrosamine bioactivation. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan WQ, Cohen SB, Man SF, Sin DD. Sex-related differences in serum cotinine concentrations in daily cigarette smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:1293–1300. doi: 10.1080/14622200802239132. [DOI] [PubMed] [Google Scholar]
- 13.Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. Eur J Clin Pharmacol. 2010;66:239–251. doi: 10.1007/s00228-009-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, et al. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. American journal of public health. 1990;80:1053–1056. doi: 10.2105/ajph.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 17.Oscarson M, McLellan RA, Asp V, Ledesma M, Bernal Ruiz ML, Sinues B, et al. Characterization of a novel CYP2A7/CYP2A6 hybrid allele (CYP2A6*12) that causes reduced CYP2A6 activity. Hum Mutat. 2002;20:275–283. doi: 10.1002/humu.10126. [DOI] [PubMed] [Google Scholar]
- 18.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 19.Fukami T, Nakajima M, Yoshida R, Tsuchiya Y, Fujiki Y, Katoh M, et al. A novel polymorphism of human CYP2A6 gene CYP2A6*17 has an amino acid substitution (V365M) that decreases enzymatic activity in vitro and in vivo. Clin Pharmacol Ther. 2004;76:519–527. doi: 10.1016/j.clpt.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Mwenifumbo JC, Al Koudsi N, Ho MK, Zhou Q, Hoffmann EB, Sellers EM, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum Mutat. 2008;29:679–688. doi: 10.1002/humu.20698. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18:67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C, Rao YS, Xu B, Hoffmann E, Jones J, Sellers EM, et al. An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles. Biochem Biophys Res Commun. 2002;290:318–324. doi: 10.1006/bbrc.2001.6209. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Liang Q, Mendes P, Sarkar M. Is 24h nicotine equivalents a surrogate for smoke exposure based on its relationship with other biomarkers of exposure? Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2011;16:144–154. doi: 10.3109/1354750X.2010.536257. [DOI] [PubMed] [Google Scholar]
- 26.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer research. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StHelen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, 3rd, Benowitz NL. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem Res Toxicol. 2012;25:952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz NL, Renner C, Lanier A, Tyndale RF, Hatsukami DK, Lindgren BR, et al. Exposure to Nicotine and Carcinogens among South Western Alaskan Native Cigarette Smokers and Smokeless Tobacco User. Cancer Epidemiol Biomarkers Prev. 2012;21:934–942. doi: 10.1158/1055-9965.EPI-11-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swan GE, Benowitz NL, Lessov CN, Jacob P, 3rd, Tyndale RF, Wilhelmsen K. Nicotine metabolism: the impact of CYP2A6 on estimates of additive genetic influence. Pharmacogenetics and genomics. 2005;15:115–125. doi: 10.1097/01213011-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Swan GE, Benowitz NL, Jacob P, 3rd, Lessov CN, Tyndale RF, Wilhelmsen K, et al. Pharmacogenetics of nicotine metabolism in twins: methods and procedures. Twin Res. 2004;7:435–448. doi: 10.1375/1369052042335269. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 32.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., 3rd Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19:3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature reviews Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 34.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le CT, Zhang Y, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamangar F, Strickland PT, Pourshams A, Malekzadeh R, Boffetta P, Roth MJ, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer research. 2005;25:425–428. [PubMed] [Google Scholar]
- 36.Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mwenifumbo JC, Myers MG, Wall TL, Lin SK, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenet Genomics. 2005;15:189–192. doi: 10.1097/01213011-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic Variation in Nicotine Metabolism Predicts the Efficacy of Extended-Duration Transdermal Nicotine Therapy. Clin Pharmacol Ther. 2010;87:553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernert JT, Alexander JR, Sosnoff CS, McGuffey JE. Time course of nicotine and cotinine incorporation into samples of nonsmokers' beard hair following a single dose of nicotine polacrilex. Journal of analytical toxicology. 2011;35:1–7. doi: 10.1093/anatox/35.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Jacob P, 3rd, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 42.Jacob P, 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–8121. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 44.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 45.Benowitz NL, Kuyt F, Jacob P, 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clinical pharmacology and therapeutics. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 46.Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–1941. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 48.Raunio H, Rautio A, Gullsten H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52:357–363. doi: 10.1046/j.0306-5251.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al Koudsi N, Tyndale RF. Hepatic CYP2B6 is altered by genetic, physiologic, and environmental factors but plays little role in nicotine metabolism. Xenobiotica. 2010;40:381–392. doi: 10.3109/00498251003713958. [DOI] [PubMed] [Google Scholar]
- 50.Haberl M, Anwald B, Klein K, Weil R, Fuss C, Gepdiremen A, et al. Three haplotypes associated with CYP2A6 phenotypes in Caucasians. Pharmacogenet Genomics. 2005;15:609–624. doi: 10.1097/01.fpc.0000171517.22258.f1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.