Abstract
Guanylyl cyclase C (GUCY2C) has canonical centrality in defense of key intestinal homeostatic mechanisms, encompassing fluid and electrolyte balance, epithelial dynamics, antitumorigenesis, and intestinal barrier function. Recent discoveries expand the homeostatic role of GUCY2C to reveal a novel gut-brain endocrine axis regulating appetite, anchored by hypothalamic GUCY2C which is responsive to intestine-derived uroguanylin. Thus, GUCY2C may represent a new target for anti-obesity pharmacotherapy. Moreover, the coincident regulation of energy balance and tumor suppression by a single hormone receptor system suggests that the GUCY2C axis might contribute to the established relationship between obesity and colorectal cancer. This confluence suggests that hormone supplementation to reconstitute GUCY2C signaling may be an elegant strategy to reverse both pathophysiologic processes.
Keywords: Guanylyl cyclase C (GUCY2C), uroguanylin, hypothalamus, obesity, colorectal cancer, pharmacotherapy
GUCY2C is a guardian of homeostatic integrity
GUCY2C is a transmembrane receptor whose expression has been canonically confined to the intestinal epithelium, with high expression all along the rostral-caudal axis from duodenum to distal colon [1–3]. GUCY2C is activated by two known endogenous peptide hormones, guanylin and uroguanylin, which also are produced in the intestinal epithelium and activate GUCY2C in a paracrine fashion. GUCY2C is also activated by the exogenous ligand, ST, which is the heat-stable enterotoxin produced by enterotoxigenic E. coli responsible for traveler’s or secretory diarrhea [4]. Binding by any of these three known cognate ligands activates GUCY2C to convert guanosine-5'-triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and increases intracellular cGMP levels, by which GUCY2C mediates its effects (Box 1). The increase in cGMP levels results in activation of downstream effectors, including cGMP-dependent protein kinases (PKGs), phosphodiesterases (PDEs) and cyclic nucleotide-gated (CNG) channels. These functions comprise a host of homeostatic mechanisms which defend critical physiologic processes vital to intestinal health, including electrolyte and fluid balance, proliferation and differentiation along the intestinal crypt-villus axis, genomic integrity via DNA damage repair, cellular metabolism, and the tight junctional barrier function of the intestinal epithelium (Figure 1) [5].
Text Box 1: The second messenger cGMP.
The second messenger cGMP is generated from GTP by soluble guanylyl cyclase (sGC) or particulate guanylyl cyclases (pGCs) [2]. Soluble GC is activated by nitric oxide (NO) or carbon monoxide (CO). Particulate GCs are activated by natriuretic peptides (NPs) consisting of atrial, brain, and C-type natriuretic peptides (ANP, BNP, and CNP, respectively), or intestinal peptides guanylin, uroguanylin, and bacterial ST. Cyclic GMP is degraded by cGMP-hydrolyzing phosphodiesterases (PDEs). Cyclic GMP affects multiple signaling pathways via three classes of cGMP receptor proteins: cGMP-regulated PDEs, which hydrolyze cAMP and/or cGMP; CNG cation channels; and PKGs. Cyclic GMP might also affect the activity of cAMP-dependent protein kinase (PKA) by direct cross-activation in the presence of high cGMP concentrations or indirectly by modulating the activity of cAMP-hydrolyzing PDEs. Nonetheless, PKG is the main intracellular mediator of cGMP signaling. PKG I acts as a soluble intracellular modulator of intracellular [Ca2+], while PKG II regulates fluid homeostasis at the cell membrane [2].
Cyclic GMP mediates a host of processes, including vascular smooth muscle relaxation, regulation of platelet activation, electrolyte and water secretion in intestine, phototransduction at the level of rod and cone photoreceptors, and odor sensation from olfactory receptor cells [2]. Modulating cGMP levels continues to be a focus of interventional pharmacology: nitrates such as glycerol trinitrate are used to induce vasodilation and treat angina, hypertension, and myocardial infarction; PDE inhibition is used to sustain cGMP levels and treat pulmonary hypertension and erectile dysfunction. The discovery that GUCY2C mediates intestinal homeostatic mechanisms, suppresses tumorigenesis, and regulates feeding behavior [5, 6], has expanded our knowledge of cGMP function. Moreover, the existence of an established market for drugs targeting cGMP pathways suggests the possibility of applying off-the-shelf pharmacological approaches to the treatment of cancer or obesity. Reciprocally, GUCY2-Crelated physiologic processes represent new targets that extend the scope of cGMP-centered pharmacotherapy.
Figure 1. GUCY2C is a guardian of homeostatic integrity.
GUCY2C is a transmembrane receptor with high expression in intestinal epithelial cells. GUCY2C is activated by guanylin (yellow) and uroguanylin (blue), and the exogenous ligand, ST (red), a heat-stable enterotoxin produced by enterotoxigenic E. coli. Upon activation GUCY2C converts cGMP from GTP (yellow arrow) and increases intracellular cGMP concentrations resulting in activation of downstream effectors, including PKGs, the main intracellular mediator of cGMP signaling. GUCY2C regulates proliferation by modulating AKT signaling. Increased cGMP levels raise the expression and activity of PTEN, which reduces the levels of phosphorylated AKT. By decreasing AKT activation, GUCY2C signaling suppresses cell proliferation and helps protect tight junctional protein concentrations and intestinal barrier integrity. GUCY2C-induced AKT inhibition also prevents the mouse double minute 2 (MDM2)-dependent degradation of the tumor suppressor p53 and promotes DNA damage repair and genomic stability. Further, GUCY2C signaling reduces levels of key proteins promoting cell proliferation and cell-cycle progression, including β-catenin (β-cat) and cyclin D1, which inhibits cell proliferation and induces cytostasis via a G1/S delay in the cell cycle. GUCY2C signaling also promotes mitochondrial biogenesis and oxidative phosphorylation. Silencing GUCY2C signaling reduces mitochondria number, oxygen consumption, and lactate dehydrogenase (LDH) activity, and elevates glucose transporter 1 (GLUT1) expression, glucose uptake, aerobic glycolysis, and lactate accumulation, which recapitulates a tumor metabolic phenotype. The nucleus is represented by the dashed red line. Tight junction proteins are represented by white elongated ovals. Blue arrows indicate activation. Red bar-headed lines indicate inhibition. Small blue circles labeled with a “p” indicate phosphorylation. The lightning bolt indicates DNA damage. Abbreviations: ATM/ATR, ataxia telangiectasia mutated/ataxia telangiectasia and Rad3-related; CHK1/2, checkpoint kinase 1/2; CHK2, checkpoint kinase 2; H2AX, H2A histone family, member X.
Recent discoveries have revealed a novel GUCY2C function in the mouse brain and expanded our awareness of GUCY2C-mediated homeostasis. GUCY2C represents one end of a gut-brain endocrine axis, anchored by expression in the hypothalamus, the key site for homeostatic coordination and regulation, and completed by uroguanylin, which is produced in the intestine and released postprandially into the systemic circulation to travel to and activate GUCY2C in the hypothalamus to signal energy levels (Figure 2) [6]. This satiety circuit mirrors other known gut-brain endocrine axes involving such well-characterized hormones as peptide YY (PYY), leptin, and ghrelin [7–11].
Figure 2. Endocrine axes in energy homeostasis.
Uroguanylin is one of many endogenous peptide hormones peripherally synthesized and released into the systemic circulation to signal energy status. These hormones travel to their cognate receptors expressed in regulatory centers in the central nervous system (CNS), including the hypothalamus (blue) in the ventral diencephalon and the dorsal vagal complex (DVC, light yellow) in the medulla. These hormones include: adiponectin and leptin from adipose tissue (red arrows); CCK, GLP-1, OXM, PYY, and uroguanylin from the intestine (blue arrows); amylin, insulin, and PP from the pancreas (yellow arrows); and ghrelin from the stomach (green arrow). Among these hormones, only ghrelin is orexigenic, and is the only known orexigenic gut hormone. The others, including uroguanylin, are anorexigenic. Communication between the gastrointestinal system and the brainstem also occurs through vagal connections (purple).
Moreover, this GUCY2C axis extends evolutionarily-conserved primitive cGMP-dependent circuits regulating feeding behavior across the phylogenetic continuum [12, 13]. Signaling programs mediated by cGMP regulate food acquisition, feeding, and satiety across diverse taxa, including worms, flies, bees, and ants [12]. In Caenorhabditis elegans, neurons that control feeding express membrane-bound guanylyl cyclases that specifically mediate the cessation of food intake (quiescence) [13]. These guanylyl cyclases share sequence homology with mammalian particulate guanylyl cyclases, with highest similarity to the natriuretic peptide receptors, GC-A and GC-B [14–16]. Elimination of cGMP signaling produces loss of appetite regulation and quiescence, excess consumption of nutrients, and accumulation of fat [13, 17]. These actions in the worm precisely recapitulate the excess nutrient consumption, blunting of satiety, and resultant obesity seen in mice with silenced GUCY2C expression [6, 17]. Evolutionary conservation of these pathways across the phylogenetic tree highlights the robust solution offered by cGMP signaling to the essential challenge of regulating nutrient acquisition in multicellular organisms [12].
The discovery that GUCY2C signaling mediates satiety reveals not only a new GUCY2C-mediated physiologic function but also integrates appetite regulation into the known homeostatic mechanisms, notably those suppressing tumorigenesis, that are dependent on the integrity of the GUCY2C signaling axis [18–21]. GUCY2C inhibits tumor initiation and growth by maintaining DNA damage repair mechanisms and restricting proliferation [19, 22]. The loss of GUCY2C paracrine hormone expression is a universal early event in colonic neoplasia. Thus, GUCY2C signaling drives both appetite regulation and tumor suppression, and may underlie the well-documented but unsolved connection between obesity and colorectal cancer [23, 24]. The correlation between obesity and cancer has been robust but underlying mechanisms have been elusive. In fact, one of the 24 Provocative Questions posed by Dr. Harold Varmus, Director of the National Cancer Institute (NCI), to tackle the most important cancer research questions is to determine the mechanistic link between obesity and cancer (Box 2) [25, 26].
Text Box 2: The National Cancer Institute’s Provocative Questions.
The goal of the NCI in its Provocative Questions (PQs) initiative is to stimulate specific areas of cancer research deemed understudied or difficult to address in the past [18]. The 24 PQs have been grouped into four thematic categories: cancer prevention and risk (which includes the PQ, How does obesity contribute to cancer risk?); mechanisms of tumor development or recurrence; tumor detection, diagnosis, and prognosis; and cancer therapy and outcomes. This project was intended to assemble a list of important but non-obvious questions to stimulate the NCI’s research communities to use laboratory, clinical, and population sciences in especially effective and imaginative ways. Not merely restatements of long-term goals of the National Cancer Program, which are to improve the prevention, detection, diagnosis, and treatment of all forms of cancer, the PQs are instead intended to build on specific advances in the understanding of cancer and cancer control; address broad issues in the biology of cancer that have proven difficult to resolve; take into consideration the likelihood of progress in the foreseeable future; and address ways to overcome obstacles to achieving long-term goals.
Under the leadership of NCI Director Dr. Harold Varmus, the NCI is attempting to define more potentially game-changing scientific questions that could influence the future directions taken by NCI-sponsored research. The initiative is envisioned as a middle ground between the larger, overarching research directions mandated by the NCI, and traditional investigator-initiated proposals [19]. The collaborative process of formulating the PQs engaged the NCI’s scientific community and many constituencies (advocacy groups, health professionals, Members of Congress), and developed into a strategy for funding new grants. The 24 PQs were culled from over 100 submitted. More than 35,000 scientists throughout the world visited the NCI’s provocative questions website. Over 750 grant applications were submitted for the $15M set aside for funding the 25 standard four-year R01 awards and 10 exploratory two-year R21 awards in 2012 [18, 19]. Critics and skeptics have some concerns: given the limited resources and high demand, the review process may have been constrained by current tendencies to prioritize projects based on track record, accomplishments, and preliminary results, despite the ostensible goal of fostering innovation; funding this initiative means that other programs lose funding and may be compromised; and success is not only uncertain, but defining success itself may be difficult for a program of this experimental nature [19].
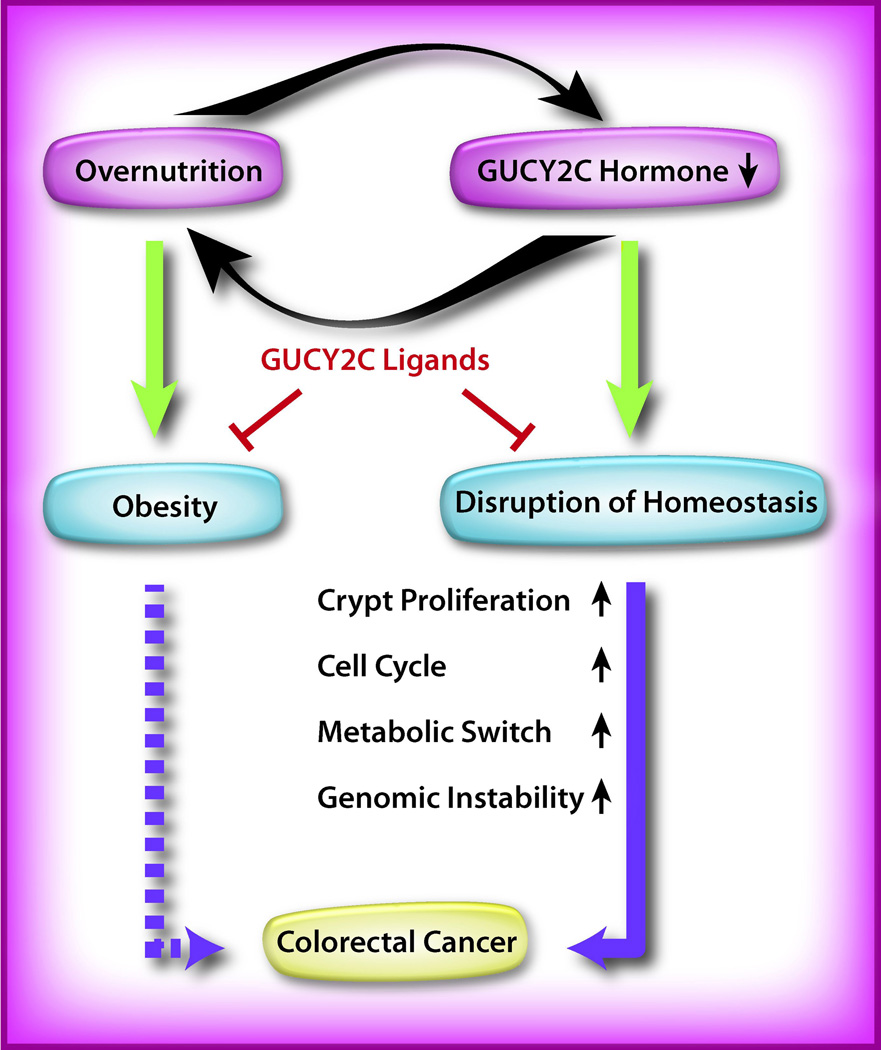
In this context, does a deficiency of intestinal GUCY2C hormones lie at the pathophysiologic intersection of these diseases? Studies have demonstrated that a deficiency in intestinal paracrine signaling contributes to tumorigenesis [18–21, 27]. Does a deficiency in intestinal endocrine signaling also promote hyperphagia and obesity? The intriguing correlative hypothesis suggests that paracrine and endocrine supplementation of GUCY2C ligands might benefit obese patients at risk for colorectal cancer by coordinately restoring epithelial homeostasis and suppressing appetite, and thus reversing obesity (Figure 3). These considerations underscore the significance of further exploring the pathophysiological role of the GUCY2C hormone axes in mechanisms underlying colorectal cancer and obesity.
Figure 3. GUCY2C signaling, obesity, and colorectal cancer.
Hyperphagia and overnutrition may suppress GUCY2C hormone expression by the intestinal epithelium. Reciprocally (curved black arrows), GUCY2C hormone loss abrogates an endocrine signal to the hypothalamus, which reduces satiety, and reinforces hyperphagia and obesity. Moreover, hormone suppression silences GUCY2C, a tumor suppressor, producing epithelial dysfunction resulting in neoplasia (solid purple arrow). Obesity further amplifies transformation through established endocrine mechanisms, e.g., hyperinsulinemia (dashed purple arrow). Hormone supplementation pharmacotherapy with GUCY2C ligands (red bar-headed lines) may oppose obesity and tumorigenesis by restoring the homeostasis of the intestinal epithelium. Green arrows represent the development of pathophysiology.
The unmet clinical need
Obesity is a major health problem, both here in the U.S. and worldwide [28, 29]. Two-thirds of the U.S. adult population is overweight (BMI > 25 kg/m2), and half of that overweight population is further classified as obese (BMI > 30 kg/m2) (Glossary) [30, 31]. Global figures, at 1.5 billion overweight and 500 million obese [32], are staggering as well. Obesity is accompanied by serious co-morbidities that degrade health, reduce life expectancy, and incur sizable treatment costs, including breast and colon cancer, depression, type 2 diabetes, heart disease, hypertension, infertility, osteoarthritis, and stroke [33].
A host of therapeutic modalities exist to combat obesity, but success has been extremely limited. Lifestyle changes with improvements in diet and exercise are effective in reducing weight and minimizing co-morbid conditions, but achieving a sustained commitment to a healthier lifestyle has proved elusive [34–36]. Bariatric surgery, which restricts the amount of food intake by gastric banding, gastric bypass, transoral gastroplasty, or sleeve gastrectomy, is the most successful anti-obesity treatment in sustained weight loss and amelioration of co-morbidities [35, 37]. Nevertheless, bariatric surgery is costly and accompanied by serious risks, which limit its recommendation to the morbidly obese (BMI > 40 kg/m2), and those with a BMI as low as 30 kg/m2 and an obesity-related co-morbidity [35, 38].
Pharmacotherapy can be effective in inducing weight loss, but drugs, too, come with associated risks [35, 39, 40]. For example, drugs that target monoamine neurotransmission can suppress appetite and produce weight loss, but also have demonstrable psychological and cardiovascular risks [41]. In fact, the persistence of accompanying risks has decimated the field of anti-obesity pharmacotherapy [42]. Until recently, due to safety concerns over anti-obesity drugs that had been in clinical use or were awaiting approval from the US Food and Drug Administration (FDA), only one drug, the lipase inhibitor orlistat (Alli™, GlaxoSmithKline; Xenical®, Roche), had been approved for long-term weight loss [43]. In 2012, after previous rejections in 2010, lorcaserin (Belviq®, Arena Pharmaceuticals) and phentermine/topiramate (Qsymia™, Vivus) were approved by the FDA for obesity treatment, though recommendations for post-marketing safety studies and their failure to receive approval from the European Medicines Agency (EMA) highlight the continuing concerns over cardiovascular and other risks [43–45]. These observations underscore the pressing need for new therapeutic targets in obesity.
The GUCY2C-uroguanylin endocrine axis
The hypothalamus integrates neurohormonal signaling from the periphery, namely gut and adipose tissue to regulate food intake and energy homeostasis (Figure 2) [10, 11, 46, 47]. Such hormones include adiponectin and leptin from adipose tissue [9, 11, 48]; cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), oxyntomodulin, and peptide YY (PYY) from the intestine [9, 11, 48]; amylin, glucagon, insulin, and pancreatic peptide (PP) from the pancreas [9, 11, 48]; and ghrelin from the stomach [9, 11, 48]. It is this growing list of peripherally sourced and centrally signaling appetite hormones that uroguanylin joins.
GUCY2C is expressed in the mouse hypothalamus [6]. Its expression is detectable at both mRNA and protein levels. Functional studies confirm that this non-canonically expressed GUCY2C mediates satiety signaling: i) Hypothalamic extracts are responsive to specific GUCY2C ligand administration and produce cGMP, as measured by cGMP accumulation assays. ii) Intravenous (IV) administration of GUCY2C ligands via the tail vein activates a satiety reflex in mice, whereas a control peptide does not, indicating that this effect is mediated by a GUCY2C cognate ligand. iii) Intracerebroventricular (ICV) administration of ST or prouroguanylin, the prohormone precursor of uroguanylin, directly into the third ventricle reduces feeding, indicating that this effect is mediated by GUCY2C signaling in the brain. Importantly, this satiety response is absent in GUCY2C knockout mice (Gucy2c−/−) regardless of the peptide used, or if ligand is administered orally in wildtype mice (Gucy2c+/+), indicating that this effect is specifically mediated by extra-intestinally expressed GUCY2C. iv) Serum levels of prouroguanylin rise rapidly following the ingestion of a meal in both humans and mice, and the time course of elevation in the serum prouroguanylin concentration matches the drop-off in food intake. v) Antibody-mediated depletion of circulating prouroguanylin eliminates normal satiety reflexes and increases food consumption [6].
Recent studies have revealed that Gucy2c−/− mice have dysregulated energy homeostasis, compared to wildtype mice (Gucy2c+/+) [6]. Gucy2c−/− mice are hyperphagic and display an increased rate of weight gain over time. They lack nutrient-induced satiety responses, and their hyperphagia is exacerbated by fasting, which suggests a defect in mechanisms regulating central satiety. They display obesity-associated disorders, including elevated adiposity, hypertriglyceridemia, hyperleptinemia, hyperinsulinemia, and impaired glycemic control, and develop metabolic disease including fulminant diabetes, left ventricular hypertrophy, and hepatic steatosis [6]. As diet-induced obese (DIO) mice also demonstrate increased feeding and diminished satiety responses, one corollary would anticipate that obesity may compromise GUCY2C signaling.
Potential molecular mechanisms underlying GUCY2C-mediated satiety
How might hypothalamic GUCY2C signaling regulate central satiety pathways? One possibility is through depolarization or hyperpolarization of neuronal membrane potentials, thereby modulating action potentials. In the intestine, GUCY2C-activated cGMP signaling regulates ion channels including the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel and CNG cation channels [4]. GUCY2C also inhibits sodium absorption from the intestinal lumen by sodium-hydrogen exchangers in brush border membranes [2, 4, 49]. In the brain, cGMP-dependent activation of CNG channels regulates neurotransmitter release and potentiates synaptic transmission through Ca2+ influx [50, 51]. Therefore, it is conceivable that GUCY2C might suppress or potentiate neuronal activation in the hypothalamus through the regulation of electrolyte flux via cGMP-gated or -regulated channels. Possible downstream effects of a GUCY2C-mediated increase in cGMP concentrations may include the depolarization of neurons expressing the anorexigenic neuropeptides pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) [11, 46, 47], which would increase the firing of anorexigenic neurons, and the hyperpolarization of neurons expressing the orexigenic neuropeptide Y (NPY) and agouti-related protein (AgRP) [11, 46, 47], which would decrease the firing of orexigenic neurons. One or both of these actions would increase satiety signaling.
Also, GUCY2C may regulate appetite by potentiating or suppressing known hormonal signaling pathways. For example, leptin and insulin are peripheral hormones which, like prouroguanylin, travel to the brain via the circulation to bind and activate their cognate receptors and suppress appetite, mirroring the uroguanylin-GUCY2C endocrine axis [52–55]. Leptin and insulin receptors are expressed in the hypothalamus, which is also the site of GUCY2C expression. Therefore, downstream signal transducers of GUCY2C activation may interact with leptin or insulin signaling intermediates and integrate GUCY2C signaling into established satiety circuits. Leptin signals through the leptin receptor/Janus kinase 2/signal transducer and activator of transcription 3 (OBR/JAK2/STAT3) pathway, and insulin signals through the insulin receptor/insulin receptor substrates/phosphoinositide 3-kinase/v akt murine thymoma viral oncogene homolog/forkhead box O1 (IR/IRS/PI3K/AKT/FOXO1) pathway [55, 56]. Signaling through particulate guanylyl cyclases and their effectors cGMP and PKG is known to activate AKT in neurons and cardiomyocytes [57, 58]. Furthermore, the ghrelin endocrine axis is similar to those of leptin and insulin in that the hormone, ghrelin, is peripherally sourced and deployed in response to nutrient levels, and this hormone signal is relayed to the brain via the circulation to activate hypothalamic growth hormone secretagogue receptors (GHSR) and regulate appetite [11, 52]. A difference is that ghrelin signaling is orexigenic, not anorexigenic. Therefore, if GUCY2C signaling intersects with the ghrelin pathway, it would most likely do so in an antagonistic or suppressive manner.
Intriguingly, receptors for leptin, insulin, and ghrelin are expressed in not only the hypothalamus, but also the dopamine neurons of the midbrain, including the ventral tegmental area (VTA) and substantia nigra (SN) [46, 59, 60]. The midbrain is another key regulatory site for energy homeostasis via reward circuits [46, 59, 60]. The discovery of functional, physiologically relevant GUCY2C in the hypothalamus, and the detection of GUCY2C transcript in the midbrain by the Allen Institute for Brain Science’s Mouse Brain Atlas project [61], make GUCY2C function in the midbrain a possibility. If proven, this would establish GUCY2C function in two key areas underpinning appetite regulation, mirroring the pattern of other known receptors in energy regulation.
Further, GUCY2C activation and the resulting increase in intracellular cGMP may induce activation and nuclear translocation of PKG. RNA studies indicate that GUCY2C activation leads to higher POMC mRNA levels in the hypothalamus [6]. One mechanism responsible for this result may be the induction of PKG nuclear translocation. PKG is usually cytosolic, but activation via cGMP has been shown to move PKG into the nucleus in some cell types, including neurons, to regulate gene expression [62, 63]. One effect may be to modulate transcription of key satiety neurotransmitters, such as POMC. Also, intracellular calcium is a known regulator of vesicle secretion in neurons [64]. Binding of cGMP to CNG calcium channels may allow the entrance of calcium and result in an increase in intracellular calcium concentrations. This may lead to the release of the anorexigenic neuropeptide α-melanocyte stimulating hormone (α-MSH) [7] or other neurotransmitters that regulate appetite.
Obesity and colorectal cancer
In light of the nearly universal loss of guanylin and uroguanylin expression in colorectal tumors [19], we speculate that the discovery that one arm of energy homeostasis is dependent on intestinal GUCY2C ligand expression implicates GUCY2C hormone loss as a heretofore unknown basis for the well-documented association between obesity and colon cancer. It also underscores the concept that GUCY2C signaling serves a conserved homeostatic role with manifold functions [18–21, 27]. Based on these findings, we hypothesize that not only is GUCY2C in the colon a target for colorectal cancer prevention and treatment, but also GUCY2C in the hypothalamus may be a previously unappreciated therapeutic target for regulating appetite and treating obesity.
In the intestinal epithelium, intracellular cGMP regulates proliferation by modulating AKT signaling, a canonical driver of cell proliferation (Figure 1) [65, 66]. Increased cGMP levels raise the expression and activity of the tumor suppressor phosphatase and tensin homolog (PTEN), which reduces the levels of phosphorylated AKT [5, 65]. By decreasing AKT activation, GUCY2C signaling suppresses cell proliferation. Tellingly, the GUCY2C hormone guanylin is the most commonly lost gene product in sporadic colorectal cancer in mice and humans [49, 67]. GUCY2C silencing produces epithelial dysfunction, which is characterized by defective differentiation, an accelerated cell cycle, hyperproliferation, increased DNA damage, reprogramming of cell metabolism, and increased susceptibility to tumorigenesis induced by carcinogens or inherited germline mutations, resulting in increased intestinal tumorigenesis [5, 67].
These observations suggest a model of cancer risk in which the suppression of hormone expression silences the GUCY2C tumor suppressor and disrupts epithelial homeostasis, increasing tumorigenesis. Might the pathogenesis or pathologic state of obesity induce epithelial dysfunction and drive guanylin loss? Such a mechanism would implicate obesity-related epithelial dysfunction in not only tumorigenesis but the exacerbation or progression of obesity itself. The importance of establishing a mechanistic link between obesity and cancer risk is perhaps best illustrated by its impact on potential therapeutic strategies. Oral GUCY2C ligand supplementation may have utility in preventing and treating colorectal cancer. We surmise that the reconstitution of GUCY2C signaling via hormone replacement may also represent a therapeutic goal for obesity prevention and treatment, and further define the connection between obesity and cancer (Figure 3).
GUCY2C, guanylin, and uroguanylin in human studies
Reports of GUCY2C mutations in humans have been rare, and most human GUCY2C studies have focused on its persistence in colorectal tumors and the utility of targeting GUCY2C as a tumor marker in molecular staging and tumor immunotherapy [67, 68]. Mutations in GUCY2C causing disease in humans have been identified in two recent studies: a gain-of-function missense mutation in the catalytic domain (c.2519G>T, p.S840I) causing early-onset mild chronic diarrhea in a Norwegian family [69]; and two loss-of-function mutations, including a missense mutation in the extracellular ligand-binding domain (c.1160A>G, p.D387G) and an insertion causing premature translational termination and the abrogation of the catalytic domain (c.2270dupA, p.N757Kfs*2), causing meconium ileus in two unrelated Bedouin kindreds [70]. The c.2519G>T mutation caused higher GUCY2C activation and increased cGMP accumulation in vitro. The authors speculated that this may lead to hyperactivation of CFTR and increased chloride and water secretion from enterocytes, thus explaining the chronic diarrhea in the affected family members. The c.1160A>G mutation caused lower GUCY2C activation and decreased cGMP production in vitro. As GUCY2C activates CFTR through cGMP generation, the authors speculated that they had identified the likely causative mutations for these cases of meconium ileus, which is more commonly caused by mutations in CFTR itself. It is unknown what effect these mutations had on hypothalamic GUCY2C in appetite regulation. These human studies on GUCY2C mutations did not provide information regarding the subjects’ appetite, body weight, adiposity, or metabolism. This is not surprising, since the existence of GUCY2C in the brain and its endocrine function in appetite regulation has only recently come to light [6]. Does hyperactivatable GUCY2C cause leanness in humans? Is it more resistant to stressors such as a sustained hypercaloric diet? Does a loss-of-function mutation portend excess food intake and body weight? Potentially, future studies in humans with GUCY2C mutations could assess satiety reflexes and energy homeostasis.
Guanylin and uroguanylin are the most commonly lost gene products in colorectal cancer [67]. Downregulation of guanylin and uroguanylin is also seen in biopsies from ulcerative colitis patients [71]. Uroguanylin has also been postulated to have function in sites other than the intestine and brain, notably the kidney. Uroguanylin is expressed in the kidney, and studies have demonstrated that IV uroguanylin administration increases renal sodium excretion [49]. Thus, uroguanylin has been hypothesized to be a natriuretic factor, acting in sodium homeostasis. Also, intestinal prouroguanylin is secreted systemically and circulates to the kidney, where it is processed into uroguanylin and excreted in the urine, and it has been reported that an equivalent sodium load is more rapidly excreted when administered orally than when delivered intravenously [49, 72]. Thus, a gastrointestinal-renal natriuretic axis signaling through uroguanylin has been proposed to regulate sodium excretion in response to acute sodium ingestion [49, 72]. However, GUCY2C is not expressed in the kidney, and another receptor for uroguanylin has not been identified [2, 49]. Moreover, a recent human study found that there was no difference in sodium excretion following equivalent oral or intravenous sodium loads during high- or low-sodium diets, and that serum levels of prouroguanylin and proguanylin did not increase, did not differ following oral or intravenous sodium, and did not correlate with sodium excretion [72]. Thus, the precise role of uroguanylin in human natriuresis remains undefined.
Concluding remarks and future perspectives
The discovery of a novel gut-brain axis regulating appetite, body weight, and metabolism through GUCY2C signaling [6] offers a potential new target for the development of pharmacotherapies to treat obesity (Box 3). Targeting GUCY2C signaling is particularly attractive because it leverages an endogenous endocrine mechanism mediating energy homeostasis. Moreover, GUCY2C hormone supplementation may be achievable with reformulations of existing drugs like the ST-analog linaclotide (Linzess™, Ironwood Pharmaceuticals, Forest Laboratories), which has already been approved by the FDA and EMA to treat disorders of gastrointestinal motility (chronic idiopathic constipation, irritable bowel syndrome with constipation) [73, 74]. In clinical trials, patients with chronic constipation improved after 12 or 26 weeks of treatment with 290 ug oral linaclotide, which had no systemic exposure, was well-tolerated, and had minimal adverse effects, diarrhea being the most common [74]. Thus, GUCY2C hormone supplementation may offer the efficacy and safety in practice that has eluded nearly all other candidates that have failed in this important therapeutic area.
Outstanding questions Box.
Can targeting hypothalamic GUCY2C with hormone supplementation or replacement produce clinical improvements in body weight and metabolism to reverse obesity?
Would increasing the serum concentrations of circulating prouroguanylin, e.g., by potentiating intestinal release or suppressing clearance, have efficacy in promoting satiety?
Are there ways to therapeutically increase active uroguanylin levels at the hypothalamus, e.g., by enhancing proteolytic cleavage of the propeptide prouroguanylin?
Does a deficiency of intestinal GUCY2C hormones lie at the pathophysiologic intersection of obesity and colorectal cancer?
Does obesity cause intestinal epithelial dysfunction and drive GUCY2C hormone loss?
Beyond direct GUCY2C ligand supplementation, therapeutic strategies might investigate mechanisms underlying the intestinal release of prouroguanylin to elevate circulating hormone levels. Reciprocally, inhibiting the proteolytic pathways mediating prouroguanylin clearance from the circulation might enhance its effective circulating concentration. Furthermore, as the propeptide prouroguanylin is cleaved to the biologically active uroguanylin at the target site in the brain [6], potentiation of this proteolysis may serve to increase satiety.
Intriguingly, the loss of intestinal guanylin and uroguanylin expression in colorectal tumorigenesis not only deprives intestinal GUCY2C of its paracrine hormones but would also serve to silence the GUCY2C endocrine axis regulating energy homeostasis via the hypothalamus. We posit that this potential molecular basis for the association between obesity and colon cancer highlights the protective role of GUCY2C signaling underlying intact homeostatic physiology. Furthermore, the pathologic mechanism of hormone silencing in colorectal cancer offers a model for a potential mechanism underlying obesity itself. Therefore, we surmise that the reconstitution of GUCY2C signaling by therapeutic administration of GUCY2C hormones may restore satiety reflexes corrupted in obesity, and drive reductions in appetite, caloric intake, body weight, and the metabolic disturbances of obesity. Moreover, the possibility that obesity pathologically contributes to intestinal hormone loss would mean that restoring appetite regulation and reversing obesity may defend against increases in both tumorigenesis and appetite. The substantial implications for improvements in patient care underscore the importance of the pharmacotherapeutic potential of GUCY2C ligand supplementation.
Acknowledgements
These studies were supported by grants from NIH (R01 CA75123, R01 CA95026, RC1 CA146033, P30 CA56036, R01 CA170533), the Pennsylvania Department of Health (SAP #4100059197, SAP #4100051723), and Targeted Diagnostic and Therapeutics Inc. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. JEL was the recipient of the Young Investigator Award from the American Society for Clinical Pharmacology and Therapeutics. SAW is the Samuel MV Hamilton Professor.
Glossary
- Anorexigenic
suppressing appetite.
- Body mass index (BMI)
a measure of body fat based on height and weight that applies to adults. BMI is defined as body mass (kg) divided by the square of height (m2). Weight status categories: underweight = <18.5 kg/m2; normal weight = 18.5–24.9; overweight = 25.0–29.9; obese (Class I) = 30.0–34.9; obese (Class II) = 35.0–39.9; obese (Class III) = >40.0.
- Guanylyl cyclase C (GUCY2C)
a transmembrane enzyme expressed in intestine and brain. In intestine, it is a key receptor for heat-stable enterotoxins responsible for acute secretory diarrhea. GUCY2C has an extracellular ligand-binding domain, a single transmembrane region, a region with sequence similarity to that of protein tyrosine kinases, and a C-terminal guanylyl cyclase domain.
- Linaclotide
a 14-residue, peptide agonist of GUCY2C. Linaclotide (290 µg, oral, once daily) significantly improved abdominal and bowel symptoms associated with irritable bowel syndrome with constipation (IBS-C) over 12 or 26 weeks of treatment. Adverse effects include diarrhea. Linaclotide, marketed as Linzess™ by Ironwood Pharmaceuticals and Forest Laboratories, was approved by the FDA and EMA in 2012 for constipation treatment.
- Lorcaserin
a selective 5-HT2C serotonin receptor agonist with serotonergic and anorexigenic properties. Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating 5-HT2C receptors on hypothalamic POMC neurons. The exact mechanism of action is unknown. Adverse effects include tumorigenesis in rodents. Lorcaserin, marketed as Belviq® by Arena Pharmaceuticals, was approved by the FDA in 2012 for chronic weight management.
- Orexigenic
stimulating appetite.
- Orlistat
a lipase inhibitor used in obesity treatment that inhibits gastric and pancreatic lipases and prevents fat absorption, thereby reducing caloric intake. Adverse effects include steatorrhea. Marketed as a prescription drug in the US since 1999, orlistat was approved by the FDA in 2007 and EMA in 2009 for over-the-counter sale without a prescription. Orlistat is marketed as Alli™ by GlaxoSmithKline and Xenical® by Roche.
- Phentermine/topiramate
a combination of phentermine, a sympathomimetic amine anorexigenic, and topiramate extended-release, an antiepileptic drug, indicated for chronic weight management. The effect of phentermine is likely mediated by release of catecholamines in the hypothalamus, resulting in reduced appetite and decreased feeding. The exact mechanism of action is unknown. Topiramate’s effect may be due to its effects on appetite suppression and satiety enhancement, induced by a combination of pharmacologic effects including augmenting the activity of the neurotransmitter gamma-aminobutyrate (GABA), modulation of voltage-gated ion channels, inhibition of AMPA/kainate excitatory glutamate receptors, or inhibition of carbonic anhydrase. The precise mechanism of action is unknown. Adverse effects include heart palpitations, fetal toxicity, and suicidal ideation. Phentermine/topiramate, marketed as Qsymia™ by Vivus, was approved by the FDA in 2012 for chronic weight management.
- Prouroguanylin
a 112-residue prohormone precursor of uroguanylin, and the predominant form found in circulation and biological fluids. Prouroguanylin is cleaved to produce the mature, biologically active uroguanylin.
- Uroguanylin
a 16-residue peptide agonist of GUCY2C. It is secreted by enterochromaffin cells in its propeptide form, with its highest expression in the proximal small intestine, and regulates electrolyte and water transport in intestinal and renal epithelia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulz S, et al. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 2.Lucas KA, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 3.Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arshad N, Visweswariah SS. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett. 2012;586:2835–2840. doi: 10.1016/j.febslet.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Lin JE, et al. Guanylyl cyclase C in colorectal cancer: susceptibility gene and potential therapeutic target. Future Oncol. 2009;5:509–522. doi: 10.2217/fon.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentino MA, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578–3588. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GW, et al. Regulation of appetite to treat obesity. Expert Rev Clin Pharmacol. 2011;4:243–259. doi: 10.1586/ecp.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem V, Bloom SR. Approaches to the pharmacological treatment of obesity. Expert Rev Clin Pharmacol. 2010;3:73–88. doi: 10.1586/ecp.09.54. [DOI] [PubMed] [Google Scholar]
- 9.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9:489–498. doi: 10.1016/j.cmet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin BE, et al. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152:2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coll AP, et al. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaun KR, et al. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J Exp Biol. 2007;210:3547–3558. doi: 10.1242/jeb.006924. [DOI] [PubMed] [Google Scholar]
- 13.You YJ, et al. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baude EJ, et al. The cloning of a Caenorhabditis elegans guanylyl cyclase and the construction of a ligand-sensitive mammalian/nematode chimeric receptor. J Biol Chem. 1997;272:16035–16039. doi: 10.1074/jbc.272.25.16035. [DOI] [PubMed] [Google Scholar]
- 15.Birnby DA, et al. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, et al. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci U S A. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroetz SM, et al. The cGMP signaling pathway affects feeding behavior in the necromenic nematode Pristionchus pacificus. PLoS One. 2012;7:e34464. doi: 10.1371/journal.pone.0034464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, et al. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Waldman SA. Corruption of homeostatic mechanisms in the guanylyl cyclase C signaling pathway underlying colorectal tumorigenesis. Cancer Biol Ther. 2010;10:211–218. doi: 10.4161/cbt.10.3.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JE, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–254. doi: 10.1053/j.gastro.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitari GM, et al. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Printz C. NCI initiative focuses on obesity-cancer link: the initiative's multidisciplinary approach may help unravel the complex connection. Cancer. 2012;118:579–580. doi: 10.1002/cncr.27412. [DOI] [PubMed] [Google Scholar]
- 24.Eheman C, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varmus H, Harlow E. Science funding: Provocative questions in cancer research. Nature. 2012;481:436–437. doi: 10.1038/481436a. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz S. Provocative questions initiative to fund innovative cancer research. J Natl Cancer Inst. 2012;104:966–967. doi: 10.1093/jnci/djs308. [DOI] [PubMed] [Google Scholar]
- 27.Lin JE, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 29.Hofbauer KG, et al. The obesity epidemic: current and future pharmacological treatments. Annual review of pharmacology and toxicology. 2007;47:565–592. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, et al. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 31.Flegal KM, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 32.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(Suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 34.Jakicic JM, et al. Effect of a stepped-care intervention approach on weight loss in adults: a randomized clinical trial. JAMA. 2012;307:2617–2626. doi: 10.1001/jama.2012.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diet, drugs and surgery for weight loss. Treat Guidel Med Lett. 2011;9:17–22. quiz 12 p following 22. [PubMed] [Google Scholar]
- 36.Sarwer DB, et al. Behavior therapy for obesity: where are we now? Current opinion in endocrinology, diabetes, and obesity. 2009;16:347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 37.Adams TD, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitka M. FDA action allows more obese patients to qualify for a bariatric procedure. JAMA. 2011;305:1287–1288. doi: 10.1001/jama.2011.377. [DOI] [PubMed] [Google Scholar]
- 39.Aronne LJ, et al. Emerging pharmacotherapy for obesity. Expert opinion on emerging drugs. 2011;16:587–596. doi: 10.1517/14728214.2011.609168. [DOI] [PubMed] [Google Scholar]
- 40.Rucker D, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sargent BJ, Moore NA. New central targets for the treatment of obesity. Br J Clin Pharmacol. 2009;68:852–860. doi: 10.1111/j.1365-2125.2009.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nature reviews. Drug discovery. 2012;11:675–691. doi: 10.1038/nrd3739. [DOI] [PubMed] [Google Scholar]
- 43.Colman E, et al. The FDA's Assessment of Two Drugs for Chronic Weight Management. N Engl J Med. 2012 doi: 10.1056/NEJMp1211277. [DOI] [PubMed] [Google Scholar]
- 44.Morrato EH, Allison DB. FDA approval of obesity drugs: a difference in risk-benefit perceptions. JAMA. 2012;308:1097–1098. doi: 10.1001/jama.2012.10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.2 New drugs for weight loss. The Medical letter on drugs and therapeutics. 2012;54:69–71. [PubMed] [Google Scholar]
- 46.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain SS, Bloom SR. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.93. [DOI] [PubMed] [Google Scholar]
- 48.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual review of psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 49.Rahbi H, et al. The uroguanylin system and human disease. Clinical science. 2012;123:659–668. doi: 10.1042/CS20120021. [DOI] [PubMed] [Google Scholar]
- 50.Wright CL, et al. Cyclic GMP alters the firing rate and thermosensitivity of hypothalamic neurons. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1704–R1715. doi: 10.1152/ajpregu.00714.2007. [DOI] [PubMed] [Google Scholar]
- 51.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav. 2010;97:15–24. doi: 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers MG, Jr, et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15:150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers MG, Jr, et al. Obesity and leptin resistance: distinguishing cause from effect. Trends in endocrinology and metabolism: TEM. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nature reviews. Neuroscience. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 56.Caricilli AM, et al. Topiramate treatment improves hypothalamic insulin and leptin signaling and action and reduces obesity in mice. Endocrinology. 2012;153:4401–4411. doi: 10.1210/en.2012-1272. [DOI] [PubMed] [Google Scholar]
- 57.Swartling FJ, et al. Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene. 2009;28:3121–3131. doi: 10.1038/onc.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashikawa-Hobara N, et al. The Akt-nitric oxide-cGMP pathway contributes to nerve growth factor-mediated neurite outgrowth in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2011;338:694–700. doi: 10.1124/jpet.111.181487. [DOI] [PubMed] [Google Scholar]
- 59.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nature reviews. Neuroscience. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 60.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones AR, et al. The Allen Brain Atlas: 5 years and beyond. Nature reviews. Neuroscience. 2009;10:821–828. doi: 10.1038/nrn2722. [DOI] [PubMed] [Google Scholar]
- 62.Hao Y, et al. Nuclear cGMP-dependent kinase regulates gene expression via activity-dependent recruitment of a conserved histone deacetylase complex. PLoS genetics. 2011;7 doi: 10.1371/journal.pgen.1002065. e1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casteel DE, et al. cGMP-dependent protein kinase anchoring by IRAG regulates its nuclear translocation and transcriptional activity. Cell Signal. 2008;20:1392–1399. doi: 10.1016/j.cellsig.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter AM, et al. Multiple Ca2+ sensors in secretion: teammates, competitors or autocrats? Trends Neurosci. 2011;34:487–497. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Cao I, et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin JE, et al. Bacterial heat-stable enterotoxins: translation of pathogenic peptides into novel targeted diagnostics and therapeutics. Toxins (Basel) 2010;2:2028–2054. doi: 10.3390/toxins2082028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waldman SA, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 2009;301:745–752. doi: 10.1001/jama.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiskerstrand T, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 70.Romi H, et al. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893–899. doi: 10.1016/j.ajhg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu F, et al. Genome-wide gene expression differences in Crohn's disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflammatory bowel diseases. 2007;13:807–821. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 72.Preston RA, et al. Sodium challenge does not support an acute gastrointestinal-renal natriuretic signaling axis in humans. Kidney international. 2012;82:1313–1320. doi: 10.1038/ki.2012.269. [DOI] [PubMed] [Google Scholar]
- 73.Ray K. IBS: Linaclotide approved for constipation-predominant IBS. Nature reviews. Gastroenterology & hepatology. 2012 doi: 10.1038/nrgastro.2012.194. [DOI] [PubMed] [Google Scholar]
- 74.McWilliams V, et al. Linaclotide: first global approval. Drugs. 2012;72:2167–2175. doi: 10.2165/11470590-000000000-00000. [DOI] [PubMed] [Google Scholar]