Abstract
Background
Little is known about the impact of knowledge of CDKN2A and MC1R genotype on melanoma prevention behaviors like sun avoidance and skin examination in the context of familial melanoma.
Methods
73 adults with a family history of melanoma were randomly assigned to be offered individualized CDKN2A and MC1R genotyping results in the context of a genetic counseling session, or the standard practice of not being offered counseling or disclosure of genotyping results. Mixed effects or longitudinal logistic models were used to determine whether the intervention affected change in sun protection habits, skin examinations and perception and beliefs related to melanoma risk, prevention, and genetic counseling.
Results
All participants in the intervention group who attended genetic counseling sessions chose to receive their test results. From baseline to follow-up, participants in the intervention group reported an increase in the frequency of skin self-examinations compared to a slight decrease in the control group (p=0.002). Participants in the intervention group reported a smaller decrease in frequency of wearing a shirt with long sleeves than did participants in the control group (p =0.047). No effect of the intervention was noted for other outcomes.
Conclusions
Feedback of CDKN2A and MC1R genotype among families without known pathogenic CDKN2A mutations does not appear to decrease sun protection behaviors.
Impact
While disclosure of CDKN2A and MC1R genotype did not have negative effects on prevention, the benefits of communicating this information remain unclear. The small number of families who tested positive for CDKN2A mutations in this study is a limitation.
Keywords: skin cancer, genetic testing, sun exposure, sun protection, surveys
INTRODUCTION
Malignant melanoma, the most serious form of skin cancer, is the fifth most common cancer for males and sixth most common for females in the United States [1]. Melanoma incidence has continued to rise in recent years at a faster rate than the seven most common cancers [2]. Between 1992 and 2008, the incidence of melanoma increased 2.4% annually on average [3]. In 2012, there were an estimated 76,250 new cases of melanoma reported in the United States, with nearly 9,180 resulting in death [1]. Skin cancer, including melanoma, is considered to be one of the most preventable types of cancer, and for that reason it is important to improve adherence to melanoma prevention and early detection behaviors. Prevention practices recommended by the American Cancer Society include: seeking shade, wearing broad spectrum sunscreen, covering up with a shirt and/or a hat, and wearing sunglasses [1]. Additionally, early detection practices, including skin self-examinations and yearly skin examinations by medical professionals, have the potential to save lives due to the exceptionally good outcome for thin melanomas.
An estimated 5% to 10% of all melanomas are hereditary, and of those, up to 40% are explained by mutations in the cyclin-dependent kinase inhibitor 2a (CDKN2A) gene [4]. In the United States, individuals who test positive for a mutation in CDKN2A have a 76% estimated lifetime risk of developing melanoma [4], compared to a lifetime risk of 2% in the general population [5].
Carriage of variants in the melanocortin-1 receptor (MC1R) gene also confers risk of melanoma, yet to a lesser degree than inheritance of a mutation in CDKN2A [6]. Although MC1R is highly associated with pigmentation characteristics, its association with melanoma risk is evident even after adjustment for pigmentation measures or among individuals otherwise considered to be at low risk for skin cancer [7–9]. Recent research argues that risk of melanoma imparted by MC1R is greater in individuals with darker hair, eyes, and skin color, which suggests that knowledge of MC1R genotype is important in assessing an individual’s level of risk [8].
Although the evidence linking genotype to melanoma risk has grown, the use of genetic testing and counseling for melanoma risk remains controversial because of uncertainty about its effects on adherence to behavioral prevention recommendations for sun avoidance/protection and self-examination [10–14]. Specifically, will individuals who test positive for these genetic mutations increase their sun avoidance and skin examination behaviors? Will those whose genetic test results are negative or inconclusive be less vigilant because they feel they are not at increased risk for melanoma?
Genetic testing within melanoma kindreds is debated because of the inability to affect meaningful change to clinical care for family members with CDKN2A mutations, with the possible exception of recommending screening for pancreatic cancer [12, 15–16]. To date, only two studies have examined the behavioral effects of testing. Aspinwall and associates conducted a prospective study looking at the effect of CDKN2A genetic testing on melanoma early detection intentions and behaviors among a high-risk research population in Utah (N=77). Changes from baseline in early detection behaviors (total body skin examinations by a medical professional and skin self-examinations) were evaluated among three groups: CDKN2A+ with a personal history of melanoma, CDKN2A + with no personal history of melanoma, and CDKN2A−. These researchers found that the CDKN2A + participants without a personal history of melanoma reported a significant increase in monthly skin self-examination intentions and behaviors at follow up [14]. Another study conducted by Bergenmar and associates measured anxiety and depression, risk perception, and sun-related habits among 11 unaffected members of melanoma-prone families at four time points: before genetic testing, at disclosure of genetic test results, six months post-disclosure, and one year post-disclosure. Four participants tested positive for a mutation in CDKN2A. Results from this study showed that disclosure of genetic test results did not change perceived risk for melanoma nor did it prompt behavioral changes relating to skin cancer prevention [17]. Both of these studies were prospective observational studies but did not include groups who did not receive genetic counseling and test results.
Given this background, we conducted a randomized controlled trial to compare the impact of a strategy of offering CDKN2A counseling and test results to a strategy of not offering genetic counseling and test results (the current standard) on attitudes, perceived risk of melanoma, and sun avoidance/protection and self-examination behaviors among individuals in hereditary melanoma families.
MATERIALS AND METHODS
Design Overview
This study used a block randomized trial design to investigate the effect of offering genetic counseling and testing on behavior and behavior-related outcomes including attitudes, perceived risk of melanoma and current and intended sun avoidance/protection and skin examination behaviors. Participants were adults who were defined as at high risk for melanoma due to previous personal history of melanoma or multiple cases of melanoma in their family. After completing a baseline interview, they were randomized to an offer of genetic testing and counseling or to usual care. A follow-up survey was completed about four months later.
Parent Familial Melanoma Study
Potential participants were identified from among individuals previously enrolled at the University of Pennsylvania into a familial melanoma study under the auspices of GenoMEL, the melanoma genetics consortium [4, 18]. Probands were patients seen at the University of Pennsylvania’s Pigmented Lesion Clinic who reported a family history of melanoma; probands did not necessarily have a personal history of melanoma. Attempts were made to recruit all first degree relatives of family members affected with melanoma and any interconnecting unaffected family members. All participants completed a full body skin examination of moles, and they were asked to provide a blood and/or buccal swab sample for CDKN2A and MC1R genotyping for research purposes only. The GenoMEL protocol at the University of Pennsylvania did not incorporate feedback of individual test results. No clinical recommendations including guidance on risk reduction were provided to participants by study staff. All participating family members signed informed consent that included permission to be re-contacted for future studies.
Recruitment and Procedures
From among enrolled individuals in melanoma families, defined as three or more cases of melanoma (verified to best local standards) on the same side of a family or two or more cases of melanoma (verified to best local standards) in first degree relatives, research staff contacted by telephone potential primary contacts who were over the age of 18 to introduce the intervention study, confirm eligibility, and collect information on family structure. Verification was based on pathologically confirmed melanoma diagnoses, though in some cases where pathology reports were not available, they were confirmed by clinical notes, physician letter, or self-report. If primary contacts were interested in participating and had eligible family members, they were asked to provide names and contact information for those family members, most of whom also were participants in the parent GenoMEL study.
On the initial telephone call, the informed consent document was reviewed and verbal consent was obtained. Once enrolled, all participants completed the baseline survey by telephone. This initial survey was completed between June and August 2010, and took about 20–25 minutes to complete. All eligible and interested family members of the primary contact were then enrolled. After completing the baseline survey, primary contacts were randomized within blocks defined by CDKN2A mutation status (positive for known pathogenic mutation vs. not positive for known pathogenic mutation) to intervention or usual care groups using a random number generator. Other enrolled family members were assigned to the treatment group of the primary contact. This was done to avoid contamination due to cross-talk within families, and because family counseling sessions are common and used when possible. Primary contacts randomized to the intervention group and their family members were invited to attend one genetic counseling session with a genetic counselor. Primary contacts randomized to the usual care (control) group and their family members received a one-page generic skin cancer prevention brochure via mail. All participants were contacted approximately four months after completion of the baseline survey to complete a follow-up survey (median 139 days, range 101–203 days). Participants were given gift card incentives after completion of the baseline and follow-up surveys ($10 at baseline and $15 for the follow-up survey). The study procedure was approved by the institutional review board at the University of Pennsylvania.
Intervention: Genetic counseling session
The counseling appointment was made with the primary contact, and family members were welcome to accompany him or her. The sessions were conducted by a genetic counselor at the University of Pennsylvania between August and October 2010. Each session lasted approximately 45–60 minutes. The genetic counselor provided background information on genotyping and genetic testing for melanoma, including a description of CDKN2A and MC1R. Participants were then given the option of receiving their CDKN2A and MC1R genotyping results. If an individual had tested positive for a CDKN2A mutation or a high risk MC1R variant, the genetic counselor reviewed risks associated with these genes. If individuals tested positive for a CDKN2A mutation, they were offered the opportunity for a second test for clinical confirmation of the results (per health system policy). This was provided free of charge as part of this study. All questions and concerns raised by the primary contact and family members were answered by the genetic counselor before the session ended. Counseling attendees also received a one-page skin cancer prevention brochure.
Measures: Survey instruments, process evaluation
Data collected in the surveys included: demographic characteristics, personal and family history of skin cancer, skin cancer risk factors, whether the participant had ever conducted a thorough skin self-examination (and if so, how recently), sun exposure and sun protection habits, risk perception, awareness of genetic counseling; and perceived benefits and barriers of sun protection, genetic counseling, and genetic testing.
The baseline and follow-up surveys were constructed using previously developed questions. Key sources of items in the survey were the Brief skin cancer Risk Assessment Tool (BRAT) [19], Sun Habits Survey [20], and Health Information National Trends Survey [21], which have been shown to be both valid and reliable in previous studies. The follow-up survey asked the same questions as the baseline, but questions relating to background information were removed and questions about reactions to genetic counseling were added to the survey for the intervention group participants. (See Appendix 1 for details of constructs and survey items). Genetic counselors’ chart notes on reactions of intervention participants and their families provided additional process evaluation data.
Statistical analysis
Descriptive statistics were obtained for baseline and follow-up measures. The baseline characteristics of the control and intervention groups were compared using bivariate statistics (Pearson chi-square tests for categorical variables, t-tests for ordinal and continuous variables). Mixed effects models were used to estimate associations between the main outcome measures and the following predictors: Time point (baseline vs. follow-up), treatment, sex, and a dichotomized version of self-reported baseline general health (fair/good vs. very good/excellent); interactions of time point by each of the other predictors were also included. Sex and general health were included in the models as covariates because the control and intervention groups differed significantly on these characteristics (see Table 1). Family history of melanoma was not included as a covariate because all participants were at high risk due to either personal or family history of melanoma. All models were repeated including family as a random effect (random intercept). For all outcomes, the goodness of fit of the random effect model was slightly worse than that of the fixed effect model, and the p-values for the fixed effects were virtually identical; therefore, fixed-effects-only results are reported here. The effect of primary interest was the interaction of time point by treatment, i.e., the extent to which receipt of genetic test results affected the change in each outcome measure from baseline to follow-up. The procedure described above was used for all outcome measures except skin examination by a medical professional, which was dichotomous; for this outcome, a longitudinal logistic regression was performed, using the same predictors as described above.
Table 1.
Baseline Characteristics of Participants, by Treatment Group
| CHARACTERISTIC | Control (N=38) | Intervention (N=35) | TOTAL (N=73) | p-valuea |
|---|---|---|---|---|
|
| ||||
| % (n) or Mean (SD) | ||||
|
DEMOGRAPHICS
| ||||
| Gender (% Female) | 57.9% (22) | 80.0% (28) | 68.5% (50) | 0.04 |
| Age, y (range = 24–87) | 56.9 (16.1) | 62.4 (14.6) | 59.5 (15.5) | 0.14 |
| Ethnicity (% Caucasian) | 100.0% (38) | 100.0% (35) | 100.0% (73) | n.s. |
| % Graduated College | 68.4% (26) | 80.0% (28) | 74.0% (54) | 0.26 |
|
| ||||
|
HEALTH
| ||||
| BMI (% overweight/obese) | 65.8% (25) | 40.0% (14) | 53.4% (39) | 0.03 |
| % High Risk | 97.4% (37) | 97.1% (34) | 97.3% (71) | 0.95 |
| % Ever diagnosed with melanoma | 68.4% (26) | 65.7% (23) | 67.1% (49) | 0.69 |
| % With family history of melanoma | 52.6% (20) | 25.7% (9) | 39.7% (29) | 0.02 |
| Self Rated Health (% Very Good or Excellent) | 52.7% (20) | 88.5% (31) | 69.9% (51) | <0.01 |
| % Last routine checkup ≤ 12 months ago | 68.4% (26) | 71.4% (25) | 69.9% (51) | 0.13 |
| % Ever health professional skin exam | 78.9% (30) | 94.3% (33) | 86.3% (63) | 0.06 |
| % Health professional skin exam ≤ 6 months | 47.4% (18) | 57.1% (20) | 52.1% (38) | 0.40 |
| % Ever genetic testing | 13.2% (5) | 8.6% (3) | 11.0% (8) | 0.53 |
| % Family member ever genetic testing | 13.2% (5) | 14.3% (5) | 13.7% (10) | 0.72 |
| % One or more sunburn last summer | 47.3% (18) | 40.0% (14) | 43.8% (32) | 0.53 |
|
| ||||
|
BEHAVIOR
| ||||
| Skin Self-Examination (% in last 1 mo.) | 63.2% (24) | 60.0% (21) | 61.6% (45) | 0.49 |
| Sun Protection Habits Index (M+SD; range 1–4) | 2.85 (0.59) | 3.01 (0.51) | 2.93 (0.56) | 0.22 |
| Wear Sunscreen (M+SD; range 1–4) | 3.34 (0.85) | 3.29 (0.86) | 3.32 (0.85) | 0.78 |
| Seek Shade (M+SD; range 1–4) | 2.55 (0.86) | 2.88 (0.95) | 2.71 (0.91) | 0.13 |
| Wear Sunglasses (M+SD; range 1–4) | 3.13 (1.21) | 3.26 (0.85) | 3.19 (1.05) | 0.61 |
| Wear a Shirt (M+SD; range 1–4) | 3.00 (1.06) | 2.80 (0.99) | 2.90 (1.03) | 0.41 |
| Wear a Hat (M+SD; range 1–4) | 2.34 (1.32) | 2.77 (0.91) | 2.55 (1.16) | 0.11 |
| Limit Midday Hours in the Sun (M+SD; range 1–4) | 2.74 (1.11) | 3.06 (0.80) | 2.89 (0.98) | 0.16 |
|
| ||||
|
ATTITUDES
| ||||
| Perceived Risk of Skin Cancer (M+SD; range 1–5) | 4.87 (0.41) | 4.63 (0.87) | 4.75 (0.68) | 0.13 |
| Perceived Benefits of Sun Protection (M+SD; range 1–4) | 3.62 (0.36) | 3.76 (0.29) | 3.69 (0.34) | 0.09 |
| Perceived Barriers of Sun Protection (M+SD; range 1–5) | 2.59 (0.87) | 2.00 (0.78) | 2.30 (0.87) | 0.03 |
| Awareness of Genetic Counseling (M+SD; range 1–4) | 2.22 (0.76) | 2.36 (0.83) | 2.29 (0.79) | 0.48 |
| Perceived Benefits of Genetic Counseling (M+SD; range 1–5) | 3.55 (0.84) | 4.10 (0.66) | 3.82 (0.80) | <0.01 |
| Perceived Benefits of Genetic Testing (M+SD; range 1–5) | 3.51 (0.87) | 3.59 (0.64) | 3.55 (0.77) | 0.66 |
| Perceived Barriers of Genetic Testing (M+SD; range 1–5) | 1.93 (0.74) | 1.48 (0.50) | 1.71 (0.67) | <0.01 |
The X2 test was used to assess relationships for categorical variables, and the t test was used to assess relationships for continuous variables.
RESULTS
Participation and Sample Characteristics
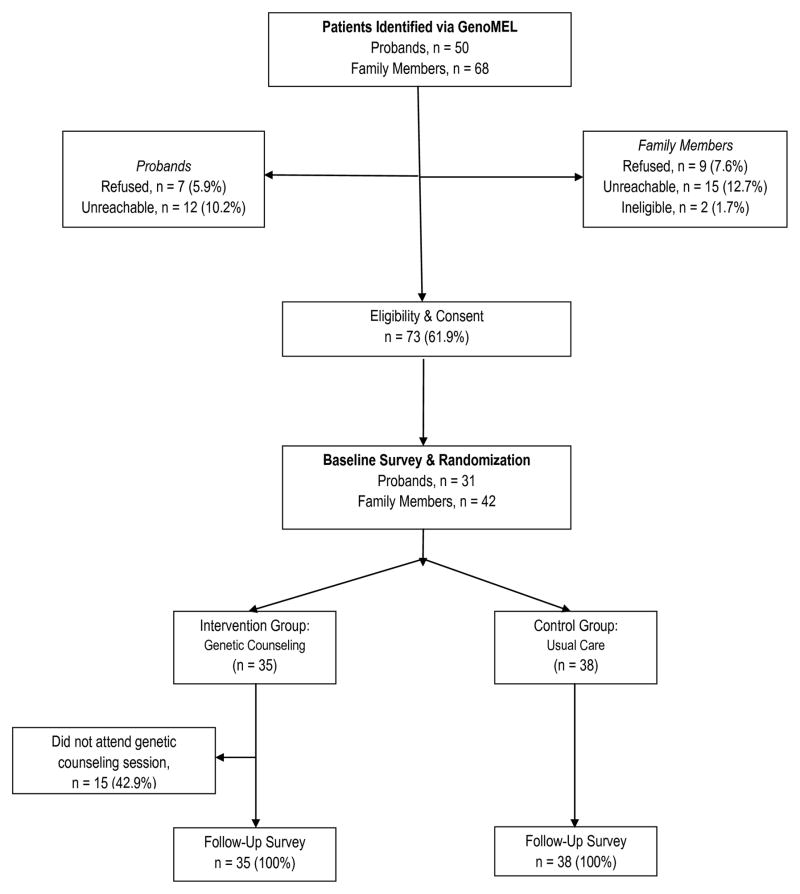
In total, 118 individuals enrolled in the parent GenoMEL study were targeted for participation in the intervention study. Of this group, 91 individuals were successfully contacted, 2 were ineligible, 16 refused, and 73 (80.2%) agreed to participate. 31 primary contacts and 42 family members completed a baseline survey, were randomized (15 families in the intervention group and 16 families in the usual care group), and were later contacted to complete a follow-up survey. Twenty of the 35 intervention group participants (15 primary contacts and 5 family members; 57.1%) attended a genetic counseling session; the main reason for non-attendance was the travel distance to the university site for the meeting. The study completion rate for the follow-up survey was 100% (n=73). The consort diagram in Figure 1 shows participation rates throughout the study.
Figure 1.
Study Consort Diagram.
Participants were white (100%), with more than half being female (Table 1). The mean age was 59.5 years and almost three-quarters (74%) of the study population were college-educated. More than 90% were at high risk for developing skin cancer, as indicated by the BRAT score, and more than two-thirds reported a previous diagnosis of melanoma in their lifetime. 43.8% of the study population reported having had at least one sunburn the previous summer. Over three-quarters (86.3%) of the participants reported having had a health professional examine their skin for signs of skin cancer in their lifetime. More than sixty percent of the participants had conducted a skin self-examination in the last month. Self-reported sun protection habits ranged from sometimes practiced to usually practiced.
There were no significant differences between intervention and control groups in age, education, percent at high risk, percent previously diagnosed with melanoma, percent ever getting genetic testing, or percent with a family member who had ever had genetic testing. However, significant differences were found between intervention and control groups in gender, body mass index (BMI), family history of melanoma (other than self), and self-reported general health. Participants in the intervention group were more likely to be female with a self-reported health score of very good or excellent. This group was also less likely to be overweight or obese and less likely to report a family history of melanoma.
Main Outcomes
Mean values at baseline and follow-up by intervention group on the main outcome measures are presented in Table 2. In the multivariate models of these measures, the interaction of time by treatment group was significant for two outcomes – wearing a shirt with long sleeves (F (1,69) = 4.089, p = .047) and most recent skin self-exam (F (1,69) = 10.107, p = .002). For the former outcome, higher scores reflect greater frequency of the behavior; as shown in Table 2, mean scores decreased from baseline to follow-up in both groups, but to a greater extent in the control group. For the latter outcome, lower scores reflect more recent self-exam; mean scores increased slightly in the control group, and decreased in the intervention group. The interaction of time by treatment was nonsignificant in the models of the other outcomes (.090 < p < .918). The analyses were repeated for only those subjects who did not have a positive genetic test, without a change in key findings.
Table 2.
Changes in Behaviors and Attitudes from Baseline to Follow-Up, by Treatment Group
| Range | Control | Intervention | Time by treatment | |||
|---|---|---|---|---|---|---|
| F | p-value | |||||
| Mean (SD) | ||||||
| CONSTRUCT | ||||||
| Prevention & Detection Behaviors | ||||||
| Sun Protection Habits | [1 – 4] | BL | 2.85 (0.59) | 3.01 (0.51) | 2.956 | 0.090 |
| FU | 2.80 (0.59) | 3.05 (0.55) | ||||
| Wear Sunscreen | [1 – 4] | BL | 3.34 (0.85) | 3.29 (0.86) | 1.978 | 0.164 |
| FU | 3.45 (0.83) | 3.60 (0.70) | ||||
| Wear Sunglasses | [1 – 4] | BL | 3.13 (1.21) | 3.26 (0.85) | 0.232 | 0.631 |
| FU | 3.24 (1.13) | 3.37 (0.88) | ||||
| Wear Shirt | [1 – 4] | BL | 3.00 (1.07) | 2.80 (0.99) | 4.089 | 0.047 |
| FU | 2.39 (1.10) | 2.60 (0.95) | ||||
| Seek Shade | [1 – 4] | BL | 2.55 (0.86) | 2.88 (0.95) | 0.003 | 0.958 |
| FU | 2.71 (0.87) | 2.89 (0.99) | ||||
| Wear a Hat | [1 – 4] | BL | 2.34 (1.32) | 2.77 (0.91) | 0.245 | 0.622 |
| FU | 2.29 (1.23) | 2.71 (1.05) | ||||
| Limit Hours in the Sun | [1 – 4] | BL | 2.74 (1.11) | 3.06 (0.80) | 0.747 | 0.391 |
| FU | 2.73 (1.02) | 3.11 (0.83) | ||||
| Skin Self-Examination | [1 – 4] | BL | 1.71 (1.09) | 1.89 (1.25) | 10.107 | 0.002 |
| FU | 1.84 (1.15) | 1.23 (0.60) | ||||
| Total Skin Examination by a Medical Professional | [0 – 1] | BL | 0.79 (0.41) | 0.94 (0.24) | 1.559 | 0.216 |
| FU | 0.55 (0.50) | 0.60 (0.50) | ||||
| Sun Exposure: Weekday | [1 – 4] | BL | 2.11 (1.09) | 2.18 (1.04) | 0.212 | 0.647 |
| FU | 1.71 (0.98) | 1.86 (1.03) | ||||
| Sun Exposure: Weekend | [1 – 4] | BL | 2.79 (1.12) | 2.56 (1.11) | 0.008 | 0.931 |
| FU | 2.26 (1.08) | 2.03 (1.12) | ||||
| Sunburn Frequency | [0 – 3] | BL | 0.43 (0.73) | 0.51 (0.85) | 0.246 | 0.621 |
| FU | 0.45 (0.72) | 0.37 (0.73) | ||||
| Perceptions & Beliefs | ||||||
| Perceived Skin Cancer Risk | [1 – 5] | BL | 4.87 (0.41) | 4.63 (0.87) | 0.156 | 0.694 |
| FU | 4.83 (0.44) | 4.54 (0.88) | ||||
| Perceived Benefits of Sun Protection | [1 – 5] | BL | 3.62 (0.36) | 3.76 (0.29) | 0.309 | 0.580 |
| FU | 3.72 (0.29) | 3.79 (0.36) | ||||
| Perceived Barriers to Sun Protection | [1 – 5] | BL | 2.59 (0.87) | 2.00 (0.78) | 1.531 | 0.220 |
| FU | 2.44 (0.91) | 1.99 (0.61) | ||||
| Awareness of Genetic Counseling (in general) | [1 – 5] | BL | 2.22 (0.76) | 2.36 (0.83) | 0.031 | 0.861 |
| FU | 2.50 (0.62) | 2.77 (0.74) | ||||
| Perceived Benefits of Genetic Counseling | [1 – 5] | BL | 3.55 (0.84) | 4.10 (0.66) | 0.011 | 0.918 |
| FU | 3.45 (0.78) | 3.88 (0.71) | ||||
Reactions to Genetic Counseling Sessions
All intervention group participants who attended the genetic counseling sessions chose to receive their test results. Two individuals had deleterious mutations in CDKN2A, two individuals carried a high risk MC1R allele, and one individual had both a deleterious CDKN2A mutation and a high risk MC1R allele. Those who tested negative for a CDKN2A mutation expressed surprise and relief upon receipt of their genotype results. Those who received positive test results were not surprised, as they already believed they were at high risk for melanoma. Most of the participants were relatively knowledgeable about sun protection and interested, but not knowledgeable about melanoma genetics. Participants indicated that they learned a lot of new information from the counseling sessions (62.5%), and rated the materials as 4.4 and the counselors as 4.7 (on a scale of 1 to 5, where 5 is the most positive rating). Ratings of the written skin cancer prevention materials showed that participants found them to be relatively easy to understand (mean = 4.7), useful (mean = 3.6), and personally relevant (mean = 3.9).
DISCUSSION
The clinical use of genetic testing for melanoma susceptibility remains controversial. Although there is established evidence demonstrating that mutations in CDKN2A and MC1R are associated with an increased risk of developing melanoma, it remains uncertain if knowledge of one’s CDKN2A and MC1R genotype impacts upon sun protection behaviors, the key component of melanoma prevention [10, 12–13]. In this study, the first randomized controlled trial of genetic counseling/testing for melanoma susceptibility, genetic testing and counseling had little impact on a wide range of sun protection behaviors and melanoma-related perceptions. Because the study included only three individuals with deleterious mutations in the intervention group, these results provide relatively little insight into the effect of mutation testing on behaviors of individuals who test positive. However, they provide important evidence that genetic testing and counseling do not lead to false reassurance and reductions in sun protection behaviors among individuals who test negative.
Concerns about false reassurance from the use of cancer screening or risk assessment tests have been noted in a variety of settings, including mammography screening and genetic susceptibility testing [22–23]. However, the great majority of these concerns have arisen from studies using hypothetical scenarios. Most studies that have examined actual behavior following participation in cancer screening or genetic susceptibility testing have found little evidence that false reassurance from a negative test leads to a reduction in preventive behaviors [24–25]. However, observational studies are limited by potential confounding if individuals who have different test results also differ in their predilection for different preventive behaviors. To our knowledge, this study is the only randomized controlled trial of genetic counseling/testing for cancer susceptibility specifically addressing the question of whether negative test results lead to reduced vigilance in prevention behaviors, further adding to the evidence that false reassurance and associated reductions in preventive behavior are not significant adverse outcomes from cancer risk assessment and counseling.
The results of this study provide another piece of the chain of evidence needed to support the use of genetic testing for melanoma susceptibility in clinical practice. Multiple studies have demonstrated that mutations in CDKN2A are associated with a substantially increased risk of melanoma - demonstrating the clinical validity of mutation testing. However, the clinical utility of testing remains controversial as it is uncertain whether test results will influence prevention behaviors. Although this study was not designed to determine the effect of testing on prevention behaviors among individuals who are found to have a mutation (a potential benefit of testing), the evidence that prevention behaviors and melanoma risk perceptions do not decline among individuals who are found not to carry a mutation (a potential harm of testing) is also an important step towards demonstrating the clinical utility of testing. Further studies are needed to address the questions of whether testing leads to reductions in the burden of melanoma among mutation carriers, and who should undergo testing if it is found to be beneficial.
This study has several limitations. The sample size was relatively small, particularly for mutation carriers, thereby limiting conclusions about that group. Furthermore, we focused on a high risk population that had previously been enrolled in research through GenoMEL in order to maximize the study efficiency and to address the population that often raises the greatest concerns about responses to negative mutation test results. However, the results in this population may not generalize to other populations at lower risk or who had not previously participated in genetic research. The study follow-up was limited and it is possible that longer follow-up would have identified changes in behavior that were not apparent in this time period. Because participants completed follow-up surveys in the fall/winter, their responses may have been affected by recall bias regarding behaviors in warm weather. Also, more than forty percent of the intervention group participants did not attend a genetic counseling session. Finally, the genetic counseling protocol was based upon the standard principles of genetic counseling for cancer susceptibility, but its effectiveness is likely to be determined in part by the specific genetic counselors involved. Thus, it is possible that the effects of the intervention would not be able to be replicated if the protocol were delivered by different counselors.
Supplementary Material
Acknowledgments
Financial Support: NIH Grant #5UC2CA148310-02
Footnotes
Conflict of Interest statement: The authors declare no conflicts of interest.
References
- 1.American Cancer Society [ACS] Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Horner MJ. SEER cancer statistics review, 1975–2007. Bethesda MD: National Institutes of Health; 2010. [Google Scholar]
- 3.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone A, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(7):2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, et al. Features associated with germline CDKN2A mutations: A GenoMEL study of melanoma prone families from three continents. J Med Genet. 2007;44(2):99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute [Internet] SEER Stat Fact Sheets: Melanoma of the Skin. [updated 2012 Aug 20; cited 2012 Dec 20]. Available from: http://seer.cancer.gov/statfacts/html/melan.html.
- 6.Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, et al. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int J Cancer. 2008;122(12):2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- 7.Box NF, Duffy DL, Chen W, Stark M, Martin NG, Sturm RA, Hayward NK. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69(4):765–773. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanetsky PA, Panossian S, Elder DE, Guerry DP, Ming ME, Schuchter L, Rebbeck TR. Does MC1R genotype convey information about melanoma risk beyond risk phenotypes? Cancer. 2010;116(10):2416–2428. doi: 10.1002/cncr.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherer D, Nagore E, Bermejo JL, Figl A, Botella-Estrada R, Thirumaran RK, et al. Melanocortin receptor 1 variants and melanoma risk: A study of 2 European populations. Int J Cancer. 2009;125(8):1868–1875. doi: 10.1002/ijc.24548. [DOI] [PubMed] [Google Scholar]
- 10.de Snoo FA, Bergman W, Gruis NA. Familial melanoma: a complex disorder leading to controversy on DNA testing. Fam Cancer. 2003;2(2):109–116. doi: 10.1023/a:1025758527675. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CB, Wadge LM, Lowstuter K, Boucher K, Leachman SA. Clinical germline genetic testing for melanoma. Lancet Oncol. 2004;5(5):314–319. doi: 10.1016/S1470-2045(04)01469-X. [DOI] [PubMed] [Google Scholar]
- 12.Leachman SA, Carucci J, Kohlmann W, Banks KC, Asgari MM, Bergman W, et al. Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol. 2009;61(4):677.e1–14. doi: 10.1016/j.jaad.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen KD, Roberts JS, Shalowitz DI, Everett JN, Kim SY, Raskin L, et al. Disclosing individual CDKN2A research results to melanoma survivors: interest, impact, and demands on researchers. Cancer Epidemiol Biomarkers Prev. 2011;20(3):522–529. doi: 10.1158/1055-9965.EPI-10-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aspinwall LG, Leaf SL, Dola ER, Kohlmann W, Leachman SA. CDKN2A genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1510–1519. doi: 10.1158/1055-9965.EPI-08-0010. [DOI] [PubMed] [Google Scholar]
- 15.Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically disposed to melanoma: A consensus statement of the Melanoma Genetics Consortium. J Clin Oncol. 1999;17(10):3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- 16.Kefford R, Newton Bishop JA, Tucker M, Bressac-de Paillerets B, Bianchi-Scarra G, Bergman W, et al. Genetic testing for melanoma. Lancet Oncol. 2002;3(11):653–654. doi: 10.1016/s1470-2045(02)00894-x. [DOI] [PubMed] [Google Scholar]
- 17.Bergenmar M, Hansson J, Brandberg Y. Family members’ perceptions of genetic testing for malignant melanoma-A prospective interview study. Eur J Oncol Nurs. 2009;13(2):74–80. doi: 10.1016/j.ejon.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.GenoMEL – the Melanoma Genetics Consortium [Internet] [cited 2012 Dec 20]. Available from: http://genomel.org/
- 19.Glanz K, Schoenfeld E, Weinstock MA, Layi G, Kidd J, Shigaki DM. Development and reliability of a brief skin cancer risk assessment tool. Cancer Detect Prev. 2003;27(4):311–315. doi: 10.1016/s0361-090x(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 20.Glanz K, Schoenfeld ER, Steffen A. A randomized trial of tailored skin cancer prevention messages for adults: Project SCAPE. Am J Public Health. 2010;100(4):735–41. doi: 10.2105/AJPH.2008.155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson D, Kreps G, Hesse B, Croyle R, Willis G, Arora N, et al. The health information national trends survey (HINTS): Development, design, and dissemination. J Health Commun. 2004;9(5):443–60. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- 22.Lerman C, Marshall J, Audrain J, Gomez-Caminero A. Genetic testing for colon cancer susceptibility: Anticipated reactions of patients and challenges to providers. Int J Cancer. 1996;69(1):58–61. doi: 10.1002/(SICI)1097-0215(19960220)69:1<58::AID-IJC15>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong K, Moye E, Williams S, Berlin J, Reynolds E. Screening mammography in women 40 to 49 years of age: A Systematic review for the American College of Physicians. Ann Intern Med. 2007;146(7):516–26. doi: 10.7326/0003-4819-146-7-200704030-00008. [DOI] [PubMed] [Google Scholar]
- 24.de Gelder R, van As E, Tilanus-Linthorst M, Bartels C, Boer R, Draisma G, et al. Breast cancer screening: Evidence for false reassurance? Int J Cancer. 2008;123(3):680–6. doi: 10.1002/ijc.23540. [DOI] [PubMed] [Google Scholar]
- 25.Dorval M, Gauthier G, Maunsell E, Dugas M, Rouleau I, Chiquette J, et al. No evidence of false reassurance among women with an inconclusive BRCA1/2 genetic test result. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2862–7. doi: 10.1158/1055-9965.EPI-05-0512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.