Abstract
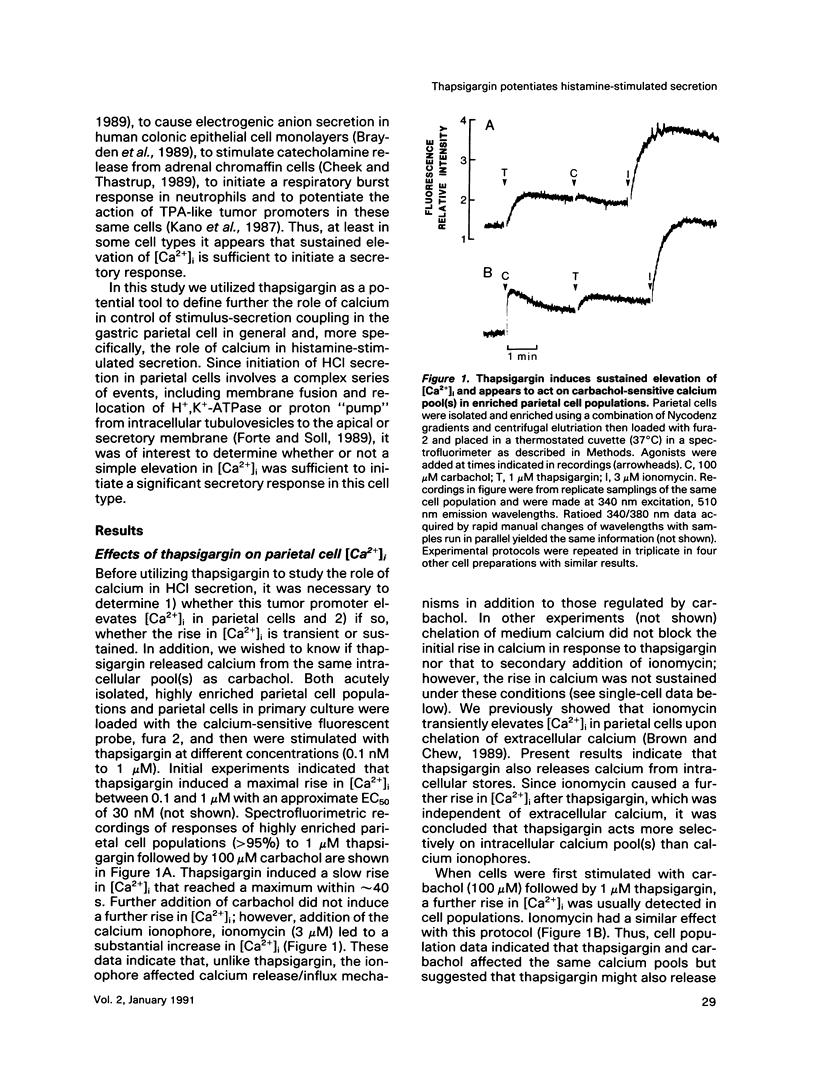
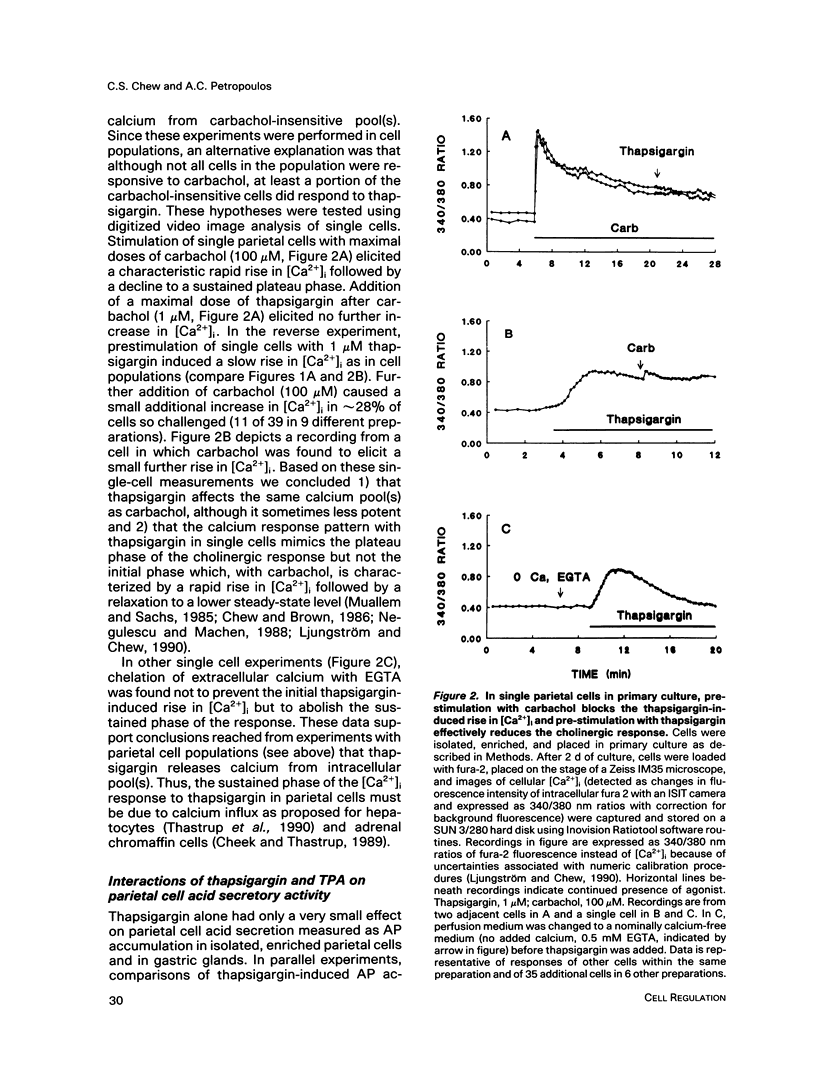
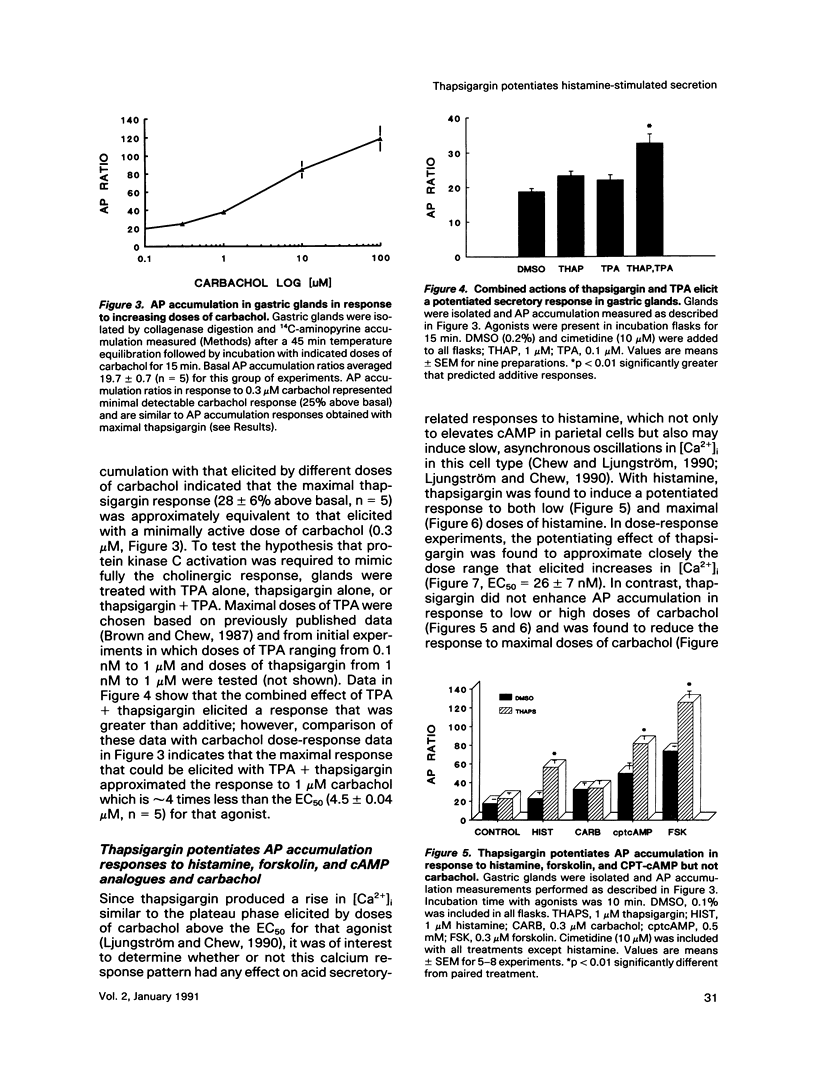
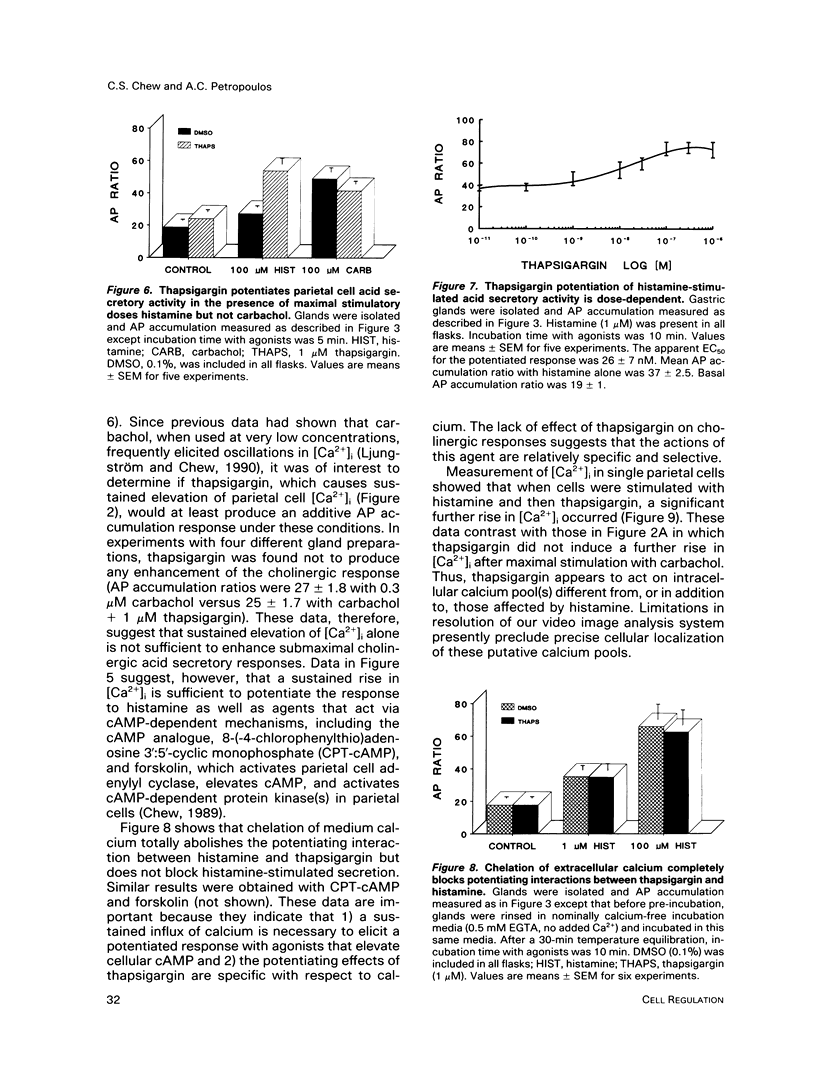
The role of calcium in control of HCl secretion by the gastric parietal cell was examined using a recently available intracellular calcium-releasing agent, thapsigargin, which has been shown, in some cell types, to induce sustained elevation of intracellular calcium ([Ca2+]i), an action that appears to be independent of inositol lipid breakdown and protein kinase C activation and to be mediated, at least partially, by selective inhibition of endoplasmic reticulum Ca2(+)-ATPase. Using the calcium-sensitive fluorescent probe, fura-2, in combination with digitized video image analysis of single cells as well as standard fluorimetric techniques, we found that thapsigargin induced sustained elevation of [Ca2+]i in single parietal cells and in parietal cells populations. Chelation of medium calcium led to a transient rise and fall in [Ca2+]i, indicating that the sustained elevation in [Ca2+]i in response to thapsigargin was due to both intracellular calcium release and influx. Although thapsigargin appeared to affect the same calcium pool(s) regulated by the cholinergic agonist, carbachol, and the pattern of thapsigargin-induced increases in [Ca2+]i were similar to the plateau phase of the cholinergic response, thapsigargin did not induce acid secretory responses of the same magnitude as those initiated by carbachol (28 vs 600% of basal). The protein kinase C activator, 12-O-tetradecanoyl phorbol-13-acetate (TPA) potentiated the secretory response to thapsigargin but this combined response also did not attain the same magnitude as the maximal cholinergic response. In the presence but not the absence of medium calcium, thapsigargin potentiated acid secretory responses to histamine, which elevate both cyclic AMP (cAMP) and [Ca2+]i in parietal cells, as well as forskolin and cAMP analogues but had no effect on submaximal and an inhibitory effect on maximal cholinergic stimulation. Furthermore, thapsigargin did not fully mimic potentiating interactions between histamine and carbachol, either in magnitude or in the pattern of temporal response. Assuming that the action of thapsigargin is specific for intracellular calcium release mechanisms, these data suggest that 1) sustained influx of calcium is necessary but not sufficient for cholinergic activation of parietal cell HCl secretion and for potentiating interactions between cAMP-dependent agonists and carbachol; 2) mechanisms in addition to elevated [Ca2+]i and protein kinase C activation may be involved in cholinergic regulation; and 3) increases in [Ca2+]i in response to histamine are not directly involved in the mechanism of histamine-stimulated secretion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Christensen S. B., Foreman J. C., Pearce F. L., Piotrowski W., Thastrup O. The ability of thapsigargin and thapsigargicin to activate cells involved in the inflammatory response. Br J Pharmacol. 1985 Jul;85(3):705–712. doi: 10.1111/j.1476-5381.1985.tb10567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Hanson P. J. Inhibition of gastric acid secretion by a phorbol ester: effect of 12-O-tetradecanoylphorbol-13-acetate on aminopyrine accumulation by rat parietal cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):566–572. doi: 10.1016/0006-291x(84)90219-5. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Brayden D. J., Hanley M. R., Thastrup O., Cuthbert A. W. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989 Nov;98(3):809–816. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Chew C. S. Multiple effects of phorbol ester on secretory activity in rabbit gastric glands and parietal cells. Can J Physiol Pharmacol. 1987 Sep;65(9):1840–1847. doi: 10.1139/y87-286. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Brown M. R. Release of intracellular Ca2+ and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta. 1986 Aug 29;888(1):116–125. doi: 10.1016/0167-4889(86)90077-7. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J. Gastrin stimulation of isolated gastric glands. Am J Physiol. 1982 May;242(5):G504–G512. doi: 10.1152/ajpgi.1982.242.5.G504. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J., Sachs G., Berglindh T. Histamine responsiveness of isolated gastric glands. Am J Physiol. 1980 Apr;238(4):G312–G320. doi: 10.1152/ajpgi.1980.238.4.G312. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Ljungström M. HCl secretion and [Ca2+]i in cultured parietal cells. J Intern Med Suppl. 1990;732:9–15. doi: 10.1111/j.1365-2796.1990.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Ljungström M., Smolka A., Brown M. R. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol. 1989 Jan;256(1 Pt 1):G254–G263. doi: 10.1152/ajpgi.1989.256.1.G254. [DOI] [PubMed] [Google Scholar]
- Chew C. S. Parietal cell protein kinases. Selective activation of type I cAMP-dependent protein kinase by histamine. J Biol Chem. 1985 Jun 25;260(12):7540–7550. [PubMed] [Google Scholar]
- Demarest J. R., Loo D. D. Electrophysiology of the parietal cell. Annu Rev Physiol. 1990;52:307–319. doi: 10.1146/annurev.ph.52.030190.001515. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Ito S., Berglindh T., Sachs G. Cellular site of gastric acid secretion. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6689–6693. doi: 10.1073/pnas.76.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foder B., Scharff O., Thastrup O. Ca2+ transients and Mn2+ entry in human neutrophils induced by thapsigargin. Cell Calcium. 1989 Oct;10(7):477–490. doi: 10.1016/0143-4160(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hakii H., Fujiki H., Suganuma M., Nakayasu M., Tahira T., Sugimura T., Scheuer P. J., Christensen S. B. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111(3):177–181. doi: 10.1007/BF00389230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S., Iizuka T., Ishimura Y., Fujiki H., Sugimura T. Stimulation of superoxide anion formation by the non-TPA type tumor promoters palytoxin and thapsigargin in porcine and human neutrophils. Biochem Biophys Res Commun. 1987 Mar 13;143(2):672–677. doi: 10.1016/0006-291x(87)91406-9. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Ruiz M. C., Fernández E., Ciarrocchi A. Role of Ca2+ in H+ transport by rabbit gastric glands studied with A23187 and BAPTA, an incorporated Ca2+ chelator. Biochim Biophys Acta. 1989 Jul 24;983(1):82–90. doi: 10.1016/0005-2736(89)90383-0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Fimmel C. J., Pandol S. J., Sachs G. Regulation of free cytosolic Ca2+ in the peptic and parietal cells of the rabbit gastric gland. J Biol Chem. 1986 Feb 25;261(6):2660–2667. [PubMed] [Google Scholar]
- Muallem S., Sachs G. Ca2+ metabolism during cholinergic stimulation of acid secretion. Am J Physiol. 1985 Feb;248(2 Pt 1):G216–G228. doi: 10.1152/ajpgi.1985.248.2.G216. [DOI] [PubMed] [Google Scholar]
- Naor Z. Further characterization of protein kinase-C subspecies in the hypothalamo-pituitary axis: differential activation by phorbol esters. Endocrinology. 1990 Mar;126(3):1521–1526. doi: 10.1210/endo-126-3-1521. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Release and reloading of intracellular Ca stores after cholinergic stimulation of the parietal cell. Am J Physiol. 1988 Apr;254(4 Pt 1):C498–C504. doi: 10.1152/ajpcell.1988.254.4.C498. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Reenstra W. W., Machen T. E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C241–C251. doi: 10.1152/ajpcell.1989.256.2.C241. [DOI] [PubMed] [Google Scholar]
- Nylander O., Bergqvist E., Obrink K. J. Dual inhibitory actions of somatostatin on isolated gastric glands. Acta Physiol Scand. 1985 Sep;125(1):111–119. doi: 10.1111/j.1748-1716.1985.tb07697.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K., Sugawara T., Watanabe M., Hirasawa N., Tsurufuji S., Fujiki H., Christensen S. B., Sugimura T. Analysis of the stimulative effect of thapsigargin, a non-TPA-type tumour promoter, on arachidonic acid metabolism in rat peritoneal macrophages. Br J Pharmacol. 1988 Jul;94(3):917–923. doi: 10.1111/j.1476-5381.1988.tb11604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K., Takahashi C., Hirasawa N., Watanabe M., Fujiki H., Tsurufuji S. Stimulation of histamine release and arachidonic acid metabolism in rat peritoneal mast cells by thapsigargin, a non-TPA-type tumor promoter. Biochim Biophys Acta. 1989 May 15;1003(1):9–14. doi: 10.1016/0005-2760(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Park J., Chiba T., Yamada T. Mechanisms for direct inhibition of canine gastric parietal cells by somatostatin. J Biol Chem. 1987 Oct 15;262(29):14190–14196. [PubMed] [Google Scholar]
- Parker P. J., Kour G., Marais R. M., Mitchell F., Pears C., Schaap D., Stabel S., Webster C. Protein kinase C--a family affair. Mol Cell Endocrinol. 1989 Aug;65(1-2):1–11. doi: 10.1016/0303-7207(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B., Thastrup O., Hofmann B., Møller J., Ryder L. P., Jacobsen K. D., Langhoff E., Dickmeiss E., Christensen S. B. Effect of thapsigargin on cytoplasmic Ca2+ and proliferation of human lymphocytes in relation to AIDS. Biochim Biophys Acta. 1988 Dec 9;972(3):257–264. doi: 10.1016/0167-4889(88)90200-5. [DOI] [PubMed] [Google Scholar]
- Soll A. H. Extracellular calcium and cholinergic stimulation of isolated canine parietal cells. J Clin Invest. 1981 Jul;68(1):270–278. doi: 10.1172/JCI110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Thastrup O., Putney J. W., Jr Calcium efflux across the plasma membrane of rat parotid acinar cells is unaffected by receptor activation or by the microsomal calcium ATPase inhibitor, thapsigargin. Cell Calcium. 1990 Jan;11(1):11–17. doi: 10.1016/0143-4160(90)90044-u. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]



