Abstract
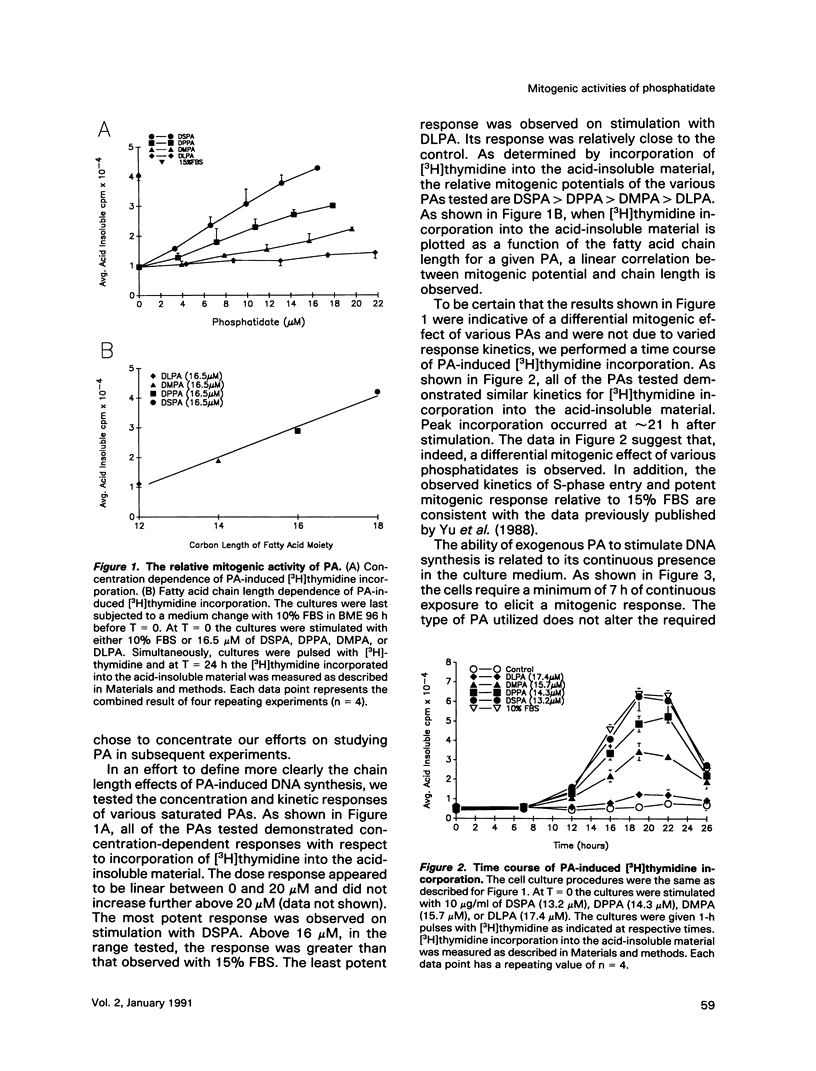
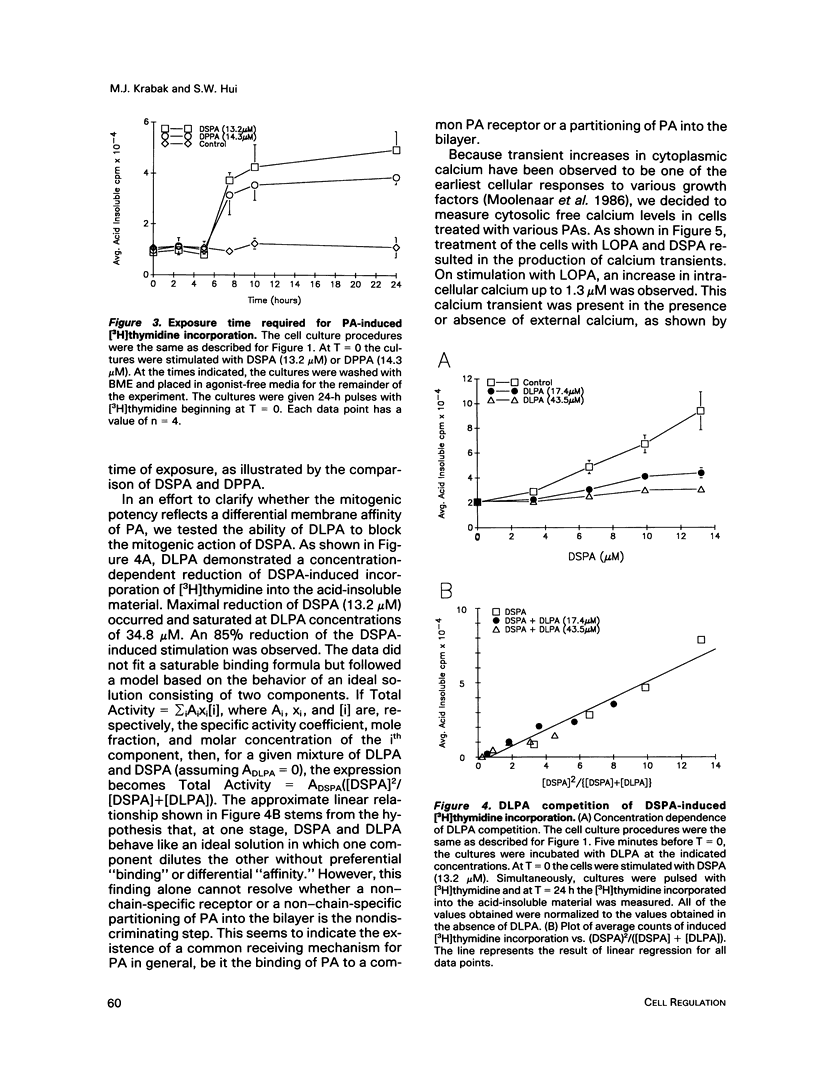
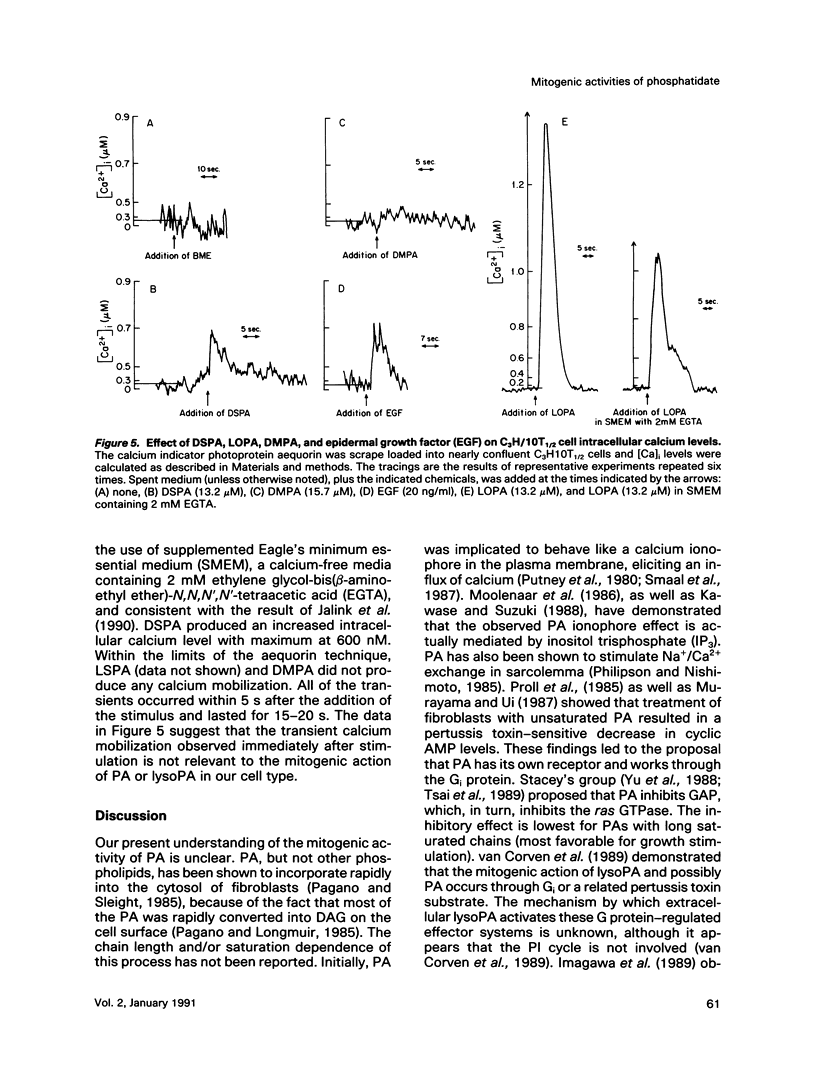
Phosphatidates (PA or phosphatidic acid) were shown to have mitogenic properties, including the stimulation of DNA synthesis and calcium mobilization in C3H/10T1/2 cells. Their continuous presence for a minimum of 7 h induced DNA synthesis with kinetics similar to that observed when 10% fetal bovine serum was used as a mitogen. PAs with long chain saturated fatty acid moieties were more mitogenic, in a dose-dependent fashion, than PAs with short saturated or unsaturated fatty acid moieties. When compared with lysostearoyl-PA (LSPA), distearoyl-PA (DSPA) was as potent with respect to the induction of DNA synthesis. Lysooleoyl-PA (LOPA) was slightly more potent than dioleoyl-PA (DOPA), but much weaker than DSPA and LSPA. Preincubation with dilauroyl-PA (DLPA) reduces the mitogenic effect of DSPA by 85%. The pattern of mitogenic inhibition suggests that a chain-length-independent, yet PA-specific, mechanism is involved. Both DSPA and DLPA are equally taken up by the cells after 30 min. LOPA, but not LSPA, produced a large calcium transient (1.3 microM), which we found to be derived from intracellular sources. DSPA, the most mitogenic PA tested, produced a weaker transient (0.6 microM). Interestingly, LSPA did not produce any detectable calcium transient. These results suggest that the chain-length-specific step in the signaling mechanism of PA occurs after the initial chain-length-independent partitioning and/or binding to the membrane and that the induction of DNA synthesis is not related to the observed calcium transients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- Cutry A. F., Kinniburgh A. J., Krabak M. J., Hui S. W., Wenner C. E. Induction of c-fos and c-myc proto-oncogene expression by epidermal growth factor and transforming growth factor alpha is calcium-independent. J Biol Chem. 1989 Nov 25;264(33):19700–19705. [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. Structural requirements for diacylglycerols to mimic tumor-promoting phobol diester action on the epidermal growth factor receptor. J Biol Chem. 1985 May 10;260(9):5315–5322. [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Effects of phosphatidic acid on islet cell phosphoinositide hydrolysis, Ca2+, and adenylate cyclase. Diabetes. 1989 Sep;38(9):1187–1192. doi: 10.2337/diab.38.9.1187. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Konda T. S., Davis J. S., Standaert M. L., Pollet R. J., Cooper D. R. Insulin rapidly increases diacylglycerol by activating de novo phosphatidic acid synthesis. Science. 1987 May 1;236(4801):586–589. doi: 10.1126/science.3107122. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Mordan L. J., Lau A. F., Kanemitsu M. Y., Boynton A. L. PDGF-induced activation of phospholipase C is not required for induction of DNA synthesis. Science. 1990 Jun 29;248(4963):1660–1663. doi: 10.1126/science.2163545. [DOI] [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Wallace D., Nandi S. Phospholipids containing polyunsaturated fatty acyl groups are mitogenic for normal mouse mammary epithelial cells in serum-free primary cell culture. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4122–4126. doi: 10.1073/pnas.86.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kawase T., Suzuki A. Phosphatidic acid-induced calcium mobilization in osteoblasts. J Biochem. 1988 Apr;103(4):581–582. doi: 10.1093/oxfordjournals.jbchem.a122309. [DOI] [PubMed] [Google Scholar]
- Lacal J. C. Diacylglycerol production in Xenopus laevis oocytes after microinjection of p21ras proteins is a consequence of activation of phosphatidylcholine metabolism. Mol Cell Biol. 1990 Jan;10(1):333–340. doi: 10.1128/mcb.10.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmuir K. J., Malinick L. A. Transfer of phosphatidic acid from liposomes to cells is collision dependent. Am J Physiol. 1989 Mar;256(3 Pt 1):C522–C531. doi: 10.1152/ajpcell.1989.256.3.C522. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Taylor D. L. Aequorin entrapment in mammalian cells. Cell Calcium. 1985 Apr;6(1-2):83–93. doi: 10.1016/0143-4160(85)90036-3. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Onuma E. K., Hui S. W. Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol. 1988 Jun;106(6):2067–2075. doi: 10.1083/jcb.106.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J. Phosphorylation, transbilayer movement, and facilitated intracellular transport of diacylglycerol are involved in the uptake of a fluorescent analog of phosphatidic acid by cultured fibroblasts. J Biol Chem. 1985 Feb 10;260(3):1909–1916. [PubMed] [Google Scholar]
- Pagano R. E., Sleight R. G. Defining lipid transport pathways in animal cells. Science. 1985 Sep 13;229(4718):1051–1057. doi: 10.1126/science.4035344. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nishimoto A. Y. Stimulation of Na+-Ca2+ exchange in cardiac sarcolemmal vesicles by phospholipase D. J Biol Chem. 1984 Jan 10;259(1):16–19. [PubMed] [Google Scholar]
- Proll M. A., Clark R. B., Butcher R. W. Phosphatidate and monooleylphosphatidate inhibition of fibroblast adenylate cyclase is mediated by the inhibitory coupling protein, Ni. Mol Pharmacol. 1985 Oct;28(4):331–337. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaal E. B., Nicolay K., Mandersloot J. G., de Gier J., de Kruijff B. 2H-NMR, 31P-NMR and DSC characterization of a novel lipid organization in calcium-dioleoylphosphatidate membranes. Implications for the mechanism of the phosphatidate calcium transmembrane shuttle. Biochim Biophys Acta. 1987 Mar 12;897(3):453–466. doi: 10.1016/0005-2736(87)90442-1. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pérez-Rodríguez R., Franchi A., Chambard J. C., Pouysségur J. Analysis of growth factor "relaxation" in Chinese hamster lung fibroblasts required for tumoral expression. J Cell Physiol. 1983 May;115(2):123–130. doi: 10.1002/jcp.1041150204. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]


