Abstract
Background
There is a lack of outcome data beyond local recurrence rates after primary treatment in rectal cancer, despite more information being necessary for clinical decision-making. We sought to determine patient selection, therapeutic modalities and outcomes of locally recurrent rectal cancer treated with curative intent.
Methods
We searched MEDLINE (1990–2010) using the medical subject headings “rectal neoplasms” and “neoplasm recurrence, local.” Selection of cohort studies was based on the primary intention of treatment and availability of at least 1 outcome variable.
Results
We included 55 cohort studies comprising 3767 patients; 8 studies provided data on the rate of intentionally curative treatment from an unselected consecutive cohort of patients (481 of 1188 patients; 40%). Patients were symptomatic with pain in 50% (796 of 1607) of cases. Overall, 3088 of 3767 patients underwent resection. The R0 resection rate was 56% (1484 of 2637 patients). The rate of external beam radiotherapy was 100% in 9 studies, 0% in 5 studies, and ranged from 12% to 97% in 37 studies. Overall postoperative mortality was 2.2% (57 of 2515 patients). Five-year survival was at least 25%, with an upper limit of 41% in 11 of 18 studies including at least 50 resections. We found a significant increase in reported survival rates over time (r2 = 0.214, p = 0.007).
Conclusion
More uniformity in treatment protocols and reporting on outcomes for locally recurrent rectal cancer is warranted. The observed improvement of reported survival rates in time is probably related to better patient selection and optimized multimodality treatment in specialized centres.
Abstract
Contexte
À part les taux de récurrences locales, on constate un manque de données relatives aux résultats des premiers traitements pour cancer rectal, alors que la prise de décision clinique nécessiterait des renseignements plus complets. Nous avons voulu déterminer comment s’effectuaient la sélection des patients et le choix des modalités thérapeutiques et quelle était l’issue des récurrences locales de cancer rectal soumises à un traitement curatif.
Méthodes
Nous avons interrogé le réseau MEDLINE (1990–2010) à partir des rubriques « rectal neoplasms » (cancers rectaux) et « neoplasm recurrence, local » (récurrence locale de cancer). La sélection des études de cohorte a été faite en fonction de la visée principale du traitement et de la présence d’au moins 1 variable liée à l’issue du traitement.
Résultats
Nous avons inclus 55 études de cohorte regroupant 3767 patients; 8 études ont fourni des données sur les taux de traitement à visée curative pour une cohorte de patients consécutifs non sélectionnés (481 patients sur 1188; 40 %). Les patients présentaient des symptômes de douleur dans 50 % des cas (796 sur 1607). Dans l’ensemble, 3088 patients sur 3767 ont subi une résection. Le taux de résection R0 était de 56 % (1484 patients sur 2637). Le taux de radiothérapie transcutanée était de 100 % dans 9 études, de 0 % dans 5 études et allait de 12 % à 97 % dans 37 études. La mortalité postopératoire globale était de 2,2 % (57 patients sur 2515). La survie à 5 ans était d’au moins 25 %, avec une limite supérieure de 41 % dans 11 études sur 18 incluant au moins 50 résections. Nous avons observé une augmentation significative des taux de survie rapportés dans le temps (r2 = 0,214, p = 0,007).
Conclusion
Les protocoles thérapeutiques et la présentation des rapports sur l’issue des récurrences locales de cancer rectal gagneraient à être uniformisés. L’amélioration des taux de survie observée dans le temps est probablement liée à une sélection plus circonspecte des patients et à une amélioration des traitements multimodaux prodigués dans des centres spécialisés.
Substantial progress has been made in local control of rectal cancer in the past decades. First, anatomic consideration of the mesorectal fascia has led to the development of total mesorectal excision (TME). This surgical technique encompasses sharp dissection under direct vision resulting in resection of the rectum and mesorectum, ideally with an intact visceral pelvic fascia covering the resection specimen. The TME technique has become widely accepted, as local recurrence rates declined from greater than 20% with conventional blunt dissection to 5%–10% with TME.1 Second, the use of neoadjuvant therapy further reduced local recurrence rates, as shown in a meta-analysis of randomized controlled trials.2
The local recurrence rate is the most relevant end point for interventional studies on neoadjuvant treatment in patients with rectal cancer, because survival is either not or only marginally improved.2 However, there is a lack of adequate data beyond local recurrence rates, despite more information being necessary for clinical decision-making. Therefore, the aim of this systematic review was to analyze the recent literature on intentionally curative treatment of locally recurrent rectal cancer.
Methods
We searched MEDLINE using the following medical subject headings: “rectal neoplasms” and “neoplasm recurrence, local.” The search was carried out for the period 1990–2010 using the limits “humans” and “all adult: 19+ years.” We reviewed all retrieved articles and systematically screened the reference lists of selected articles for additional studies of interest. The date of the most recent search was Oct. 1, 2010.
Selection of cohort studies in patients with locally recurrent rectal cancer was based on the primary intention of treatment and availability of at least 1 outcome variable, either local control or survival. Multiple studies from the same institution were included only if there were different inclusion criteria based on patient characteristics or type of treatment and if there was no substantial overlap.
We extracted the following data from the included papers (when provided): study period, selection criteria, treatment characteristics of primary rectal cancer, characteristics of local recurrence (symptom score S0–2), synchronous metastases, time interval from primary tumour to local recurrence, completeness of resection (R status), percentage of sacral resection, application of external beam radiotherapy (EBRT) for local recurrence (timing, dose, reirradiation, concurrent chemotherapy), use of intraoperative radiotherapy (IORT), operative mortality, follow-up, crude distant metastasis and local control rate, actuarial local control and survival and median survival.
Statistical analysis
We used descriptive statistics to determine overall results for evaluable studies. Analyses were performed using SPSS for Windows version 16.0.2. (SPSS Inc.).
Results
Description of selected cohorts
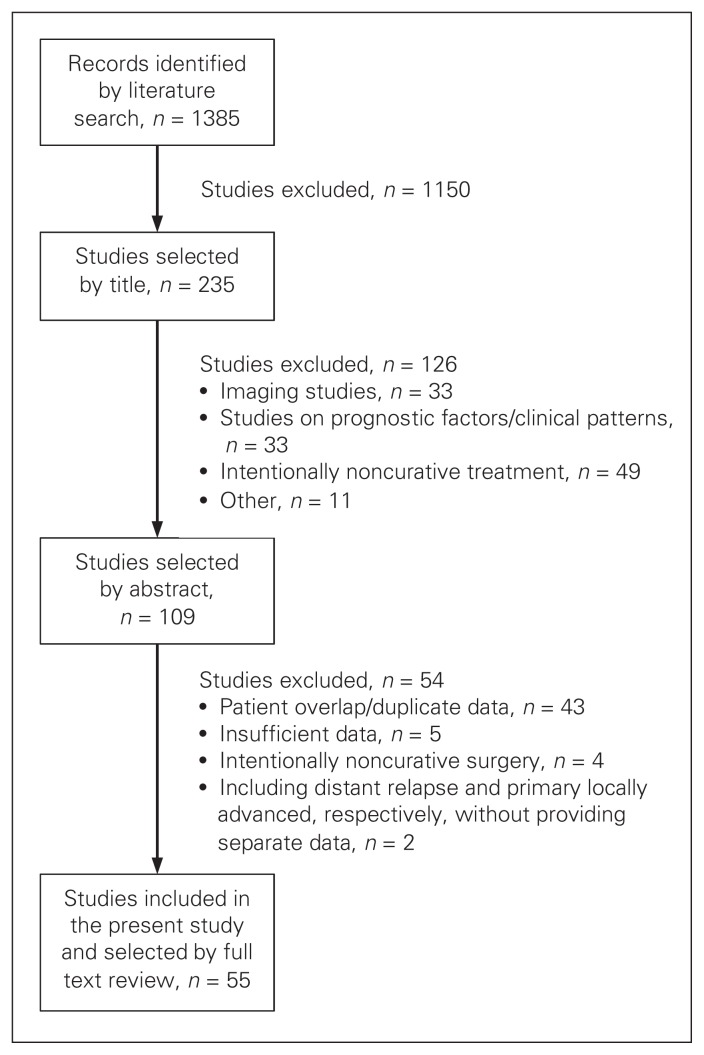
We selected 55 cohort studies comprising 3767 patients for detailed analysis (Fig. 1 and Table 1). Twenty-four studies provided data on patient selection leading to the final cohort (Table 1). The authors used several selection criteria based on type of treatment for primary rectal cancer, characteristics of local recurrence, type of treatment for local recurrence and intraoperative findings. In 8 studies,9,13,16,20,42–44,54 the total number of patients undergoing treatment with curative intent from an unselected consecutive cohort of patients could be determined: 481 of 1188 patients (40%). The median period of patient inclusion was 13.3 (range 3.9–34) years in 51 studies (Table 1).
Fig. 1.
Selection of studies on intentionally curative treatment of locally recurrent rectal cancer for inclusion in our systematic review.
Table 1.
Locally recurrent rectal cancer with curative intent: cohort selection, primary therapy and clinical presentation
| Study | Year | Period, yr | Selection | Primary therapy, % | Characteristics of local recurrence, %* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Ni | Inclusion/exclusion | Ns | APR | RT | CT | S0 | S1 | S2 | M1 | M1res | Interval, mo* | |||
| Abuchaibe et al.3 | 1993 | 6.3 | — | All IORT | 27 | — | 26 | 37 | 0 | 30 | 70 | 0 | 0 | — |
|
| ||||||||||||||
| Estes et al.4 | 1993 | — | — | All exenterations | 16 | 19 | 50 | — | 0 | 0 | 100 | 0 | 0 | 8 |
|
| ||||||||||||||
| Gagliardi et al.5 | 1995 | 19 | 84 | No irresectable, advanced M1 | 49 | 18 | — | — | 16 | 35 | 49 | 27 | 13 | (13) [2–81] |
|
| ||||||||||||||
| Gunderson et al.6 | 1996 | 14.3 | 182 | No prior RT, all IORT | 123 | — | 0 | 24 | — | — | — | 8 | 6 | — |
|
| ||||||||||||||
| Bussières et al.7 | 1996 | — | — | All IORT | 73 | 33 | 51 | 10 | — | — | — | 14 | — | < 2 yr 54% |
|
| ||||||||||||||
| Knol et al.8 | 1997 | 15 | 292 | Complete follow-up | 280 | 44 | — | — | 15 | 21 | 64 | 41 | 0 | (12) [3–96] |
|
| ||||||||||||||
| Bozetti et al.9 | 1997 | 34 | 213 | Suitable for reoperation | 45 | 24 | 4 | 18 | — | — | — | 0 | 0 | (13) [4–50] |
|
| ||||||||||||||
| Maetani et al.10 | 1998 | 18.1 | 59 | 59 | 61 | — | — | — | — | — | 20 | 15 | — | |
|
| ||||||||||||||
| Eble et al.11 | 1998 | 3.9 | — | All IORT | 31 | — | — | 19 | — | — | 81 | — | — | — |
|
| ||||||||||||||
| Wanebo et al.12 | 1999 | 23 | 95 | All sacral resection | 53 | 55 | 100 | — | — | — | — | 4 | 4 | < 12 mo 28% |
|
| ||||||||||||||
| Salo et al.13† | 1999 | 9.9 | 194 | No intestinal bypass | 131 | 27 | 39 | — | 44 | 29 | 27 | 5 | 5 | 21 |
|
| ||||||||||||||
| Zacherl et al.14 | 1999 | 9 | — | All sacral resection | 12 | 42 | — | — | 0 | 0 | 100 | 0 | 0 | (41) [6–84] |
|
| ||||||||||||||
| Hashiguchi et al.15 | 1999 | 22.1 | 51 | 51 | 57 | 0 | — | 22 | 33 | 45 | 37 | 12 | (32) [5–199] | |
|
| ||||||||||||||
| Adachi et al.16 | 1999 | 11 | 21 | No irresectable | 9 | 22 | 0 | — | 56 | 33 | 11 | 0 | 0 | 35 [8–61] |
|
| ||||||||||||||
| Law et al.17 | 2000 | 10.7 | 47 | 47 | 30 | — | — | — | — | — | — | — | 18 | |
|
| ||||||||||||||
| Rodel et al.18 | 2000 | 3.9 | 35 | 35 | 17 | 0 | 17 | — | — | — | 9 | 9 | (22) [4–112] | |
|
| ||||||||||||||
| Lindel et al.19 | 2001 | 18.7 | 69 | All IORT, no M1 | 49 | 28 | 13 | — | — | — | — | 0 | 0 | (24) [4–123] |
|
| ||||||||||||||
| Lopez-Kostner et al.20 | 2001 | 13.9 | 117 | No irresectable or M1 | 43 | — | — | — | 16 | 42 | 42 | — | — | (20) [2–86] |
|
| ||||||||||||||
| Garcia-Aguilar et al.21 | 2001 | 11 | 87 | 87 | 28 | 32 | 32 | — | — | — | 0 | 0 | 25 | |
|
| ||||||||||||||
| Yamada et al.22 | 2001 | 16.9 | 83 | 83 | 53 | 0 | — | — | — | — | 0 | 0 | (20) [11–31] | |
|
| ||||||||||||||
| Bergamaschi et al.23 | 2001 | 23 | 91 | No M1, all initial LAR | 35 | 0 | 0 | — | 43 | — | — | 3 | 3 | (16) [3–58] |
|
| ||||||||||||||
| Huguier et al.24 | 2001 | 30 | 80 | All initial local excision | 38 | 21 | 3 | — | 71 | — | — | 34 | 26 | 15 [9–x] |
|
| ||||||||||||||
| Shoup et al.25 | 2002 | 10.5 | 634 | No irresectable, all IORT | 100 | 16 | 50 | — | — | — | — | — | — | 21 |
|
| ||||||||||||||
| Friel et al.26 | 2002 | 12 | — | All initial local excision | 29 | 0 | 7 | — | 66 | — | — | 7 | 3 | (26) [5–89] |
|
| ||||||||||||||
| Mohiuddin et al.27 | 2002 | 13 | — | All prior RT | 103 | — | 100 | — | — | — | — | — | — | (19) [2–86] |
|
| ||||||||||||||
| Pezner et al.28 | 2002 | 10 | — | All prior RT, all IORT | 15 | 27 | 100 | 80 | — | — | 33 | 7 | 7 | (25) [0–62] |
|
| ||||||||||||||
| Hahnloser et al.29‡ | 2003 | 15.5 | 429 | No M1 after preoperative RT | 304 | 29 | — | — | 23 | 23 | 54 | 0 | 0 | (33) [2–175] |
|
| ||||||||||||||
| Saito et al.30 | 2003 | 14.3 | — | No anastomotic recurrence | 85 | 61 | 0 | — | — | — | — | 31 | 0 | (22) [10–56] |
|
| ||||||||||||||
| Kakuda et al.31 | 2003 | 12.5 | — | All exenteration | 22 | — | — | — | 14 | 38 | 48 | 9 | 0 | — |
|
| ||||||||||||||
| Bakx et al.32 | 2004 | 15 | — | No initial local excision | 40 | 28 | 55 | — | 38 | 28 | 34 | 5 | 5 | (17) [5–188] |
|
| ||||||||||||||
| Moriya et al.33 | 2004 | 18 | 163 | All sacral resection | 57 | 51 | 4 | — | 0 | 0 | 100 | 9 | 9 | (23) [7–102] |
|
| ||||||||||||||
| Reerink et al.34 | 2004 | 9.6 | 50 | No palliative intent | 40 | 20 | 0 | 0 | — | — | — | 0 | 0 | (17) [5–74] |
|
| ||||||||||||||
| Weiser et al.35 | 2005 | 33 | — | All initial R0 local excision | 50 | 0 | 44 | — | 49 | — | — | 16 | 16 | (20) [4–70] |
|
| ||||||||||||||
| Vermaas et al.36 | 2005 | 18 | 117 | Not fit enough, M1 | 92 | 25 | — | — | — | — | 31 | 2 | — | (15) [2–186] |
|
| ||||||||||||||
| Boyle et al.37 | 2005 | 7 | 64 | 64 | 34 | 58 | — | 33 | 31 | 36 | — | — | 31 | |
|
| ||||||||||||||
| Henry et al.38 | 2005 | 15 | 90 | 19 no data on hydronephrosis | 71 | 27 | 58 | 69 | 51 | — | — | 4 | 4 | 24 |
|
| ||||||||||||||
| Rudmik et al.39 | 2005 | 9 | — | All intraluminal recurrence | 9 | 0 | 11 | 11 | 56 | 44 | 0 | 0 | 0 | (21) [8–53] |
|
| ||||||||||||||
| Melton et al.40 | 2006 | 17 | — | All sacral resection | 29 | 45 | 93 | 76 | — | — | — | 10 | 10 | (44) [15–294] |
|
| ||||||||||||||
| Valentini et al.41 | 2006 | 4.2 | — | All prior RT | 59 | 24 | 100 | 75 | — | — | 41 | 0 | 0 | (27) [9–106] |
|
| ||||||||||||||
| Bedrosian et al.42 | 2006 | 10 | 134 | No palliative intent (R2 or M1) | 85 | 23 | — | — | — | — | — | 0 | 0 | (22) [1–113] |
|
| ||||||||||||||
| Asoglu et al.43 | 2007 | 7 | 72 | No irresectable or M1 | 50 | 34 | 100 | — | 0 | 48 | 52 | 0 | 0 | (24) [4–113] |
|
| ||||||||||||||
| Palmer et al.44 | 2007 | 10 | 141 | All surgical exploration | 57 | 28 | 30 | — | 35 | — | — | 18 | 2 | (16) [3–79] |
|
| ||||||||||||||
| Wells et al.45 | 2007 | 7.9 | 52 | 52 | 23 | 46 | 62 | — | — | — | 12 | 0 | (21) [3–166] | |
|
| ||||||||||||||
| Wiig et al.46 | 2008 | 13.3 | 204 | No prior RT | 150 | 27 | 0 | — | 37 | — | — | 5 | 0 | (19) [3–161] |
|
| ||||||||||||||
| Heriot et al.47 | 2008 | 16.7 | 160 | 160 | 7 | 6 | — | 30 | — | — | — | — | (23) [1–159] | |
|
| ||||||||||||||
| Schurr et al.48 | 2008 | — | 72 | 72 | 28 | 76 | 69 | — | — | — | 27 | 13 | 42 | |
|
| ||||||||||||||
| Tanaka et al.49 | 2008 | 18 | 43 | 43 | — | 0 | 70 | — | — | — | 19 | 0 | (26) [3–100] | |
|
| ||||||||||||||
| Rades et al.50 | 2008 | 11 | — | All RT for recurrence | 94 | — | 0 | 18 | — | — | — | 33 | 0 | — |
|
| ||||||||||||||
| Sagar et al.51§ | 2009 | 6 | — | All sacral resection | 40 | 40 | 38 | 45 | — | — | — | 5 | 5 | (35) [12–104] |
|
| ||||||||||||||
| Kusters et al.52 | 2009 | 13.9 | 209 | No irresectable | 170 | 31 | 51 | — | 25 | 35 | 40 | 0 | 0 | (29) [3–283] |
|
| ||||||||||||||
| Fujii et al.53 | 2009 | 20 | 76 | No R2 resection or M1 | 61 | — | — | — | — | — | — | 0 | 0 | — |
|
| ||||||||||||||
| de Chaisemartin et al.54 | 2009 | — | 18 | — | 18 | — | 22 | 33 | 44 | 34 | 22 | 22 | 0 | (14) [3–60] |
|
| ||||||||||||||
| Park et al.55 | 2009 | 7 | — | No T1, M1 or local excision | 62 | 48 | 19 | 100 | — | — | — | 0 | 0 | 28 |
|
| ||||||||||||||
| Pacelli et al.56 | 2010 | 15.9 | 157 | No irresectable or M1 | 58 | 17 | 33 | 59 | 50 | 21 | 29 | 0 | 0 | — |
|
| ||||||||||||||
| Das et al.57 | 2010 | 4 | — | All prior RT | 50 | — | 100 | — | — | — | — | 26 | 0 | (28) [5–354]¶ |
|
| ||||||||||||||
| Total | 3767 | |||||||||||||
APR = abdominoperineal resection; CT = adjuvant chemotherapy; Interval = mean (median) [range] time interval from primary tumour; IORT = intraoperative radiotherapy; M1 = distant metastasis either before or synchronous with local recurrence; M1res = resected distant metastasis; Ni = initial population; Ns = study population after selection; RT = radiotherapy with or without concurrent chemotherapy, either pre- or postoperative; S0 = no symptoms; S1 = symptomatic without pain; S2 = symptomatic with pain.
Unless otherwise indicated.
Including 123 patients described by Gunderson et al.6
7 patients already described by Boyle et al.37
Interval between RT treatments (7 patients previous RT for other cancer).
Therapy for primary rectal cancer
The surgical procedure for the initial tumour was described in 44 studies (Table 1). The median abdominoperineal resection (APR) rate was 27% (range 0%–62%) in these studies. Local excision was specified as the primary surgical treatment in 13 studies; 2 of them26,35 exclusively included patients who had undergone local excision, and the median local excision rate was 10% (range 6%–30%) in the remaining 11 studies.4,7,17,20,25,29,34,40,44,45,47 Included patients had undergone EBRT as part of the primary treatment in a median of 28% (range 0%–100%) of cases in 43 studies (Table 1). Previous radiotherapy dose was specified in 10 studies,4,7,9,12,19,27,28,41,55,57 with a total dose ranging from 10 to 110 Gy. The median percentage of patients who underwent chemotherapy as part of the treatment for the primary tumour was 37% (range 0%–100%) in 21 evaluable studies (Table 1).
Clinical presentation of local recurrence
Patients were asymptomatic (S0) when presenting with locally recurrent rectal cancer in 29% (571 of 2000) of cases in 27 studies (Table 1). Symptoms were classified according to presence of pain in 23 studies (Table 1), and 50% (796 of 1607) of patients were symptomatic with pain (S2). Five studies4,14,31,33,39 reporting on symptoms had a clinically relevant patient selection (sacral resection or intraluminal recurrence only). After exclusion of these studies, the rates of S0 and S2 clinical presentation were 30% (563 of 1884) and 47% (700 of 1491), respectively. The median interval between primary tumour and local recurrence ranged from 12 to 44 months in 40 studies (Table 1), with an overall maximum interval of 354 months, as reported by Das and colleagues.57
Data on synchronous distant metastases were provided in 48 studies (Table 1). In 18 of these, there was no clinical sign of distant dissemination at the time of treatment for local recurrence. The proportion of patients in whom distant metastases were identified ranged from 2% to 41% in the other 30 studies, with a reported percentage below 10% in 14 of these studies (Table 1). All distant metastases were resected with curative intent in 11 of these 30 studies (3%–16% of included patients), while selected patients received surgical treatment for distant disease in another 8 studies (2%–26% of included patients; Table 1).
Surgery for local recurrence
All patients from 30 studies underwent resection of the pelvic recurrence (Table 2). In the remaining 25 studies, the percentage of resection varied from 17% to 99%, with a resection rate above 70% in 15 of these 25 studies (Table 2). Differences in resection rates were mainly explained by the fact that patients with unresectable disease or intraoperatively detected distant disease were not excluded from the initial study population in some studies. The overall number of patients who underwent resection was 3088 of 3767 in the 55 selected studies. In the 51 studies specifying the duration of the study period, the median number of resections performed per year was 3.6 (range 0.8–19.6; Table 2). After excluding multicentre studies, only 12 centres performed at least 5 resections per year. The median reported R0 resection rate of 46 studies was 59% (range 14%–100%; Table 2). The overall calculated R0 resection rate was 56% (1484 of 2637 patients). A macroscopically complete resection (R0/R1) could be achieved in a median of 85% (range 31%–100%) of patients in 38 studies (Table 2). Part of the sacrum was included in the resection in all patients in 5 studies12,14,33,40,51 and in no patients in another 17 studies (Table 2). In the remaining 20 studies reporting on sacral resection, the median rate was 13% (range 2%–73%; Table 2).
Table 2.
Locally recurrent rectal cancer with curative intent: therapy for local recurrence
| Study | Characteristics of surgery for local recurrence | Perioperative treatment for local recurrence, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Resection | R0, % | R0/R1, % | Sacral, % | Preop EBRT | IORT | Postop EBRT | Total EBRT | Re-EBRT | CRT | CT | ||
|
| ||||||||||||
| No. (%) | No./yr | |||||||||||
| Abuchaibe et al.3 | 26 (96) | 4.1 | 31 | 31 | 0 | 31 | 100 | 46 | 77 | 4 | 27 | — |
|
| ||||||||||||
| Estes et al.4 | 16 (100) | — | 100 | — | 19 | 25 | 0 | 0 | 25 | 0 | 0 | — |
|
| ||||||||||||
| Gagliardi et al.5 | 49 (100) | 2.6 | 27 | 51 | — | 2 | 0 | 61 | 63 | — | 0 | — |
|
| ||||||||||||
| Gunderson et al.6 | 122 (99) | 8.5 | 14 | 47 | — | 84 | 100 | 23 | 98 | 0 | 75 | 2 |
|
| ||||||||||||
| Bussières et al.7 | 63* (86) | — | — | 67 | 0 | 25 | 100 | 16 | 41† | 0 | 0 | 16 |
|
| ||||||||||||
| Knol et al.8 | 48 (17) | 3.2 | — | 42 | — | 25 | 0 | 77 | 100 | — | 58 | — |
|
| ||||||||||||
| Bozetti et al.9 | 45 (100) | 1.3 | 47 | — | 0 | 0 | 0 | 38 | 38 | 0 | 11 | — |
|
| ||||||||||||
| Maetani et al.10 | 59 (100) | 3.3 | — | — | 73 | 44 | 0 | 0 | 44 | — | — | — |
|
| ||||||||||||
| Eble et al.11 | 31 (100) | 7.9 | 45 | 74 | 0 | 71 | 100 | 29 | 100 | — | 71 | — |
|
| ||||||||||||
| Wanebo et al.12 | 53 (100) | 2.3 | 85 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | — | — |
|
| ||||||||||||
| Salo et al.13 | 103 (79) | 10.4 | 69 | 82 | 3 | 21 | 40‡ | 28 | 49 | 0 | — | — |
|
| ||||||||||||
| Zacherl et al.14 | 12 (100) | 1.3 | 100 | 100 | 100 | 33 | 0 | 0 | 33 | — | 8 | — |
|
| ||||||||||||
| Hashiguchi et al.15 | 51 (100) | 2.3 | 24 | 53 | — | 41 | 53 | 43 | 75§ | 0 | — | — |
|
| ||||||||||||
| Adachi et al.16 | 9 (100) | 0.8 | 78 | 78 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | — |
|
| ||||||||||||
| Law et al.17 | 47 (100) | 4.4 | 51 | — | 11 | — | 0 | — | — | — | — | — |
|
| ||||||||||||
| Rodel et al.18 | 26 (74) | 6.7 | 65 | 100 | 12 | 100 | 0 | 0 | 100 | 0 | 100 | 66 |
|
| ||||||||||||
| Lindel et al.19 | 49 (100) | 2.6 | 51 | 69 | — | 94† | 100 | 3 | 97† | 7 | 52 | — |
|
| ||||||||||||
| Lopez-Kostner et al.20 | 43 (100) | 3.1 | — | — | — | — | 0 | — | — | — | — | — |
|
| ||||||||||||
| Garcia-Aguilar et al.21 | 51 (59) | 4.6 | 82 | — | — | 0 | 0 | 0 | 0 | 0 | — | 0 |
|
| ||||||||||||
| Yamada et al.22 | 60 (72) | 3.6 | — | — | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||
| Bergamaschi et al.23 | 35 (100) | 1.5 | 34 | 100 | 0 | 0 | 0 | 60 | 60 | 0 | 0 | — |
|
| ||||||||||||
| Huguier et al.24 | 38 (100) | 1.3 | — | — | — | 0 | 16 | 79 | 79 | 0 | 32 | 8 |
|
| ||||||||||||
| Shoup et al.25 | 100 (100) | 9.5 | 64 | 94 | — | 37 | 100 | 0 | 37 | 0 | — | 58 |
|
| ||||||||||||
| Friel et al.26 | 29 (100) | 2.4 | 72 | 83 | 0 | 41 | 0 | 17 | 59 | 0 | 0 | — |
|
| ||||||||||||
| Mohiuddin et al.27 | 34 (33) | 2.6 | — | — | 0 | 100 | 0 | 0 | 100 | 100 | 100 | — |
|
| ||||||||||||
| Pezner et al.28 | 15 (100) | 1.5 | 27 | 80 | 0 | 0 | 100 | 20 | 20 | 20 | 0 | 13 |
|
| ||||||||||||
| Hahnloser et al.29 | 304 (100) | 19.6 | 45 | 54 | — | 0 | 43 | 80 | 80 | — | 55 | — |
|
| ||||||||||||
| Saito et al.30 | 57 (67) | 4.0 | 75 | — | 37¶ | 40 | 0 | 0 | 40¶ | 0 | 0 | 0 |
|
| ||||||||||||
| Kakuda et al.31 | 22 (100) | 1.8 | 55 | 77 | 4 | — | 32 | — | — | — | — | — |
|
| ||||||||||||
| Bakx et al.32 | 40 (100) | 2.7 | 40 | 100 | 20 | 10 | 0 | 25 | 33§ | — | 0 | — |
|
| ||||||||||||
| Moriya et al.33 | 57 (100) | 3.2 | 84 | 100 | 100 | 40 | 16 | 0 | 40 | 0 | — | — |
|
| ||||||||||||
| Reerink et al.34 | 25 (63) | 2.6 | 68 | — | 0 | 52 | 0 | 48 | 100 | 0 | 12 | — |
|
| ||||||||||||
| Weiser et al.35 | 49 (98) | 1.5 | 96 | 100 | 12 | 29 | 26 | 14 | 42 | — | 42 | 49** |
|
| ||||||||||||
| Vermaas et al.36 | 92 (100) | 5.1 | 58 | 85 | 13 | 64 | 29 | 32 | 96 | — | 0 | 10 |
|
| ||||||||||||
| Boyle et al.37 | 57 (89) | 8.1 | 42 | 86 | 12 | 42 | 0 | 0 | 42† | 0 | — | — |
|
| ||||||||||||
| Henry et al.38 | 71 (100) | 4.7 | 59 | 83 | — | — | 37‡ | — | 56 | — | 41 | 10 |
|
| ||||||||||||
| Rudmik et al.39 | 9 (100) | 1.0 | 100 | — | 0 | 0 | 0 | 22 | 22 | — | 22 | 0 |
|
| ||||||||||||
| Melton et al.40 | 29 (100) | 1.7 | 62 | 97 | 100 | 0 | 41 | 14 | 14 | 3 | 0 | 45 |
|
| ||||||||||||
| Valentini et al.41 | 39* (66) | 9.3* | 54 | 62 | 0 | 100 | 0 | 0 | 100 | 100 | 100 | 77 |
|
| ||||||||||||
| Bedrosian et al.42 | 85 (100) | 8.5 | 76 | 100 | — | 27 | 42 | 0 | 27 | 2 | 27 | 53** |
|
| ||||||||||||
| Asoglu et al.43 | 36 (72) | 5.1 | 67 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — |
|
| ||||||||||||
| Palmer et al.44 | 50* (88) | 5.0* | 50 | — | 0 | 28 | 22 | 0 | 28 | 0 | 0 | — |
|
| ||||||||||||
| Wells et al.45 | 52* (100) | 6.6* | 80 | 100 | 54 | 50 | 0 | 0 | 50 | 0 | 38 | — |
|
| ||||||||||||
| Wiig et al.46 | 139 (93) | 10.5 | 47 | 89 | 4 | 100 | — | 0 | 100 | 0 | 11 | — |
|
| ||||||||||||
| Heriot et al.47 | 153* (96) | 9.2* | 64 | 90 | 19 | 61 | 8 | 3 | 63† | 6 | 44 | 0 |
|
| ||||||||||||
| Schurr et al.48 | 45 (63) | — | — | 82 | 24 | 16 | 0 | 0 | 16 | 0 | 16 | 18** |
|
| ||||||||||||
| Tanaka et al.49 | 35 (81) | 1.9 | 77 | — | 0 | — | 0 | — | — | 0 | — | — |
|
| ||||||||||||
| Rades et al.50 | 46 (49) | 4.2 | 52 | 85 | — | 0 | 0 | 100 | 100 | 0 | 67 | 67 |
|
| ||||||||||||
| Sagar et al.51 | 40 (100) | 6.7 | 50 | 98 | 100 | 60 | 0 | 3 | 63 | 0 | 60 | 13 |
|
| ||||||||||||
| Kusters et al.52 | 170 (100) | 12.2 | 55 | — | 25 | 85 | 91 | 0 | 85 | 42 | 62 | — |
|
| ||||||||||||
| Fujii et al.53 | 61 (100) | 3.1 | — | 100 | 0 | 11 | 0 | 0 | 12 | 0 | 0 | 30†† |
|
| ||||||||||||
| de Chaisemartin et al.54 | 11 (61) | — | 100 | — | 0 | 45 | 0 | 18 | 63 | 0 | — | — |
|
| ||||||||||||
| Park et al.55 | 38 (61) | 5.4 | 61 | — | 11 | 39¶ | 0 | 0 | 39 | — | 39 | — |
|
| ||||||||||||
| Pacelli et al.56 | 44 (76) | 2.8 | 57 | 80 | 2 | 79 | 50 | 0 | 79 | — | 79 | 44 |
|
| ||||||||||||
| Das et al.57 | 18 (36) | 4.5 | 39 | 100 | 0 | 100 | 50 | 0 | 100 | 100 | 96 | — |
|
| ||||||||||||
| Total | 3088 | |||||||||||
CT = adjuvant systemic chemotherapy; CRT = concurrent chemotherapy during EBRT as percentage of total resections; EBRT = external beam radiotherapy; IORT = intraoperative radiotherapy; Postop = postoperative; Preop = preoperative; re-EBRT = EBRT for both primary tumour and local recurrence; Sacral = abdominosacral resection.
Percentage of study population.
28 of 52 patients and 24 of 26 patients brachytherapy, respectively.
5 and 1 patients both preoperative and postoperative EBRT, respectively.
Percentage of R0 resections.
Including some patients with preoperative chemotherapy.
Intraoperative hyperthermic intraperitoneal chemotherapy.
Perioperative treatment for local recurrence
External beam radiotherapy was uniformly performed either in the preoperative or in the postoperative setting for all patients in 9 studies, whereas no patients in 5 of the studies underwent EBRT. The rate of EBRT reported in 37 other studies ranged from 12% to 97% (Table 2). External beam radiotherapy was given preoperatively in 37 studies; in 20 of these, radiotherapy was uniformly applied in the preoperative setting (Table 2). Postoperative EBRT was described in 25 studies, and irradiation occurred exclusively in the postoperative setting in 8 of these 25 studies (Table 2). Radiotherapy dose was specified in 28 studies.3–9,11,18,19, 28,29, 30,33–37,41,44,46,47,50–53,56,57 Without prior EBRT, the total dose was 40–60 Gy in most studies, with an overall range of 15–80 Gy. Conventional fractionation (1.8–2.0 Gy per fraction) was uniformly applied in 13 studies,3,6,7,9,11,18,19,28,30,36,46,50,52 and selective use of a hypofractionated schedule (fractions of 5 Gy) was described in 2 studies.34,44 In 3 studies,27,41,57 all patients had undergone EBRT for the primary tumour; the median (and range) rates were 50.4 (30–55) Gy, 50.4 (30–74) Gy and 47 (25–70) Gy, respectively. Reirradiation schedules were as follows: 40.8 Gy in daily fractions of 1.2 Gy, median 34.8 (range 15–49.2) Gy in daily fractions of 1.2 or 1.8 Gy, and 39 Gy in fractions of 1.5 Gy twice daily, respectively.27,41,57 An additional 8 studies3,19,28,40,42,47,52,56 described the use of re-EBRT, 7 of which specified the rate.3,19,28,40,42,47,52 The radiotherapy schedule was specified in 3 of these studies,28,52,56 with a total dose ranging from 23.4 to 30.6 Gy in daily fractions of 1.2 or 1.8 Gy. External beam radiotherapy was part of a chemoradiotherapy regimen, mostly fluorouracil-based, in 27 studies (Table 2); routine use of chemoradiotherapy was reported in only 3 studies.18,27,41
Intraoperative radiotherapy (IORT) was routinely used as part of the treatment protocol in 7 studies; in another 16 studies, the rate of IORT varied from 8% to 91% (Table 2). Thirteen studies3,6,7,11,15,19,25,28,35,36,47,52,57 specified the total dose of IORT, which mostly ranged from 10 to 20 Gy.
Outcome after treatment with curative intent
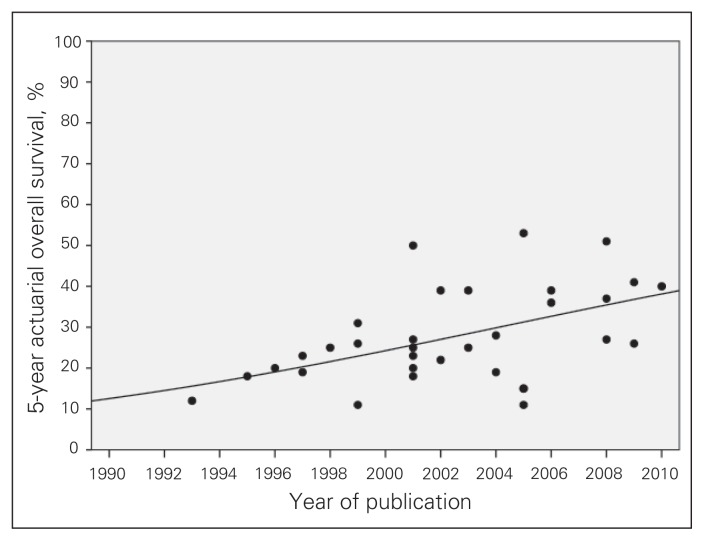
Postoperative mortality was 2.2% (57 of 2515 patients), across 46 studies (Table 3), most of which defined postoperative mortality as in-hospital mortality or 30-day mortality; 2 studies48,52 defined it as 60-d and 90-d mortality, respectively. The median follow-up was less than 36 months in 24 of 37 studies with available data (Table 3). The observed crude rate of distant metastases during follow-up varied between 9% and 68%, with a median rate of 41% across 29 studies (Table 3). The crude local control rate in patients who underwent R0 resection ranged between 29% and 100%; the rate was above 75% in 9 of 14 studies (Table 3). The actuarial local control rate for R0 resection was more than 55% in 7 of 8 studies (Table 3). The most consistently reported outcome parameter was 5-year survival, which ranged from 11% to 51%. Five-year survival was at least 25%, with an upper limit of 41%, in 11 of 18 studies including at least 50 resections (Table 3). Figure 2 displays the reported 5-year survival rates of series without selection for type of surgical resection, by year of publication. The curve fit model demonstrates a significant increase in reported survival rates over time (r2 = 0.214, p = 0.007).
Table 3.
Locally recurrent rectal cancer with curative intent: outcome after treatment
| Study | Operative mortality, % | Follow-up, median [range] mo | M1, % | Crude local control, % | Actuarial 3-yr local control, % | Actuarial overall survival, % | Overall survival, median mo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||
| R0 | R0/R1 | All | R0 | All | R0 5-yr | All 3-yr | All 5-yr | R0 | All | |||||
| Abuchaibe et al.3 | 0 | (25) [2–82] | 41 | 50 | 50 | 26 | 56* | 26* | 29 | — | 12 | — | 22 | |
|
| ||||||||||||||
| Estes et al.4 | 6.3 | — | — | — | — | — | — | — | 49 | — | 49 | — | — | |
|
| ||||||||||||||
| Gagliardi et al.5 | 0 | (28) | — | — | — | 47 | — | — | — | 33 | 18 | — | — | |
|
| ||||||||||||||
| Gunderson et al.6 | — | — | 52 | 93 | 74 | 70 | 100 | 62 | 44† | 39 | 20 | 30 | 28 | |
|
| ||||||||||||||
| Bussières et al.7 | 0 | (30) | — | — | — | 71 | — | 31 | — | 31 | — | — | — | |
|
| ||||||||||||||
| Knol et al.8 | — | (11) [1–118] | — | — | — | — | — | — | — | — | 23 | — | — | |
|
| ||||||||||||||
| Bozetti et al.9 | 0 | (40) | — | — | — | — | — | — | — | — | 19 | — | — | |
|
| ||||||||||||||
| Maetani et al.10 | — | — | 49 | — | — | 39 | — | — | — | — | 25 | — | — | |
|
| ||||||||||||||
| Eble et al.11 | 0 | (28) [24–x] | 32 | 79 | — | 71 | 78† | — | — | 58‡ | — | — | — | |
|
| ||||||||||||||
| Wanebo et al.12 | 7.5 | — | 55 | — | — | 47 | — | — | — | 1 | 31 | — | 36 | |
|
| ||||||||||||||
| Salo et al.13 | 0.8 | (54) | — | — | — | — | — | — | 35 | — | 31 | 42 | 28 | |
|
| ||||||||||||||
| Zacherl et al.14 | 0 | — | — | — | — | — | — | — | 17 | 17 | — | 22§ | 22§ | |
|
| ||||||||||||||
| Hashiguchi et al.15 | 7.8 | (30) [5–128] | — | — | — | — | — | — | 0 | 25 | 11 | — | — | |
|
| ||||||||||||||
| Adachi et al.16 | 0 | — | — | 29 | 29 | — | — | — | — | — | 26 | — | — | |
|
| ||||||||||||||
| Law et al.17 | 0 | — | 46 | 87 | — | — | — | — | 39 | — | — | 25 | — | |
|
| ||||||||||||||
| Rodel et al.18 | 0 | (27) [6–48] | 35 | 82 | — | — | — | — | 82† | 63 | — | — | — | |
|
| ||||||||||||||
| Lindel et al.19 | 0 | (x) [4–150] | 67 | — | — | — | 56¶ | 35¶ | 40 | — | 27 | — | — | |
|
| ||||||||||||||
| Lopez-Kostner et al.20 | 4.7 | (33) | 40 | — | — | 76 | — | — | — | — | 50** | — | — | |
|
| ||||||||||||||
| Garcia-Aguilar et al.21 | 0 | (28) | 10 | 76 | — | — | — | — | 35 | — | 23 | — | 22 | |
|
| ||||||||||||||
| Yamada et al.22 | 3.3 | — | — | — | — | — | — | — | — | 30 | 18 | — | — | |
|
| ||||||||||||||
| Bergamaschi et al.23 | 2.8 | — | 46 | — | — | 27 | — | — | 80 | — | 25 | NR | 26 | |
|
| ||||||||||||||
| Huguier et al.24 | 0 | — | — | — | — | — | — | — | — | — | 20 | — | — | |
|
| ||||||||||||||
| Shoup et al.25 | — | (23.2) | 40 | — | — | 67 | — | — | 51** | — | 39** | — | 53 | |
|
| ||||||||||||||
| Friel et al.26 | 0 | (39) [2–147] | 31 | — | — | 83 | — | — | — | — | — | — | — | |
|
| ||||||||||||||
| Mohiuddin et al.27 | — | (24) [3–84] | — | — | — | 44 | — | — | — | — | 22 | — | 44 | |
|
| ||||||||||||||
| Pezner et al.28 | 6.6 | (x) [20–x] | 62 | — | — | 38 | — | 25 | — | 29 | — | — | — | |
|
| ||||||||||||||
| Hahnloser et al.29 | 0.3 | — | — | — | — | — | — | — | 37 | 43 | 25 | — | 31 | |
|
| ||||||||||||||
| Saito et al.30 | 0 | (40) [3–150] | 37 | 77 | — | — | — | — | 39 | 46 | 39 | — | — | |
|
| ||||||||||||||
| Kakuda et al.31 | 4.5 | (17) | — | — | — | 68 | — | — | — | — | 12 | 23 | 13 | |
|
| ||||||||||||||
| Bakx et al.32 | 5.0 | (100) [4–200] | 23 | — | — | 50 | — | — | — | — | 28 | — | 25 | |
|
| ||||||||||||||
| Moriya et al.33 | 3.5 | (42) [17–163] | — | — | — | — | — | 57 | 42** | 54** | 36** | — | — | |
|
| ||||||||||||||
| Reerink et al.34 | 0 | (81) [20–134] | 44 | — | — | — | — | 49 | — | 36 | 19 | — | 26 | |
|
| ||||||||||||||
| Weiser et al.35 | 0 | (33) | — | — | — | — | — | — | 59 | — | 53 | — | — | |
|
| ||||||||||||||
| Vermaas et al.36 | EBRT | 3.3 | (16) [4–156] | — | — | — | — | 31 | 28 | 21 | 34 | 11 | — | — |
|
| ||||||||||||||
| No EBRT | 13 | 30 | 15 | |||||||||||
|
| ||||||||||||||
| Boyle et al.37 | 1.6 | — | — | 50 | 47 | — | — | — | — | — | — | — | 34 | |
|
| ||||||||||||||
| Henry et al.38 | Hydro | 4.3 | (27) | — | — | — | 61 | — | — | — | — | 15 | 57 | 31 |
|
| ||||||||||||||
| No hydro | 45 | 78 | 43 | |||||||||||
|
| ||||||||||||||
| Rudmik et al.39 | 0 | (30) [6–59] | 22 | 100 | — | 100 | — | — | — | — | — | 33 | 33 | |
|
| ||||||||||||||
| Melton et al.40 | 3.4 | (23) [0.3–88] | 50 | — | — | 40 | — | 38¶ | — | — | 20** | 49 | 33 | |
|
| ||||||||||||||
| Valentini et al.41 | 2.6 | (36) [9–69] | 68 | — | — | — | 69¶ | 39¶ | 67 | — | 39 | — | 42 | |
|
| ||||||||||||||
| Bedrosian et al.42 | — | (43) [1–149] | 31 | — | 64 | 64 | — | 51¶ | 43 | — | 36 | — | — | |
|
| ||||||||||||||
| Asoglu et al.43 | 0 | — | 54 | 67 | — | — | — | — | — | — | — | 28 | 19 | |
|
| ||||||||||||||
| Palmer et al.44 | 5.3 | (12) [0–103] | 24 | 80 | — | — | — | — | 57 | — | — | — | 21 | |
|
| ||||||||||||||
| Wells et al.45 | 0 | (29) [3–72] | 60 | — | 33 | 33 | — | — | 47‡ | 41‡ | — | — | 40 | |
|
| ||||||||||||||
| Wiig et al.46 | 0.7 | (23) [1–150] | — | — | — | — | 73¶ | 48¶ | 52 | — | 27 | 74 | — | |
|
| ||||||||||||||
| Heriot et al.47 | 0.6 | (20) [0–177] | — | — | — | — | — | — | 48 | 56 | 37 | — | 43 | |
|
| ||||||||||||||
| Schurr et al.48 | 8.8 | — | — | — | — | — | — | — | — | — | — | — | 55 | |
|
| ||||||||||||||
| Tanaka et al.49 | 0 | (44) [1–146] | — | — | — | 70 | — | — | — | 55 | 51 | — | — | |
|
| ||||||||||||||
| Rades et al.50 | — | (19) [6–66] | 41 | — | — | — | — | 55 | — | 51 | — | — | — | |
|
| ||||||||||||||
| Sagar et al.51 | 2.5 | (25) [4–64] | 18 | — | — | 72 | — | — | — | — | — | — | — | |
|
| ||||||||||||||
| Kusters et al.52 | 8.2 | (35) [3–146] | 52 | — | — | — | 68¶ | 54¶ | 58** | — | 41** | — | — | |
|
| ||||||||||||||
| Fujii et al.53 | — | — | — | — | 49 | 49 | — | — | — | — | 26 | — | — | |
|
| ||||||||||||||
| de Chaisemartin et al.54 | 0 | (23) [7–58] | 9 | 73 | — | 73 | — | — | 100 | — | — | — | — | |
|
| ||||||||||||||
| Park et al.55 | 0 | (31) [8–70] | — | — | — | — | — | — | 35 | — | — | — | — | |
|
| ||||||||||||||
| Pacelli et al.56 | 6.8 | (82) [8–157] | 37 | 88 | 74 | — | — | 40†† | — | — | 40 | — | — | |
|
| ||||||||||||||
| Das et al.57 | — | (25) [0–71] | — | — | — | — | — | 47 | — | 66 | — | — | 60 | |
EBRT = external beam radiotherapy; Hydro = hydronephrosis; M1 = crude rate of patients with distant metastases during follow-up; NR = not reached.
2-year,
3-year,
4-year,
mean,
5-year,
cancer-specific,
5-year for R0/R1 resections.
Fig. 2.
Reported actuarial 5-year survival after treatment with curative intent for locally recurrent rectal cancer during the past 2 decades (curve fit model: r2 = 0.214, p = 0.007). Selected series based on type of resection were excluded.
Topographical differences
We grouped studies into 4 categories according to the geographic areas from which they originated: Europe, the United States, Japan and the remaining countries. As shown in Table 4, the highest rate of prior EBRT was found in the United States (50%), and EBRT was most frequently used to treat local recurrence in Europe. Japan had the lowest rates of EBRT and IORT, but a more aggressive surgical approach was suggested, with 39% of resections including part of the sacrum.
Table 4.
Differences in treatment characteristics for locally recurrent rectal cancer among studies conducted in Europe, the United States, Japan and other countries
| Treatment characteristic | Location; no. (%) | Total no. (%) | |||
|---|---|---|---|---|---|
| Europe, n = 25 studies | United States, n = 17 studies | Japan, n = 9 studies | Other,*n = 5 studies | ||
| Prior EBRT | 412/1165 (35) | 459/926 (50) | 2/328 (1) | 46/283 (16) | 919/2702 (34) |
| EBRT for local recurrence | 919/1278 (72) | 726/1175 (62) | 111/340 (33) | 138/244 (57) | 1894/3037 (62) |
| IORT for local recurrence | 341/1068 (32) | 572/1178 (49) | 36/436 (8) | 12/295 (4) | 961/2977 (32) |
| Sacral resection | 117/1026 (11) | 95/368 (26) | 145/371 (39) | 61/252 (24) | 418/2017 (21) |
| R0 resection | 517/1013 (51) | 634/1116 (57) | 161/256 (63) | 172/252 (68) | 1484/2637 (56) |
EBRT = external beam radiotherapy, IORT = intraoperative radiotherapy.
Studies from Chile (n = 1), New Zealand (n = 1), Canada (n = 2) and Korea (n = 1).
Discussion
In general, local recurrence is the reflection of an aggressive biological behaviour of the primary tumour as it is accompanied by synchronous distant disease in a high percentage of patients. In the Dutch TME trial,58 83 of 129 patients (63%) with local recurrence also had distant metastases. The Swedish Rectal Cancer Trial and Stockholm I trial reported distant disease in 66 of 143 patients (46%) and 86 of 156 patients (55%) with local recurrence, respectively.59,60 Our systematic review suggests that 40% of unselected consecutive patients with locally recurrent rectal cancer are candidates for intentionally curative treatment. Curative treatment of both local and distant recurrence is achievable in only a small subgroup of patients (Table 1). Probably such treatment should only be considered in patients with indolent tumour behaviour based on a long disease-free interval (at least 2 yr) from primary treatment.
Overall, half of the patients were classified as having S2 clinical presentation (symptomatic with pain). Although these were selected patients who were candidates for intentionally curative treatment, this finding is concordant with an unselected cohort of 156 patients with locally recurrent rectal cancer from the Stockholm Rectal Cancer Study: the rates of S0, S1 and S2 clinical presentation were 13%, 33% and 54%, respectively.60 Intractable pain associated with a pelvic recurrence is an awful clinical condition. While distant disease is the determining factor for prognosis in most of these patients, local recurrence will generally affect quality of life.61,62 Complete or partial initial relief of pain after radiotherapy alone or after multimodality treatment, including surgery, is reported in up to 83% of patients, although the rate of long-term pain-free survival is about 30%.27,41,63
The 2 most important predictors of radical resection of local recurrence are previous anterior resection instead of APR and the absence of pain at the time of recurrence.43,64,65 Intraluminal recurrences, especially after initial local excision, are separated from the bony pelvis and sacral nerves by remaining soft tissue, thereby not resulting in pain and enabling resection with adequate margins in almost all patients.26,35,39 Given the worse outcome for local recurrence after prior APR, optimal primary treatment of distal cancers is of utmost importance. The pelvis becomes narrower at the level of the levator ani. When following this natural curve, the surgeon will end up with a so-called coning resection, thereby increasing the risk of tumour-positive margins. The extralevatoric APR with en bloc removal of the levator muscle in combination with downstaging by neoadjuvant therapy will improve local control in distal rectal cancers.66,67
We found a wide variety in treatment protocols with regard to perioperative radiotherapy for locally recurrent rectal cancer reported in the studies we reviewed (Tables 2 and 4). Some institutes did not include EBRT or IORT in their protocols, while the indication for radiotherapy ranged from highly selective to routine use at other centres. In addition, EBRT had been applied either in the preoperative or in the postoperative setting. Chemotherapy was not always added as radiosensitizer, especially in series published before 2005. The indication for EBRT did not depend only on prior EBRT; comparing data on EBRT from Tables 1 and 2 revealed that some patients received no irradiation during the entire treatment, neither for the primary tumour, nor for the local recurrence. On the other hand, there is a tendency toward reirradiation in patients with locally recurrent rectal cancer after prior pelvic radiotherapy.
There is no concluding evidence to determine the most optimal treatment strategy for locally recurrent rectal cancer. This is also related to the heterogeneity of the disease, as shown in the present review, based on type of primary surgery, previous radiotherapy, extent of recurrent disease (i.e., fixation grade, extension to pelvic sidewall) and the presence of symptoms or distant metastases. The available data do not enable pooling of data from several subgroups to compare different treatment approaches among different disease entities. However, there is increasing consensus that EBRT should be given preoperatively with concurrent chemotherapy, as demonstrated by papers published since 2006 (Table 2). This recommendation is based on the need for optimal preoperative downsizing and downstaging to maximize the chance of an R0 resection, which is the most important predictor for survival after treatment for locally recurrent rectal cancer.68,69 The calculated overall R0 resection rate of 56% leads us to conclude that there is room for improvement. The use of IORT is still controversial, and data from randomized controlled trials are lacking.
There is a need for more complete and uniform reporting on outcome parameters (Table 3). Better comparison of data can be achieved by determining outcomes for similar groups of patients (e.g., those who undergo R0 or R0/R1 resection) using standardized parameters, such as 3- and 5-year local control and overall survival. Missing follow-up data and inappropriate length of follow-up in most of the remaining studies reflect the low quality of available data on the treatment of locally recurrent rectal cancer.
The use of EBRT for primary rectal cancer has increased during the past decades. Without previous radio-therapy, patients with locally recurrent rectal cancer can be optimally treated by full-dose (chemo)radiotherapy. In the Dutch TME trial, radiotherapy at a dose of 45 Gy or higher was applied in 42% of patients for local recurrence after TME alone, whereas the rate was only 4% for the preoperative radiotherapy group.69 A radiotherapy dose less than 45 Gy was associated with shorter survival after local recurrence in both univariable and multivariable analysis. Long-term follow-up of the Swedish and Dutch rectal cancer trials showed that time from local recurrence to death was significantly shorter in the irradiation group than in patients who underwent surgery alone.59,69 Thus, radiotherapy does not affect systemic dissemination and, therefore, local recurrences after radiotherapy are more often concomitant with distant disease, leading to a worse prognosis from time of local recurrence. From these data, it can be concluded that radiotherapy for primary resectable rectal cancer mostly prevents potentially curable local recurrence. More selective application of neoadjuvant therapy for primary resectable rectal cancer can minimize early and late adverse effects, and broadens therapeutic options if local recurrence develops.
Conclusion
If intentionally curative treatment in a patient with locally recurrent rectal cancer is considered by the multidisciplinary team, a standardized approach with optimal neoadjuvant treatment is indicated. Full-dose chemoradiotherapy or an adapted schedule depending on previous EBRT maximizes the chance of an R0 resection, which is the most important prognostic factor. Surgery should be performed in specialized centres by an experienced surgeon. The distant metastasis rate during follow-up supports the use of adjuvant chemotherapy, although there is no conclusive evidence. Systemic chemotherapy preceding or following (re)irradiation in a neoadjuvant setting is probably worthwhile to explore in future studies given the systemic nature of the disease. Finally, prospective data collection should ideally be performed in the setting of a trial and/or (inter)national cancer registry. Standardized reporting of actuarial local control and survival for predefined categories (i.e., completeness of resection) based on these data will improve available evidence.
Footnotes
Competing interests: None declared.
Contributors: All authors helped design the study, analyzed data and approved publication of the article. P.J. Tanis acquired the data and wrote the article. A. Doeksen and J.J.B. van Lanschot reviewed the article.
References
- 1.Peeters KC, van de Velde CJ. Surgical quality assurance in rectal cancer treatment: the key to improved outcome. Eur J Surg Oncol. 2005;31:630–5. doi: 10.1016/j.ejso.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Wong RK, Tandan V, De Silva S, et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007:CD002102. doi: 10.1002/14651858.CD002102.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Abuchaibe O, Calvo FA, Azinovic I, et al. Intraoperative radiotherapy in locally advanced recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 1993;26:859–67. doi: 10.1016/0360-3016(93)90502-m. [DOI] [PubMed] [Google Scholar]
- 4.Estes NC, Thomas JH, Jewell WR, et al. Pelvic exenteration: a treatment for failed rectal cancer surgery. Am Surg. 1993;59:420–2. [PubMed] [Google Scholar]
- 5.Gagliardi G, Hawley PR, Hershman MJ, et al. Prognostic factors in surgery for local recurrence of rectal cancer. Br J Surg. 1995;82:1401–5. doi: 10.1002/bjs.1800821035. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson LL, Nelson H, Martenson JA, et al. Intraoperative electron and external beam irradiation with or without 5-fluorouracil and maximum surgical resection for previously unirradiated, locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1379–95. doi: 10.1007/BF02054527. [DOI] [PubMed] [Google Scholar]
- 7.Bussières E, Gilly FN, Rouanet P, et al. Recurrences of rectal cancers: results of a multimodal approach with intraoperative radiation therapy. French Group of IORT. Intraoperative Radiation Therapy. Int J Radiat Oncol Biol Phys. 1996;34:49–56. doi: 10.1016/0360-3016(95)02048-9. [DOI] [PubMed] [Google Scholar]
- 8.Knol HP, Hanssens PE, Rutten HJ, et al. Effect of radiation therapy alone or in combination with surgery and/or chemotherapy on tumor and symptom control of recurrent rectal cancer. Strahlenther Onkol. 1997;173:43–9. doi: 10.1007/BF03039193. [DOI] [PubMed] [Google Scholar]
- 9.Bozzetti F, Bertario L, Rossetti C, et al. Surgical treatment of locally recurrent rectal carcinoma. Dis Colon Rectum. 1997;40:1421–4. doi: 10.1007/BF02070705. [DOI] [PubMed] [Google Scholar]
- 10.Maetani S, Onodera H, Nishikawa T, et al. Significance of local recurrence of rectal cancer as a local or disseminated disease. Br J Surg. 1998;85:521–5. doi: 10.1046/j.1365-2168.1998.00602.x. [DOI] [PubMed] [Google Scholar]
- 11.Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol. 1998;49:169–74. doi: 10.1016/s0167-8140(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 12.Wanebo HJ, Antoniuk P, Koness RJ, et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438–48. doi: 10.1007/BF02235044. [DOI] [PubMed] [Google Scholar]
- 13.Salo JC, Paty PB, Guillem J, et al. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171–7. doi: 10.1007/s10434-999-0171-8. [DOI] [PubMed] [Google Scholar]
- 14.Zacherl J, Schiessel R, Windhager R, et al. Abdominosacral resection of recurrent rectal cancer in the sacrum. Dis Colon Rectum. 1999;42:1035–9. doi: 10.1007/BF02236698. [DOI] [PubMed] [Google Scholar]
- 15.Hashiguchi Y, Sekine T, Sakamoto H, et al. Intraoperative irradiation after surgery for locally recurrent rectal cancer. Dis Colon Rectum. 1999;42:886–93. doi: 10.1007/BF02237096. [DOI] [PubMed] [Google Scholar]
- 16.Adachi W, Nishio A, Watanabe H, et al. Reresection for local recurrence of rectal cancer. Surg Today. 1999;29:999–1003. doi: 10.1007/s005950050635. [DOI] [PubMed] [Google Scholar]
- 17.Law WL, Chu KW. Resection of local recurrence of rectal cancer: results. World J Surg. 2000;24:486–90. doi: 10.1007/s002689910077. [DOI] [PubMed] [Google Scholar]
- 18.Rödel C, Grabenbauer GG, Matzel KE, et al. Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Dis Colon Rectum. 2000;43:312–9. doi: 10.1007/BF02258294. [DOI] [PubMed] [Google Scholar]
- 19.Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83–7. doi: 10.1016/s0167-8140(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Kostner F, Fazio VW, Vignali A, et al. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44:173–8. doi: 10.1007/BF02234289. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Aguilar J, Cromwell JW, Marra C, et al. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1743–8. doi: 10.1007/BF02234449. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Ishizawa T, Niwa K, et al. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–93. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 23.Bergamaschi R, Pessaux P, Burtin P, et al. Abdominoperineal resection for locally recurrent rectal cancer. Tech Coloproctol. 2001;5:97–102. doi: 10.1007/pl00012131. [DOI] [PubMed] [Google Scholar]
- 24.Huguier M, Houry S, Barrier A. Local recurrence of cancer of the rectum. Am J Surg. 2001;182:437–9. doi: 10.1016/s0002-9610(01)00748-6. [DOI] [PubMed] [Google Scholar]
- 25.Shoup M, Guillem JG, Alektiar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585–92. doi: 10.1007/s10350-004-6250-9. [DOI] [PubMed] [Google Scholar]
- 26.Friel CM, Cromwell JW, Marra C, et al. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875–9. doi: 10.1007/s10350-004-6320-z. [DOI] [PubMed] [Google Scholar]
- 27.Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144–50. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 28.Pezner RD, Chu DZ, Ellenhorn JD. Intraoperative radiation therapy for patients with recurrent rectal and sigmoid colon cancer in previously irradiated fields. Radiother Oncol. 2002;64:47–52. doi: 10.1016/s0167-8140(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 29.Hahnloser D, Nelson H, Gunderson LL, et al. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502–8. doi: 10.1097/01.SLA.0000059972.90598.5F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito N, Koda K, Takiguchi N, et al. Curative surgery for local pelvic recurrence of rectal cancer. Dig Surg. 2003;20:192–9. doi: 10.1159/000070385. [DOI] [PubMed] [Google Scholar]
- 31.Kakuda JT, Lamont JP, Chu DZ, et al. The role of pelvic exenteration in the management of recurrent rectal cancer. Am J Surg. 2003;186:660–4. doi: 10.1016/j.amjsurg.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Bakx R, van Tinteren H, van Lanschot JJ, et al. Surgical treatment of locally recurrent rectal cancer. Eur J Surg Oncol. 2004;30:857–63. doi: 10.1016/j.ejso.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Moriya Y, Akasu T, Fujita S, et al. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047–53. doi: 10.1007/s10350-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 34.Reerink O, Mulder NH, Botke G, et al. Treatment of locally recurrent rectal cancer, results and prognostic factors. Eur J Surg Oncol. 2004;30:954–8. doi: 10.1016/j.ejso.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005;48:1169–75. doi: 10.1007/s10350-004-0930-3. [DOI] [PubMed] [Google Scholar]
- 36.Vermaas M, Ferenschild FT, Nuyttens JJ, et al. Preoperative radio-therapy improves outcome in recurrent rectal cancer. Dis Colon Rectum. 2005;48:918–28. doi: 10.1007/s10350-004-0891-6. [DOI] [PubMed] [Google Scholar]
- 37.Boyle KM, Sagar PM, Chalmers AG, et al. Surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2005;48:929–37. doi: 10.1007/s10350-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 38.Henry LR, Sigurdson E, Ross E, et al. Hydronephrosis does not preclude curative resection of pelvic recurrences after colorectal surgery. Ann Surg Oncol. 2005;12:786–92. doi: 10.1245/ASO.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Rudmik LR, Buie WD, Heine JA. Reoperation for intraluminal rectal cancer recurrence. Dis Colon Rectum. 2005;48:1752–4. doi: 10.1007/s10350-005-0070-4. [DOI] [PubMed] [Google Scholar]
- 40.Melton GB, Paty PB, Boland PJ, et al. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum. 2006;49:1099–107. doi: 10.1007/s10350-006-0563-9. [DOI] [PubMed] [Google Scholar]
- 41.Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–39. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Bedrosian I, Giacco G, Pederson L, et al. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum. 2006;49:175–82. doi: 10.1007/s10350-005-0276-5. [DOI] [PubMed] [Google Scholar]
- 43.Asoglu O, Karanlik H, Muslumanoglu M, et al. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33:1199–206. doi: 10.1016/j.ejso.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Palmer G, Martling A, Cedermark B, et al. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447–54. doi: 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 45.Wells BJ, Stotland P, Ko MA, et al. Results of an aggressive approach to resection of locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:390–5. doi: 10.1245/s10434-006-9119-4. [DOI] [PubMed] [Google Scholar]
- 46.Wiig JN, Larsen SG, Dueland S, et al. Preoperative irradiation and surgery for local recurrence of rectal and rectosigmoid cancer. Prognostic factors with regard to survival and further local recurrence. Colorectal Dis. 2008;10:48–57. doi: 10.1111/j.1463-1318.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 47.Heriot AG, Byrne CM, Lee P, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–91. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 48.Schurr P, Lentz E, Block S, et al. Radical redo surgery for local rectal cancer recurrence improves overall survival: a single center experience. J Gastrointest Surg. 2008;12:1232–8. doi: 10.1007/s11605-008-0517-8. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka K, Noura S, Ohue M, et al. Doubling time of carcinoembryonic antigen is a significant prognostic factor after the surgical resection of locally recurrent rectal cancer. Dig Surg. 2008;25:319–24. doi: 10.1159/000158597. [DOI] [PubMed] [Google Scholar]
- 50.Rades D, Kuhn H, Schultze J, et al. Prognostic factors affecting locally recurrent rectal cancer and clinical significance of hemoglobin. Int J Radiat Oncol Biol Phys. 2008;70:1087–93. doi: 10.1016/j.ijrobp.2007.07.2364. [DOI] [PubMed] [Google Scholar]
- 51.Sagar PM, Gonsalves S, Heath RM, et al. Composite abdominosacral resection for recurrent rectal cancer. Br J Surg. 2009;96:191–6. doi: 10.1002/bjs.6464. [DOI] [PubMed] [Google Scholar]
- 52.Kusters M, Dresen RC, Martijn H, et al. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:1444–9. doi: 10.1016/j.ijrobp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Fujii S, Shimada H, Yamagishi S, et al. Surgical strategy for local recurrence after resection of rectal cancer. Hepatogastroenterology. 2009;56:667–71. [PubMed] [Google Scholar]
- 54.de Chaisemartin C, Penna C, Goere D, et al. Presentation and prognosis of local recurrence after total mesorectal excision. Colorectal Dis. 2009;11:60–6. doi: 10.1111/j.1463-1318.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 55.Park JK, Kim YW, Hur H, et al. Prognostic factors affecting oncologic outcomes in patients with locally recurrent rectal cancer: impact of patterns of pelvic recurrence on curative resection. Langenbecks Arch Surg. 2009;394:71–7. doi: 10.1007/s00423-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 56.Pacelli F, Tortorelli AP, Rosa F, et al. Locally recurrent rectal cancer: prognostic factors and long-term outcomes of multimodal therapy. Ann Surg Oncol. 2010;17:152–62. doi: 10.1245/s10434-009-0737-5. [DOI] [PubMed] [Google Scholar]
- 57.Das P, Delclos ME, Skibber JM, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys. 2010;77:60–5. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 58.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 59.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–50. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 60.Holm T, Cedermark B, Rutqvist LE. Local recurrence of rectal adenocarcinoma after ‘curative’ surgery with and without preoperative radiotherapy. Br J Surg. 1994;81:452–5. doi: 10.1002/bjs.1800810344. [DOI] [PubMed] [Google Scholar]
- 61.Frykholm GJ, Pahlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol. 1995;34:185–94. doi: 10.1016/0167-8140(95)01519-m. [DOI] [PubMed] [Google Scholar]
- 62.Meagher AP, Ward RL. Current evidence does not support routine adjuvant radiotherapy for rectal cancer. ANZ J Surg. 2002;72:835–40. doi: 10.1046/j.1445-2197.2002.02543.x. [DOI] [PubMed] [Google Scholar]
- 63.Miner TJ, Jaques DP, Paty PB, et al. Symptom control in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2003;10:72–9. doi: 10.1245/aso.2003.03.040. [DOI] [PubMed] [Google Scholar]
- 64.Dresen RC, Peters EE, Rutten HJ, et al. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071–7. doi: 10.1016/j.ejso.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Huguier M, Houry S. Treatment of local recurrence of rectal cancer. Am J Surg. 1998;175:288–92. doi: 10.1016/s0002-9610(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 66.den Dulk M, Marijnen CA, Putter H, et al. Risk factors for adverse outcome in patients with rectal cancer treated with an abdominoperineal resection in the total mesorectal excision trial. Ann Surg. 2007;246:83–90. doi: 10.1097/01.sla.0000259432.29056.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West NP, Anderin C, Smith KJ, et al. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588–99. doi: 10.1002/bjs.6916. [DOI] [PubMed] [Google Scholar]
- 68.Caricato M, Borzomati D, Ausania F, et al. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126–32. doi: 10.1016/j.ejso.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 69.van den Brink M, Stiggelbout AM, van den Hout WB, et al. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol. 2004;22:3958–64. doi: 10.1200/JCO.2004.01.023. [DOI] [PubMed] [Google Scholar]