Abstract
The oxysterol binding protein family are amphitropic proteins that bind oxysterols, sterols, and possibly phosphoinositides, in a conserved binding pocket. The Saccharomyces cerevisiae oxysterol binding protein family member Kes1 (also known as Osh4) also binds phosphoinositides on a distinct surface of the protein from the conserved binding pocket. In this study, we determine that the oxysterol binding protein family member Kes1 is required to maintain the ratio of complex sphingolipids and levels of ceramide, sphingosine-phosphate and sphingosine. This inability to maintain normal sphingolipid homeostasis resulted in misdistribution of Pma1, a protein that requires normal sphingolipid synthesis to occur to partition into membrane rafts at the Golgi for its trafficking to the plasma membrane.
Introduction
Numerous regulatory mechanisms ensure proper levels of lipids within cells and organelles, with loss of control leading to various disease states. Glycerophospholipid and sterol synthesis occurs in the endoplasmic reticulum (ER), as do the first steps in the synthesis of sphingolipids. All three classes of lipids are transported to the Golgi apparatus where phospholipids can be further modified, and also serve as substrates for the final steps in the synthesis of sphingolipids. Lipid composition changes throughout the secretory pathway from a low concentration of sterols and sphingolipids in the ER with progressively higher levels in the Golgi and ultimately the plasma membrane. Membrane nanodomains or “rafts” formed by sphingolipids, sterols, saturated glycerophospholipids, and specific proteins have been proposed to be involved in the generation of this lipid gradient between the Golgi and the plasma membrane [1]. The processes integrating lipid metabolism with the formation of lipid rafts in the Golgi, and their subsequent transport out of this organelle are unclear. Lipid-binding proteins have been proposed to coordinate lipid metabolism with vesicular trafficking [2].
Yeast Osh proteins are part of an enigmatic class of lipid-binding proteins found throughout Eukarya united by a β-barrel structure that binds sterols, oxysterols, and phosphoinositides (PIPs) [3], [4]. The disruption of any six OSH genes in Saccharomcyes cerevisiae has minimal to mild affects on cellular growth [5]. However, inactivation of all seven OSH genes results in a substantial decrease in growth, indicating that Osh proteins most likely share an overlapping essential function [5]. Kes1 (Osh4) is the most well studied member of this family and numerous genetic and cell biology studies suggest Kes1 inhibits vesicular trafficking at the trans-Golgi. The mechanism is not clear, although it is known that Kes1 inhibits vesicular trafficking in a phosphatidylinositol (PI) 4-phosphate (PI-4P) dependent manner [6], [7], [8], [9], [10], [11], [12]. In vitro, Kes1 and other Osh proteins have been demonstrated to transfer sterols between membranes and a role for these proteins as direct non-vesicular sterol transporters has been proposed [9], [11]. Recent in vivo evidence is at odds with a role for Osh proteins in non-vesicular trafficking of sterols as cells diminished for function of all seven yeast Osh proteins displayed minimal defects in the intracellular transport of sterols [13], [14].
In this study, we reveal that Kes1 is required for maintenance of the normal ratio of complex sphingolipids and for maintenance of proper levels of sphingosine, sphingosine-phosphate, and ceramides. In the absence of Kes1 function, the localization of Pma1, a plasma membrane proton transporter whose trafficking from the Golgi to the plasma membrane requires sphingolipid synthesis, is compromised. We suggest that oxysterol binding protein superfamily members integrate sphingolipid metabolism with vesicular trafficking events.
Materials and Methods
Yeast strains and media
Rich medium was yeast extract protein dextrose (YEPD, 1% bacto-yeast extract, 2% bacto-peptone, 2% dextrose). Minimal medium was synthetic complete (SC, 0.67% bacto-yeast nitrogen base without amino acids, 2% dextrose, and nutrients as described). The S. cerevisiae strains used in this study are listed in Table 1. The CMY306 strain was constructed using standard molecular and yeast genetic methods whereby the KES1 gene was inactivated in the SEY6210-PMA1-dsRFP strain. The kes1Δ::HIS3 DNA region was amplified by polymerase chain reaction (PCR) from cells of strain CMY136 using primers hybridizing 500 base pairs (bp) upstream and downstream of the KES1 open reading frame (ORF). The linear PCR product was transformed into SEY6210-PMA1-RFP cells and His+ transformants were screened by PCR of genomic DNA using primers hybridizing 600 bp upstream and downstream of the KES1 ORF for confirmation of kes1Δ::HIS3.
Table 1. Yeast strains used in this study.
| Yeast Strain | Genotype | Source |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| kes1Δ | BY4741 kes1Δ::KanMx4 | Euroscarf |
| SEY6210 | MATα leu2-3, 112 ura3-52 his3-100 trp1-901 lys2-801 suc2-179 | Emr lab [24] |
| SEY6210-PMA1-dsRFP | SEY6210 PMA1-dsRFP::KANMX4 | McMaster lab [19] |
| CMY136 | MATα sec14ts ura3 his3 trp1 leu2 GAL+ kes1Δ::HIS3 | McMaster lab [7] |
| CMY306 | SEY6210 PMA1-dsRFP kes1Δ::HIS3 | This study |
Analysis of sphingolipids
For steady-state labeling cells were grown overnight to saturation. Cells were then seeded into low-density cultures and grown for at least six generations in the presence of 5 µCi/ml [3H]inositol. Aliquots of 5·107 cells per point were harvested and pipetted into five volumes of ice-cold 10% tricholoroacetic acid solution. Lipids were extracted and resolved by thin layer chromatography in chloroform:methanol:NH4OH (9∶7∶2). Bands were visualized by exposure X-ray film, scraped into vials, and quantified by liquid scintillation counting. Liquid chromatography-mass spectrometry sphingolipid measurements were performed as described [15].
Live cell imaging
Live cells were observed using a Zeiss Axiovert 200 M microscope fitted with a plan-neofluor 100× oil immersion lens. Images were captured using a Zeiss Axio Cam HR using Axiovision 4.5 software. Cells were visualized using differential interference contrast (DIC) and filters for rhodomine (for red fluorescent protein (RFP).
Results
The oxysterol binding protein Kes1 regulates complex sphingolipid metabolism
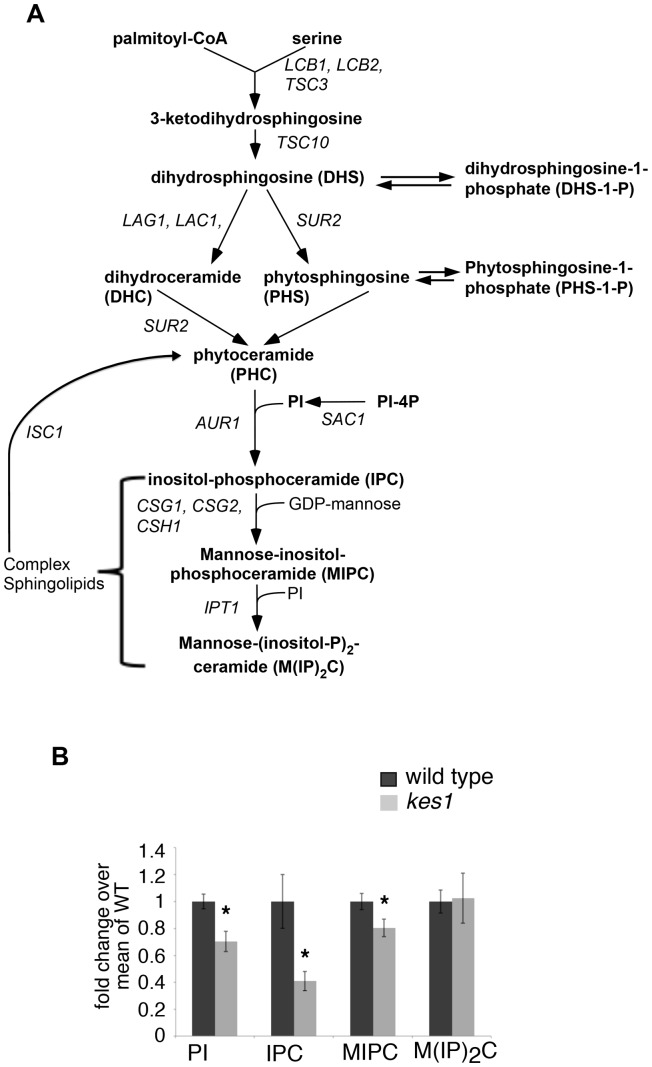
PI serves as the donor of head groups of the synthesis of complex sphingolipids in S. cerevisiae (Fig. 1A). The oxysterol binding protein Kes1 has been recently determined to enhance the conversion of PI-4P to PI by the PI-4P phosphatase Sac1 [10]. This prompted us to investigate if in cells with an inactivated KES1 gene there were altered levels of sphingolipids. We labeled cells with radiolabeled inositol to steady state and determined its incorporation into the metabolites of the sphingolipid biosynthetic pathway of S. cerevisiae to determine relative levels of complex sphingolipids. We observed that in cells with an inactivated KES1 gene there was a 30% decrease in radiolabel incorporation into PI, a 60% decrease into the sphingolipid inositol-phosphoceramide (IPC), and a 20% decrease in mannosylinositolphosphorylceramide (MIPC), while labeling of mannosyldiinositolphosphorylceramide M(IP)2C was unchanged (Fig. 1B). These data indicate that Kes1 is required to maintain a normal cellular sphingolipid composition.
Figure 1. Kes1 enhances complex sphingolipid metabolism.
(A) Phosphoinositides plays a central role in the synthesis of sphingolipids in yeast. Gene names are shown in italics. Metabolic intermediates and complex sphingolipids are shown in bold font. Inactivation of KES1 inhibits the synthesis of sphingolipids. (B) Cells were grown to late logarithmic phase and inoculated into medium containing myo-[3H]inositol, grown to mid-logarithmic phase and lipids were extracted and resolved by thin layer chromatography. Radioactivity of the resolved lipids was measured for PI, IPC, MIPC and M(IP)2C. Values were normalized to cell number and are presented as fold-change over wild type. Data represent mean +/− SE of four independent experiments performed in triplicate. *P<0.05 respective to control.
The levels of sphingoid bases and ceramides are altered by Kes1
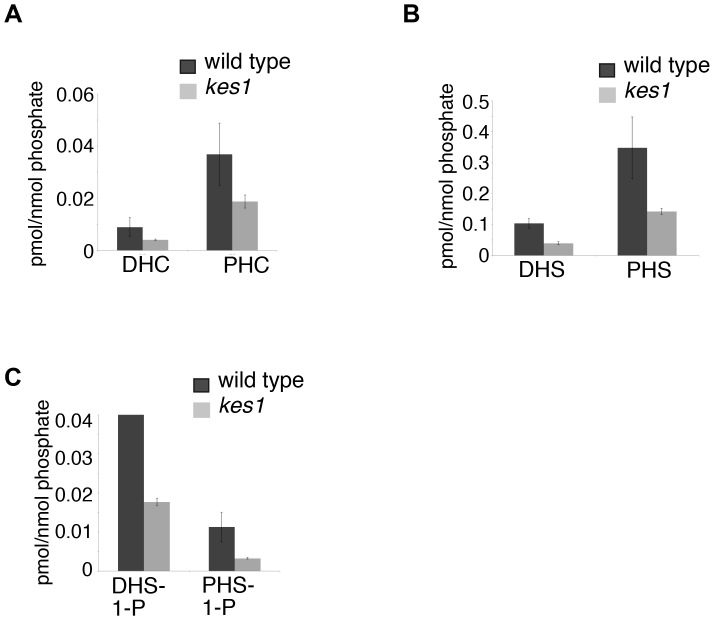
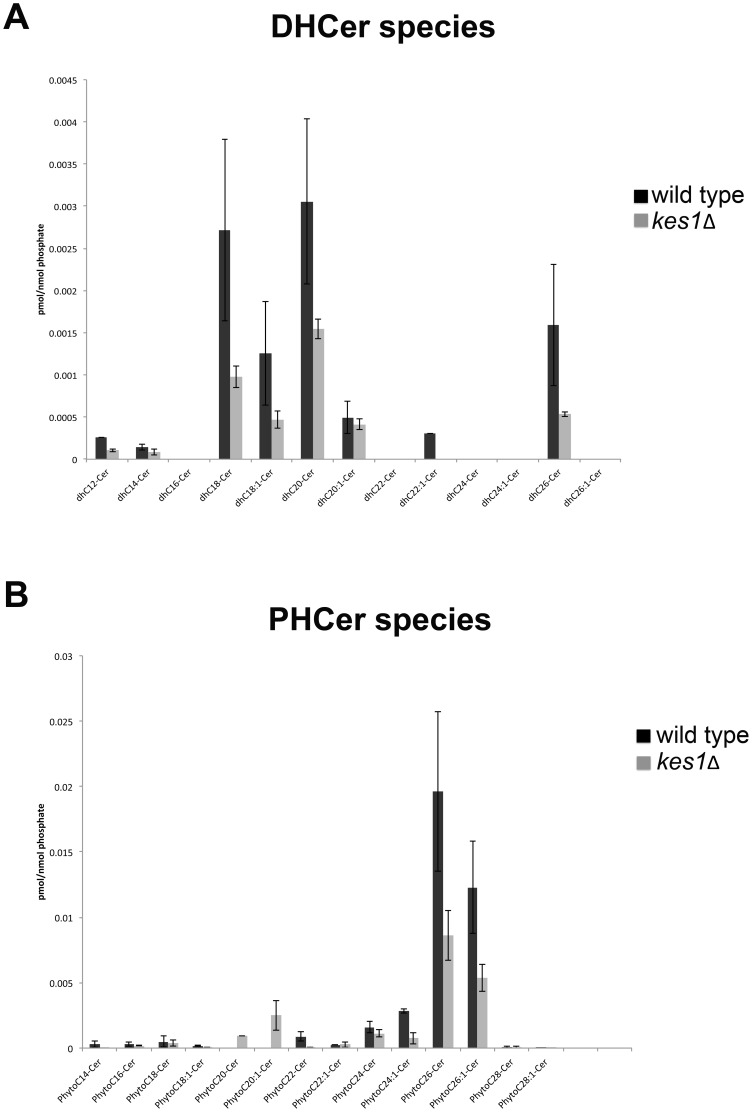
There is interconversion between complex sphingolipids, ceramides, and sphingoid bases with each having important cell signaling roles. We analyzed the level of sphingoid bases and ceramides using mass spectrometry to determine if Kes1 affected their levels and fatty acid composition. Inactivation of KES1 resulted in a 50% reduction in the level of sphingosines, sphingosine- phosphates and ceramides (Fig. 2A–C). An analysis of the composition of fatty acids within yeast ceramides revealed an overall decrease in all acyl species (Fig. 3). Kes1 is required to maintain sphingoid base, ceramide and complex sphingolipid levels.
Figure 2. Kes1 affects sphingoid base and ceramide levels.
Sphingolipids were extracted and measured by liquid chromatography-mass spectrometry and normalized to total inorganic phosphate. Total lipid levels representing all chain lengths are given for (A) dihydroceramide (DHC) and phytoceramide (PHC), (B) dihydrosphingosine (DHS) and phytosphingosine (PHS) and (C) dihydrosphingosine-1-phosphate (DHS-1-P) and phytosphingosine-1-phosphate (PHS-1-P). Data are expressed as a mean +/− SE of a minimum of three separate experiments.
Figure 3. Ceramide species is unchanged in cells lacking Kes1.
Lipids were extracted from cells and (A) dihydroceramide (DHCer) and (B) phytoceramide (PHCer) measured by liquid chromatography-mass spectrometry and normalized to total inorganic phosphate.
Kes1 is required for proper localization of the lipid raft protein Pma1
We assessed if the altered sphingolipid levels in cells lacking KES1 had a physiological effect by determining if there was altered localization of a Pma1-RFP fusion protein. Normal sphingolipid synthesis is required to properly partition this endogenous membrane raft associated protein into lipid rafts at the Golgi for subsequent delivery to the plasma membrane [16]. As expected, in wild type cells Pma1-RFP was at the plasma membrane while in cell with an inactivated KES1 gene the majority of Pma1-RFP was intracellular (Fig. 4). Quantification of this distribution determined that Pma1-RFP was primarily intracellular in 81% of cells with an inactivated KES1 gene, compared to 6% in wild type cells. This implies that the defects in sphingolipid levels in kes1Δ cells affect trafficking and localization of Pma1p.
Figure 4. Kes1 regulates Pma1 localization.
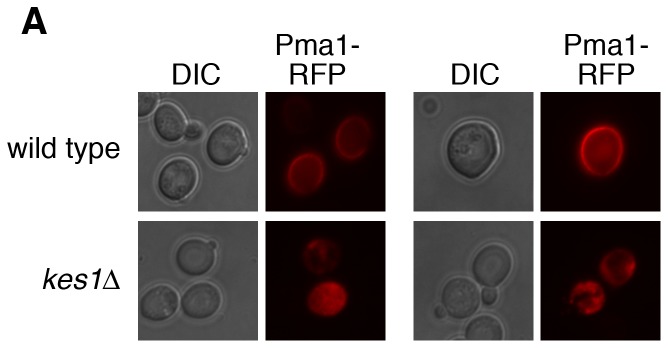
SEY6210-PMA1-dsRFP (wild type) and SEY6210-PMA1-dsRFP kes1Δ (CMY306) cells were grown to mid-logarithmic phase in SC medium at 25°C and visualized using a RFP filter. For quantification, 150 and 120 cells were counted for wild type and kes1Δ cells, respectively, over three different experiments to determine the percentage of cells with Pma1-RFP intracellular accumulation (6% for wild type and 81% for kes1Δ). Representative images are shown.
Discussion
A major finding from this study is that Kes1 regulates the levels of complex sphingolipids, ceramides, sphingosine-phosphate, and sphingosine. The ratio of complex sphingolipids was skewed in cells with an inactivated KES1 gene such that those upstream in this section of the sphingolipid biosynthetic pathway were decreased the most. This is consistent with the recent discovery that Kes1 can activate the PI-4P phosphatase Sac1 to produce PI [10], with the PI produced being preferentially used for the synthesis of complex sphingolipids [17]. Inactivation of the KES1 gene did not affect the overall fatty acid composition within ceramide species, however, an unanticipated finding was that Kes1 was required to maintain appropriate levels of sphingosines, sphingosine-phosphates, and ceramides. A physiological effect upon inactivation of the KES1 gene was abnormal distribution of the endogenous lipid raft localized protein Pma1, with Pma1 found primarily intracellularly rather than at the plasma membrane.
Complex sphingolipid synthesis takes place in the Golgi while sphingoid base and ceramide synthesis occurs in the ER. Proper Pma1 localization can be affected by defects in sphingolipid synthesis [16], [18], or by perturbing lipid raft composition at the plasma membrane [19]. It is unclear from the current study which of these is occurring. However, we had previously determined that Kes1 localizes to the Golgi [8]. This suggests that partitioning of Pma1 into appropriate vesicles for trafficking from the Golgi to the plasma membrane is likely being affected. Indeed, results from a genome-wide screen demonstrated that yeast with an inactivated KES1 gene, or defective in sterol and sphingolipid synthesis, were unable to traffic Fus-Mid-GFP. Fus-Mid-GFP is an engineered protein composed of the extracellular region of Fus1p fused to the transmembrane domain and cytoplasmic tail of Mid2p followed by GFP. Fus-Mid-GFP has to associate with lipid rafts to exit the Golgi and to traffic to the plasma membrane [20]. Previous data showed that inactivation of the KES1 gene did not alter the secretion of invertase [20], supporting a notion that Kes1 regulates specific vesicular pathways out of the trans-Golgi. We also noted that inactivation of KES1 did not affect FM4-64 trafficking from the plasma membrane to the vacuole (data not shown) consistent with this notion.
Large scale genome-wide association studies have determined that inactivation of the KES1 gene is synthetic sick with genes of the sterol biosynthetic pathway in yeast, but not those of the sphingolipid pathway [21]. This is consistent with Kes1 affecting the level of sphingolipid synthesis, a process that works in parallel with the sterol synthesis pathway for a shared downstream function. This interpretation is also in agreement with a study where it was identified that Kes1 inhibits phospholipid flippase activity at the trans-Golgi, likely indirectly, to relax membrane curvature to establish an ideal environment for sterol-rich rafts and vesicles to form [12]. The above observations combined with the data here that determined that Kes1 is necessary for normal sphingolipid levels and distribution of the lipid raft protein Pma1, imply that Kes1 may aid in assembly of membrane rafts at the Golgi for subsequent trafficking of rafts and their cargo to the plasma membrane.
Interestingly, in contrast to that observed for inactivation of the KES1 gene where we observed a decrease in the level of sphingosine, sphingosine-phosphate, and ceramides, inactivation of SAC1 resulted in an elevation of these lipids [17]. Sac1 localizes to both the Golgi and ER while Kes1 has only been reported to associate with the Golgi. Sphingoid bases are synthesized in the ER where Sac1 was reported to be part of the SPOTS complex comprised of the first enzyme in the sphingoid base pathway serine palmitoyltransferase, Tsc3 (a regulatory subunit of serine palmitoyltransferase), and Orm1/2 [22], [23]. Similar to the inactivation of SAC1, the loss of Orm function results in an accumulation of sphingoid bases. In addition, deletion of the ORM1 and ORM2 genes is synthetic lethal with deletion of SAC1 consistent with their regulating sphingoid base synthesis as part of a protein complex. Unlike Sac1, Kes1 is not found at the ER and is not part of the SPOTS complex. How Kes1 regulates sphingoid base levels will require further work.
Funding Statement
This work was supported by CIHR (http://www.cihr-irsc.gc.ca/e/193.html), NIH (http://www.nih.gov/), VA (http://www.research.va.gov/funding/), and NSERC (http://www.nserc-crsng.gc.ca/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, et al. (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. The Journal of cell biology 185: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Matteis MA, Di Campli A, D'Angelo G (2007) Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochimica et biophysica acta 1771: 761–768. [DOI] [PubMed] [Google Scholar]
- 3. de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, et al. (2011) Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. The Journal of cell biology 195: 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Im YJ, Raychaudhuri S, Prinz WA, Hurley JH (2005) Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 437: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beh CT, Cool L, Phillips J, Rine J (2001) Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 157: 1117–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, et al. (2007) A general amphipathic alpha-helical motif for sensing membrane curvature. Nature structural & molecular biology 14: 138–146. [DOI] [PubMed] [Google Scholar]
- 7. Fairn GD, Curwin AJ, Stefan CJ, McMaster CR (2007) The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proceedings of the National Academy of Sciences of the United States of America 104: 15352–15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeBlanc MA, McMaster CR (2010) Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. The Journal of biological chemistry 285: 33875–33884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raychaudhuri S, Im YJ, Hurley JH, Prinz WA (2006) Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. The Journal of cell biology 173: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, et al. (2011) Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144: 389–401. [DOI] [PubMed] [Google Scholar]
- 11. Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, et al. (2009) Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. The Journal of cell biology 187: 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muthusamy BP, Raychaudhuri S, Natarajan P, Abe F, Liu K, et al. (2009) Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Molecular biology of the cell 20: 2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beh CT, McMaster CR, Kozminski KG, Menon AK (2012) A detour for yeast oxysterol binding proteins. The Journal of biological chemistry 287: 11481–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, et al. (2011) Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic 12: 1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowart LA, Okamoto Y, Lu X, Hannun YA (2006) Distinct roles for de novo versus hydrolytic pathways of sphingolipid biosynthesis in Saccharomyces cerevisiae. The Biochemical journal 393: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Chang A (2002) Sphingoid base synthesis is required for oligomerization and cell surface stability of the yeast plasma membrane ATPase, Pma1. Proceedings of the National Academy of Sciences of the United States of America 99: 12853–12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brice SE, Alford CW, Cowart LA (2009) Modulation of sphingolipid metabolism by the phosphatidylinositol-4-phosphate phosphatase Sac1p through regulation of phosphatidylinositol in Saccharomyces cerevisiae. The Journal of biological chemistry 284: 7588–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Sitaraman S, Chang A (2006) Multiple degradation pathways for misfolded mutants of the yeast plasma membrane ATPase, Pma1. The Journal of biological chemistry 281: 31457–31466. [DOI] [PubMed] [Google Scholar]
- 19. Zaremberg V, Gajate C, Cacharro LM, Mollinedo F, McMaster CR (2005) Cytotoxicity of an anti-cancer lysophospholipid through selective modification of lipid raft composition. The Journal of biological chemistry 280: 38047–38058. [DOI] [PubMed] [Google Scholar]
- 20. Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, et al. (2005) A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proceedings of the National Academy of Sciences of the United States of America 102: 17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, et al. (2010) The genetic landscape of a cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, et al. (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463: 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han S, Lone MA, Schneiter R, Chang A (2010) Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proceedings of the National Academy of Sciences of the United States of America 107: 5851–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson JS, Klionsky DJ, Banta LM, Emr SD (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Molecular and cellular biology 8: 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]