Abstract
S. agalactiae (group B streptococci, GBS) is a major microbial pathogen in human neonates and causes invasive infections in pregnant women and immunocompromised individuals. The S. agalactiae β-hemolysin is regarded as an important virulence factor for the development of invasive disease. To examine the role of β-hemolysin in the interaction with professional phagocytes, the THP-1 monocytic cell line and human granulocytes were infected with a serotype Ia S. agalactiae wild type strain and its isogenic nonhemolytic mutant. We could show that the nonhemolytic mutants were able to survive in significantly higher numbers than the hemolytic wild type strain, in THP-1 macrophage-like cells and in assays with human granulocytes. Intracellular bacterial multiplication, however, could not be observed. The hemolytic wild type strain stimulated a significantly higher release of Tumor Necrosis Factor-α than the nonhemolytic mutant in THP-1 cells, while similar levels of the chemokine Interleukin-8 were induced. In order to investigate bacterial mediators of IL-8 release in this setting, purified cell wall preparations from both strains were tested and found to exert a potent proinflammatory stimulus on THP-1 cells. In conclusion, our results indicate that the β-hemolysin has a strong influence on the intracellular survival of S. agalactiae and that a tightly controlled regulation of β-hemolysin expression is required for the successful establishment of S. agalactiae in different host niches.
Introduction
The Gram positive pathogen Streptococcus agalactiae or group B streptococcus (GBS) is the leading microbial agent of neonatal pneumonia, sepsis and meningitis presenting as early or late-onset disease in human newborns [1] [2]. In most cases, vertical transmission of S. agalactiae to the neonate occurs during delivery from colonized mothers. But bacteria can also be acquired through horizontal transfer from external sources, for example, in the intensive care unit of the hospital [3]. A rising incidence of invasive infections in recent years has been described for nonpregnant adults as well as elderly and immunocompromised populations [4].
The S. agalactiae β-hemolysin is regarded as an important virulence factor for the development of invasive disease. Several studies have determined the role of the S. agalactiae surface-associated β-hemolysin as a membrane destabilizing toxin in lung epithelial cells [5], and brain endothelial cells [6] which contributes to disease pathogenesis. However, controversial reports exist regarding the role of β-hemolysin for the survival of S. agalactiae in phagocytes. Liu et al. found that a deletion in the cylE gene renders the pathogen sensitive to host phagocytic clearance mechanisms [7]. On the other hand, Sendi et al. [8] have shown the critical involvement of the S. agalactiae CovR/S (also called CsrR/S) [9] two-component global regulatory system in virulence. Mutations in the cov gene lead to an increased β-hemolytic activity with lower capsule expression. The resultant phenotype showed impaired intracellular survival in neutrophils in contrast to the LH/HC phenotype. Nevertheless, which role the β-hemolysin plays for the observed effect remains to be determined.
The cyl locus of Streptococcus agalactiae encodes β-hemolysin activity and consists of a cluster of genes. While a typical ABC transporter for the extrusion of the β-hemolysin is present in this gene cluster, the other genes appear to encode proteins with similarities to fatty acid synthesis enzymes [10] [11]. In this study, we have used a wild type strain and an isogenic mutant deficient in cylA that encodes the ATP- binding domain of the β-hemolysin transporter [11]. We investigated the role of the β-hemolysin for survival of S. agalactiae within THP-1 monocytic cells and primary human granulocytes as relevant host cells of the innate immune system.
Materials and Methods
Streptococcal Strains and Growth Conditions
The bacterial strains and plasmids used are listed in Table 1. BSU 6 a S. agalactiae serotype Ia strain served as the wild type. The isogenic β-hemolysin deletion mutant of this strain, designated as BSU 281, was generated by deleting the cylA gene that encodes the ATP-binding domain of the β-hemolysin transporter as described in an earlier publication [11]. For immunofluorescence microscopy, both streptococcal strains were transformed with the reporter plasmid pBSU101 as described previously [12]. The plasmid carries a copy of the enhanced green fluorescent gene egfp under the control of the S. agalactiae CAMP- factor gene (cfb) promoter. The bacterial strains carrying pBSU101 were designated as BSU 98 (parent strain: BSU 6) and BSU 453 (parent strain: BSU 281).
Table 1. Bacterial strains and plasmids.
| S. agalactiae strains | Description | Source or reference |
| BSU 6 serotype Ia strain | Clinical isolate; Hly+ | Ulm collection |
| BSU 281 | BSU 6 derivative; ΔcylA; Hly− | Gottschalk et al., 2006 [11] |
| BSU 98 | BSU 6 derivative carrying plasmid pBSU101; Hly+ | This study |
| BSU 453 | BSU 281 derivative carrying plasmid pBSU101; Hly− | This study |
| Plasmids | ||
| pAT28 | Specr; ori pUC; ori pAMβ1 | Trieu-Cuot et al., 1990 [39] |
| pBSU101 | pAT28 derivative carrying egfp under the control of the cfb promoter | Aymanns et al., 2011 [12] |
Hly+ refers to hemolytic isolates.
Hly−refers to nonhemolytic isolates.
The bacteria were grown at 37°C in Todd-Hewitt broth (THB; Oxoid, Wesel, Germany) supplemented with 0.5% yeast extract (Difco) containing 120 mg/l Spectinomycin. For experiments, bacteria were adjusted to 107 colony forming units (CFU)/ml, washed in phosphate-buffered saline (PBS, pH 7.0) and resuspended in RPMI-1640 cell culture medium (Sigma-Aldrich, Deisenhofen, Germany).
THP-1 Cells as a Model for Human Macrophages
THP-1 (ATCC, East Greenwich, RI, USA) is a human acute monocytic leukemia cell line. Morphologically they appear as large, round single, non adherent cells. They were grown at a density of 2×105 cells/ml in RPMI1640 medium supplemented with 10% γ-irradiated FBS, 50 µM β-mercaptoethanol, 2 mM L-Glutamine, 100 U/100 µg/ml Penicillin/Streptomycin, 2 mM HEPES (all from Biochrom, Berlin, Germany). Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C.
Cells were passaged when density reached 8×105 cells/ml and media were changed every three days. For experiments, 106 cells were seeded into each well of a 6-well tissue culture plates (Becton Dickinson) and kept in the presence of 10 ng/ml Phorbol 12-myristate 13-acetate (PMA, Sigma, Deisenhofen, Germany) overnight at 37°C and 5% CO2 [13]. On the next day, THP-1 monocytes were completely differentiated into adherent macrophages; a phenomenon controlled via light microscopy. THP-1 macrophages were washed three times with RPMI 1640 medium without antibiotics followed by adding the same medium for the rest of the infection assays.
Isolation of Human Peripheral Blood Granulocytes
Peripheral blood was collected by venipuncture from healthy adult volunteers who gave informed written consent to donate blood specifically for the purpose of the study. The ethics committee at the University of Ulm approved this procedure. Granulocytes were isolated as described elsewhere [14]. Briefly, a two-layer density gradient consisting of a bottom layer of Histopaque 1119 (Sigma-Aldrich, Deisenhofen, Germany) and an upper layer of lymphocyte separation medium 1077 (PAA, Pasching, Austria) was prepared. Blood was layered carefully on top and centrifuged for 5 min at 1000 rpm, followed by 25 min at 2200 rpm at room temperature. The pink layer at the interface between the two gradients, which is formed mainly by granulocytes, was collected and washed with 1×PBS (PAA, Austria) and resuspended in RPMI 1640 medium. The cells were further fractioned on a discontinuous Percoll (Amersham Biosciences, Uppsala, Sweden) gradient consisting of layers with densities of 1.105 g/ml (85%), 1.100 g/ml (80%), 1.087 g/ml (70%), and 1.081 g/ml (65%) at 2200 rpm for 25 min. Purified granulocytes forming approximately 2–3 interfaces between 70% to 80% of Percoll layers were collected. The cells were washed with PBS and resuspended in RPMI 1640 medium supplemented with 10% γ-irradiated FBS. The cell preparations contained mostly granulocytes as determined by morphological examination of the cells after Giemsa-staining. Trypan blue exclusion confirmed >99% viability of cells by this procedure.
Intracellular S. agalactiae Survival Assay in THP-1 Macrophages
In order to quantify the intracellular bacteria, 106 THP-1 macrophages per well were infected with the hemolytic (BSU 98) and nonhemolytic (BSU 453) strain at a multiplicity of infection (MOI) of 1∶1 for 0.75 and 1.5 h. Extracellular bacteria were killed using 1 µg/ml Penicillin G and 100 µg/ml Gentamicin (both from Sigma-Aldrich) for 1 h. Subsequently, the medium was removed from each well and cold distilled water was added to lyse the cells with repeated pipetting. The lysates were plated, in various dilutions, on THY agar plates (containing 120 mg/l Spectinomycin) and incubated overnight at 37°C with 5% CO2. Colony counts were performed to determine the number of intracellular bacteria.
To assess the effect of inhibitors of the eukaryotic cytoskeleton, assays were performed in the presence of Cytochalasin D (Sigma) at final concentrations of 0.5, 1, 2.5 and 5 µg/ml. Cytochalasin D was added to each well 30 min before infection and was present during the entire experiment.
S. agalactiae Survival Assay in Human Granulocytes
Infection of 105 freshly isolated granulocytes with hemolytic and non hemolytic S. agalactiae strains was carried out at a MOI of 1∶1 and incubated at 37°C with 5% CO2 for 2 h. In order to determine the total number of viable bacteria, eukaryotic cells were collected by centrifugation at 4000 rpm for 10 min. The pellet was resuspended in 5 ml of ice cold distilled water to lyse granulocytes. The lysate was plated, in various dilutions, on THY agar plates (containing 120 mg/l Spectinomycin) and incubated overnight at 37°C with 5% CO2. Colony counts were performed to determine the total number of viable bacteria.
Lactate Dehydrogenase (LDH) Cytotoxicity Assay
Bacterial cell-mediated cytotoxicity to THP-1 macrophages and granulocytes was detected by measuring LDH activity present in the culture supernatant. For LDH measurements, the Cytotoxicity detection kit (Clontech, USA) was used according to the manufacturer’s instructions. The amount of enzyme was determined in a microplate reader (absorbance at 492 nm, reference wavelength at 620 nm).
Visualizing Intracellular S. agalactiae by Fluorescence Microscopy
0.5×106 THP-1 cells were seeded in each well of a 12-well tissue culture plate. In the presence of 10 ng/ml PMA, overnight cultures of monocytic cells differentiate into macrophages and become adherent on coverslips present at the bottom of the well. On the next day, the cells were washed three times with RPMI 1640 medium without antibiotics followed by adding the same medium for the rest of the assay. The macrophages were infected with the hemolytic (BSU 98) and nonhemolytic (BSU 453) strain at a MOI 1∶1 and incubated at 37°C with 5% CO2 for 1.5 h. The cells were washed three times with 1×PBS followed by fixation with 4% formaldehyde for 20 min. After washing with PBS, the coverslips were air-dried. The cells were stained with Evans Blue (bioMérieux, France) for 30 min in the dark. After washing with PBS, the coverslips were air-dried again in the dark. To mount the cells on a microscopic glass slide, VECTASHIELD mounting medium with DAPI (Vector Laboratories, CA) was used. In this way, the slides can be stored at 4°C in the dark for weeks. Intracellular bacteria within THP-1 macrophages were analyzed by fluorescence microscopy with a Zeiss Axioskop-2® fluorescence microscope fitted with a Axiocam HR camera and Axiovison software version 4.8.
Intracellular S. agalactiae Multiplication Assay in THP-1 Macrophages
106 THP-1 macrophages per well were infected with the hemolytic (BSU 98) and non-hemolytic (BSU 453) strain at a MOI of 1∶1 for 1.5 h. Both Penicillin G (1 µg/ml) and Gentamicin (100 µg/ml) were added to each well and incubated for 1, 2, 3, 4, 5, and 24 h at 37°C with 5% CO2 to allow S. agalactiae multiplication. The medium was removed from each well and cold distilled water was added to lyse the cells with repeated pipetting. The lysates were plated on THY Agar plates (containing 120 mg/l Spectinomycin) to determine the number of intracellular bacteria.
Cell Wall Preparations
Purified cell walls were prepared as described elsewhere [15]. Briefly, S. agalactiae strains BSU 6 and BSU 281 were grown in THY broth (Todd-Hewitt broth supplemented with 0.5% yeast extract) until mid-logarithmic phase. Bacteria were harvested by centrifugation at 10000 rpm for 10 min at 4°C. At this stage, the pellet can be stored at −20°C. The bacterial pellets were resuspended in cold 50 mM tris-HCl (pH = 7.0) and boiled with sodium dodecyl sulfate (SDS) for 15 min. The resulting denatured bacterial suspensions were then mixed with acid-washed glass beads (150–212 µm, Sigma-Aldrich) and subjected to mechanical breaking (8–10 times in a ribolyser at 14000 rpm for 10 min). The supernatants from all the ribolyser tubes were pooled together and the crude cell wall fragments were obtained by centrifugation at 15000 rpm for 15 min at 4°C. Further purification was achieved by treating the cell wall pellet with DNase (10 mg/ml, Sigma-Aldrich), RNase (50 mg/ml, Sigma-Aldrich) and 20 mM MgSO4 for 2 h at 37°C followed by adding 10 mM CaCl2 and trypsin (100 µg/ml) and kept for overnight shaking at 37°C. After centrifugation, the cell wall pellet was incubated first with 8 M LiCl followed by 100 mM EDTA for 15 min each at 37°C and then washed at each step with sterile distilled water. A last short centrifugation step in the presence of acetone was performed and the pellet was washed with sterile distilled water. Finally, the suspension containing the cell wall was lyophilized and stored at −20°C for future use. Lyophilized cell wall appears as a white cotton like substance.
For stimulation experiments, lyophilized cell wall was weighed and resuspended to 1 mg/ml in RPMI 1640 medium.
TNF-α and IL-8 Assays
For cytokine induction assays, 106 THP-1 macrophages per well were infected with the hemolytic (BSU 98) and nonhemolytic strain (BSU 453) at a MOI of 1∶1 for 0, 0.75, 1.5, and 3 h. Following the indicated incubation times, extracellular bacteria were killed using Penicillin G (1 µg/ml) and Gentamicin (100 µg/ml) for 1 h. The cell culture supernatants were collected; cells were removed by centrifugation and stored at −20°C until measurement. Uninfected THP-1 macrophages in medium, bacterial cells in medium without macrophages and medium alone served as controls. Release of Tumor Necrosis Factor-α (TNF-α) and Interleukin-8 (IL-8) was measured by a sandwich-ELISA method using Human IL-8 Cytoset™ (Invitrogen) and Human TNF-α Cytoset™ (Invitrogen).
Additionally, 106 THP-1 macrophages were challenged with various concentrations of S. agalactiae cell wall preparations and incubated for 2.45, 6, and 18 h for assays of both cytokines at 37°C with 5% CO2. Cell free supernatants were stored at −20°C until cytokine assays were performed.
Statistical Analysis
The data were analyzed using SPSS18.0 software. The Mann-Whitney U test was performed for at least three independent samples for statistical analysis. Data are expressed as average ± SD. Data were considered significant for p-values <0.05.
Results
Effect of β-hemolysin on Survival of S. agalactiae in Professional Phagocytes
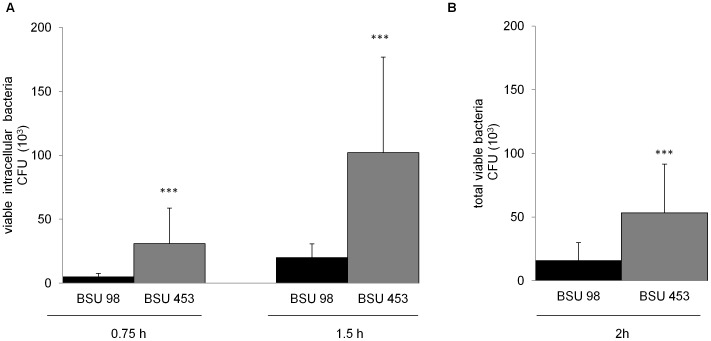
A previous study from Sendi et al. showed that the hyperhemolytic S. agalactiae phenotype (with low capsule expression) displayed impaired survival within human neutrophils as compared to the low hemolytic S. agalactiae phenotype (with high capsule expression) [8]. To elucidate the role of the β-hemolysin in this context, we investigated the survival of a hemolytic wild type strain and an isogenic nonhemolytic S. agalactiae mutant in phagocytic cells. Intracellular bacterial counts and total survival of the S. agalactiae hemolytic wild type (BSU 98) and the nonhemolytic (BSU 453) mutant strain within THP-1 macrophages and human granulocytes were quantified by CFU determination respectively. After infection with the hemolytic wild type strain,the number of intracellular bacteria recovered from THP-1 macrophages was significantly lower in comparison to the nonhemolytic mutant (for 0.75 h and 1.5 h of infection) (Fig. 1A). An MOI of 1 was chosen to ensure sub-cytolytic conditions for the hemolytic as well as the nonhemolytic strain. Similar results were obtained for higher MOI (5 and 10) but under these conditions, increased lysis was observed in THP-1 cells following the incubation with the hemolytic strain BSU 98 (data not shown). A significant difference in survival of the two S. agalactiae strains was also observed when incubated with human granulocytes for 2 h without extracellular bacterial killing by antibiotics (Fig. 1B). The hemolytic wild type bacteria display impaired survival in the presence of professional phagocytes as compared to a nonhemolytic S. agalactiae mutant strain.
Figure 1. Survival of S. agalactiae and β-hemolysin expression in professional phagocytes.
The monocyte-derived macrophage cell line THP-1 or freshly isolated granulocytes were infected with hemolytic (BSU 98) and nonhemolytic (BSU 453) bacteria at a MOI of 1∶1 for indicated time points. A) Intracellular bacteria were quantified after killing the extracellular bacteria using Penicillin (1 µg/ml) and Gentamicin (100 µg/ml) for additional 1 h. B) Total viable bacteria after incubation with granulocytes without killing of extracellular bacteria. Data shown are the mean ± SD of six independent experiments. Data is considered extremely significant for p values <0.001 (***).
Cytotoxicity of β-hemolysin on Eukaryotic Host Cells
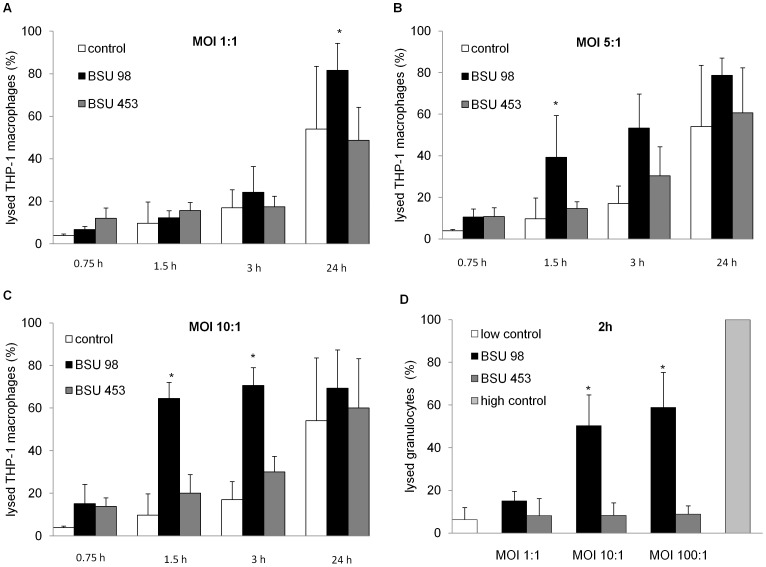
Since the group B streptococcal β-hemolysin is associated with injury of various eukaryotic cell types including macrophages [5] [16] we quantified the cytotoxic effect of the β-hemolysin on macrophages and granulocytes. We hypothesized that the enhanced lysis of eukaryotic cells infected with the hemolytic strain (BSU 98) could decrease the number of recovered bacteria in comparison to eukaryotic cells infected with the nonhemolytic strain (BSU 453). Lactate Dehydrogenase (LDH) assays were carried out with THP-1 macrophages at MOI of 1, 5 and 10 for 0.75, 1.5, 3 and 24 h. MOI 1, 10 and 100 were tested using human granulocytes for 2 h. The bacterial cell-mediated cytotoxicity can be measured as the amount of the intracellular enzyme LDH released into the culture supernatant by the damaged cells. The LDH release can be directly correlated with the percentage of lysed eukaryotic cells. The results show that at an MOI of 1, strain BSU 98 produced no significant injury to macrophages till 1.5 h, similar to BSU 453 (Fig. 2A). At higher MOIs (5 and 10) the hemolytic strain BSU 98 induced a significant lysis of macrophages compared to BSU 453 (Fig. 2B and 2C). Coincubation of granulocytes with strains BSU 98 and BSU 453 for 2 hours induces basal level of cytotoxicity at an MOI of 1. Higher MOIs (10 and 100) resulted in a more than five-fold increase in the percentage of granulocytes lysed by BSU 98 as compared to the nonhemolytic strain BSU 453 (Fig. 2D).
Figure 2. Effect of bacterial cell mediated cytotoxicity as measured by LDH Cytotoxicity Assay.
A–C) THP-1 macrophages were infected at indicated multiplicity of infections and time points to measure the LDH release into the supernatant. The amount of LDH released is proportional to the percentage of lysed eukaryotic cells. D) Human granulocytes were infected at indicated multiplicity of infections for 2 h to measure the LDH release into the supernatant. High control corresponds to maximum lysis achieved using 2% of Triton X-100. Uninfected cells served as control. Data shown are the mean ± SD of three independent experiments. Data is considered significant for p values <0.05 (*).
Effect of Cytochalasin D on Bacterial Uptake by Macrophages
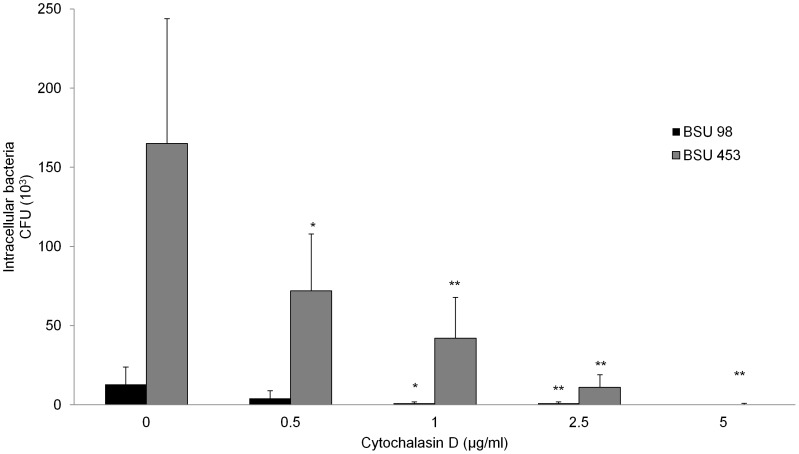
Being an actin depolymerizing agent, Cytochalasin D can inhibit actin dependent uptake of S. agalactiae by macrophages. To investigate if Cytochalasin D reduces the internalization of the nonhemolytic strain BSU 453 by THP-1 cells to the levels observed for the wild type strain BSU 98, Cytochalasin D was used in a range from 0.5–5 µg/ml. In Fig. 3, Cytochalasin D inhibits the uptake of bacteria and therefore CFU of both strains decreased in a dose-dependent manner. As observed in the previous assays, a significant difference in intracellular colony counts of BSU 98 and BSU 453 is still visible at 0.5 and 1 µg/ml of Cytochalasin D. However, at 5 µg/ml, Cytochalasin D completely inhibited the uptake of both S. agalactiae strains, confirming that the number of internalized bacteria in this assay is dependent on the uptake of bacteria into the intracellular compartment.
Figure 3. Effect of Cytochalasin D on invasion capacity of S. agalactiae in macrophages.
Cytochalasin D treated THP-1 macrophages were infected with hemolytic (BSU 98) and nonhemolytic (BSU 453) bacteria for 90 min followed by adding antibiotics for 1 h to kill extracellular bacteria. Infected THP-1 macrophages without Cytochalasin D treatment served as positive control. Data shown are the mean ± SD of six independent experiments. Data is considered significant for p values <0.05 (*) and highly significant for p values <0.01 (**).
Microscopic Evaluation of Intracellular S. agalactiae Localization
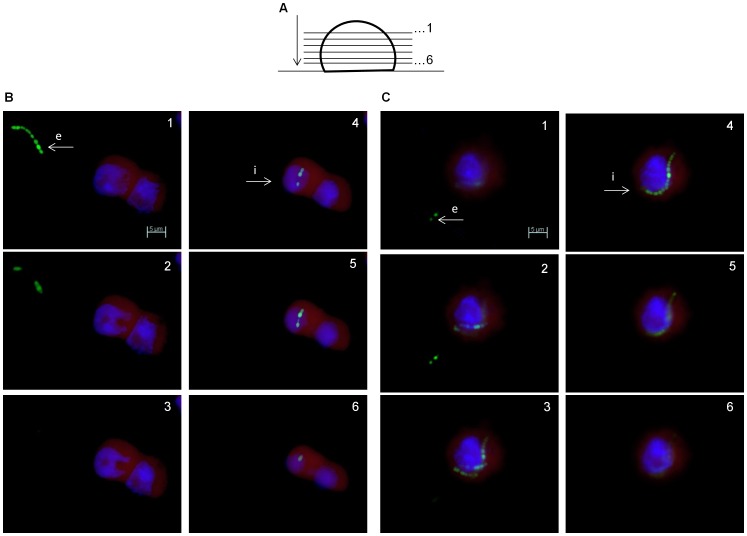
Using a Zeiss Axioskop-2® fluorescence microscope we visualized the intracellular presence of S. agalactiae in eukaryotic cells. To document the subcellular localization of the bacteria within THP-1 macrophages, series of images were acquired from a specimen at equally spaced focus points by moving it along the Z-axis of the microscope. This was combined with three channel fluorescence (blue, green and red) to obtain the resultant Z-stack multichannel images. Distance between the z-planes was set to 1 µm.
As depicted in Fig. 4B and 4C, image series of an infected THP-1 macrophage clearly demonstrate that some bacteria (marked i) are intracellular, whereas others (marked e) are extracellular. On the basis of qualitative comparison, analysis of the infected cells illustrate that within THP-1 macrophages multiple chains of the nonhemolytic bacteria were found more often than in macrophages infected with the hemolytic strain.
Figure 4. Microscopic evaluation of intracellular S. agalactiae localization.
A) A schematic representation of Z-stacking in Zeiss Axioskop-2® fluorescence microscope. As depicted, images are acquired for six z-stacks through macrophages on a microscopic glass slide with a 63× objective. THP-1 macrophages are infected with BSU 98 (B) and BSU 453 (C) at a MOI of 1∶1 for 1.5 h. “e” and “i” refer to extracellular and intracellular bacteria respectively. Nuclear staining with DAPI (blue), both S. agalactiae strains are EGFP labeled (green) and cytoplasm staining of macrophages with Evans blue (red). Scale Bar: 5 µm (for all images).
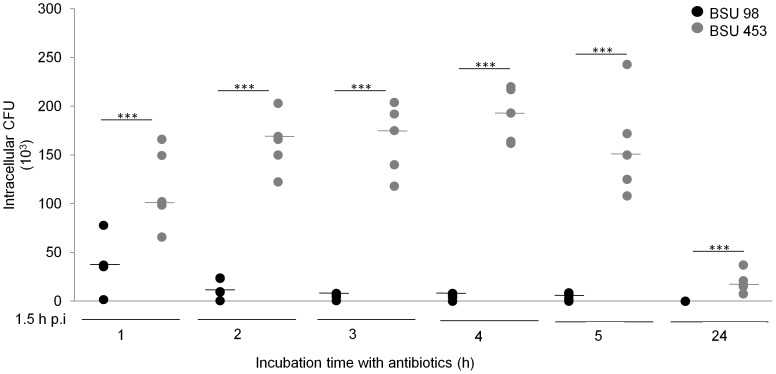
Intracellular S. agalactiae Multiplication
Previous literature showed that hemolytic S. agalactiae strains do not multiply within the eukaryotic host cell [17]. To analyze if the higher colony counts of S. agalactiae strain BSU 453 in our assays were caused by intracellular multiplication of the nonhemolytic mutant, we tested the ability of both strains to multiply within the THP-1 macrophages. As shown in Fig. 5, no significant increase in intracellular CFU was observed between 1 and 5 h of infection. At 24 h, no viable bacteria were recovered, indicating that both S. agalactiae strains did not multiply and are eventually killed by the macrophages. These data confirm the enhanced intracellular bacterial counts of BSU 453 in human macrophages, without evidence of a significant intracellular multiplication within phagocytes.
Figure 5. S. agalactiae does not multiply within macrophages.
THP-1 macrophages were infected with hemolytic (BSU 98) and nonhemolytic (BSU 453) bacteria for 90 min. After this, antibiotics were added into the medium for the rest of the assay. Macrophages were lysed at indicated time points and lysate were plated. Data shown are the values from five independent experiments with median. Data is considered extremely significant for p values <0.001 (***).
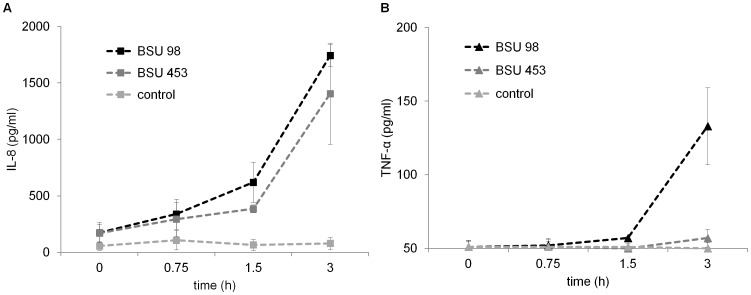
Cytokine Induction by Type Ia Group B Streptococci
The strength and efficiency of the immune response of the host is dependent on the release of proinflammatory cytokines. We investigated the induction of TNF-α and IL-8 from S. agalactiae infected THP-1 macrophages. Both BSU 98 and BSU 453 induce marked production of IL-8; however there was no overall difference in the release by macrophages (Fig. 6A). Nevertheless, the production of TNF-α from the infected macrophages in response to S. agalactiae is delayed. However a significant difference in the ability of the two S. agalactiae strains to produce TNF-α was observed after 3 hours of incubation, suggesting a functional role of TNF-α in S. agalactiae pathogenesis (Fig. 6B).
Figure 6. Intracellular S. agalactiae induced cytokine expression in THP-1 macrophages.
THP-1 macrophages were infected with hemolytic (BSU 98) and nonhemolytic (BSU 453) GBS at a MOI of 1∶1 at indicated time points. Extracellular bacteria were killed by antibiotics for an additional 1 h. A) IL-8 and B) TNF-α levels were measured in the supernatant by ELISA. Uninfected cells served as control. Data shown are the mean ± SD of three independent experiments.
Induction of Proinflammatory Cytokines by S. agalactiae Cell Wall Preparations
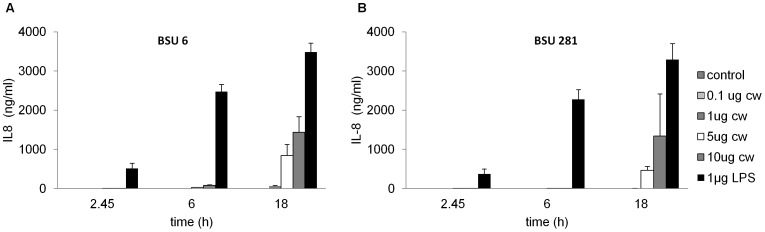
S. agalactiae molecules located on the cell surface or secreted into the supernatant play an important role for the pathogenicity of the bacteria. Previous literature showed that the β-hemolysin contributes to the stimulation of IL-8 release [8] [16]. Because a high IL-8 release from THP-1 macrophages was also observed following stimulation with strain BSU 98 as well as the nonhemolytic strain BSU 453 (Fig. 6A), we hypothesize that the presence of β-hemolysin in BSU 98 may not be the sole mediator for IL-8 release, suggesting the involvement of other bacterial factors that could trigger the release of proinflammatory cytokines. The bacterial cell wall components of gram positive bacteria are a major proinflammatory stimulus and trigger the innate immune system similarly to lipopolysaccharide (LPS) of gram negative bacteria [15] [18]. To investigate the role of cell wall, THP-1 macrophages were stimulated with S. agalactiae cell wall preparations from hemolytic and nonhemolytic strains.
A dose and time dependency was observed in the production of IL-8 following stimulation with 0.1, 1, 5 and 10 µg/ml of cell wall preparations. Maximum stimulation was observed for 1 µg of LPS that served as a positive control in the assay. As β-hemolysin activity is lost during the cell wall isolation method, the resultant cell wall fragments from BSU 6 and BSU 281 show a similar response towards the production of IL-8 as shown in Fig. 7A and 7B.
Figure 7. Similar IL-8 expression pattern in response to purified cell wall preparations from hemolytic and nonhemolytic S. agalactiae strains.
106 THP-1 macrophages/well stimulated with 0.1, 1, 5 and 10 µg/ml of cell wall preparations from (A) BSU 6 (hemolytic) and (B) BSU 281 (nonhemolytic) for indicated time points. Treatment of cells with 1 µg/ml of lipopolysaccharide served as positive control, unstimulated cells in medium as negative control. Data shown are the mean ± SD of three independent experiments.
Discussion
Severe invasive S. agalactiae infections are not only a major cause of infections in newborns but also in nonpregnant adults. Bacterial invasion and disease pathogenesis is a complex process that is achieved through numerous virulence factors. The S. agalactiae β-hemolysin is considered as one of the most important virulence factors in this context. Invasive S. agalactiae infections are almost exclusively caused by β-hemolytic strains. However, the role of the S. agalactiae β-hemolysin in the molecular interaction with host cells is not completely understood.
Interestingly, the cov (or csr) system as a major regulator of S. agalactiae virulence genes suppresses β-hemolysin expression [19] [9]. A recent study by Sendi et al. found that a strain carrying a cov mutation resulting in a high hemolytic S. agalactiae variant with low capsule expression showed low intracellular survival in human neutrophils in contrast to the low hemolytic variant with high capsule expression [8]. To elucidate whether the observed difference is due to β-hemolysin or capsule expression, we used a serotype Ia strain and its isogenic nonhemolytic mutant to investigate the potential factors responsible for the different survival in eukaryotic host cells. The β-hemolytic S. agalactiae wild type strain was found in lower numbers in the intracellular compartment of THP-1 macrophages in comparison to the nonhemolytic mutant strain. With increasing incubation time (from 0.75 to 1.5 h), the number of recovered intracellular bacteria increases most probably due to additional uptake. Higher MOIs (5 and 10) and higher incubation times (>2 h) lead to enhanced lysis of the eukaryotic cells attributed to the cytolytic activity of β-hemolysin in the wild type strain and were therefore not included in the analysis. In contrast to this, the nonhemolytic strain caused damage of eukaryotic cells only at long-term incubation (24 h) which may be caused by the induction of apoptosis [20] [21]. To investigate if an improved survival of the nonhemolytic strain could also be observed in the interaction with granulocytes as described by Sendi et al., the hemolytic strain and its nonhemolytic isogenic mutant were tested for survival (Fig. 1) following 2 h incubation with primary human granulocytes under sub-cytolytic conditions. In these settings, significantly higher numbers of the nonhemolytic strains were recovered which is compatible with the results of Sendi et al. obtained for S. agalactiae CovR/S mutants. While the major phenotypic difference between the two strains we tested is the loss of hemolysis in the mutant strain, we can currently not exclude the possibility that the mutation of the hemolysin transporter causes an altered expression of other virulence determinants, which may contribute to the increased intracellular persistence, we observed.
It is intriguing to see that an important virulence regulator of S. agalactiae suppresses the β-hemolysin expression. The clinical observation that cov mutation and resulting hyperhemolysis are associated with devastating fulminant invasive disease offers a possible explanation for this phenomenon [8]. S. agalactiae is most often a colonizing bacterial pathogen causing invasive disease mainly in neonates and immunocompromised patients. Maximal expression of virulence factors does not appear to be beneficial in all stages of the course of an infection. The improved survival within professional macrophages may provide advantages like the escape from antibody attacks or the use of these host cells in the sense of a Trojan horse, as it has been observed in other pathogens [22] [23]. A recent publication describing the increased expression of the cov regulator in S. pyogenes recovered from the intracellular environment of macrophages supports this line of argument [24]. While investigating the survival of S. aureus agr mutants in murine models of infection, Schwan et al. also suggested a possible role of hemolysin expression in providing a growth advantage in S. aureus mixed cultures within abscesses and wounds [25]. Littmann et al. investigated the role of bacteria-bound pneumolysin in the survival of S. pneumoniae in human dendritic cells in vitro [26]. Recovery of higher numbers of the pneumolysin-deficient strain from human dendritic cells as compared to the wild type strain seems to be advantageous because the hemolytic activity of pneumolysin was not found to be essential for invasive disease in their experimental setup. Using a murine model of pneumococcal bacteremia, Harvey et al. found an enhanced in vivo survival of the pneumolysin deficient strain [27]. Independent observations from both groups about the pneumolysin expression dependent survival of S. pneumoniae support our findings suggesting that a low or absent hemolysin activity may, for some streptococcal strains, be critical for its survival within professional phagocytes.
In our experiments, we could demonstrate that the survival of S. agalactiae is dependent on the uptake into THP-1 macrophages. We were able to show that entry of S. agalactiae into THP-1 macrophages is inhibited by Cytochalasin D in a dose dependent manner, indicating that the uptake of S. agalactiae occurs through phagocytosis, a mechanism that actively involves actin polymerization (Fig. 3). A similar finding was made by Valentin-Weigand et al. for serotype III Group B streptococci using the murine macrophage-like cell line J774 [28]. Our results also support the findings of Fettuciari et al. who showed in a recent publication that the S. agalactiae β-hemolysin activates calpain leading to a severe disturbance of the cytoskeleton and may thus prevent the uptake of S. agalactiae into eukaryotic cells [29].
Our fluorescence microscopy data clearly distinguishes between intracellular and extracellular bacteria. However, the current study does not address the location of bacteria within the macrophages. Previous electron micrograph and fluorescence microscopic studies on various GBS serotypes have reported the location of GBS within the membrane-bound vacuole in a variety of host-cell types, including epithelial cells [17], endothelial cells [30] [31], dendritic cells [32] and macrophages [33] [29] [34]. In Listeria monocytogenes, the hemolysin is important in phagosomal escape and hemolysin negative mutants are thus impaired in intracellular survival [35]. However, in a recent investigation of the subcellular localization of S. agalactiae within J774 macrophages, no indication for the escape of S. agalactiae from the phagosome was observed [33]. The vast majority of S. agalactiae could be observed within phagosomes and survival of S. agalactiae under these conditions was even found to be dependent on proper acidification of the vacuoles. It is therefore not surprising to see that in contrast to findings in Listeria the ß-hemolysin does not appear to be crucial for intracellular survival of S. agalactiae.
The strength and efficiency of the immune response of the host is dependent on the release of cytokines. Previous literature has shown that group B streptococci stimulate the release of various cytokines and chemokines from human mononuclear cells and murine macrophages [36] [37]. Antibodies against TNF-α in an experimental neonatal rat model of GBS sepsis significantly increased their survival, suggesting a contribution of TNF-α in GBS pathogenesis [38]. In line with this, our results showed the release of TNF-α by THP-1 macrophages when stimulated with S. agalactiae serotype Ia strains. Higher TNF-α release was observed in macrophages exposed to the hemolytic strain (BSU 98) suggesting a possible role of β-hemolysin expression in the induction of TNF-α at 3 h. The chemokine IL-8 is an important marker of bacterial infections. Previous reports described β-hemolysin as a major inducer of IL-8 [16]. However, we could not find a significant difference in the release of IL-8 by the hemolytic and nonhemolytic bacteria. Use of different S. agalactiae strains and cell lines could account for this difference and hence the results are only partially comparable. The wild type strain we used displays only moderate β-hemolytic activity. In addition, the higher number of nonhemolytic bacteria may have compensated for the IL-8 production and therefore a comparable release of IL-8 is observed. Since a major effect of the β-hemolysin on IL-8 release could not be observed, we investigated other proinflammatory stimuli that are present in both hemolytic and nonhemolytic strains of S. agalactiae and may account for the observed release of IL-8. In this regard the gram positive cell wall is a likely proinflammatory candidate.
The bacterial cell wall isolation method inactivates and removes β-hemolysin resulting in a cell wall preparation consisting of peptidoglycan and lipoteichoic acids. Previous studies have shown the effect of individual S. agalactiae cell wall components on the release of host-derived proinflammatory cytokines, particularly TNF-α on cord blood monocytes [18]. Compatible with these results, we found that the presence of cell wall components from both hemolytic and nonhemolytic bacteria induces similar levels of IL-8 in a concentration dependent manner.
In conclusion, we were able to show that the absence of S. agalactiae β-hemolysin may enable bacteria to survive in higher numbers inside professional phagocytes, an ability which may be beneficial at certain stages of S. agalactiae infections. This explanation is partly supported by the previous report indicating that the S. pyogenes gene expression profile does not remain the same at all infection stages. Two-component regulatory systems allow bacteria to adapt to changing environmental conditions. In particular, later phases of infection are potentially influenced by the CovR/S two component system in S. pyogenes [24]. In line with this, the S. agalactiae CovR/S two component system could up or down regulate the expression of its target genes in a way that proves favorable for bacterial growth inside the host [9]. While our investigation does not target the CovR/S system, it is well known that this regulator suppresses β-hemolysin expression [9]. In addition, a recent publication shows the importance of the CovR/S regulator for intracellular survival of S. agalactiae [33]. Our data support the hypothesis that invasive S. agalactiae infections represent a multifunctional process that is achieved by an intricate control and regulation of specific virulence factors.
Funding Statement
The work of AS and BS was supproted by Deutsche Forschungsgemeinschaft (DFG) grant GSC270. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Luck S, Torny M, d'Agapeyeff K, Pitt A, Heath P, et al. (2003) Estimated early-onset group B streptococcal neonatal disease. Lancet 361: 1953–1954. [DOI] [PubMed] [Google Scholar]
- 2. Zangwill K, Schuchat A, Wenger JD (1992) Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. MMWR 41(No (SS-6) 25–32. [PubMed] [Google Scholar]
- 3. Morinis J, Shah J, Murthy P, Fulford M (2011) Horizontal transmission of group B streptococcus in a neonatal intensive care unit. Paediatr Child Health 16: e48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farley MM (1995) Group B streptococcal infection in older patients. Spectrum of disease and management strategies. Drugs Aging 6: 293–300. [DOI] [PubMed] [Google Scholar]
- 5. Nizet V, Gibson R, Chi E, Framson P, Hulse M, et al. (1996) Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun 64: 3818–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doran KS, Liu GY, Nizet V (2003) Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 112: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, et al. (2004) Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A 101: 14491–14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sendi P, Johansson L, Dahesh S, Van-Sorge NM, Darenberg J, et al. (2009) Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis 15: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR (2005) Regulation of virulence by a two-component system in group B streptococcus. J Bacteriol 187: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spellerberg B, Pohl B, Haase G, Martin S, Weber-Heynemann J, et al. (1999) Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J Bacteriol 181: 3212–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottschalk B, Broker G, Kuhn M, Aymanns S, Gleich-Theurer U, et al. (2006) Transport of multidrug resistance substrates by the Streptococcus agalactiae hemolysin transporter. J Bacteriol 188: 5984–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aymanns S, Mauerer S, van Zandbergen G, Wolz C, Spellerberg B (2011) High-level fluorescence labeling of gram-positive pathogens. PLoS One 6: e19822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwende H, Fitzke E, Ambs P, Dieter P (1996) Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol 59: 555–561. [PubMed] [Google Scholar]
- 14. Esmann L, Idel C, Sarkar A, Hellberg L, Behnen M, et al. (2010) Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol 184: 391–400. [DOI] [PubMed] [Google Scholar]
- 15. Heumann D, Barras C, Severin A, Glauser MP, Tomasz A (1994) Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun 62: 2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V (2002) Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis 185: 196–203. [DOI] [PubMed] [Google Scholar]
- 17. Rubens CE, Smith S, Hulse M, Chi EY, van Belle G (1992) Respiratory cell invasion by group B streptococci. Infect Immun 60: 5157–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vallejo JG, Bake rCJ, Edwards MS (1996) Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect Immun 64: 5042–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, et al. (2004) CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol 54: 1250–1268. [DOI] [PubMed] [Google Scholar]
- 20. Fettucciari K, Rosati E, Scaringi L, Cornacchione P, Migliorati G, et al. (2000) Group B Streptococcus induces apoptosis in macrophages. J Immunol 165: 3923–3933. [DOI] [PubMed] [Google Scholar]
- 21. Ulett GC, Maclean KH, Nekkalapu S, Cleveland JL, Adderson EE (2005) Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J Immunol 175: 2555–2562. [DOI] [PubMed] [Google Scholar]
- 22. Laskay T, van Zandbergen G, Solbach W (2003) Neutrophil granulocytes–Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol 11: 210–214. [DOI] [PubMed] [Google Scholar]
- 23. Thwaites GE, Gant V (2011) Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol 9: 215–222. [DOI] [PubMed] [Google Scholar]
- 24. Hertzen E, Johansson L, Kansal R, Hecht A, Dahesh S, et al. (2012) Intracellular Streptococcus pyogenes in human macrophages display an altered gene expression profile. PLoS One 7: e35218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwan WR, Langhorne MH, Ritchie HD, Stover CK (2003) Loss of hemolysin expression in Staphylococcus aureus agr mutants correlates with selective survival during mixed infections in murine abscesses and wounds. FEMS Immunol Med Microbiol 38: 23–28. [DOI] [PubMed] [Google Scholar]
- 26. Littmann M, Albiger B, Frentzen A, Normark S, Henriques-Normark B, et al. (2009) Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression. EMBO Mol Med 1: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harvey RM, Ogunniyi AD, Chen AY, Paton JC (2011) Pneumolysin with low hemolytic activity confers an early growth advantage to Streptococcus pneumoniae in the blood. Infect Immun 79: 4122–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valenti-Weigand P, Benkel P, Rohde M, Chhatwal GS (1996) Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun 64: 2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fettucciari K, Quotadamo F, Noce R, Palumbo C, Modesti A, et al. (2011) Group B Streptococcus (GBS) disrupts by calpain activation the actin and microtubule cytoskeleton of macrophages. Cell Microbiol 13: 859–884. [DOI] [PubMed] [Google Scholar]
- 30. Gibson RL, Lee MK, Soderland C, Chi EY, Rubens CE (1993) Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun 61: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greco R, DeMartino L, Donnarumma G, Conte M, Seganti L, et al. (1995) Invasion of cultured human cells by Streptococcus pyogenes. Res Microbiol 146: 551–560. [DOI] [PubMed] [Google Scholar]
- 32. Lemire P, Houde M, Lecours MP, Fittipaldi N, Segura M (2012) Role of capsular polysaccharide in Group B Streptococccus interactions with dendritic cells. Microbes Infect 14: 1064–1076. [DOI] [PubMed] [Google Scholar]
- 33. Cumley NJ, Smith LM, Anthony M, May RC (2012) The CovS/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infect Immun 80: 1650–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teixeira CF, Azevedo NL, Carvalho TM, Fuentes J, Nagao PE (2001) Cytochemical study of Streptococcus agalactiae and macrophage interaction. Microsc Res Tech 54: 254–259. [DOI] [PubMed] [Google Scholar]
- 35. Portnoy DA, Chakraborty T, Goebel W, Cossart P (1992) Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun 60: 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan H, Williams DL, Zingarelli B, Breuel KF, Teti G, et al. (2007) Differential regulation of lipopolysaccharide and Gram-positive bacteria induced cytokine and chemokine production in macrophages by Galpha(i) proteins. Immunology 122: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwak DJ, Augustine NH, Borges WG, Joyner JL, Green WF, et al. (2000) Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun 68: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Givner LB, Gray L, O'Shea TM (1995) Antibodies to tumor necrosis factor-alpha: use as adjunctive therapy in established group B streptococcal disease in newborn rats. Pediatr Res 38: 551–554. [DOI] [PubMed] [Google Scholar]
- 39. Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P (1990) A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in Gram-positive bacteria. Nucleic Acids Res 18: 4296. [DOI] [PMC free article] [PubMed] [Google Scholar]