Abstract
Background
Gastric carcinoma (GC) is one of the highest cancer-mortality diseases with a high incidence rate in Asia. For surgically unfit but medically fit patients, palliative chemotherapy is the main treatment. The chemotherapy regimen of docetaxel, cisplatin and 5-fluorouracil (DCF) has been used to treat the advanced stage or metastatic GC. It is necessary to compare effectiveness and toxicities of DCF regimen with non-taxane-containing palliative chemotherapy for GC.
Methods
PubMed, EmBase, Cochrane Central Register of Controlled Trials and China National Knowledge Infrastructure databases were searched to select relative randomized controlled trials (RCTs) comparing DCF to non-taxane-containing chemotherapy for patients with palliatively resected, unresectable, recurrent or metastatic GC. Primary outcome measures were 1-year and 2-year overall survival (OS) rates. Secondary outcome measures were median survival time (MST), median time to progression (TTP), response rate and toxicities.
Results
Twelve RCTs were eligible and 1089 patients were analyzed totally (549 in DCF and 540 in control). DCF regimen increased partial response rate (38.8% vs 27.9%, p = 0.0003) and reduced progressive disease rate (18.9% vs 33.3%, p = 0.0005) compared to control regimen. Significant improvement of 2-year OS rate was found in DCF regimen (RR = 2.03, p = 0.006), but not of 1-year OS rate (RR = 1.22, p = 0.08). MST was significantly prolonged by DCF regimen (p = 0.039), but not median TTP (p = 0.054). Both 1-year OS rate and median TTP had a trend of prolongation by DCF regimen. Chemotherapy-related mortality was comparable (RR = 1.23, p = 0.49) in both regimens. In grade I-IV toxicities, DCF regimen showed a major raise of febrile neutropenia (RR = 2.33, p<0.0001) and minor raises of leucopenia (RR = 1.25, p<0.00001), neutropenia (RR = 1.19, p<0.00001), and diarrhea (RR = 1.59, p<0.00001), while in other toxicities there were no significant differences.
Conclusion
DCF regimen has better response than non-taxane containing regimen and could potentially improve the survival outcomes. The chemotherapy-related toxicity of DCF regimen is acceptable to some extent.
Introduction
Gastric carcinoma is one of the highest cancer-mortality diseases [1]–[3] with a high incidence rate in Asia [4]. A lot of patients are diagnosed at advanced even end stage carcinoma, indicating poor outcomes [5]. For resectable diseases, surgery is considered as the mainstream treatment [6]–[9]. Adjuvant or neo-adjuvant chemotherapy has also been proven to benefit the survival rate in some studies and meta-analyses [10]–[14]. For surgically unfit but medically fit patients, palliative chemotherapy is the main treatment [6], [12], [15]–[18]. The regimens of cisplatin and 5-fluorouracil (CF) or epirubicin, cisplatin and 5-fluorouracil (ECF) have been used widely [19], and were often considered as the reference regimens for advanced gastric cancer [20]. Among new generation chemotherapy regimens, docetaxel, which is a semisynthetic taxane, promoting the assembly and stabilization of microtubules to inhibit the depolymerization [21], has been used more and more extensively with more potent effects [18], [22]–[25]. The chemotherapy based on docetaxel may be effective [26], because docetaxel lacks cross-resistance with other anti-tumor drugs [23]. The chemotherapy regimen of docetaxel, cisplatin and 5-fluorouracil (DCF) has been used to treat the advanced stage or metastatic gastric carcinoma with encouraging survival outcomes [27]–[33] and better quality of life [34], [35] in several studies. However, it was reported in some researches [36]–[38] that more toxicity, such as hematotoxicity, happened in DCF than in other regimens. Therefore, evaluation of benefits against the chemotherapy-related toxicities was needed. Present systematic review and meta-analysis were done to evaluate the survival outcomes and toxicities of DCF for palliatively resected, unresectable, recurrent or metastatic gastric carcinoma, compared with those of non-taxane-containing regimens.
Methods
No protocol was registered.
Search Strategy
We searched the electronic databases of PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/), EmBase (http://www.embase.com/home), Cochrane Central Register of Controlled Trials (http://ovidsp.tx.ovid.com/sp-3.4.1b/ovidweb.cgi), and China National Knowledge Infrastructure Database (http://acad.cnki.net/Kns55/brief/result.aspx?dbPrefix=CJFQ) up to July 31, 2011. The search strategy in PubMed was as follows, (“docetaxel”[Supplementary Concept] OR docetaxel[Text Word]) AND (“stomach neoplasms”[MeSH Terms] OR (“stomach”[All Fields] AND “neoplasms”[All Fields]) OR “stomach neoplasms”[All Fields] OR (“gastric”[All Fields] AND “cancer”[All Fields]) OR “gastric cancer”[All Fields]) AND (“humans”[MeSH Terms] AND (Clinical Trial[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp] OR Review[ptyp]) AND English[lang]). The search strategy was also referred in other electronic databases.
Inclusion and Exclusion Criteria
Only randomized controlled trials (RCTs) were eligible for inclusion. Included patients were diagnosed with palliatively resected, unresectable, recurrent or metastatic gastric carcinoma. Both, patients with previous surgery and without, were acceptable. DCF palliative chemotherapy could be administrated as the first-line regimen. If the control arm was blank or contaminated with taxane, the trials were excluded. Response, survival outcomes or toxicities were mandatory to be reported.
Selection, Assessment and Data Extraction
Two independent reviewers (Chen XL, Yang C) read the title and abstract of every searched citation to select eligible studies for further assessment. Full text of potentially eligible citation was retrieved and determined for inclusion. Jadad scale described by Jadad, et al was used to assess the quality of RCTs [39]. Data was extracted independently by two reviewers mentioned above. Primary outcome measures included 1) 1-year and 2-year overall survival (OS) rates. Secondary outcome measures were 2) median survival time (MST), 3) time to progression (TTP), 4) response rate (WHO Criteria) [40] containing complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD) and overall response rate (ORR), 5) toxicities (grade I–IV) and 6) chemotherapy-related death. ORR meant the combination of CR and PR. Study sample and regimen details were also extracted. Any disagreements in studies assessment and data collection were discussed and resolved by a third party (Hu JK, Chen XZ) as the referees.
Statistical Analysis
The statistical analysis was performed by Reviewer Manager (RevMan) software, version 5.0 offered by The Cochrane Collaboration. The Mantel-Haenszel (M-H) test was used for comparison of dichotomous data and risk ratio (RR) or risk difference (RD) estimate. The 95% confidence interval (CI) of RR or RD was also calculated. A two-sided P value less than 0.05 was considered as a significant difference. Between-trials heterogeneity was evaluated by the Chi-square test; P value less than 0.1 was considered as significant heterogeneity [41]. Providing heterogeneity existed, random effect model was used for meta-analysis; otherwise fixed effect model was used.
Results
Literatures Search and Selection
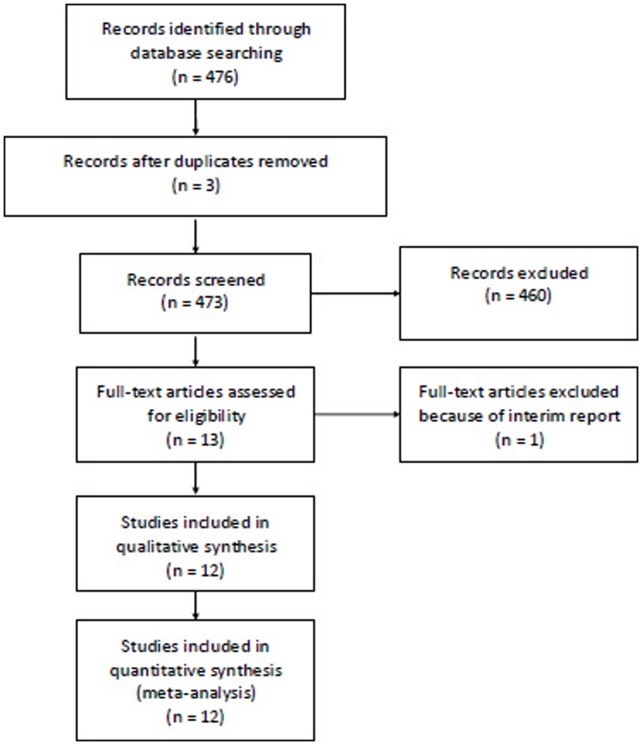
The literature search and selection procedure were shown in Figure 1. Totally, 12 RCTs were eligible for analysis and 1089 patients with palliatively resected, unresectable, recurrence or metastatic gastric cancer were involved (549 in DCF vs 540 in control) [35], [38], [42]–[51] (Table1). The sample size of individual RCT ranged from 36 to 445. There were no significant differences in the baselines between DCF and controlled group in these studies, as reported. In these studies, DCF were compared with cisplatin and fluorouracil (CF), epirubicin, cisplatin and fluorouracil (ECF), oxaliplatin and fluorouracil (FOLFOX4), etoposide and fluorouracil (EF) regimens.
Figure 1. Literature search and selection procedure.
Table 1. Summary information of included RCTs.
| Studies | Demographic data | Intervention& control | Outcome measures | Jadad scores |
| Chu JH, et al [42]20061 center in China | 40 patients with recurrence or metastaticgastric carcinoma chemotherapy-naïvewithin 1 month. | DCF: docetaxel 25 mg/m2, cisplatin 35 mg/m2, 5-FU750 mg/m2.3–4 cycles.CF: cisplatin 20 mg/m2, 5-FU 1000 mg/m2. 3–4 cycles. | ORR, MST, TTP andtoxicities | 1 |
| Van Cutsem E, et al [43] 200672 centers in 16 countries. | 457 patients with metastaticor locally advanced/recurrentgastric cancer.12 patients without treatmentwere excluded. | DCF: docetaxel 75 mg/m2, cisplatin 75 mg/m2 and 5-FU750 mg/m2. Median6 (1–16) cycles. CF: cisplatin100 mg/m2 and 5-FU1000 mg/m2. Median 4 (1–12) cycles. | ORR,TTP, OS, toxicities and QOL | 2 |
| Sadighi S, et al [35]20061 center in Iran | 86 patients with primary or recurrent gastric cancer(III–IV stage). 15 patients did not completethe questionnaires andwere excluded in QOL analyses. | DCF: docetaxel 60 mg/m2, cisplatin 60 mg/m2 and 5-FU750 mg/m2. 3–6 cyclesECF: epirubicin 60 mg/m2, cisplatin 60 mg/m2 and 5-FU750 mg/m2. 3–6cycles. | ORR and QOL | 2 |
| Li XQ, et al [44]20071 center in China | 60 patients with stage IV gastric carcinoma. | DCF: docetaxel 25 mg/m2, cisplatin 6 mg/m2 and 5-FU200 mg/m2. 2 cycles.CF: cisplatin 6 mg/m2 and 5-FU 200 mg/m2. 2 cycles. | ORR, MST andtoxicities | 1 |
| Roth AD, et al [38]200713 centers in 4countries | 121 patients with unresectablegastric cancer,metastatic or locally carcinoma. 2 patients withouttreatment were excluded. | DCF: docetaxel 85 mg/m2, cisplatin 75 mg/m2, and5-FU300 mg/m2. Median 4 cycles.ECF: epirubicin 50 mg/m2, cisplatin 60 mg/m2, 5-FU 200 mg/m2.Median 5.5 cycles. | ORR, OS, toxicitiesand QOL | 2 |
| Wu GC, et al [45]20081 center in China | 58 patients with stage III–IV gastriccarcinoma received first orsecondary treatment. | DCF: docetaxel 75 mg/m2, cisplatin 75 mg/m2, 5-FU 750 mg/m2.2 cycles.CF: cisplatin 75 m/m2, 5-FU 750 mg/m2. 2 cycles | ORR, MST, andtoxicities | 1 |
| Zhang FL, et al [46]20081 center in China | 50 chemotherapy-naive patients with localrecurrence or metastatic carcinoma. | DCF: docetaxel 75 mg/m2, cisplatin 25 mg/m2, 5-FU 500 mg/m2. More than2 cycles.CF: cisplatin 25 m/m2, 5-FU 500 mg/m2.More than 2 cycles. | ORR and Toxicities | 2 |
| Hou AJ, et al [47]20091 center in China | 40 patients with stage IIIB–IV after gastrectomy orpalliative surgery. 4 patients could not be evaluated. | DCF: docetaxel 40 mg/m2, cisplatin 30 mg/m2 and 5-FU 200 mg/m2.More than 2 cycles.ELF: etoposide 120 mg/m2, 5-FU 500 mg/m2. More than 2 cycles. | ORR, OS, MST andQOL | 3 |
| Zhao F, et al [48]20091 center in China | 31 gastric cancer patients in DCF arm and32 in FOLFOX4 arm with recurrenceafter radical gastrectomy or withoutsurgery because ofmetastasis. | DCF: docetaxel 75 mg/m2, cisplatin 20 mg/m2 and 5-FU 350 mg/m2. Median 3.1 cycles.FOLFOX4: oxaliplatin 100 mg/m2 and5-FU 400 mg/m2.Median 3.2 cycles. | ORR, TTP and MST | 1 |
| Shen YC, et al [49]20091 center in China. | 48 chemotherapy-naive patients with late stage gastric carcinoma after noor palliative surgery. | DCF: docetaxel 35 mg/m2, cisplatin 6 mg/m2 and 5-FU250 mg/m2. 3–4 cyclesCF: cisplatin 75 mg/m2 and 5-FU 1000 mg/m2. 3–4 cycles | ORR, TTP andtoxicities | 1 |
| Liang B, et al [50]20101 center in China | 58 patients in DCF arm and control armwith advanced gastric cancer expectedto survive more than3 months. | DCF: docetaxel 75 mg/m2, cisplatin 75 mg/m2 and 5-FU 300 mg/m2.More than 2 cycles.ECF: epirubicin 50 mg/m2, cisplatin 60 mg/m2, 5-FU 200 mg/m2. Morethan 2 cycles. | ORR and toxicities | 1 |
| Gao H, et al [51]20101 center in China | 64 patients with stage IIIB–IV gastric carcinoma. | DCF: docetaxel 60 mg/m2, cisplatin 25 mg/m2, 5-FU 1000 mg/m2. More than 2 cycles.ECF: epirubicin 50 mg/m2, cisplatin 25 mg/m2, 5-FU 1000 mg/m2. Morethan 2 cycles. | ORR, OS and QOL | 3 |
Abbreviations: DCF: docetaxel, cisplatin and fluorouracil; CF: cisplatin and fluorouracil; ECF: epirubicin, cisplatin and fluorouracil; FOLFOX4: oxaliplatin and fluorouracil; EF: etoposide and fluorouracil; FU: fluorouracil; ORR: overall response rate; TTP: time to progression; QOL: quality of life; MST: median survival time; OS: overall survival.
Response
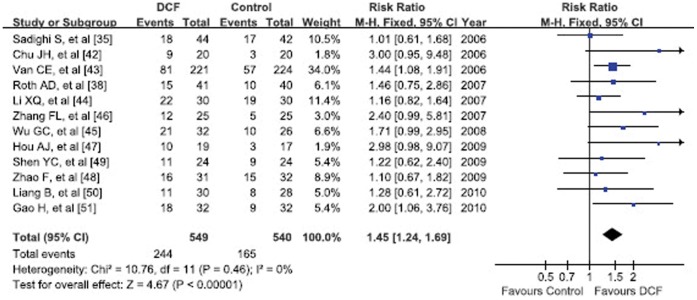
Response rate was based on WHO Criteria [40]. The overall response rate (ORR) combined of CR and PR, was 44.4% (244/549) vs 30.6% (165/540) in DCF and non-taxane-containing regimens, respectively. Its meta-analysis showed significantly better ORR of DCF regimen (RR = 1.45, 95% CI 1.24–1.69, p<0.00001) (Figure 2). The subgroup meta-analysis results of CR, PR, SD and PD were listed in Table 2. CR and SD rates were not significantly different between two groups (Table 2). DCF regimen was able to noticeably increase PR rate (38.8% vs 27.9%, RR = 1.39, 95%CI 1.16–1.65, p = 0.0003), as well as reduce PD rate (18.9% vs 33.3%, RR = 0.65, 95%CI 0.51–0.83, p = 0.0005) (Forest plots not shown).
Figure 2. Forest plot of overall response rate.
Table 2. Response comparison between DCF and non-taxane-containing chemotherapy.
| Response | Study | DCF | Non-taxane-containing | PooledRR | 95% CI | P value | Model | References | ||||
| counts | Events | Total | Accumulated | Events | Total | Accumulated | ||||||
| percentage | percentage | |||||||||||
| CompleteResponse (CR) | 11 | 25 | 505 | 5.0% | 14 | 498 | 2.8% | 1.69 | 0.91–3.14 | 0.10 | Fixed | [38], [42]–[51] |
| PartialResponse (PR) | 11 | 196 | 505 | 38.8% | 139 | 498 | 27.9% | 1.39 | 1.16–1.65 | 0.0003 | Fixed | [38], [42]–[51] |
| StableDisease (SD) | 9 | 122 | 444 | 27.5% | 136 | 438 | 31.1% | 0.88 | 0.72–1.08 | 0.23 | Fixed | [43]–[51] |
| ProgressiveDisease (PD) | 9 | 84 | 444 | 18.9% | 146 | 438 | 33.3% | 0.65 | 0.51–0.83 | 0.0005 | Fixed | [43]–[51] |
Abbreviations: DCF: docetaxel, cisplatin and fluorouracil; RR: risk ratio.
Survival Outcomes
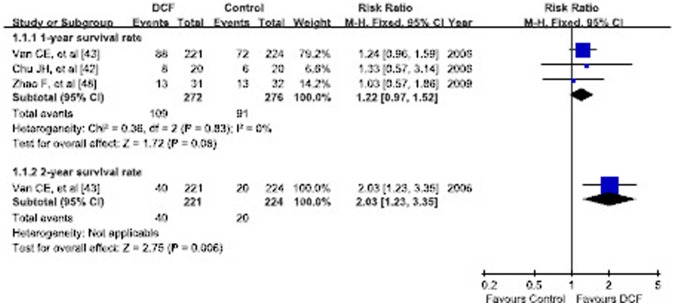
Only 3 RCTs reported the 1-year OS rate and its details ranging from 40.0% to 41.9% in DCF and 30.0% to 40.0% in non-taxane-containing regimens [42], [43], [48]. The cumulative 1-year OS rate was 40.1% (109/272) in DCF and 33.0% (91/276) in non-taxane-containing regimens. Meta-analysis demonstrated no significant difference in 1-year OS rate (RR = 1.22, 95% CI 0.97–1.52, p = 0.08). However only one RCT analyzed 2-year OS rate showing significantly better 2-year OS rate in DCF regimen (18.0% vs 9.0%, RR = 2.03, p = 0.006) [38] (Figure 3).
Figure 3. Forest plot of subgroup analysis of 1-year and 2-year overall survival rates.
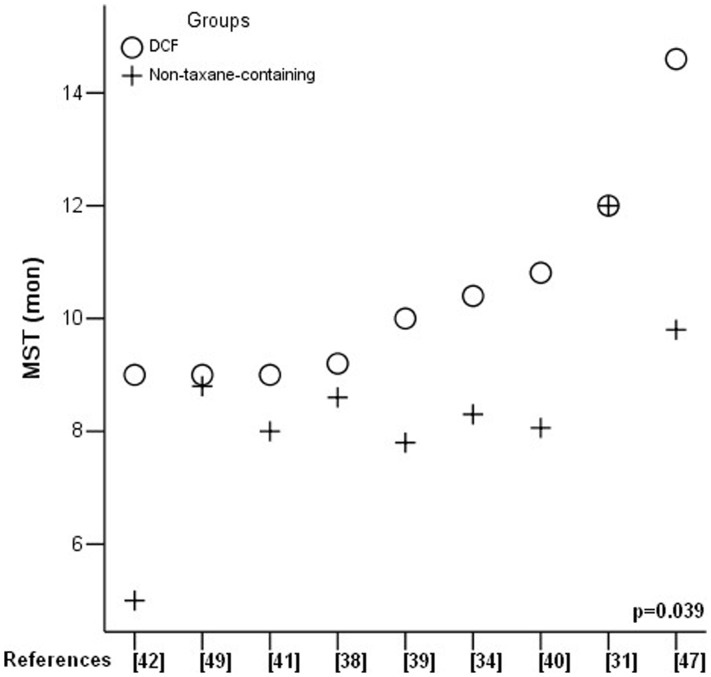
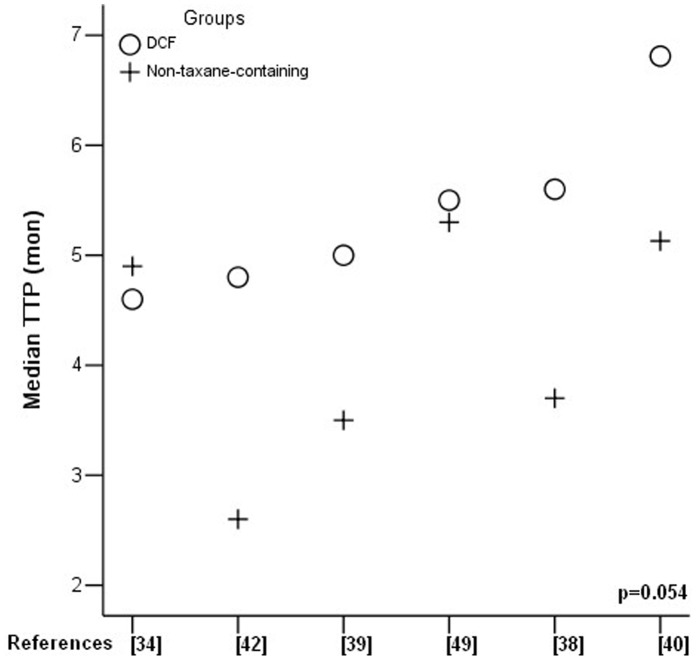
MST was reported in 9 RCTs and it ranged from 9.0 to 14.6 months in DCF and 5.0 to 12.0 months in non-taxane-containing regimens (Table 3). Six RCTs analyzed median TTP which ranged from 4.6 to 6.8 months in DCF and 2.6 to 5.5 months in non-taxane-containing regimens (Table 3). Comparison of MST and median TTP between two groups by one-way ANOVA test (SPSS 13.0) demonstrated significantly prolonged MST in DCF regimen (p = 0.039) (Figure 4). Median TTP showed a trend of prolongation in DCF regimen without reaching statistically significant difference (p = 0.054) (Figure 5).
Table 3. Details information of MST and TTP reported by some RCTs.
| Studies | MST (range) | P value | Median TTP (range) | P value | ||
| DCF | Non-taxane-containing | DCF | Non-taxane-containing | |||
| Chu JH, et al [42] | 10.0 | 7.8 | – | 5.0 | 3.5 | – |
| Van CE, et al [43] | 9.2 (8.4–10.6) | 8.6 (7.2–9.5) | 0.02 | 5.6 (4.9–5.9) | 3.7 (3.4–4.5) | <0.001 |
| Sadighi S, et al [35] | 12.0 (7–17) | 12.0 (8–14) | – | – | – | – |
| Li XQ, et al [44] | 9.0 | 5.0 | <0.05 | 4.8 | 2.6 | <0.05 |
| Roth AD, et al [38] | 10.4 (8.3–12.0) | 8.3 (7.2–13.0) | – | 4.6 (3.5–5.6) | 4.9 (3.2–6.1) | – |
| Wu GC, et al [45] | 14.6 | 9.8 | <0.05 | – | – | – |
| Hou AJ, et al [47] | 9.0 (2–18) | 8.0 (2–18) | >0.05 | – | – | – |
| Zhao F, et al [48] | 9.0 | 8.8 | >0.05 | 5.5 | 5.3 | >0.05 |
| Gao H, et al [51] | 10.81 (8.42–13.20) | 8.06 (6.46–9.67) | 0.038 | 6.81 (5.52–8.11) | 5.13 (4.18–6.07) | 0.041 |
Abbreviation: MST: median survival time; TTP: time to progression; DCF: docetaxel, cisplatin and fluorouracil.
Figure 4. Median survival time (MST) comparison.
Figure 5. Median time-to-progression (TTP) comparison.
Toxicities
In these studies, the toxicities were graded from I to IV according to WHO Criteria, National Cancer Institute-Common Terminology Criteria and National Cancer Institute of Canada Common Toxicity Criteria. These three different criteria are comparable. We compared grade I–IV and grade III–IV in both arms according to reports information (Table 4). The major grade I–IV toxicities (accumulated rate >5%) of DCF regimen were hematological toxicity including leucopenia (81.7%), neutropenia (84.1%), febrile neutropenia (27.3%), thrombocytopenia (24.7%), anemia (81.6%), and digestive systemic toxicity including diarrhea (58.9%), nausea/vomiting (59.2%), stomatitis (56.2%), anorexia (45.8%), constipation (26.3%), liver damage (7.3%). Other toxicities were neurological damage (33.7%), alopecia (73.5%), anaphylaxis (11.8%), infection (16.2%), fatigue (54.6%) and fluid retention (6.7%).
Table 4. Toxicities comparison between DCF and non-taxane-containing chemotherapy.
| Toxicities | Study | DCF | Non-taxane-containing | Pooled | 95% CI | P value | Model | References | ||||
| counts | Events | Total | Accumulated | Events | Total | Accumulated | RR/RD | |||||
| percentage | percentage | |||||||||||
| Leucopenia (I–IV) | 6 | 282 | 345 | 81.7% | 228 | 346 | 65.9% | RR = 1.25 | 1.15–1.35 | <0.00001 | Fixed | [42]–[44], [47], [49], [51] |
| III–IV | 5 | 185 | 342 | 54.1% | 97 | 342 | 28.4% | RR = 1.72 | 1.15–2.56 | 0.008 | Random | [38], [43], [44], [47], [51] |
| Neutropenia (I–IV) | 4 | 254 | 302 | 84.1% | 215 | 303 | 71.0% | RR = 1.19 | 1.11–1.28 | <0.00001 | Fixed | [43], [47], [48], [51] |
| III–IV | 4 | 185 | 302 | 61.3% | 128 | 303 | 42.2% | RR = 1.46 | 1.28–1.66 | <0.00001 | Fixed | [43], [47], [48], [51] |
| Febrile neutropenia (I–IV) | 2 | 69 | 253 | 27.3% | 30 | 256 | 11.7% | RR = 2.33 | 1.57–3.44 | <0.0001 | Fixed | [43], [51] |
| III–IV | 2 | 17 | 73 | 23.3% | 7 | 72 | 9.7% | RR = 2.37 | 1.10–5.09 | 0.03 | Fixed | [38], [51] |
| Thrombocytopenia (I–IV) | 5 | 82 | 332 | 24.7% | 102 | 334 | 30.5% | RR = 1.22 | 0.64–2.32 | 0.55 | random | [43], [44], [47], [48], [51] |
| III–IV | 6 | 19 | 373 | 5.1% | 48 | 357 | 13.4% | RR = 0.60 | 0.33–1.08 | 0.09 | Fixed | [38], [43], [44], [47], [48], [51] |
| Anemia (I–IV) | 5 | 271 | 332 | 81.6% | 265 | 334 | 79.3% | RR = 1.03 | 0.97–1.10 | 0.28 | Fixed | [43], [44], [47], [48], [51] |
| III–IV | 5 | 47 | 332 | 14.2% | 61 | 334 | 18.3% | RR = 0.73 | 0.48–1.12 | 0.15 | Fixed | [43], [44], [47], [48], [51] |
| Diarrhea (I–IV) | 5 | 192 | 326 | 58.9% | 122 | 330 | 37.0% | RR = 1.59 | 1.36–1.87 | <0.00001 | Fixed | [42]–[44], [48], [49] |
| III–IV | 4 | 49 | 323 | 15.2% | 20 | 326 | 6.1% | RR = 2.82 | 1.62–4.89 | 0.0002 | Fixed | [38], [43], [44], [48] |
| Nausea/vomiting (I–IV) | 7 | 223 | 377 | 59.2% | 235 | 379 | 62.0% | RR = 0.96 | 0.86–1.07 | 0.42 | Fixed | [42]–[44], [47]–[49], [51] |
| III–IV | 6 | 49 | 374 | 13.1% | 51 | 375 | 13.6% | RR = 0.97 | 0.68–1.38 | 0.85 | Fixed | [38], [43], [44], [47], [48], [51] |
| Stomatitis (I–IV) | 2 | 141 | 251 | 56.2% | 142 | 254 | 55.9% | RR = 1.01 | 0.86–1.17 | 0.94 | Fixed | [43], [44] |
| III–IV | 3 | 49 | 292 | 16.8% | 63 | 294 | 21.4% | RR = 0.79 | 0.57–1.09 | 0.15 | Fixed | [38], [43], [44] |
| Anorexia (I–IV) | 2 | 116 | 253 | 45.8% | 116 | 256 | 45.3% | RR = 1.01 | 0.84–1.22 | 0.90 | Fixed | [43], [51] |
| III–IV | 2 | 28 | 253 | 11.1% | 23 | 256 | 9.0% | RR = 1.23 | 0.73–2.08 | 0.44 | Fixed | [43], [51] |
| Constipation (I–IV) | 1 | 5 | 19 | 26.3% | 2 | 17 | 11.8% | RR = 2.24 | 0.50–10.06 | 0.29 | Fixed | [47] |
| III–IV | 1 | 0 | 19 | 0.0% | 0 | 17 | 0.0% | RD = 0.15 | −0.10–0.40 | 0.25 | Fixed | [47] |
| Liver damage (I–IV) | 3 | 6 | 82 | 7.3% | 7 | 81 | 8.6% | RR = 0.84 | 0.29–2.42 | 0.75 | Fixed | [47], [48], [51] |
| III–IV | 3 | 0 | 82 | 0.0% | 0 | 81 | 0.0% | RD = 0.00 | −0.04–0.04 | 1.00 | Fixed | [47], [48], [51] |
| Neurological | 2 | 85 | 252 | 33.7% | 66 | 256 | 25.8% | RR = 0.41 | 0.02–9.09 | 0.58 | Random | [43], [48] |
| damage(I–IV) | ||||||||||||
| III–IV | 3 | 19 | 293 | 6.5% | 8 | 296 | 2.7% | RR = 2.39 | 1.07–5.36 | 0.03 | Fixed | [38], [43], [48] |
| Alopecia (I–IV) | 2 | 36 | 49 | 73.5% | 7 | 47 | 14.9% | RR = 3.75 | 1.01–13.84 | 0.05 | Random | [44], [47] |
| III–IV | 3 | 41 | 90 | 45.6% | 8 | 87 | 9.2% | RR = 8.48 | 0.16–461.63 | 0.29 | Random | [38], [44], [47] |
| Anaphylaxis (I–IV) | 2 | 6 | 51 | 11.8% | 1 | 49 | 2.0% | RR = 4.11 | 0.74–22.88 | 0.11 | Fixed | [47], [51] |
| III–IV | 2 | 0 | 51 | 0.0% | 0 | 49 | 0.0% | RD = 0.00 | −0.05–0.05 | 1.00 | Fixed | [47], [51] |
| Infection (I–IV) | 2 | 39 | 241 | 16.2% | 28 | 244 | 11.5% | RR = 1.41 | 0.90–2.22 | 0.13 | Fixed | [42], [43] |
| III–IV | 1 | 28 | 221 | 12.7% | 16 | 224 | 7.1% | RR = 1.77 | 0.99–3.19 | 0.06 | Fixed | [43] |
| Fatigue (I–IV) | 2 | 131 | 240 | 54.6% | 108 | 241 | 44.8% | RR = 1.22 | 1.02–1.46 | 0.03 | Fixed | [43], [47] |
| III–IV | 2 | 41 | 240 | 17.1% | 31 | 241 | 12.9% | RR = 1.34 | 0.87–2.06 | 0.18 | Fixed | [43], [47] |
| Fluid retention (I–IV) | 1 | 2 | 30 | 6.7% | 0 | 30 | 0.0% | RR = 5.00 | 0.25–99.95 | 0.29 | Fixed | [44] |
| III–IV | 1 | 0 | 30 | 0.0% | 0 | 30 | 0.0% | RD = 0.00 | −0.06–0.06 | 1.00 | Fixed | [44] |
Abbreviations: DCF: docetaxel, cisplatin and fluorouracil; RR: risk ratio; RD: risk difference.
The meta-analysis results of these toxicities were listed in Table 3 (Forest plots not shown). There was significant increase of toxicities in DCF regimen in leucopenia (I–IV RR = 1.25, p<0.00001; III–IV RR = 1.72, p = 0.008), neutropenia (I–IV RR = 1.19, p<0.00001; III–IV RR = 1.46, p<0.00001), febrile neutropenia (I–IV RR = 2.33, p<0.0001; III–IV RR = 2.37, p = 0.03), diarrhea (I–IV RR = 1.59, p<0.00001; III–IV RR = 2.82, p = 0.0002), neurological damage (III–IV RR = 2.39, p = 0.03) and fatigue (I–IV RR = 1.22, p = 0.03). In grade I–IV toxicities, DCF regimen showed major raise of febrile neutropenia and minor raise of leucopenia, neutropenia, and diarrhea. Other toxicities such as thrombocytopenia, anemia, nausea/vomiting, stomatitis, anorexia, constipation, alopecia, neurological damage and infection appeared with no significant differences in both arms. Some toxicities including febrile neutropenia, liver damage, anaphylaxis and fluid retention were rare.
One RCT reported chemotherapy-related deaths within 30 days of the last infusion which were mainly caused by infection in both groups [43]. Four RCTs reported no chemotherapy-related mortality during the treatment [38], [45], [46], [50]. The accumulated chemotherapy-related mortality rates were 6.6% (23/349) in DCF and 5.5% (19/343) in non-taxane-containing regimens without significant difference (RR = 1.23, 95%CI 0.69–2.91, p = 0.49) (Forest plot not shown).
Discussion
Although our meta-analysis did not show the significant difference of 1-year OS rate between DCF chemotherapy and non-taxane-containing regimens, we found that DCF arm was relatively better than control arms in cumulative 1-year OS rate, MST, median TTP and ORR. Only one study, which had a large sample size (445 patients), reported their 2-year OS rate (DCF 18% vs CF 9%) [43]. Many studies preferred to show the MST or OS time, TTP and especially ORR which represented the short survival outcomes. There was an interesting phenomenon in one study, in which DCF arm had shorter median TTP but longer MST than ECF arm [38]. According to our results, the difference of ORR was significant between two arms, with DCF having more benefits than control group in short time.
On the toxicity analysis, we found that DCF showed worse hematological toxicity, diarrhea, and fatigue. Many studies reported that these toxicities in both group could be accepted or controlled by granulocyte colony stimulating factors (G-CSF), antiemetic, vitamin B6 and drug discontinuance[38], [46], [49], [51]–[53]. Further, the total mortality of treatment, mainly caused by infection, was 6.6% in DCF arm and 5.5% in control arms, and showed no significant difference. Prophylactic antibiotics might be necessary for patients with severe leucopenia. Quality of life (QOL) after chemotherapy was of less focus than survival outcomes. Four RCTs demonstrated that DCF group did not have lower QOL. In fact, it even had obvious improvements in global QOL and Karnofsky performance status as well as prolonged the time to worsening of global health [35], [38], [43], [47]. Although causing more toxicities, DCF was a relatively safe and acceptable chemotherapy.
In every chemotherapy cycle for three or four weeks, the total dosage of docetaxel was from 105 mg/m2 to 300 mg/m2, cisplatin from 90 mg/m2 to 360 mg/m2, and 5-FU from 3000 mg/m2 to 15000 mg/m2 in these studies. The single dosage of docetaxel was noticeably lower in four studies from China (25 mg/m2 to 40 mg/m2) [42], [44], [47], [49], that was about one half of the dosage of three foreign studies (60 mg/m2 to 85 mg/m2) and five other Chinese studies (60 mg/m2 to 75 mg/m2) [35], [38], [43], [45], [46], [48], [50], [51] in which single dosage was similar to that recommended in National Comprehensive Cancer Network (NCCN) clinical practice guideline in oncology [54]. Similar phenomenon happened in cisplatin. Studies in China showed a noticeably lower single dosage of cisplatin (6 mg/m2 to 35 mg/m2) [35], [44], [46]–[49], [51] than foreign RCTs [35], [38], [43] (60 mg/m2 to 75 mg/m2) [45], [50] and NCCN recommendation [54]. From all these, we could see that there was a big difference in approach to dosage of DCF. The reason for this, we believe, was the individual distinctions of people in China and Western countries. Moreover, among Chinese RCTs, there was also a difference in the dosage of docetaxel and cisplatin. The question about which plan has better balance of survival outcomes and toxicities, a bigger single dosage with lower frequencies or a smaller single dosage with higher frequencies, needs further research.
A previous systematic review showed that there was significant difference in ORR but no significant difference in overall survival, when docetaxel was compared with non-taxane-containing regimens, which is in accord to our study [55]. However, in former review non-docetaxel-containing regimens might include both single and combined chemotherapy regiments. If docetaxel alone is compared with combined regimens, it might show relatively inferior efficacy of docetaxel. However, in our review, DCF was compared with other combined chemotherapy in all RCTs, which in our opinion seemed more reasonable.
In retrieved literatures of RCTs on DCF, we found that there were no studies from Japan. On one hand, it can be explained by the fact that we only retrieved the English or Chinese language articles. On the other hand, many studies [56]–[61] in Japan talked about the new generation chemotherapy drug S-1 alone or S-1 combined with other drugs, such as docetaxel, and reported promising effects in treating gastric carcinoma. However, they did not compare S-1 with DCF.
Further, we could see that DCF as palliative chemotherapy was reported in all RCTs. At present, adjuvant single or combined chemotherapy following radical surgeries included 5-FU, cisplatin, FOLFOX, capecitabine and S-1. Docetaxel was not the routine first line chemotherapy regimen. From NCCN introduction, DCF was recommended as first line chemotherapy for metastatic or locally advanced cancer [54]. It might raise the question whether or not DCF regimen could be used in postoperative adjuvant chemotherapy and have better survival outcomes than traditional adjuvant chemotherapy.
In our review, 9 out of 12 RCTs came from China, in which the population was small and follow-up time was short. Their aim was to evaluate the short term outcomes, ORR and toxicities. Only three RCTs including a foreign one reported the survival rate. This might influence the analysis in our review. More RCTs with longer follow-up time are needed.
Conclusion
DCF regimen had better response than non-taxane containing regimen and could potentially improve the survival outcomes. The chemotherapy-related toxicity of DCF regimen is also acceptable to some extent. At the same time, more high-quality RCTs are needed to decide on the effectiveness of DCF regimen.
Acknowledgments
Authors thank the substantial work of Volunteer Team of Gastric Cancer Surgery (VOLTGA) based on Multidisciplinary Team (MDT) of Gastrointestinal Tumors, West China Hospital, Sichuan University, China. Authors also sincerely appreciate Dr. Alex Zhuruk from General Surgery, University Of Western Ontario, Canada for his English language support. We also referred to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in this study [62].
Funding Statement
This research was funded by these sources: 1) National Natural Science Foundation of China (No. 81071777); 2) Outstanding Young Scientific Scholarship Foundation of Sichuan University, from the Fundamental Research Funds for the Central Universities of China (No.2011SCU04B19); 3) New Century Excellent Talents in University support program, Ministry of Education of China (2012SCU-NCET-11-0343). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wagner AD, Wedding U (2009) Advances in the pharmacological treatment of gastro-oesophageal cancer. Drugs Aging 26: 627–646. [DOI] [PubMed] [Google Scholar]
- 2. Bittoni A, Maccaroni E, Scartozzi M, Berardi R, Cascinu S (2010) Chemotherapy for locally advanced and metastatic gastric cancer: state of the art and future perspectives. Eur Rev Med Pharmacol Sci 14: 309–314. [PubMed] [Google Scholar]
- 3. Catalano V, Labianca R, Beretta GD, Gatta G, De Braud F, et al. (2009) Gastric cancer. Crit Rev Oncol Hematol 71: 127–164. [DOI] [PubMed] [Google Scholar]
- 4. Ajani JA (1998) Chemotherapy for gastric carcinoma: new and old options. Oncology (Williston Park) 12(10 Suppl 7 44–47. [PubMed] [Google Scholar]
- 5. Tsai JY, Safran H (2003) Status of treatment for advanced gastric carcinoma. Curr Oncol Rep 5: 210–218. [DOI] [PubMed] [Google Scholar]
- 6. Van Cutsem E, Van de Velde C, Roth A, Lordick F, Kohne CH, et al. (2008) Expert opinion on management of gastric and gastro-oesophageal junction adenocarcinoma on behalf of the European Organization for Research and Treatment of Cancer (EORTC)-gastrointestinal cancer group. Eur J Cancer 44: 182–194. [DOI] [PubMed] [Google Scholar]
- 7. Hu JK, Yang K, Zhang B, Chen XZ, Chen ZX, et al. (2009) D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today 39: 207–213. [DOI] [PubMed] [Google Scholar]
- 8. Yang K, Chen XZ, Hu JK, Chen ZX, Chen JP (2009) Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol 15: 5352–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen XZ, Hu JK, Zhou ZG, Rui YY, Yang K, et al. (2010) Meta-analysis of effectiveness and safety of D2 plus para-aortic lymphadenectomy for resectable gastric cancer. J Am Coll Surg 210: 100–105. [DOI] [PubMed] [Google Scholar]
- 10. Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, et al. (2007) The effectiveness of intravenous 5-Flurouracil-containing chemotherapy after curative resection for gastric carcinoma: a systematic review of published randomized controlled trials. J Chemotherapy 19: 359–374. [DOI] [PubMed] [Google Scholar]
- 11. Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, et al. (2002) Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol 8: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, et al. (2000) Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol 11: 301–306. [DOI] [PubMed] [Google Scholar]
- 13. Das P, Ajani JA (2005) Gastric and gastro-oesophageal cancer therapy. Expert Opin Pharmacother 6: 2805–2812. [DOI] [PubMed] [Google Scholar]
- 14. Chen XZ, Yang K, Liu J, Chen XL, Hu JK (2011) Neoadjuvant plus adjuvant chemotherapy benefits overall survival of locally advanced gastric cancer. World J Gastroenterol 17: 4542–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, et al. (2011) Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 29: 868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, et al. (2010) Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol 28: 2947–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, et al. (2010) Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol 66: 721–728. [DOI] [PubMed] [Google Scholar]
- 18. Takayama T, Sato Y, Sagawa T, Okamoto T, Nagashima H, et al. (2007) Phase I study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Br J Cancer 97: 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohtsu A (2005) Current status and future prospects of chemotherapy for metastatic gastric cancer: a review. Gastric Cancer 8: 95–102. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E (2004) Docetaxel in gastric cancer. Eur J Cancer (Suppl 7): 52–58.
- 21. Cortes JE, Pazdur R (1994) Docetaxel. J Clin Oncol 13: 2643–2655. [DOI] [PubMed] [Google Scholar]
- 22. Bang YJ, Kang WK, Kang YK, Kim HC, Jacques C, et al. (2002) Docetaxel 75 mg/m(2) is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol 32: 248–254. [DOI] [PubMed] [Google Scholar]
- 23. Haller DG, Misset JL (2002) Docetaxel in advanced gastric cancer. Anticancer Drugs 13: 451–460. [DOI] [PubMed] [Google Scholar]
- 24. Giuliani F, Gebbia V, De Vita F, Maiello E, Di BM, et al. (2003) Docetaxel as salvage therapy in advanced gastric cancer: a phase II study of the Gruppo Oncologico Italia Meridionale (G.O.I.M.). Anticancer Res 23: 4219–4222. [PubMed] [Google Scholar]
- 25. Chen XZ, Jiang K, Hu JK, Zhang B, Gou HF, et al. (2008) Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China. World J Gastroenterol 14: 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth AD, Ajani JA (2003) Docetaxel-based chemotherapy in the treatment of gastric cancer. Ann Oncol (Suppl 2): 41–44. [DOI] [PubMed]
- 27. Yamamoto K, Fujiwara Y, Nishida T, Takiguchi S, Nakajima K, et al. (2009) Induction chemotherapy with docetaxel, 5-FU and CDDP (DFP) for advanced gastric cancer. Anticancer Res 29: 4211–4215. [PubMed] [Google Scholar]
- 28. Ajani JA (2002) Docetaxel for gastric and esophageal carcinomas. Oncology (Williston Park) 16(6 Suppl 6 89–96. [PubMed] [Google Scholar]
- 29. Oh DY, Kim TY, Kwon JH, Lee JJ, Joh Y, et al. (2005) Docetaxel +5-fluorouracil+cisplatin 3-day combination chemotherapy as a first-line treatment in patients with unresectable gastric cancer. Jpn J Clin Oncol 35: 380–385. [DOI] [PubMed] [Google Scholar]
- 30. Oh SC, Park KH, Choi IK, Yoon SY, Kim SJ, et al. (2005) Docetaxel (Taxotere), cisplatin, UFT, and leucovorin combination chemotherapy in advanced gastric cancer. Br J Cancer 92: 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park SR, Chun JH, Kim YW, Lee JH, Choi IJ, et al. (2005) Phase II study of low-dose docetaxel/fluorouracil/cisplatin in metastatic gastric carcinoma. Am J Clin Oncol 28: 433–438. [DOI] [PubMed] [Google Scholar]
- 32. Lorenzen S, Hentrich M, Haberl C, Lorenzen S, Hentrich M, et al. (2007) Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol 18: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 33. Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, et al. (2007) Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 25: 3205–3209. [DOI] [PubMed] [Google Scholar]
- 34. Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, et al. (2007) Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 25: 3210–3216. [DOI] [PubMed] [Google Scholar]
- 35. Sadighi S, Mohagheghi MA, Montazeri A, Sadighi Z (2006) Quality of life in patients with advanced gastric cancer: a randomized trial comparing docetaxel, cisplatin, 5-FU (TCF) with epirubicin, cisplatin, 5-FU (ECF). BMC Cancer 6: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li CP, Chen JS, Chen LT, Yen CJ, Lee KD, et al. (2010) A phase II study of weekly docetaxel and cisplatin plus oral tegafur/uracil and leucovorin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer. Br J Cancer 103: 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Cutsem E (2004) The treatment of advanced gastric cancer: new findings on the activity of the taxanes. Oncologist (Suppl 2): 9–15. [DOI] [PubMed]
- 38. Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, et al. (2007) Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 25: 3217–3223. [DOI] [PubMed] [Google Scholar]
- 39. Jadad AR, Moore RA, Carroll D (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 40. Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting Results of cancer treatment. Cancer 47: 207–214. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JPT, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration. Available: http://www.cochrane-handbook.org. Accessed 2013 Mar 13.
- 42.Chu JH, Zhang Y, Liu DF, Ji HM (2006) Weekly docetaxel combined with cisplatin of fluorouracil for advanced gastric carcinoma. Chin J Clin Oncol 11: 541–542. [Chinese].
- 43. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, et al. (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24: 4991–4997. [DOI] [PubMed] [Google Scholar]
- 44.Li XQ, Gu HG, Guo JW, Zhu XX (2007) Clinical study of continuous venous infusion of low-dose 5-Fu and cisplatin combined with weekly docetaxel for treatment of advanced gastric cancer. Modern Oncol 15: 659–661. [Chinese].
- 45.Wu GC, Bian XS, Wei CH, Song ZX (2008) A comparison of TCF and FLP for late stage of gastric carcinoma. J Shandong Med 48: 73–74. [Chinese].
- 46.Zhang FL, Hu YF, Duan W (2008) Clinical observation of docetaxel combined with LFP for late stage gastric carcinoma. Chin J Clin Oncol 35: 1397–1398, 1406. [Chinese].
- 47.Hou AJ, Hu Y, Zhou W, Zhang HW, Huang YL, et al.. (2009) Weekly docetaxel, cisplatin and low dose of fluorouracil for advanced gastric carcinoma: a randomized clinical trial. Tumor 29: 160–163. [Chinese].
- 48.Zhao F, Wang Q, Zhang JW, Hang M, Chen SB (2009) Therapeutic evaluation of docetaxel-combined chemotherapy for advanced gastric carcinoma. Acta Universitatis Medicinalis NanJing (Natural Science) 29: 237–239. [Chinese].
- 49.Shen YC, Chu JH (2009) Observation of weekly dose of docetaxel combined with small doses of cisplatin, 5-fluorouracil continuous intravenous infusion treatment of advanced gastric cancer. J Basic Clin Oncol 22: 318–320. [Chinese].
- 50.Liang B, Han N, Yang JM (2010) Clinical study of comparative efficacy of DCF regimen vs. ECF regimen in treating advanced gastric cancer. Chin J Clin Oncol Rehabil 17: 59–61. [Chinese].
- 51.Gao H, Ding X, Wei D, Xu T, Cheng P, et al.. (2010) Docetaxel versus epirubic in combined with cisplatin, leucovorin and fluorouracil for advanced gastric carcinoma as first line therapy: a randomized clinical trial. Chin J Clin Oncol 15: 529–533. [Chinese].
- 52. Mavroudis D, Kourousis C, Androulakis N, Kalbakis K, Agelaki S, et al. (2000) Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): a phase II trial. Am J Clin Oncol 23: 341–344. [DOI] [PubMed] [Google Scholar]
- 53. Makatsoris T, Papakostas P, Kalofonos HP, Xanthakis I, Tsavdaridis D, et al. (2007) Intensive weekly chemotherapy with docetaxel, epirubicin and carboplatin with G-CSF support in patients with advanced gastric cancer: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. Med Oncol 24: 301–307. [DOI] [PubMed] [Google Scholar]
- 54. Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, et al. (2010) Gastric cancer. J Natl Compr Canc Netw 8: 378–409. [DOI] [PubMed] [Google Scholar]
- 55. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, et al. (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 3: CD004064. [DOI] [PubMed] [Google Scholar]
- 56. Yoshida K, Hirabayashi N, Takiyama W, Ninomiya M, Takakura N, et al. (2004) Phase I study of combination therapy with S-1 and docetaxel (TXT) for advanced or recurrent gastric cancer. Anticancer Res 24: 1843–1851. [PubMed] [Google Scholar]
- 57. Rino Y, Takanashi Y, Yukawa N, Saeki H, Wada H, et al. (2006) A phase I study of bi-weekly combination therapy with S-1 and docetaxel for advanced or recurrent gastric cancer. Anticancer Res 26: 1455–1462. [PubMed] [Google Scholar]
- 58. Yamaguchi K, Shimamura T, Hyodo I, Koizumi W, Doi T, et al. (2006) Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer 94: 1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, et al. (2006) Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 12: 3402–3407. [DOI] [PubMed] [Google Scholar]
- 60. Kakeji Y, Oki E, Egashira A, Sadanaga N, Takahashi I, et al. (2009) Phase II study of biweekly docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Oncology 77: 49–52. [DOI] [PubMed] [Google Scholar]
- 61. Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, et al. (2010) Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol 66: 721–728. [DOI] [PubMed] [Google Scholar]
- 62. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6: e1000097 doi:10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]