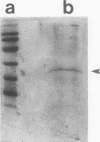
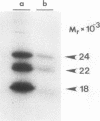
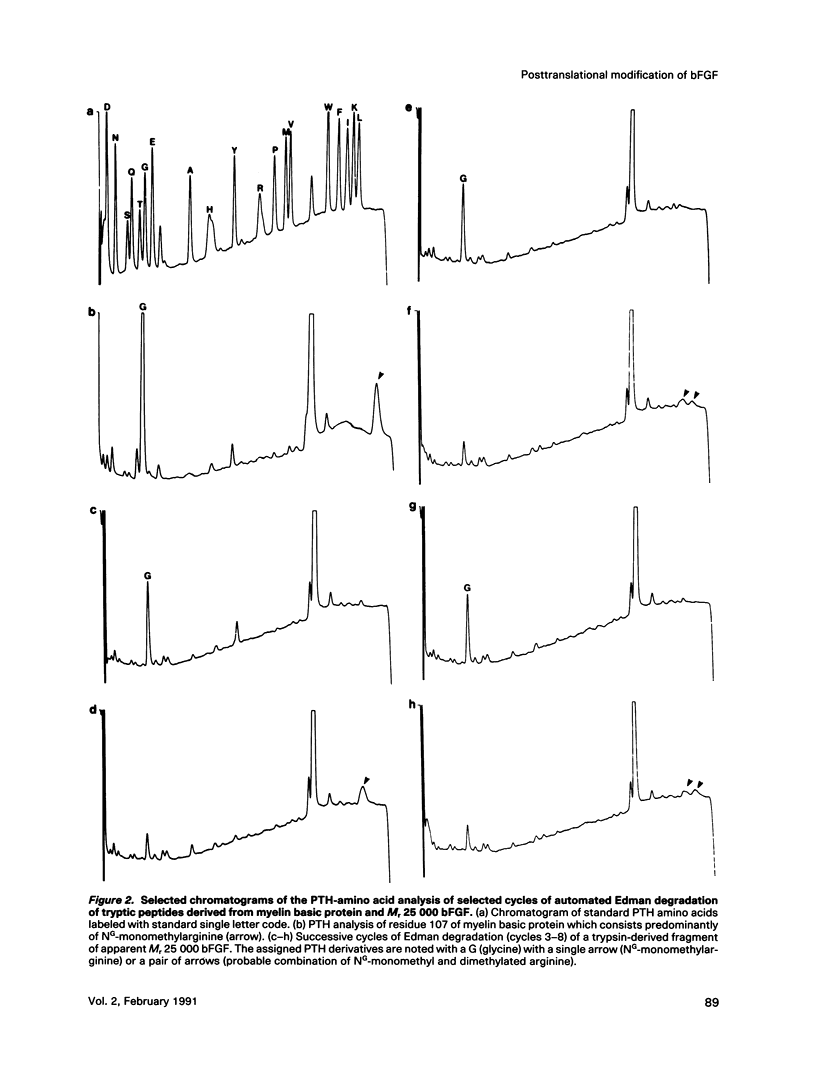
Abstract
Basic fibroblast growth factor (bFGF) is a heparin-binding angiogenic polypeptide mitogen. Protein sequence analysis of bFGF isolated from tissue sources initially established that it is composed of 146 amino acids (apparent Mr 18,000). More recently larger apparent molecular weight forms have been identified and partially characterized. In addition, these high molecular weight forms (apparent Mr 22,000 and 25,000) have been shown to localize preferentially to nuclear fractions of transfected cells. In this report we demonstrate that the higher molecular weight, amino terminally extended forms of bFGF contain methylated arginine residues. The demonstration is based on 1) amino acid sequence analysis of a protein known to contain methylated arginine (myelin basic protein) and a comparison with amino acid sequence analysis of trypsin-derived fragments of the high molecular weight bFGF purified from guinea pig brain and 2) the ability to label in vivo the high molecular weight forms of bFGF with S-adenosyl-L-(methyl-3H)-methionine, the substrate of arginine-protein methylase I. These results are suggestive of a role of arginine methylation in directing nuclear localization of certain forms of bFGF.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa L. C., Karn J., Vidali G., Allfrey V. G. Distribution of NG, NG,-dimethylarginine in nuclear protein fractions. Biochem Biophys Res Commun. 1977 Feb 7;74(3):969–976. doi: 10.1016/0006-291x(77)91613-8. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Sterner R., Vidali G., Allfrey V. G. Post-synthetic modifications of nuclear proteins. High mobility group proteins are methylated. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1322–1327. doi: 10.1016/0006-291x(79)92153-3. [DOI] [PubMed] [Google Scholar]
- Brostoff S., Eylar E. H. Localization of methylated arginine in the A1 protein from myelin. Proc Natl Acad Sci U S A. 1971 Apr;68(4):765–769. doi: 10.1073/pnas.68.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Marshak D. R., Fraser B. A., Maciag T. Structural evidence that endothelial cell growth factor beta is the precursor of both endothelial cell growth factor alpha and acidic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7216–7220. doi: 10.1073/pnas.83.19.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. N., Navickas I. J., Chang C. N., Dancis B. M. Methylation of ribosomal proteins in HeLa cells. Arch Biochem Biophys. 1976 Feb;172(2):627–633. doi: 10.1016/0003-9861(76)90117-x. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Beyer A. L., Walker B., Lestourgeon W. M. Identification of NG, NG-dimethylarginine in a nuclear protein from the lower eukaryote physarum polycephalum homologous to the major proteins of mammalian 40S ribonucleoprotein particles. Biochem Biophys Res Commun. 1977 Jan 24;74(2):621–629. doi: 10.1016/0006-291x(77)90348-5. [DOI] [PubMed] [Google Scholar]
- Cruz-Alvarez M., Pellicer A. Cloning of a full-length complementary DNA for an Artemia salina glycine-rich protein. Structural relationship with RNA binding proteins. J Biol Chem. 1987 Oct 5;262(28):13377–13380. [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F., Ghaffari S. H., Rao S. V., Dumbar T. S., Olson M. O. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa). J Biol Chem. 1986 Jul 15;261(20):9167–9173. [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Marvil D. K., Nowak L., Szer W. A single-stranded nucleic acid-binding protein from Artemia salina. I. Purification and characterization. J Biol Chem. 1980 Jul 10;255(13):6466–6472. [PubMed] [Google Scholar]
- Moscatelli D., Joseph-Silverstein J., Manejias R., Rifkin D. B. Mr 25,000 heparin-binding protein from guinea pig brain is a high molecular weight form of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5778–5782. doi: 10.1073/pnas.84.16.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Joseph-Silverstein J., Rifkin D. B. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol. 1986 Nov;129(2):273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Enzymatic methylation of protein fractions from calf thymus nuclei. Biochem Biophys Res Commun. 1967 Oct 11;29(1):14–20. doi: 10.1016/0006-291x(67)90533-5. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Wilson S. H. Studies on the structure of mouse helix-destabilizing protein-1. DNA binding and controlled proteolysis with trypsin. J Biol Chem. 1980 Dec 10;255(23):11547–11556. [PubMed] [Google Scholar]
- Presta M., Moscatelli D., Joseph-Silverstein J., Rifkin D. B. Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol. 1986 Nov;6(11):4060–4066. doi: 10.1128/mcb.6.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Brewer M. T., Thompson R. C., Moscatelli D., Presta M., Rifkin D. B. A form of human basic fibroblast growth factor with an extended amino terminus. Biochem Biophys Res Commun. 1987 Apr 29;144(2):543–550. doi: 10.1016/s0006-291x(87)80001-3. [DOI] [PubMed] [Google Scholar]
- Sommer A., Moscatelli D., Rifkin D. B. An amino-terminally extended and post-translationally modified form of a 25kD basic fibroblast growth factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1267–1274. doi: 10.1016/s0006-291x(89)80140-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]