Abstract
Background
COPD is characterized by variability in exercise capacity and physical activity (PA), and acute exacerbations (AEs). Little is known about the relationship between daily step count, a direct measure of PA, and the risk of AEs, including hospitalizations.
Methods
In an observational cohort study of 169 persons with COPD, we directly assessed PA with the StepWatch Activity Monitor, an ankle-worn accelerometer that measures daily step count. We also assessed exercise capacity with the 6-minute walk test (6MWT) and patient-reported PA with the St. George's Respiratory Questionnaire Activity Score (SGRQ-AS). AEs and COPD-related hospitalizations were assessed and validated prospectively over a median of 16 months.
Results
Mean daily step count was 5804±3141 steps. Over 209 person-years of observation, there were 263 AEs (incidence rate 1.3±1.6 per person-year) and 116 COPD-related hospitalizations (incidence rate 0.56±1.09 per person-year). Adjusting for FEV1 % predicted and prednisone use for AE in previous year, for each 1000 fewer steps per day walked at baseline, there was an increased rate of AEs (rate ratio 1.07; 95%CI = 1.003–1.15) and COPD-related hospitalizations (rate ratio 1.24; 95%CI = 1.08–1.42). There was a significant linear trend of decreasing daily step count by quartiles and increasing rate ratios for AEs (P = 0.008) and COPD-related hospitalizations (P = 0.003). Each 30-meter decrease in 6MWT distance was associated with an increased rate ratio of 1.07 (95%CI = 1.01–1.14) for AEs and 1.18 (95%CI = 1.07–1.30) for COPD-related hospitalizations. Worsening of SGRQ-AS by 4 points was associated with an increased rate ratio of 1.05 (95%CI = 1.01–1.09) for AEs and 1.10 (95%CI = 1.02–1.17) for COPD-related hospitalizations.
Conclusions
Lower daily step count, lower 6MWT distance, and worse SGRQ-AS predict future AEs and COPD–related hospitalizations, independent of pulmonary function and previous AE history. These results support the importance of assessing PA in patients with COPD, and provide the rationale to promote PA as part of exacerbation-prevention strategies.
Introduction
COPD is the fourth most common cause of death in the US, affects 5% of US adults, and accounts for a large number of hospitalizations [1], [2]. Persons with COPD have significantly reduced exercise capacity, measured by clinic-based tests such as the 6-minute walk test (6MWT), and reduced physical activity (PA), directly measured with accelerometers or assessed by questionnaires [3]–[10]. In addition, lower 6MWT distance and lower daily step count, a direct and novel measure of PA, are significant predictors of all-cause mortality in COPD [11], [12].
COPD is also characterized by acute exacerbations (AEs) which result in poorer health-related quality of life (HRQL), a faster decline in lung function, and increased mortality [13]–[17]. Hospitalizations due to AEs account for a large portion of COPD-related medical costs [18]. To date, the key known factors predicting AEs and hospitalizations are the degree of airflow obstruction and history of prior AEs [13], [19]–[23]. Since these factors are usually already maximized with medical pharmacological therapy, there is a need to identify additional factors that can be targeted for intervention [20].
Persons with COPD have a wide range of PA levels which may be potentially modifiable. The relationship between PA and risk of AEs and COPD-related hospitalizations is unclear [24]. Prior studies have been limited because they assessed PA only by self-report which is notoriously overestimated, did not account for prior AE history, or studied only severe AEs that resulted in hospitalizations [8], [9], [19], [25]–[26]. In this report, our primary aim is to examine the relationship between PA directly measured with an accelerometer and risk of moderate and severe AEs and COPD-related hospitalizations. We hypothesize that lower daily step count predicts greater risk of AEs and COPD-related hospitalizations, independent of lung function and prior AEs. As a secondary aim, we assess 6MWT, a commonly used clinic-based test of exercise capacity, and patient-report of PA with the St. George's Respiratory Questionnaire Activity Score (SGRQ-AS), and examine their relationships with risk of AEs and COPD-related hospitalizations.
Methods
Ethics Statement
The protocol was approved by the VA Boston Healthcare System Committee on Human Research, and written informed consent obtained from each participant.
Study Design and Participants
This work arises from a study which has been previously published, and the subjects reported here include the 127 subjects previously reported [27]. Between January 2009 and November 2011, we recruited eligible participants who were over 40 years of age and who had received care for COPD in the VA Boston Healthcare System general pulmonary clinics. The diagnosis of COPD was defined as having a smoking history of at least 10 pack-years and a ratio of forced expiratory volume in one second (FEV1) to forced vital capacity of <0.70 or evidence of emphysema on chest computed tomography. Exclusion criteria were inability to ambulate and occurrence of an AE within 4 weeks of enrollment [15].
At baseline, information about demographics, medical history, and medications was obtained. Subjects reported being at their usual clinical status at the time of enrollment. Participants underwent measurement of FEV1, using an Eaglet spirometer (nSpire Health, Inc.) [28]. The 6MWT was performed following ATS guidelines, except that a practice 6MWT was not done [29]. Patient-reported measures included assessments of dyspnea using the modified Medical Research Council (MMRC) scale [30], HRQL using the SGRQ, with scores ranging from 0–100 and lower scores indicating better HRQL [31], and depression using the Beck Depression Inventory [32]. The SGRQ has been recommended for use as a patient-reported measure of functional status, and we present the SGRQ-AS [3]. We considered a history of previous AEs as a potential confounder. To assess the past occurrence of AEs, participants were asked if they had received treatment with prednisone for breathing problems in the year prior to enrollment [13], [15], [23], [33].
The StepWatch Activity Monitor (SAM) (Orthocare Innovations, Seattle, WA, USA), an ankle-worn accelerometer, measures step counts from all walking–as part of PA and exercise in persons with COPD. We have shown the SAM to be accurate and valid in persons with COPD [27]. In this study, participants wore the SAM for 14 consecutive days and were instructed to perform their usual physical activities and exercise. The SAM does not provide on-instrument feedback of steps walked. Subjects returned the SAM to study staff by mail. Study staff downloaded the step counts, which are date and time stamped, via a docking station. No-wear days, defined as ones with <200 steps recorded and <8 hours of wear time, were excluded from the analysis [27], [34]. Subjects with ≥8 no-wear days were excluded.
Study Outcomes
The primary outcomes were AEs and COPD-related hospitalizations. AE was defined as “a complex of respiratory symptoms (increased or new onset) of at least two of the following: cough, sputum, wheezing, dyspnea, or chest tightness lasting 3 or more days, requiring a course of treatment with antibiotics or systemic steroids [15], [35].” We included moderate AEs that required treatment in the outpatient setting and severe AEs that required hospitalizations. We included hospitalizations due to AE and/or pneumonia as COPD-related hospitalizations. Hospitalizations due to other pulmonary or cardiac causes were excluded. After the baseline visit, participants were prospectively queried on the use of oral corticosteroids and/or antibiotics and hospitalizations for lung problems using a structured telephone interview every 3 months for a median of 16 months. Participant reports were verified with medical records. Two investigators, blinded to baseline characteristics, reviewed subject responses and medical records to determine if an AE had occurred and the primary cause of hospitalizations. Independence of events was assured by considering a new event only if subjects had been off oral corticosteroids and/or antibiotics for at least 14 days following the previous AE or COPD-related hospitalization [15], [36].
Statistical Analysis
Descriptive results are reported as means ± SD or percentages, as appropriate. Comparisons of descriptive characteristics were performed with the use of unpaired T tests or Fisher's Exact Test, as appropriate. The incidence rates of AEs and COPD-related hospitalizations were determined by dividing the numbers of AEs and COPD-related hospitalizations by the person-years of follow-up. Predictors of AEs and COPD-related hospitalizations were assessed using negative binomial models with the logarithm of observation time as an offset variable (PROC GENMOD, SAS 9.2, SAS Institute; Cary, NC) [23], [36]. In this approach, daily step count was examined as a continuous variable and the rate ratio calculated by exponentiating the regression coefficient. In separate models, daily step count was categorized in quartiles. Linear trend P values were derived using an ordinal variable coded based on the median of each step-count quartile. Variables significant at the P<0.05 level in univariate analyses were subsequently examined in multivariate models. All multivariate models included FEV1 % predicted and prednisone for AE in previous year as covariates. To put the results into clinical context, we calculated the rate ratios and 95% confidence intervals corresponding to published minimum clinically important differences (MCIDs) for the 6MWT and SGRQ-AS [4], [37]–[40].
Results
Study Participants
A total of 188 persons with COPD were enrolled. The analysis excluded 12 subjects who did not have baseline step-count data because 5 were noncompliant with step-count monitoring (had ≥8 no-wear days), 5 had no baseline step-count data due to an AE during the monitoring period, 1 lost the SAM, and 1 had SAM accuracy <90%. An additional 7 subjects did not participate in follow-up telephone calls. There were no differences in FEV1 % predicted, 6MWT distance, SGRQ Total Score (SGRQ-TS), SGRQ-AS, or MMRC dyspnea score among the 19 subjects excluded and the 169 subjects included in the analysis. In 169 persons with baseline and follow-up data, 167 were males, mean age was 71±8 years, mean FEV1 was 1.55±0.57 L (54±20% predicted) [41], and mean daily step count was 5804±3141 (Table 1). All 4 GOLD stages were represented; most subjects were GOLD II (46%) and GOLD III (33%) [2]. Twenty subjects (12%) had participated in a previous pulmonary rehabilitation program and 43 (25%) used supplemental oxygen. Four subjects died during follow-up.
Table 1. Subject Characteristics.* .
| Total | Mean Daily Step<5232+ | Mean Daily Step≥5232+ | |
| n = 169 | n = 85 | n = 84 | |
| Age† | 71±8 | 73±8 | 69±8 |
| Body-mass index† | 29±6 | 30±7 | 28±5 |
| Marital status† | |||
| Married | 76 (45) | 46 (54) | 30 (36) |
| Not married | 93 (55) | 39 (46) | 54 (64) |
| Race | |||
| White | 156 (92) | 79 (93) | 77 (92) |
| Non-White | 13 (8) | 6 (7) | 7 (8) |
| Employment status† | |||
| Full or part-time | 20 (12) | 5 (6) | 15 (18) |
| Not working | 42 (25) | 18 (21) | 24 (28) |
| Retired | 107 (63) | 62 (73) | 45 (54) |
| Education | |||
| Some/Completed high school | 76 (45) | 40 (47) | 36 (43) |
| Some/Completed college or higher | 93 (55) | 45 (53) | 48 (57) |
| Alcohol use | |||
| ≥1 day/week | 52 (31) | 30 (35) | 22 (26) |
| <1 day/week | 117 (69) | 55 (65) | 62 (74) |
| Prior participation in pulmonary rehabilitation† | 20 (12) | 16 (19) | 4 (5) |
| Supplemental oxygen use† | 43 (25) | 32 (38) | 11 (13) |
| Prednisone for AE in previous year | 51 (30) | 31 (36) | 20 (24) |
| Coronary artery disease† | 63 (37) | 39 (46) | 24 (29) |
| Congestive heart failure | 23 (14) | 15 (18) | 8 (10) |
| Diabetes mellitus | 48 (28) | 24 (28) | 24 (29) |
| Pack-years | 68±37 | 70±33 | 66±40 |
| FEV1 (liters)† | 1.55±0.57§ | 1.42±0.57 | 1.68±0.55¶ |
| FEV1, % predicted† | 54±20§ | 51±20 | 58±20¶ |
| GOLD stage† | |||
| I | 16 (10)§ | 6 (7) | 10 (12)¶ |
| II | 77 (46) | 35 (41) | 42 (51) |
| III | 56 (33) | 28 (33) | 28 (34) |
| IV | 19 (11) | 16 (19) | 3 (4) |
| 6MWT distance (meters)† | 371±100 | 319±90 | 423±81 |
| MMRC dyspnea score† | |||
| 0–1 | 68 (40) | 20 (24) | 48 (57) |
| 2–4 | 101 (60) | 65 (76) | 36 (43) |
| SGRQ-TS† | 45±20 | 49±18 | 42±20 |
| SGRQ-AS† | 63±23 | 69±19 | 57±25 |
| Beck depression index | 12±11 | 11±11 | 12±11 |
| Medication for COPD‡ | |||
| Any short-acting β2 agonist | 150 (89) | 78 (92) | 72 (86) |
| Any short-acting muscarinic antagonist | 29 (17) | 18 (21) | 11 (13) |
| Any long-acting β2 agonist | 108 (64) | 56 (66) | 52 (62) |
| Any long-acting muscarinic antagonist | 125 (74) | 63 (74) | 62 (74) |
| Any inhaled corticosteroid | 115 (68) | 61 (72) | 54 (64) |
AE denotes acute exacerbation; FEV1 forced expiratory volume in 1 second; GOLD Global Initiative for Chronic Obstructive Lung Disease; 6MWT 6-minute walk test; MMRC Modified Medical Research Council; SGRQ-TS St. George's Respiratory Questionnaire Total Score; and SGRQ-AS St. George's Respiratory Questionnaire Activity Score.
Mean ± standard deviation for continuous variables and N (%) for categorical variables.
The median average daily step count is 5,232.
P Value<0.05; Unpaired T-test (continuous variables) or Fisher's Exact Test (categorical variables).
Information on medication was self-reported; subjects may have been taking more than one medication.
N = 168.
N = 83.
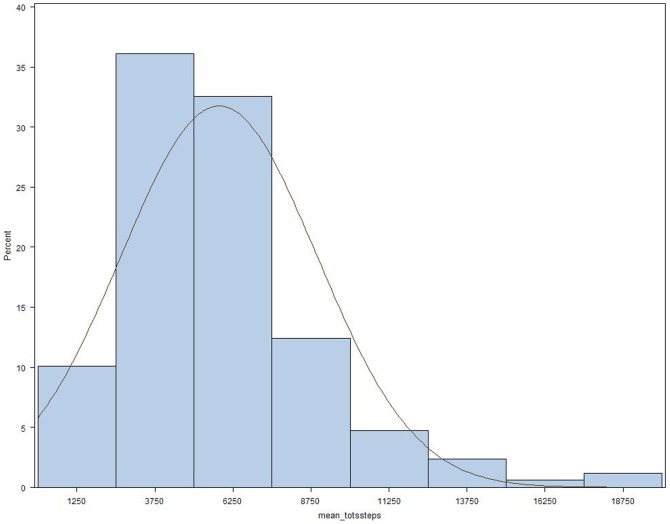
Figure 1 shows the distribution of average daily step count. Of 2,366 days monitored, only 3% (n = 81) met the definition of a no-wear day. Compared to subjects with average daily step count ≥ the median average of 5,232, those with a daily step count <5,232 were significantly older, and had higher body-mass index (BMI), lower FEV1 % predicted, lower 6MWT distance, higher MMRC dyspnea score, worse SGRQ-TS, worse SGRQ-AS, and higher frequency of supplemental oxygen use and coronary artery disease (Table 1). In the 51 subjects (30%) who had used prednisone for breathing problems in the year prior to enrollment, mean daily step count was 4956±2608, which was significantly lower than the 6170±3288 steps per day observed in the 118 subjects who had not used prednisone (P = 0.012).
Figure 1. Distribution of mean daily step count.
Median is 5,232 steps per day, N = 169.
Outcome Assessment
Over 209 person-years of follow-up, there were 263 AEs (incidence rate 1.3±1.6 per person-year) in 99 of 169 subjects (59%). Of these, 167 AEs were experienced by 54 of the 85 persons with daily step count < the median, and 96 AEs were experienced by 45 of the 84 persons with daily step count ≥ the median. There were 116 COPD-related hospitalizations (incidence rate 0.56±1.09 per person-year) in 54 of 169 subjects (32%). Of these, 79 hospitalizations were experienced by 37 persons with daily step count < the median, and 37 hospitalizations were experienced by 17 persons with daily step count ≥ the median. 224 AEs (85%) and 108 COPD-related hospitalizations (93%) were verified with medical records.
In univariate models, lower daily step count was a significant predictor of higher rates of future AEs and COPD-related hospitalizations (Table 2). There was a significant linear trend of decreasing daily step count by quartiles and increasing rate ratios for AEs (P = 0.0003) and COPD-related hospitalizations (P = 0.0003). Lower 6MWT distance and worse SGRQ-AS were also significantly associated with higher rate ratios for AEs and COPD-related hospitalizations (Table 2). Lower FEV1 % predicted and prednisone for AE in previous year were significantly associated with a higher rate ratio for AEs and COPD-related hospitalizations. Worse SGRQ-TS and supplemental oxygen use also significantly predicted future AEs. Age, BMI, pack-years, history of diabetes mellitus or coronary artery disease, MMRC dyspnea score, Beck's depression index, and season were not related to the risk of AEs and COPD-related hospitalizations.
Table 2. Univariate Associations with Number of Acute Exacerbations and COPD-Related Hospitalizations.
| Characteristics | Acute Exacerbations | COPD-Related Hospitalizations | ||||
| Rate Ratio | 95% CI | P Value | Rate Ratio | 95% CI | P Value | |
| Age (per year increase) | 1.02 | 0.99–1.04 | 0.13 | 1.03 | 0.995–1.07 | 0.08 |
| Body-mass index (per kg/m2 increase) | 0.996 | 0.96–1.03 | 0.81 | 0.96 | 0.92–1.01 | 0.16 |
| Mean Daily Step Count (per 1000 step decrease) | 1.11 | 1.04–1.19 | 0.003 | 1.29 | 1.13–1.49 | 0.0003 |
| Mean daily step Quartiles | ||||||
| (ref ≥6956) | ||||||
| <3667 | 3.00 | 1.68–5.36 | 0.0002 | 8.69 | 2.92–25.8 | <0.0001 |
| 3667≤×<5232 | 2.62 | 1.46–4.71 | 0.001 | 6.94 | 2.31–20.9 | 0.0006 |
| 5232≤×<6956 | 2.36 | 1.30–4.27 | 0.005 | 6.80 | 2.25–20.6 | 0.0007 |
| P for linear trend | 0.0003 | 0.0003 | ||||
| 6MWT distance | 1.10 | 1.03–1.17 | 0.003 | 1.21 | 1.10–1.34 | 0.0002 |
| (per 30-meter decrease§) | ||||||
| SGRQ-AS (per 4-point worsening§) | 1.07 | 1.03–1.12 | 0.0005 | 1.12 | 1.04–1.19 | 0.002 |
| FEV1, % predicted† | 1.13 | 1.02–1.25 | 0.01 | 1.22 | 1.05–1.42 | 0.008 |
| (per 10% decrease in % of predicted value) | ||||||
| Prednisone for AE in previous year (ref = no) | 2.44 | 1.66–3.58 | <0.0001 | 2.16 | 1.17–4.00 | 0.01 |
| SGRQ-TS | 1.07 | 1.03–1.12 | 0.002 | 1.09 | 1.01–1.17 | 0.02 |
| (per 4-point worsening) | ||||||
| MMRC dyspnea score 2–4 (ref = 0–1) | 1.47 | 0.97–2.22 | 0.07 | 1.67 | 0.89–3.14 | 0.11 |
| Supplemental oxygen use (ref = no) | 1.56 | 1.01–2.40 | 0.04 | 1.50 | 0.77–2.91 | 0.23 |
| Pack-years | 1.003 | 0.998–1.01 | 0.27 | 1.001 | 0.99–1.01 | 0.87 |
| Diabetes mellitus | ||||||
| (ref = no) | 1.14 | 0.73–1.79 | 0.55 | 1.23 | 0.63–2.41 | 0.54 |
| Coronary artery disease | 1.08 | 0.71–1.65 | 0.72 | 0.90 | 0.47–1.73 | 0.75 |
| (ref = no) | ||||||
| Beck depression index | 1.01 | 0.99–1.02 | 0.50 | 1.005 | 0.98–1.03 | 0.74 |
| Season of step count monitoring | ||||||
| (ref = Summer) | ||||||
| Fall | 0.85 | 0.51–1.40 | 0.52 | 0.98 | 0.45–2.13 | 0.97 |
| Winter | 0.80 | 0.40–1.61 | 0.54 | 0.61 | 0.20–1.88 | 0.39 |
| Spring | 0.79 | 0.44–1.41 | 0.43 | 1.14 | 0.48–2.73 | 0.76 |
6MWT denotes 6-minute walk test; SGRQ-AS St. George's Respiratory Questionnaire Activity Score; FEV1 forced expiratory volume in 1 second; AE acute exacerbation; SGRQ-TS St. George's Respiratory Questionnaire Total Score; MMRC Modified Medical Research Council; and ref reference group.
Rate ratios calculated for a MCID of 30 m [37] for 6MWT and 4 units [42] for SGRQ-AS. The regression coefficients (SE) in natural log risk per 30-m decrease in 6MWT predicting AEs and COPD-related hospitalizations are 0.0976 (0.0323) and 0.1946 (0.0516), respectively. The regression coefficients (SE) in natural log risk per 4-unit decrease in SGRQ-AS predicting AEs and COPD-related hospitalizations are 0.0712 (0.0206) and 0.1091 (0.0347), respectively.
N = 168.
In multivariate models adjusting for FEV1 % predicted and prednisone for AE in previous year, for each 1000 fewer steps per day walked at baseline, there was a significantly increased rate of AEs (rate ratio 1.07; 95%CI = 1.003–1.15) and COPD-related hospitalizations (rate ratio 1.24; 95%CI = 1.08–1.42) (Table 3). The rate ratio for AEs and COPD-related hospitalizations in each step-count quartile was significantly increased compared to the rate ratio for persons in the highest step-count quartile (Table 3). Compared to persons in the highest step-count quartile, persons in the lowest quartile had a rate ratio of 2.26 (95%CI = 1.25–4.08) for AEs and 6.01 (95%CI = 1.99–18.2) for COPD-related hospitalizations. There was a significant linear trend of decreasing daily step count by quartiles and increasing rate ratios for AEs (P = 0.008) and COPD-related hospitalizations (P = 0.003).
Table 3. Multivariate Models of Associations between Daily Step Count and Number of Acute Exacerbations and COPD-Related Hospitalizations Adjusting for FEV1 % Predicted and Prednisone for AE in Previous Year.* .
| Model 1† | Acute Exacerbations | COPD-Related Hospitalizations | ||||||
| Rate Ratio | 95% CI | P value | Rate Ratio | 95% CI | P value | |||
| FEV1, % predicted (per 10% increase in % of predicted value) | 1.05 | 0.95 | 1.16 | 0.33 | 1.14 | 0.98 | 1.32 | 0.09 |
| Prednisone for AE in previous year (ref = no) | 2.17 | 1.48 | 3.18 | <0.0001 | 1.72 | 0.94 | 3.13 | 0.08 |
| Mean Daily Step Count (per 1000 step decrease) | 1.07 | 1.003 | 1.15 | 0.04 | 1.24 | 1.08 | 1.42 | 0.003 |
FEV1 denotes forced expiratory volume in 1 second; AE acute exacerbation; and ref reference group.
N = 168.
Two separate multivariate models. Model 1 examines daily step count as a continuous variable. Model 2 examines daily step count in quartiles.
Similarly, in multivariate models, lower 6MWT distance and worse SGRQ-AS were significant predictors of AEs and COPD-related hospitalizations, independent of FEV1 % predicted and prednisone for AE in previous year (Tables 4 and 5). A decrease of 30 meters (37) in 6MWT distance was associated with an increased rate ratio of 1.07 (95%CI = 1.01–1.14) for AEs and 1.18 (95%CI = 1.07–1.30) for COPD-related hospitalizations. A worsening of SGRQ-AS by 4 points [42] was associated with an increased rate ratio of 1.05 (95%CI = 1.01–1.09) for AEs and 1.10 (95%CI = 1.02–1.17) for COPD-related hospitalizations. SGRQ-TS and supplemental oxygen use were not significantly associated with risk of AEs and COPD-related hospitalizations in multivariate models, adjusting for FEV1 % predicted and prednisone for AE in previous year.
Table 4. Multivariate Model of Associations between 6MWT distance and Number of Acute Exacerbations and COPD-Related Hospitalizations Adjusting for FEV1 % Predicted and Prednisone for AE in Previous Year.* .
| Acute Exacerbations | COPD-Related Hospitalizations | |||||||
| Rate Ratio | 95% CI | P value | Rate Ratio | 95% CI | P value | |||
| FEV1, % predicted (per 10% increase in % of predicted value) | 1.06 | 0.96 | 1.17 | 0.23 | 1.15 | 0.99 | 1.33 | 0.06 |
| Prednisone for AE in previous year (ref = no) | 2.14 | 1.46 | 3.14 | 0.0001 | 1.71 | 0.95 | 3.07 | 0.08 |
| 6MWT distance (per 30-meter decrease§) | 1.07 | 1.01 | 1.14 | 0.03 | 1.18 | 1.07 | 1.30 | 0.001 |
FEV1 denotes forced expiratory volume in 1 second; AE acute exacerbation; ref reference group; and 6MWT denotes 6-minute walk test.
N = 168.
Rate ratios calculated for a MCID of 30 m for 6MWT. The regression coefficients (SE) in natural log risk per 30-m decrease in 6MWT distance predicting AEs and COPD-related hospitalizations are 0.0674 (0.0308) and 0.1624 (0.0502), respectively.
Table 5. Multivariate Model of Associations between SGRQ-AS and Number of Acute Exacerbations and COPD-Related Hospitalizations Adjusting for FEV1 % Predicted and Prednisone for AE in Previous Year.* .
| Acute Exacerbations | COPD-Related Hospitalizations | |||||||
| Rate Ratio | 95% CI | P value | Rate Ratio | 95% CI | P value | |||
| FEV1, % predicted (per 10% increase in % of predicted value) | 1.07 | 0.97 | 1.17 | 0.17 | 1.17 | 1.02 | 1.36 | 0.03 |
| Prednisone for AE in previous year (ref = no) | 1.99 | 1.34 | 2.95 | 0.0006 | 1.60 | 0.87 | 2.94 | 0.13 |
| SGRQ-AS (per 4-point worsening§) | 1.05 | 1.01 | 1.09 | 0.02 | 1.10 | 1.02 | 1.17 | 0.008 |
FEV1 denotes forced expiratory volume in 1 second; AE acute exacerbation; ref reference group; and SGRQ-AS St. George's Respiratory Questionnaire Activity Score.
N = 168.
Rate ratios calculated for a MCID of 4 units for SGRQ-AS. The regression coefficients (SE) in natural log risk per 4-unit decrease in SGRQ-AS predicting AEs and COPD-related hospitalizations are 0.0484 (0.0202) and 0.0923 (0.0347), respectively.
Discussion
Our results demonstrate that persons with COPD with lower daily step count have significantly higher rate ratios for AEs and COPD-related hospitalizations, independent of FEV1 % predicted and previous exacerbation history. These novel findings are further supported by the significant linear associations over the entire range of daily step counts with rate ratios for AEs and COPD-related hospitalizations. Our results strongly support the rationale to study PA promotion as part of future exacerbation-prevention interventions in COPD.
A strength of our study is the use of 3 complementary measures of functional status to assess exacerbation risk prospectively in the same cohort. We examined daily step count as a direct measure of PA in the community, 6MWT as a clinic-based test of exercise capacity, and the SGRQ-AS as a patient-reported assessment of PA. Our data demonstrate that the relationship between PA and exacerbation risk is robust since daily step count predicts AE and COPD-related hospitalizations in a similar fashion as 6MWT distance and SGRQ-AS. Our results add to the evidence that daily step count is an important clinical characteristic of persons with COPD that can complement the 6MWT and questionnaire assessment of PA [43].
We focus on daily step count because it can be easily and directly translatable from the research to the clinical setting. Daily step count is a meaningful and relevant metric that, from the public health standpoint, can help define PA recommendations and promote PA in persons with COPD [44]. Healthcare providers and patients understand what it means to target PA goals to increase daily step counts. It is not feasible for providers to advise patients to improve their 6MWT distance since it is a clinic-based measure of exercise capacity that has little meaning to an individual. We previously published a pilot study showing that it is feasible for patients to monitor daily step count with a pedometer, and that persons with COPD can increase their walking with step-count goals [45].
In addition, directly measured daily step count overcomes limitations of questionnaire assessments of PA. First, it is well-known that persons overestimate self-reported physical activity. Second, the SGRQ-AS is used primarily in research settings and has no obvious meaning to most clinicians and all patients. Finally, prior studies using self-reported PA to examine risk for AEs/hospitalizations crudely characterized PA as ≥2 hours per week versus <2 hours per week [8], [25]. In contrast, directly measured daily step count allows accurate characterization of the relationship between PA and risk of AEs and hospitalizations so that future intervention studies can be appropriately designed.
To date, history of previous AEs has emerged as the strongest predictor of future AEs and hospitalizations, and FEV1 % predicted has been consistently found to be a significant predictor of future AEs and hospitalizations [13], [19]–[23]. However, spirometry alone is an inadequate predictor as there is a subset of patients with severely reduced FEV1 % predicted who do not experience frequent AEs [13]. We have identified daily step count, 6MWT distance, and SGRQ-AS as significant predictors of risk of AEs and COPD-related hospitalizations, independent of FEV1 % predicted. Furthermore, we show that lower daily step count, lower 6MWT distance, and worse SGRQ-AS predict future AEs and COPD-related hospitalizations, regardless of previous AE history. Our results that 6MWT and SGRQ-AS are independent predictors of risk of AEs and COPD-related hospitalizations are consistent with previously published studies. Two previous studies have shown that 6MWT distance predicts AEs in univariate, but not multivariate models [13], [46]. A lower 6MWT distance has been shown to predict hospitalizations in univariate but not multivariate models, which did not account for previous AEs or hospitalizations [19]. Epidemiological studies have shown a significant trend of decreasing physical activity, assessed by self-report, and increased risk of COPD-related admissions and hospitalization readmissions for COPD exacerbation [8], [9]. We calculated the rate ratios corresponding to a range of potentially clinically relevant changes in 6MWT to put our results into greater clinical context (Tables 4 and 6) [4], [37]–[40]. Similarly, we calculated the rate ratios that correspond to a 4-point worsening in SGRQ-AS. As MCID data for the SGRQ-AS are not available, we extrapolated the MCID of 4 units based on the SGRQ-TS [31], [42].
Table 6. Calculated Rate Ratios for Published MCIDs for 6MWT Distance.
| 6MWT Distance MCIDs | Acute Exacerbations | COPD-Related Hospitalizations | ||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | |
| Per 25 meter decrease [4], [38] | 1.058 | 1.006–1.112 | 1.145 | 1.055–1.243 |
| Per 35 meter decrease [39] | 1.082 | 1.008–1.161 | 1.209 | 1.078–1.356 |
| Per 54 meter decrease [40] | 1.129 | 1.013–1.258 | 1.340 | 1.122–1.599 |
MCID denotes minimum clinically important difference; and 6MWT 6-minute walk test.
A host of variables including chronic hypercapnia, pulmonary hypertension, hypoxemia, current smoking, older age, lower BMI, higher MMRC dyspnea score, and season have been inconsistently associated with AEs or hospitalizations in previous studies [19]–[23], [47]. We did not find an association between pack-years, age, BMI, MMRC dyspnea score, oxygen use, prior pulmonary rehabilitation, or season with AEs and COPD-related hospitalizations. Our results may reflect differences in age, gender, and FEV1 among cohorts and we accounted for potential confounding factors. Our study extends the generalizability and significance of the current literature by (1) including persons with all COPD severities, (2) studying moderate AEs as well as severe AEs, (3) directly measuring PA, and (4) including persons with COPD who have never participated in pulmonary rehabilitation [25], [26], [46].
Compared to other studies, our higher mean % predicted FEV1 is due to the fact that we included persons with all stages of COPD, including GOLD I or mild COPD. Our study was designed to be as inclusive as possible to increase generalizability, and thus included persons with all GOLD stages of COPD severity. Our cohort has similar frequencies of moderate COPD (GOLD II) and very severe (GOLD IV) as previously published clinical trials in COPD such as Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) [13] and Understanding the Potential Long-term Impacts on Function with Tiotropium (UPLIFT) [48], supporting that our cohort represents the entire range of disease severity. Interestingly, our results are obtained in a cohort that has been largely medically optimized, indicating the importance of factors other than pharmacological therapy in managing exacerbation risk. Sixty-four percent of subjects were using a long-acting β2 agonist and 74% a long-acting muscarinic antagonist.
The main strengths of our study include our use of 3 complementary measures of functional status, our validated method of measuring daily step count, our structured approach to obtaining a prospective history of AEs and COPD-related hospitalizations, and the high percentage of events confirmed with medical records. We used an a priori event-based definition of AE that was easy for patients to recall and had blinded adjudication of events [15], [36]. Given our clear definition of AEs, every 3 month follow-up, and medical chart review, it is unlikely that we missed any AEs as we defined them. Much of the current literature has reported on risk factors only for severe AEs that resulted in hospitalizations [8], [9], [19], [20], [23], [25], [26]. Our inclusion of moderate AEs that required medication treatment but not hospitalization broadens the significance of our results, as most AEs do not result in hospitalizations. It is possible that we did not capture mild AEs not requiring treatment. Nevertheless, the incidence rate for AEs of 1.3 per person-year observed in our study is comparable to incidence rates reported in the placebo groups of large COPD clinical trials [13], [33], [48].
Some limitations need to be considered. We did not capture upper extremity activities. However, total daily PA has been shown to be closely related to leg activity in persons with COPD [49]. We did not measure the intensity of walking or activities such as swimming or bicycling that do not result in step counts. Previously, devices used to assess PA have reported relatively obscure units such as “activity units” which are difficult to understand and do not allow comparison between studies. The devices used in previous studies include (1) the RT3 accelerometer that reports physical activity movements using “vector magnitude units” (VMUs); (2) the Dynaport activity monitor that reports time spent in walking, cycling, standing, sitting, or lying; and (3) the SenseWear armband that reports total daily energy expenditure which is then converted to a “physical activity level” [6], [7]. Depew et al. have shown that daily step count is a surrogate for physical activity level, with daily step count <4,580 reflecting severe inactivity or physical activity level <1.40 [50]. Since walking is a common PA and steps per day is a measure of PA that is easy to understand, steps per day is a clinically relevant and practical variable to monitor in persons with COPD.
We did not track daily step count during follow-up, but we have previously shown that daily step count does not change significantly over time in stable COPD [27]. Therefore misclassification of baseline step counts is unlikely. The follow-up period, a median of 16 months, could be considered short, but it was sufficient to see a significant association between daily step count and risk of AEs and COPD-related hospitalizations. In addition, new events, such as a stroke or hip replacement, which may affect walking are more likely to occur with a longer follow-up period, confounding the relationship between daily step count and risk of AEs and COPD-related hospitalizations.
We considered a history of previous AEs requiring therapy with corticosteroids as a potential confounder since AEs tend to recur in the same person. To assess AEs in the year prior to study enrollment, we asked, ‘Have you used prednisone for breathing problems in the past year?’ This approach of adjustment for previous exacerbations has been used in previously published studies [13], [15], [23]. The report of prednisone use before study entry is distinct from the definition of AEs assessed prospectively, “a complex of respiratory symptoms (increased or new onset) of at least two of the following: cough, sputum, wheezing, dyspnea, or chest tightness lasting 3 or more days, requiring a course of treatment with antibiotics or systemic steroids,” as previously used in large clinical studies of COPD exacerbations [13], [15], [23].
We did not collect information on the time period between participation in a pulmonary rehabilitation program and participation in this study. In case the results are biased because the 20 subjects who had ever participated in pulmonary rehabilitation had an increased daily step count, we performed a sensitivity analysis excluding the 20 subjects. We found similar results, and thus, included the 20 subjects in the final results. Finally, these results need to be confirmed in larger studies, and intervention studies are needed to assess whether increases in daily step count reduce AE and COPD-related hospitalization risk in persons with COPD.
In conclusion, these results provide evidence for the importance of daily step count as a determinant of health status and exacerbation risk in persons with COPD. Our results suggest that there is a subgroup of COPD patients with low daily step count who have significantly increased risk of AEs and COPD-related hospitalizations, regardless of their % predicted FEV1. We speculate that the “low walker” may be a novel COPD phenotype. In contrast to other proposed phenotypes defined by frequent exacerbations, radiologic differences, or persistent systemic inflammation, the PA phenotype is potentially amenable to behavioral modification [13], [47], [51].
Funding Statement
The research reported here was supported by the Department of Veteran Affairs, Veterans Health Administration, Rehabilitation Research and Development Service through a VA Career Development Award to Dr. Moy. Supported in part by CIMIT: Center for Integration of Medicine and Innovative Technology (Dr. Moy). Supported in part by VA Rehabilitation Research and Development Merit Review Grant B6618R (Dr. Garshick). This study was initiated by the investigators. The results of the present study do not constitute endorsement of the StepWatch Activity Monitor by the authors. Orthocare Innovations had no involvement in the study design, the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the paper for publication. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mannino DM, Braman S (2007) The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 4: 502–506. [DOI] [PubMed] [Google Scholar]
- 2. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. (2007) Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 3. Kocks JW, Asijee GM, Tsiligianni IG, Kerstjens HA, van der Molen T (2011) Functional status measurement in COPD: a review of available methods and their feasibility in primary care. Prim Care Respir J 20: 269–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, et al. (2011) The minimal important difference of exercise tests in severe COPD. Eur Respir J 37: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moy ML, Matthess K, Stolzmann K, Reilly J, Garshick E (2009) Free-living physical activity in COPD: assessment with accelerometer and activity checklist. J Rehabil Res Dev 46: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, et al. (2005) Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 972–977. [DOI] [PubMed] [Google Scholar]
- 7. Watz H, Waschki B, Meyer T, Magnussen H (2009) Physical activity in patients with COPD. Eur Respir J 33: 262–272. [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM (2006) Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 61: 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, et al. (2003) Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 58: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waschki B, Spruit MA, Watz H, Albert PS, Shrikrishna D, et al. (2012) Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med 106: 522–530. [DOI] [PubMed] [Google Scholar]
- 11. Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, et al. (2006) Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 173: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waschki B, Kirsten A, Holz O, Müller KC, Meyer T, et al. (2011) Physical activity is the strongest predictor of all-cause mortality in patients with COPD. A prospective cohort study. Chest 140: 331–342. [DOI] [PubMed] [Google Scholar]
- 13. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, et al. (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez-Roisin R (2000) Toward a consensus definition for COPD exacerbation. Chest 117: 398S–401S. [DOI] [PubMed] [Google Scholar]
- 15. Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, et al. (2011) Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr, Desbiens N, et al. (1996) Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med 154: 959–967. [DOI] [PubMed] [Google Scholar]
- 17. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, et al. (1998) Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157: 1418–1422. [DOI] [PubMed] [Google Scholar]
- 18. Strassels SA, Smith DH, Sullivan SD, Mahajan PS (2001) The costs of treating COPD in the United States. Chest 119: 344–352. [DOI] [PubMed] [Google Scholar]
- 19. Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E (1999) Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 158–164. [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Aymerich J, Monsó E, Marrades RM, Escarrabill J, Félez MA, et al. (2001) Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 164: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 21. Niewoehner DE, Lokhnygina Y, Rice K, Kuschner WG, Sharafkhaneh A, et al. (2007) Risk indexes for exacerbations and hospitalizations due to COPD. Chest 131: 20–28. [DOI] [PubMed] [Google Scholar]
- 22. Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, et al. (2012) Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J 39: 38–45. [DOI] [PubMed] [Google Scholar]
- 23. Wells JM, Washko GR, Han MK, Abbas N, Nath H, et al. (2012) Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 367: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seidel D, Cheung A, Suh ES, Raste Y, Atakhorrami M, et al. (2012) Physical inactivity and risk of hospitalization for chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 16: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 25. Benzo RP, Chang CCH, Farrell MH, Kaplan R, Ries A, et al. (2010) Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration 80: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, et al. (2012) Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc 13: 291–297. [DOI] [PubMed] [Google Scholar]
- 27. Moy ML, Danilack VA, Weston NA, Garshick E (2012) Daily step counts in a US cohort with COPD. Respir Med 106: 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 29. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test (2002) Am J Respir Crit Care Med 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 30. Mahler DA, Wells CK (1988) Evaluation of clinical methods for rating dyspnea. Chest 93: 580–586. [DOI] [PubMed] [Google Scholar]
- 31. Jones PW, Quirk FH, Baveystock CM, Littlejohns P (1992) A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 32. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 33. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 34. Matthews CE, Hagstromer M, Pober DM, Bowles HR (2012) Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc 44: S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA Jr, et al. (2005) Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 143: 317–326. [DOI] [PubMed] [Google Scholar]
- 36. Aaron SD, Fergusson D, Marks GB, Suissa S, Vandemheen KL, et al. (2008) Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax 63: 122–128. [DOI] [PubMed] [Google Scholar]
- 37. Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, et al. (2012) Six minute walk test in COPD: Minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 187: 382–386. [DOI] [PubMed] [Google Scholar]
- 38. Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, et al. (2010) Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 91: 221–225. [DOI] [PubMed] [Google Scholar]
- 39. Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, et al. (2008) Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 32: 637–643. [DOI] [PubMed] [Google Scholar]
- 40. Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH (1997) Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med 155: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 41. Hankinson JL, Odencrantz JR, Fedan KB (1999) Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 42. Jones PW (2005) St. George's Respiratory Questionnaire: MCID. COPD 2: 75–79. [DOI] [PubMed] [Google Scholar]
- 43. vanGestel AJR, Clarenbach CF, Stowhas AC, Rossi VA, Sievi NA, et al. (2012) Predicting daily physical activity in patients with chronic obstructive pulmonary disease. PLOS ONE 7: e48081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee IM, Buchner DM (2008) The importance of walking to public health. Med Sci Sports Exerc 40: S512–S518. [DOI] [PubMed] [Google Scholar]
- 45. Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR (2012) A pilot study of an Internet walking program and pedometer in COPD. Respir Med 106: 1342–1350. [DOI] [PubMed] [Google Scholar]
- 46. Faganello MM, Tanni SE, Sanchez FF, Pelegrino NR, Lucheta PA, et al. (2010) BODE index and GOLD staging as predictors of 1-year exacerbation risk in chronic obstructive pulmonary disease. Am J Med Sci 339: 10–14. [DOI] [PubMed] [Google Scholar]
- 47. Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, et al. (2011) Chronic obstructive pulmonary disease exacerbations in the COPD Gene study: associated radiologic phenotypes. Radiology 261: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, et al. (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 49. Walker PP, Burnett A, Flavahan PW, Calverley PMA (2008) Lower limb activity and its determinants in COPD. Thorax 63: 683–689. [DOI] [PubMed] [Google Scholar]
- 50. Depew ZS, Novotny PJ, Benzo RP (2012) How many steps are enough to avoid severe physical inactivity in patients with chronic obstructive pulmonary disease? Respirology 17: 1026–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, et al. (2012) Persistent systematic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLOS ONE 7: e37483. [DOI] [PMC free article] [PubMed] [Google Scholar]