Abstract
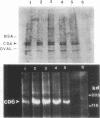
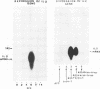
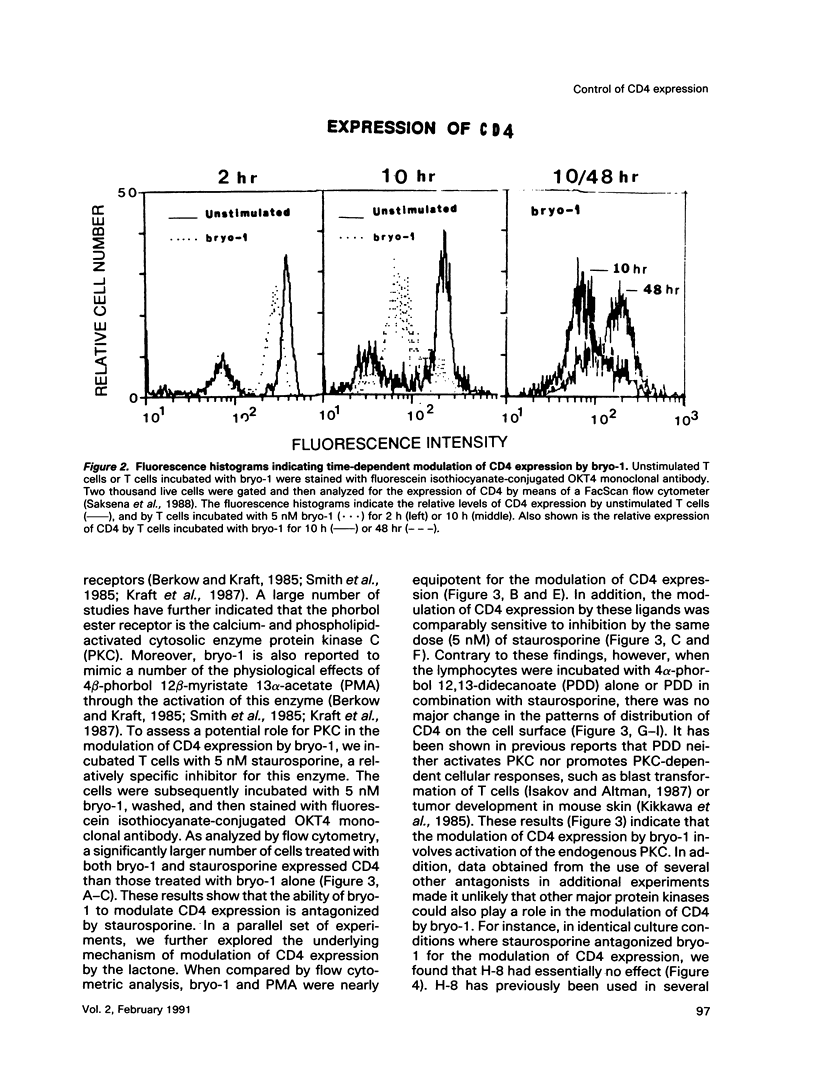
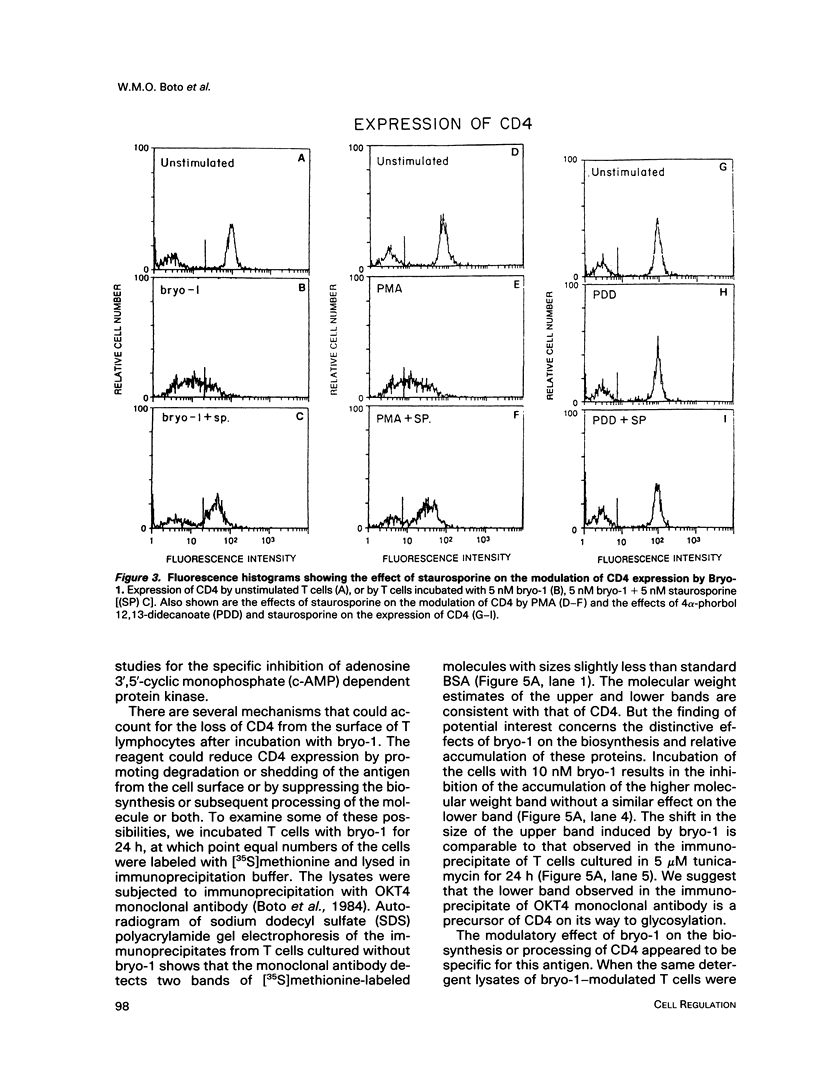
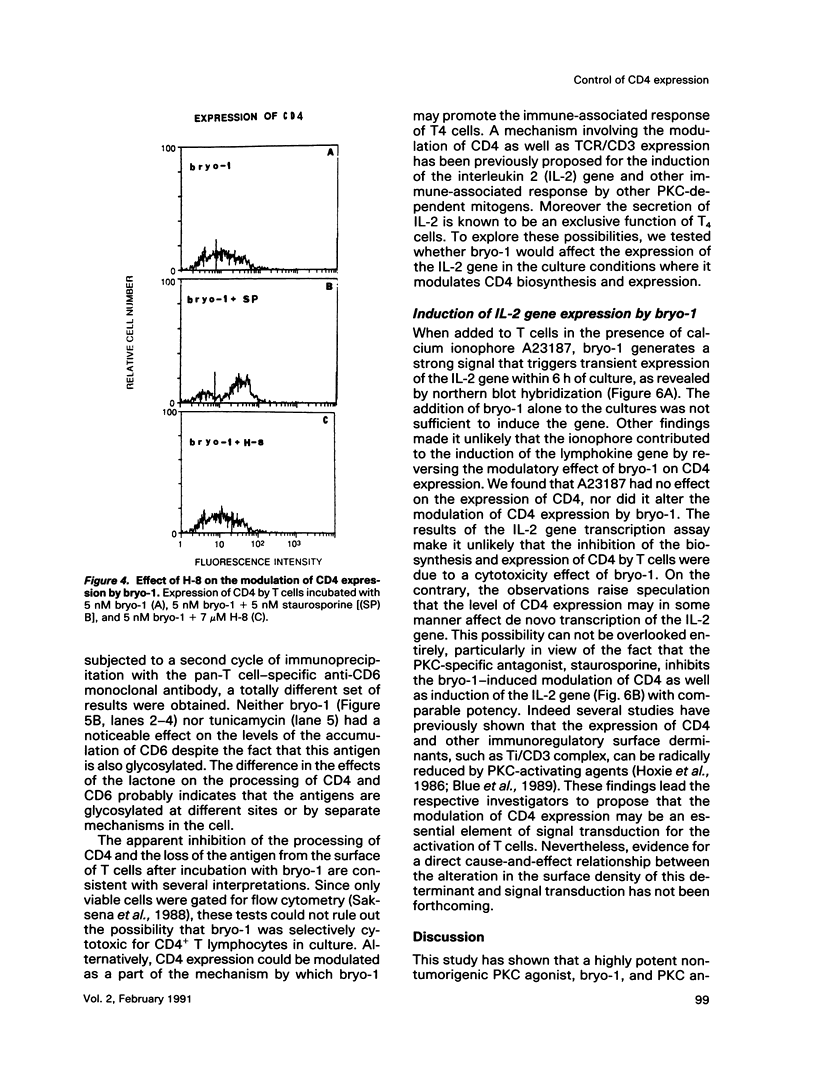
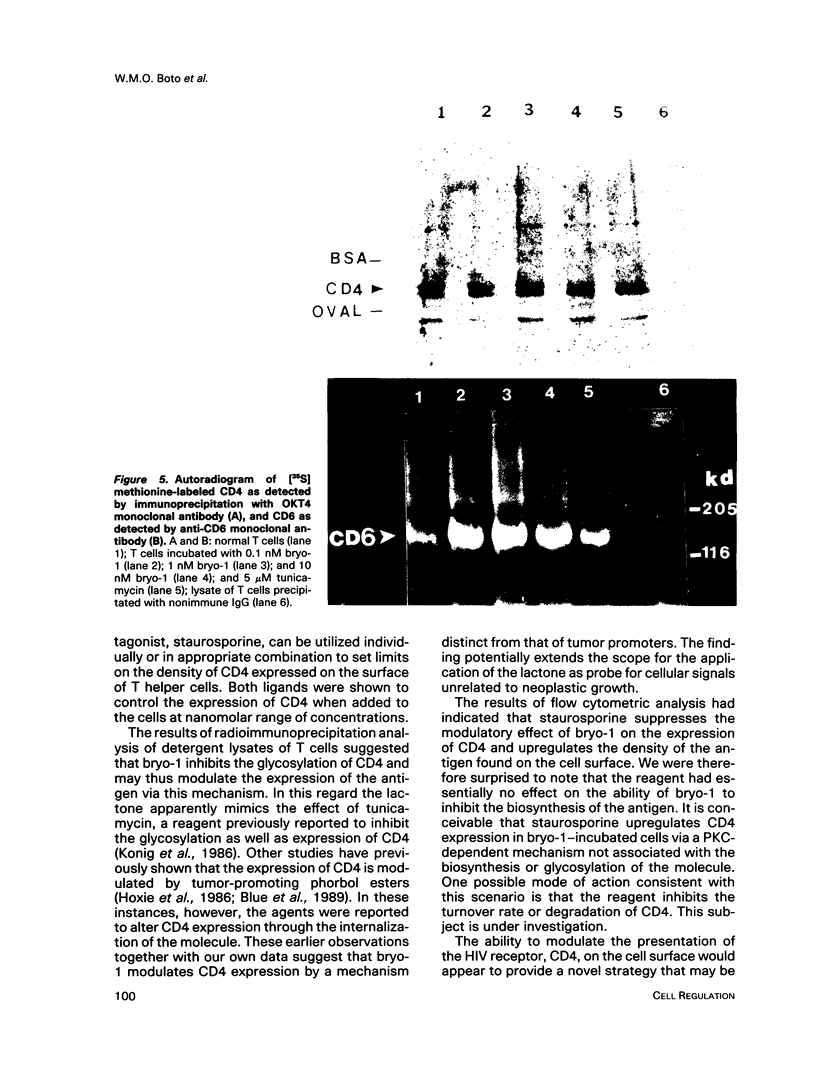
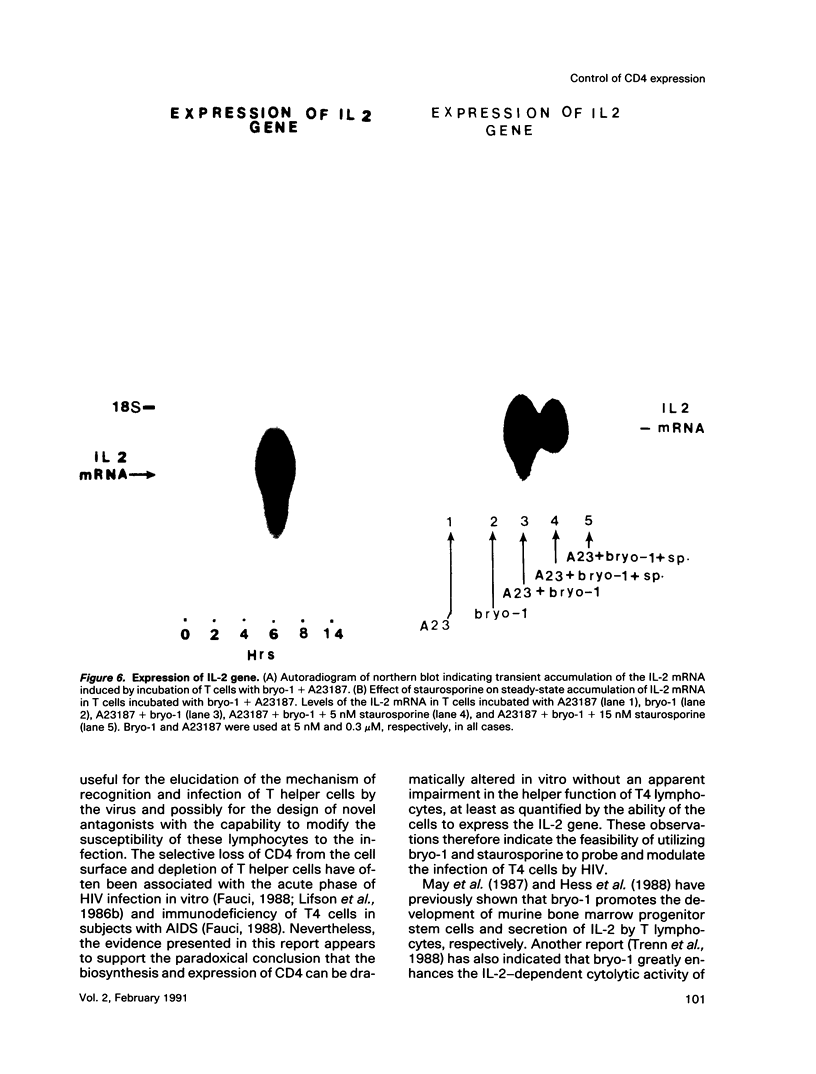
A family of structurally related macrocyclic lactones, bryostatins, have recently been shown to display several intriguing pharmacologic properties. Bryostatins are biosynthetic products of bryozoa phyllum of marine animals. To extend the analyses of the biological activities of these highly unusual biosynthetic animal products, we have examined the effect of bryostatin 1 (bryo-1) on the steady-state expression of the human immunodeficiency virus receptor, CD4, by normal peripheral blood T lymphocytes. Incubation of the cells with 5 nM bryo-1 caused a substantial loss of CD4 from the cell surface, as analyzed by flow cytometry using anti-CD4 monoclonal antibody. The modulation of CD4 expression by bryo-1 was not due to a cytotoxicity effect: in the culture conditions where it modulated CD4, bryo-1 also stimulated the expression of the interleukin 2 gene, as indicated by northern blot hybridization. In addition, incubation of the lymphocytes with nanomolar amounts of protein kinase C antagonist, staurosporine, resulted in the inhibition of the bryo-1-induced modulation of CD4 expression. The results of radioimmunoprecipitation analysis of detergent lysates of [35S] methionine-labeled lymphocytes strongly suggest that bryo-1 inhibits the glycosylation and expression of CD4 in a manner similar to that of tunicamycin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkow R. L., Kraft A. S. Bryostatin, a non-phorbol macrocyclic lactone, activates intact human polymorphonuclear leukocytes and binds to the phorbol ester receptor. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1109–1116. doi: 10.1016/0006-291x(85)90205-0. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Branton K. R., Jr, Schlossman S. F. Regulation of CD4 and CD8 surface expression on human thymocyte subpopulations by triggering through CD2 and the CD3-T cell receptor. J Immunol. 1989 Jan 15;142(2):374–380. [PubMed] [Google Scholar]
- Boto W. M., D'Antonio R., Levy D. A. Homologous and distinctive antigens of Onchocerca volvulus and Dirofilaria immitis: detection by an enzyme-linked immuno-inhibition assay. J Immunol. 1984 Aug;133(2):981–987. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dell'Aquila M. L., Nguyen H. T., Herald C. L., Pettit G. R., Blumberg P. M. Inhibition by bryostatin 1 of the phorbol ester-induced blockage of differentiation in hexamethylene bisacetamide-treated Friend erythroleukemia cells. Cancer Res. 1987 Nov 15;47(22):6006–6009. [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Matthews D. M., Callahan K. J., Cassel D. L., Cooper R. A. Transient modulation and internalization of T4 antigen induced by phorbol esters. J Immunol. 1986 Aug 15;137(4):1194–1201. [PubMed] [Google Scholar]
- Isakov N., Altman A. Human T lymphocyte activation by tumor promoters: role of protein kinase C. J Immunol. 1987 May 15;138(10):3100–3107. [PubMed] [Google Scholar]
- Jameson B. A., Rao P. E., Kong L. I., Hahn B. H., Shaw G. M., Hood L. E., Kent S. B. Location and chemical synthesis of a binding site for HIV-1 on the CD4 protein. Science. 1988 Jun 3;240(4857):1335–1339. doi: 10.1126/science.2453925. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Liskamp R. M. Computer-assisted molecular modeling of tumor promoters: rationale for the activity of phorbol esters, teleocidin B, and aplysiatoxin. Proc Natl Acad Sci U S A. 1986 Jan;83(2):241–245. doi: 10.1073/pnas.83.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Sasaki M., Yoshida H., Wigzell H., Kimura G., Nomoto K. Cytopathic effect determined by the amount of CD4 molecules in human cell lines expressing envelope glycoprotein of HIV. J Immunol. 1990 Jan 1;144(1):94–102. [PubMed] [Google Scholar]
- Kraft A. S., Smith J. B., Berkow R. L. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1334–1338. doi: 10.1073/pnas.83.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König R., Ashwell G., Hanover J. A. Glycosylation of CD4. Tunicamycin inhibits surface expression. J Biol Chem. 1988 Jul 5;263(19):9502–9507. [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Hwang K. M., Nara P. L., Fraser B., Padgett M., Dunlop N. M., Eiden L. E. Synthetic CD4 peptide derivatives that inhibit HIV infection and cytopathicity. Science. 1988 Aug 5;241(4866):712–716. doi: 10.1126/science.2969619. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- May W. S., Sharkis S. J., Esa A. H., Gebbia V., Kraft A. S., Pettit G. R., Sensenbrenner L. L. Antineoplastic bryostatins are multipotential stimulators of human hematopoietic progenitor cells. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8483–8487. doi: 10.1073/pnas.84.23.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Properties and characterization of a rat spleen cell-derived factor that induces resistance to natural killer cell lysis in YAC lymphoma cells. J Immunol. 1988 Sep 1;141(5):1782–1787. [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., Desmyter J., De Clercq E. Specific interaction of aurintricarboxylic acid with the human immunodeficiency virus/CD4 cell receptor. Proc Natl Acad Sci U S A. 1989 May;86(9):3322–3326. doi: 10.1073/pnas.86.9.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Pettit G. R. Bryostatins: potent, new mitogens that mimic phorbol ester tumor promoters. Biochem Biophys Res Commun. 1985 Nov 15;132(3):939–945. doi: 10.1016/0006-291x(85)91898-4. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Trenn G., Pettit G. R., Takayama H., Hu-Li J., Sitkovsky M. V. Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J Immunol. 1988 Jan 15;140(2):433–439. [PubMed] [Google Scholar]
- Trenn G., Pettit G. R., Takayama H., Hu-Li J., Sitkovsky M. V. Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J Immunol. 1988 Jan 15;140(2):433–439. [PubMed] [Google Scholar]