Background: Carbohydrate epitopes are often used as markers for characterization of human embryonic stem cells (hESC).
Results: Several glycosphingolipids not previously found in hESC were characterized.
Conclusion: The glycosylation of hESC is more complex than previously thought.
Significance: These findings will help to understand the immunogenicity of hESC and might impact future applications in regenerative medicine.
Keywords: Carbohydrate Structure, Embryonic Stem Cell, Glycoconjugate, Glycolipid Structure, Mass Spectrometry (MS)
Abstract
Due to their pluripotency and growth capability, there are great expectations for human embryonic stem cells, both as a resource for functional studies of early human development and as a renewable source of cells for use in regenerative medicine and transplantation. However, to bring human embryonic stem cells into clinical applications, their cell surface antigen expression and its chemical structural complexity have to be defined. In the present study, total non-acid glycosphingolipid fractions were isolated from two human embryonic stem cell lines (SA121 and SA181) originating from leftover in vitro fertilized human embryos, using large amounts of starting material (1 × 109 cells/cell line). The total non-acid glycosphingolipid fractions were characterized by antibody and lectin binding, mass spectrometry, and proton NMR. In addition to the globo-series and type 1 core chain glycosphingolipids previously described in human embryonic stem cells, a number of type 2 core chain glycosphingolipids (neo-lactotetraosylceramide, the H type 2 pentaosylceramide, the Lex pentaosylceramide, and the Ley hexaosylceramide) were identified as well as the blood group A type 1 hexaosylceramide. Finally, the mono-, di-, and triglycosylceramides were characterized as galactosylceramide, glucosylceramide, lactosylceramide, galabiaosylceramide, globotriaosylceramide, and lactotriaosylceramide. Thus, the glycan diversity of human embryonic stem cells, including cell surface immune determinants, is more complex than previously appreciated.
Introduction
Human embryonic stem cells (hESC)2 derived from the inner cell mass of blastocysts are pluripotent, i.e. have the capacity to transform into all derivatives of the three primary germ layers of the developing embryo as well as the ability to replicate indefinitely (1–4). These features make hESC excellent candidates to be used in regenerative medicine, provided that the grafted cells are tolerated by the immune system of the recipient. Thus, before hESC can be brought into the clinic, there is need for a deeper understanding of the molecular mechanisms underlying the proliferation and differentiation of hESC. Carbohydrate epitopes are often used as markers for definition and characterization of hESC and also to monitor their differentiation (5). Cell surface marker profiling of undifferentiated hESC in culture show expression of the stage-specific embryonic antigen 3 (SSEA-3) and SSEA-4 and the keratan sulfate-associated antigens TRA-1-60 and TRA-1-81 (5–8). SSEA-3 and SSEA-4 are glycosphingolipids (globopentaosylceramide and sialyl-globopentaosylceramide, respectively), since the globo carbohydrate core is only found in glycosphingolipids.
Most of the current knowledge about cell surface carbohydrates on embryonic stem cells originates from experiments performed on mouse embryonic cells. The majority of these studies have been done using immune labeling techniques, and chemical structural characterization of antigens are lacking. There are only two studies where the glycosphingolipids of human embryonic stem cells have been characterized (9, 10). By use of immunofluorescence, flow cytometry, MALDI-MS, and MS/MS analyses of glycosphingolipids from crude lipid extracts, glycosphingolipids of the globo-series (globotetraosylceramide, globopentaosylceramide/SSEA-3, and the Globo H hexaosylceramide) and with type 1 core chains (lactotetraosylceramide and fucosyl-lactotetraosylceramide/H type 1 pentaosylceramide) were identified in undifferentiated hESC, and the gangliosides found were GM3, GM1, GD1a or GD1b, sialyl-globopentaosylceramide/SSEA-4, and disialyl-globopentaosylceramide3. Differentiation into neural progenitor cells led to expression of mainly gangliosides of the ganglio-series (9, 10), whereas differentiation into endodermal cells gave a predominant expression of globotetraosylceramide (10).
In order to get a comprehensive overview of the glycosphingolipid expression of cultured hESC, we have in the present study isolated total non-acid glycosphingolipid fractions from two human embryonic stem cell lines (SA121 and SA181) using large amounts of starting material (1 × 109 cells/cell line). The total non-acid glycosphingolipid fractions and isolated subfractions were characterized with antibody and lectin binding, mass spectrometry, and proton NMR. This approach allowed an increased resolution and several non-acid glycosphingolipids not previously described in human embryonic stem cells were identified, such as type 2 core chain glycosphingolipids (the H type 2 pentaosylceramide, the Lex pentaosylceramide, and Ley hexaosylceramide) as well as a blood group A type 1 hexaosylceramide. In addition, the mono-, di-, and triglycosylceramides were characterized as galactosylceramide, glucosylceramide, lactosylceramide, galabiaosylceramide, globotriaosylceramide, and lactotriaosylceramide.
EXPERIMENTAL PROCEDURES
Expansion and Harvest of Human Embryonic Stem Cells
hESC were grown and passaged as described previously (11). In brief, two cell lines (SA121 and SA181) were generated from two separate leftover human in vitro fertilized embryos. Cells were transferred from mechanically dissected cultures grown on mouse embryonic fibroblasts to the feeder-free system and expanded for four passages to achieve a frozen working cell bank. The achieved cell banks were then quality-controlled according to conventional quality control criteria for human pluripotent stem cells. In order to obtain enough material for this study, each bank was thawed in passage five and expanded accordingly, with passages performed every third or fourth day. Dense flasks in passages 8, 9, and 10 were harvested using the phosphate-buffered saline-based (PBS; pH 7.3) enzyme-free cell dissociation buffer (Invitrogen), thus minimizing the risk of destroying outer cell membrane compounds. Each harvest generated roughly 1 × 109 undifferentiated human embryonic stem cells from 30 T175 flasks/occasion (Corning Inc.). Cells were pooled and centrifuged briefly (3 min in swing out rotor at 300 × g), after which the supernatant was decanted, and the cell pellet was stored at −80 °C.
Isolation of Glycosphingolipids from Human Embryonic Stem Cell Lines
Non-acid glycosphingolipids were isolated from the two hESC lines (SA121 and SA181), essentially as described (12, 13). The cells (1 × 109 cells from each cell line) were extracted by the addition of first 5 ml of methanol at 70 °C. After a brief centrifugation, the supernatant was removed, and the cells were extracted three times with chloroform/methanol (1:2, v/v) and three times with chloroform/methanol (2:1, v/v) in the same manner as above. The supernatants were pooled and dried. Acid and non-acid glycosphingolipid fractions were then separated by DEAE-cellulose column chromatography. The non-acid fraction was subjected to mild alkaline hydrolysis, and after dialysis, polar and non-polar lipids were separated on a silicic acid column. In order to separate the non-acid glycosphingolipids from alkali-stable phospholipids, the polar lipid fraction was acetylated and separated on a second silicic acid column, followed by deacetylation and dialysis. Final purifications were performed by chromatographies on DEAE-cellulose and silicic acid columns. Approximately 3 mg of total non-acid glycosphingolipids were obtained from each cell line, and these fractions were structurally characterized by thin-layer chromatography, binding of lectin and monoclonal antibodies, and mass spectrometry. Thereafter, partly purified subfractions were obtained by separation of the non-acid glycosphingolipids using Iatrobeads (Iatrobeads 6RS-8060, Iatron Laboratories, Tokyo) columns (0.5 g), eluted with increasing amounts of methanol in chloroform. These subfractions were further characterized by antibody binding, mass spectrometry, and proton NMR spectroscopy.
Reference Glycosphingolipids
Total acid and non-acid glycosphingolipid fractions were from various human and animal tissues (13–17). The individual glycosphingolipids obtained by repeated chromatography on silicic acid columns and by HPLC were identified by mass spectrometry (18, 19) and proton NMR spectroscopy (20).
Thin-layer Chromatography
Aluminum- or glass-backed silica gel 60 high performance thin-layer chromatography plates (Merck) were used for thin-layer chromatography and were eluted with chloroform/methanol/water (60:35:8, v/v/v) as a solvent system. The different glycosphingolipids were applied to the plates in quantities of 1–4 μg of pure glycosphingolipids and 20–40 μg of glycosphingolipid mixtures. Chemical detection was done with anisaldehyde (13).
Chromatogram Binding Assays
Erythrina cristagalli lectin was purchased from Vector Laboratories. Monoclonal anti-A (HE193), anti-B (HEB-29), and anti-H type 1 (17-206) antibodies were obtained from GeneTex/Abcam. Monoclonal anti-H type 2 (A583) and rabbit anti-mouse (Z0259) antibodies were from DakoCytomation Norden A/S, anti-Lea (78.FR2.3) was from Dominion Biologicals Limited (Dartmouth, Canada), anti-Leb (BG-6/T218) was from Signet/Covance, anti-Globo H (MBr1) was from Enzo Life Sciences, and anti-Globopenta/SSEA3 (MC-631) was from eBioscience. Monoclonal anti-Lex/SSEA-1 (P12) and anti-Ley (F3) were purchased from Calbiochem.
Binding of monoclonal antibodies to glycosphingolipids on thin-layer chromatograms was performed according to Hansson et al. (21). Chromatograms with separated glycosphingolipids were dipped for 1 min in diethylether/n-hexane (1:5, v/v) containing 0.5% (w/v) polyisobutylmethacrylate (Aldrich). After drying, the chromatograms were soaked in PBS containing 2% bovine serum albumin and 0.1% NaN3 (Solution A), for 2 h at room temperature. Suspensions of monoclonal antibodies diluted 1:50–1:500 (the dilution used for each monoclonal antibody is given in Table 1) in Solution A were gently sprinkled over the chromatograms, followed by incubation for 2 h at room temperature. After washing with PBS, there followed a second 2-h incubation with 125I-labeled (labeled by the IODO-GEN method according to the manufacturer's instructions (Pierce)) rabbit anti-mouse antibodies diluted to 2 × 106 cpm/ml in Solution A. Finally, the plates were washed six times with PBS. Dried chromatograms were autoradiographed for 12–24 h using XAR-5 x-ray films (Eastman Kodak Co.). Chromatogram binding assays with 125I-labeled lectin from E. cristagalli were done as described (16).
TABLE 1.
Monoclonal antibodies used in chromatogram binding assays
| Antibodies | Clone | Manufacturer | Dilution | Isotype |
|---|---|---|---|---|
| Anti-blood group A | HE-193 | GeneTex/Abcam | 1:500 | IgM |
| Anti-blood group B | HEB-29 | GeneTex/Abcam | 1:100 | IgM |
| Anti-H type 1 | 17-206 | GeneTex/Abcam | 1:50 | IgG3 |
| Anti-H type 2 | A583 | DakoCytomation Norden A/S | 1:100 | IgM |
| Anti-Globo H | Mbr1 | Enzo Life Science | 1:50 | IgM |
| Anti-Lewisa | 78.FR2.3 | Dominion | 1:50 | IgM |
| Anti-Lewisb | BG-6/T218 | Signet/Covance | 1:100 | IgM |
| Anti-Lewisy | F3 | Calbiochem | 1:200 | IgM |
| Anti-Lewisx/SSEA-1 | P12 | Calbiochem | 1:200 | IgM |
| Anti-Globopenta/SSEA-3 | MC-631 | eBioscience | 1:50 | IgM |
Binding of human serum to glycosphingolipids on thin-layer chromatograms was performed as described (22). The human blood group AB serum pool was collected from 21 healthy blood donors and used at a dilution of 1:5. The secondary antibodies were alkaline phosphate-conjugated goat anti-human polyvalent immunoglobulins (Sigma), and the reactions were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chromogenic substrate (Sigma).
LC-ESI/MS and ESI/MS/MS of Native Glycosphingolipids
The glycosphingolipids (dissolved in methanol/acetonitrile (75:25, v/v)) were separated on a 200 × 0.150-mm column, packed in house with 5-μm Polyamine II particles (YMC Europe GmbH, Dinslaken, Germany), and eluted with a water gradient (A, 100% acetonitrile; B, 10 mm ammonium bicarbonate). Samples were analyzed on an LTQ linear quadrupole ion trap mass spectrometer (Thermo Electron) by LC-ESI/MS at −3.5 kV. Full scan (m/z 500–1800, two microscans, maximum 100 ms, target value of 30,000) was performed, followed by data-dependent MS2 scans (two microscans, maximum 100 ms, target value of 10,000) with normalized collision energy of 35%, an isolation window of 2.5 units, an activation q = 0.25, and an activation time of 30 ms.
Endoglycoceramidase Digestion and LC/MS
Endoglycoceramidase II from Rhodococcus spp. (23) (Takara Bio Europe S.A., Gennevilliers, France) was used for hydrolysis of glycosphingolipids. Briefly, 50 μg of glycosphingolipids were resuspended in 100 μl of 0.05 m sodium acetate buffer, pH 5.0, containing 120 μg of sodium cholate, and sonicated briefly. Thereafter, 1 milliunit of enzyme was added, and the mixture was incubated at 37 °C for 48 h. The reaction was stopped by the addition of chloroform/methanol/water to the final proportions 8:4:3 (v/v/v). The oligosaccharide-containing upper phase thus obtained was separated from detergent on a Sep-Pak QMA cartridge (Waters). The eluant containing the oligosaccharides was dried under nitrogen and under vacuum.
The glycosphingolipid-derived oligosaccharides were analyzed by capillary LC/MS and MS/MS as described (19). In brief, the oligosaccharides were separated on a column (200 × 0.180 mm) packed in house with 5-μm porous graphite particles (Hypercarb, Thermo Scientific), eluted with an acetonitrile gradient (A, 10 mm ammonium bicarbonate; B, 80% acetonitrile, 10 mm ammonium bicarbonate). The saccharides were analyzed in the negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer.
Proton NMR Spectroscopy
1H NMR spectra were acquired on a Varian 600-MHz spectrometer at 30 °C. Samples were dissolved in dimethyl sulfoxide/D2O (98:2, v/v) after deuterium exchange. Two-dimensional double quantum-filtered correlated spectroscopy (DQF-COSY) spectra were recorded using the standard pulse sequence (24).
RESULTS
Isolation of Total Non-acid Glycosphingolipids from hESC
Total amounts of non-acid glycosphingolipids isolated from each hESC line SA121 and SA181 (1 × 109 cells) were 3 mg. Thin-layer chromatography using chemical staining showed that both cell lines had a number of compounds migrating in the mono- to hexaglycosylceramide regions (Fig. 1, lanes 1 and 2). The total glycosphingolipid fractions were first analyzed by binding of lectin and monoclonal antibodies, as described below.
FIGURE 1.
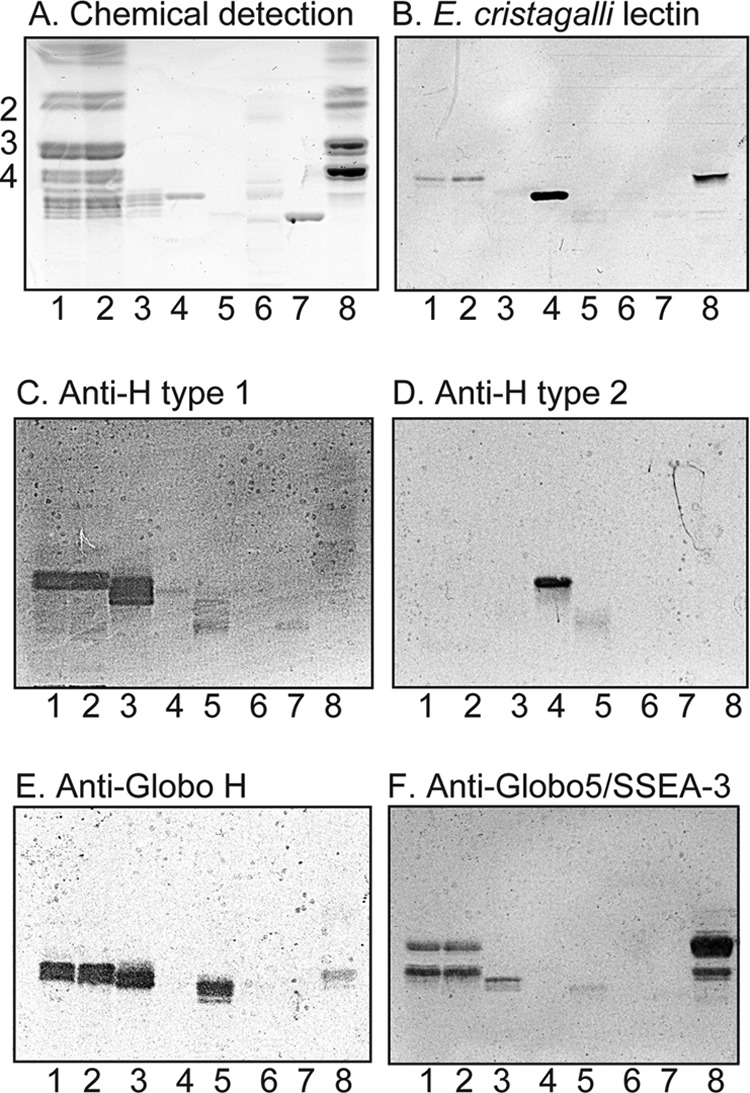
Binding of E. cristagalli lectin and monoclonal antibodies to non-acid glycosphingolipids of human embryonic stem cells. A–F, thin-layer chromatogram after detection with anisaldehyde (A) and autoradiograms obtained by binding of E. cristagalli lectin (B), the monoclonal anti-H type 1 antibody 17-206 (C), the monoclonal anti-H type 2 antibody 92FR-A2 (D), the monoclonal anti-Globo H antibody MBr1 (E), and the monoclonal anti-Globo5/SSEA-3 antibody MC-813-70 (F). Lane 1, non-acid glycosphingolipids of human embryonic stem cell line SA121 (40 μg); lane 2, non-acid glycosphingolipids of human embryonic stem cell line SA181 (40 μg); lane 3, reference H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 4, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 5, reference Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer) (4 μg); lane 6, non-acid glycosphingolipids of mouse small intestine (10 μg); lane 7, reference B type 2 hexaglycosylceramide (Galα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 8, non-acid glycosphingolipids of human kidney (blood group A) (20 μg). The numbers to the left of the chromatogram in A denote the approximate number of carbohydrate residues in the bands. The chromatograms were eluted with chloroform/methanol/water (60:35:8, v/v/v), and the binding assays were done as described under “Experimental Procedures.” Autoradiography was for 12 h.
Binding of E. cristagalli Lectin and Monoclonal Antibodies to the Total Non-acid Glycosphingolipids from hESC
The lectin from E. cristagalli binds to glycoconjugates with terminal Galβ4GlcNAc and Fucα2Galβ4GlcNAc sequences (16). In the non-acid fractions from the SA121 and SA181 cell lines, a binding of E. cristagalli lectin in the tetraglycosylceramide region was obtained (Fig. 1B), indicating the presence of a Galβ4GlcNAc-terminated glycosphingolipid (i.e. neo-lactotetraglycosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer)).
In addition, the monoclonal antibodies directed against the H type 1, Globo H, and Globopenta/SSEA3 epitopes bound to compounds migrating in the penta- and hexaglycosylceramide regions in the non-acid fractions from the SA121 and SA181 cell lines (Fig. 1, C, E, and F). This suggested the presence of H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), globopentaosylceramide (Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), and Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer).
It should here be noted that some of these monoclonal antibodies used were not entirely specific. One example is the anti-Globo H antibody, which also bound to the H type 1 glycosphingolipid (Fig. 1E, lane 3). Thus, the binding of the anti-Globo H antibody in the penta-/hexaosylceramide region in the non-acid fractions from the SA121 and SA181 cell lines could be a recognition of Globo H hexaglycosylceramide with a different ceramide than the reference Globo H but could also be a cross-reactivity with the H type 1 pentaosylceramide. Also, the anti-Globopenta/SSEA3 antibody was highly cross-reactive, and, in addition to the globopentaosylceramide, it bound to globotetraosylceramide, the H type 1 pentaosylceramide, and the Globo H hexaosylceramide (Fig. 1F). The cross-reactivities observed are summarized in Table 2.
TABLE 2.
Cross-reactivities of monoclonal antibodies
| Antibody | Clone | Claimed specificity | Cross-reactivity noted |
|---|---|---|---|
| Anti-H type 1 | 17-206 | H type 1: Fucα2Galβ3GlcNAc | Globo H: Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc |
| Anti-Globo H | MBr1 | Globo H: Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc | H type 1: Fucα2Galβ3GlcNAc |
| Anti-Lex/SSEA-1 | P12 | Lex: Galβ4(Fucα3)GlcNAc | Lea: Galβ3(Fucα4)GlcNAc |
| Anti-Ley | F3 | Ley: Fucα2Galβ4(Fucα3)GlcNAc | Lex: Galβ4(Fucα3)GlcNAc |
| Leb: Fucα2Galβ3(Fucα4)GlcNAc | |||
| H type 2: Fucα2Galβ4GlcNAc | |||
| Anti-Globopenta/SSEA-3 | MC-631 | Globopenta: Galβ3GalNAcβ3Galα4Galβ4Glc | Globotetra: GalNAcβ3Galα4Galβ4Glc |
| Globo H: Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc | |||
| H type 1: Fucα2Galβ3GlcNAc |
Separation of the Total Non-acid Glycosphingolipids from hESC
After these studies the non-acid glycosphingolipid fractions were separated on Iatrobeads columns, in order to enrich the slow migrating glycosphingolipids. Thereby, four glycosphingolipid-containing fractions were obtained from each cell line (Fig. 2, A and B). These fractions were denoted fractions 121:I–IV and 181:I–IV, respectively. Thus, fractions 121:I and 181:I (0.2 mg each) contained compounds migrating in the monoglycosylceramide region, whereas the material in fractions 121:II and 181:II (0.2 mg each) migrated as di- and triglycosylceramides. Fractions 121:III and 181:III (0.2 mg each) contained compounds migrating as triglycosylceramides, and fractions 121:IV and 181:IV (0.7 mg each) contained triglycosylceramides and more slow migrating compounds. After these eight fractions had been analyzed by binding of monoclonal antibodies, mass spectrometry, and proton NMR, fractions 121:IV and 181:IV were further separated by Iatrobeads chromatography in an attempt to obtain more structural information about the slow migrating compounds. The fractions collected were pooled according to the migration on thin-layer chromatograms, giving in each case four fractions of ∼0.1 mg. The two fractions containing the slow migrating compounds from each separation were denoted fractions 121:IV-C, 121:IV-D, 181:IV-C, and 181:IV-D, respectively.
FIGURE 2.
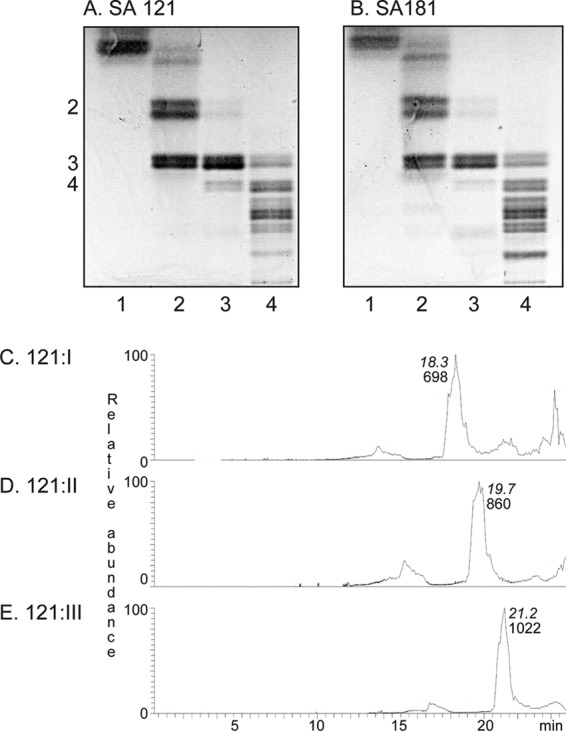
Thin-layer chromatograms of the isolated non-acid glycosphingolipid fractions from human embryonic cell lines SA121 and SA181 and ESI/MS of fractions 121:I–III (mono-, di-, and triglycosylceramides). A, lane 1, fraction 121:I from cell line SA121 (10 μg); lane 2, fraction 121:II (10 μg); lane 3, fraction 121:III (10 μg); lane 4, fraction 121:IV (20 μg). B, lane 1, fraction 181:I from cell line SA181 (10 μg); lane 2, fraction 181:II (10 μg); lane 3, fraction 181:III (10 μg); lane 4, fraction 181:IV (20 μg). The numbers to the left of the chromatogram in A denote the approximate number of carbohydrate residues in the bands. The thin-layer chromatograms were eluted with chloroform/methanol/water (60:35:8, v/v/v), and anisaldehyde was used for detection of glycosphingolipids. C, base peak chromatogram from ESI-MS of fraction 121:I from human embryonic stem cell line SA121. D, base peak chromatogram from ESI-MS of fraction 121:II from human embryonic stem cell line SA121. E, base peak chromatogram from ESI-MS of fraction 121:III from human embryonic stem cell line SA121. The retention times of the molecular ions are given in italic type.
Characterization of Mono-, Di-, and Triglycosylceramides from hESC
The base peak chromatograms from ESI-MS of the native fractions 121:I, 121:II, and 121:III from human embryonic stem cell line SA121 (Fig. 2, C–E), indicated that the major compounds of these fractions were a monohexosylceramide, a dihexosylceramide, and a trihexosylceramide, respectively, in all three cases with sphingosine long chain base and non-hydroxy 16:0 fatty acids.
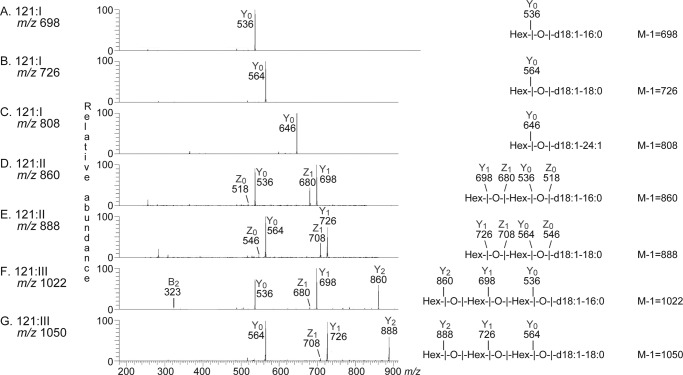
Further analyses of mass chromatograms and by MS2 confirmed the presence of monohexosylceramide with d18:1–16:04 in fraction 121:I (Fig. 3A). In addition, monohexosylceramide with d18:1–18:0 and d18:1–24:1 was found (Fig. 3, B and C).
FIGURE 3.
MS2 of mono-, di-, and triglycosylceramides from human embryonic stem cells (fractions 121:I–III). The interpretation formulas show the deduced glycosphingolipid structures. A, MS2 spectrum of the ion at m/z 698 of fraction 121:I from human embryonic stem cell line SA121 (retention time 18.2 min). B, MS2 spectrum of the ion at m/z 726 of fraction 121:I from human embryonic stem cell line SA121 (retention time 18.3 min). C, MS2 spectrum of the ion at m/z 808 of fraction 121:I from human embryonic stem cell line SA121 (retention time 18.6 min). D, MS2 spectrum of the ion at m/z 860 of fraction 121:II from human embryonic stem cell line SA121 (retention time 19.7 min). E, MS2 spectrum of the ion at m/z 888 of fraction 121:II from human embryonic stem cell line SA121 (retention time 19.9 min). F, MS2 spectrum of the ion at m/z 1022 of fraction 121:III from human embryonic stem cell line SA121 (retention time 21.2 min). G, MS2 spectrum of the ion at m/z 1050 of fraction 121:III from human embryonic stem cell line SA121 (retention time 21.1 min).
Fraction 121:II contained dihexosylceramides with d18:1–16:0 and d18:1–18:0 (Fig. 3, D and E), and fraction 121:III had trihexosylceramides with d18:1–16:0 and d18:1–18:0 (Fig. 3, F and G). Identical results were obtained by MS2 of fractions 181:I–III (data not shown).
The anomeric regions of the 600-MHz proton NMR spectra of fractions 121:I, 121:II, and 121:III are shown in Fig. 4, and identified structures as well as chemical shift data for the anomeric proton resonances are given in Table 3. Fraction 121:I thus contains two monohexosylceramide species identified as Glcβ1Cer (structure 1) (∼90%) and Galβ1Cer (structure 2) (∼10%) from the positions of the anomeric resonances at 4.075 and 4.024 ppm, respectively (25). The glucose is corroborated by the observation of its H2 resonance at 2.996 ppm (data not shown). Furthermore, the ceramide structure of both compounds is consistent with a sphingosine long-chain base, as evidenced by the presence of the H4 and H5 resonances, which are shifted downfield due to the C4=C5 double bond. The fatty acids are non-hydroxy, as demonstrated by the H2 and H3 resonances at 2.02 and 1.44 ppm (data not shown), respectively, and the presence of a cis double bond is also evident from the triplet at 5.32 ppm.
FIGURE 4.
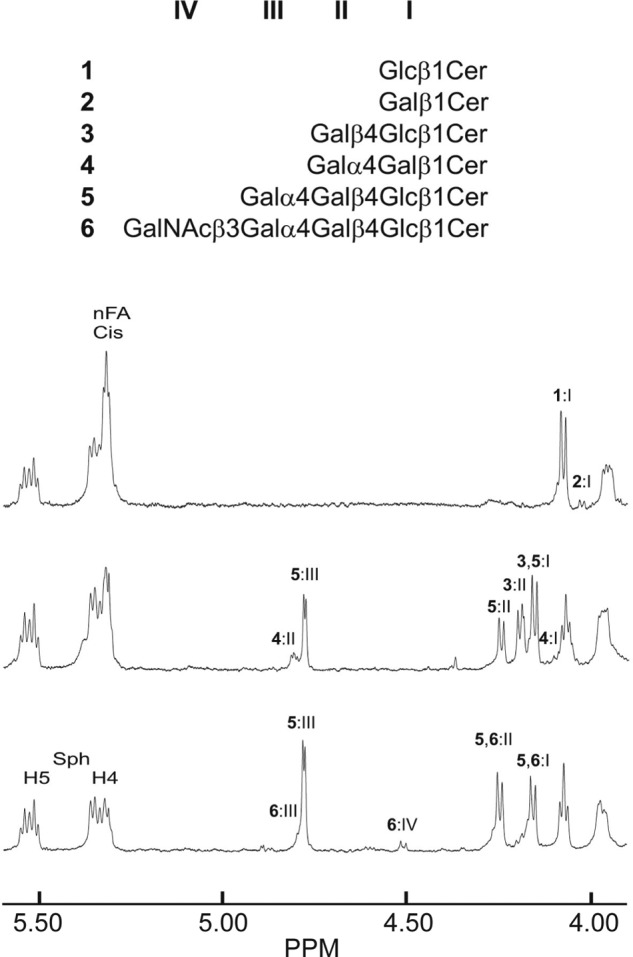
Anomeric regions of the 600-MHz proton NMR spectra (30 °C) of fractions 121:I, 121:II, and 121:III derived from the human embryonic stem cell line SA121. Samples (∼0.1 mg of each sample) were dissolved in dimethyl sulfoxide/D2O (98:2, v/v). Above the spectra the six different structures (1–6) that were identified are depicted, and anomeric resonances are labeled by Roman numerals. The presence of a triplet resonance due to unsaturated cis bonds in the non-hydroxy fatty acids (nFA) is indicated, as are the H4 and H5 resonances stemming from the sphingosine base.
TABLE 3.
Anomeric resonance shifts (ppm) of glycosphingolipid structures identified by NMR at 600 MHz and 30 °C for compounds isolated from human embryonic stem cell lines SA121 and SA181
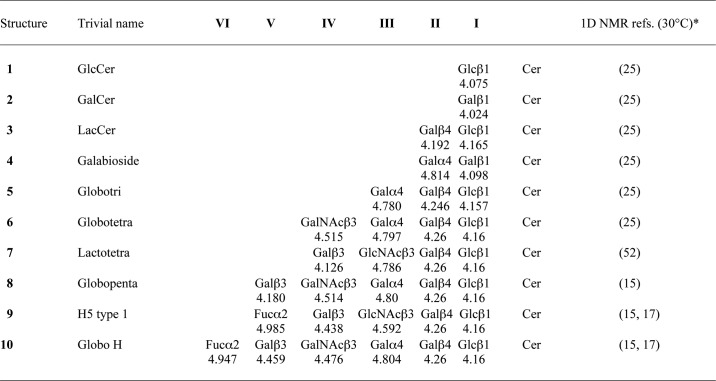
* NMR references do not refer to the original findings because most of these data were acquired at higher temperatures.
The subsequent fraction (121:II) displays anomeric resonances from three different glycosphingolipids: globotriaosylceramide (structure 5) (∼45%), as evidenced by resonances at 4.780 ppm (Galα4), 4.246 ppm (Galβ4), and 4.157 ppm (Glcβ1); galabiaosylceramide (structure 4) (∼10%) with anomeric resonances at 4.814 ppm (Galα4) and 4.098 ppm (Galβ1); and finally, lactosylceramide (structure 3) (∼45%) with resonances at 4.195 (Galβ4) and 4.155 ppm (Glcβ1), the latter completely overlapping the corresponding globotriaosylceramide resonance (25, 26). In fraction 121:III, only one additional compound is evident, namely globotetraosylceramide (structure 6), whose minor resonances (∼5% of the dominating globotriaosylceramide contribution) are found at 4.797 ppm (Galα4), 4.514 ppm (GalNAcβ3), 4.26 ppm (Galβ4), and 4.16 ppm (Glcβ1) (25). Both fractions 121:II and 121:III have ceramides with sphingosine long-chain bases and non-hydroxy fatty acids, and fraction 121:II has more cis unsaturation than fraction 121:III. The structures found in the corresponding fractions isolated from cell line SA181 were identical.
Binding of Monoclonal Antibodies to the Slow Migrating Non-acid Glycosphingolipids from hESC
Probing of the non-acid fractions 121:IV and 181:IV with monoclonal antibodies directed against the blood group A epitope gave a distinct binding in the hexaglycosylceramide region in both cell lines (Fig. 5B). No binding of the anti-Lea or the anti-Leb antibodies was obtained (data not shown). However, slow migrating compounds of both SA121:IV and SA181:IV were recognized by both the anti-Lex antibody and the highly cross-reactive anti-Ley antibody (Fig. 5, C and D).
FIGURE 5.
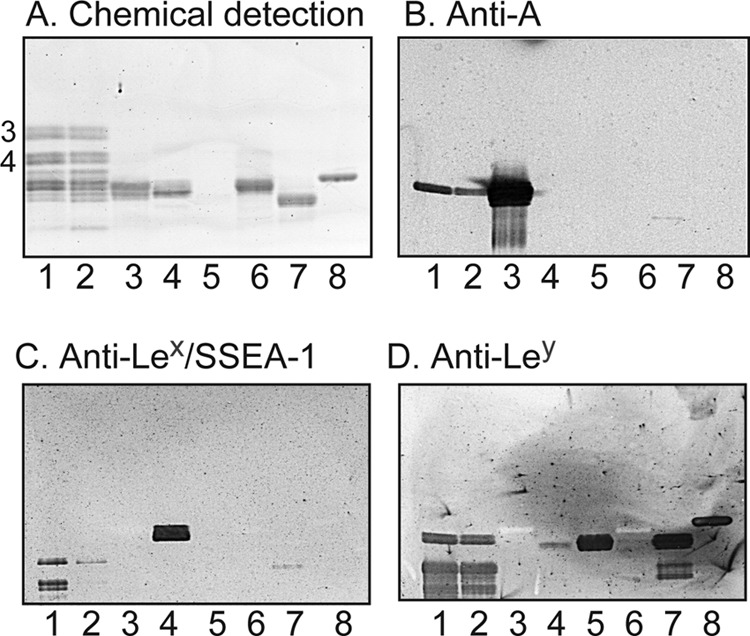
Binding of monoclonal antibodies to slow migrating non-acid glycosphingolipids of human embryonic stem cells. A–D, thin-layer chromatogram after detection with anisaldehyde (A) and autoradiograms obtained by binding of the monoclonal anti-blood group A antibody HE-193 (B), the monoclonal anti-Lex/SSEA-1 antibody P12 (C), and the monoclonal anti-Ley antibody F3 (D). A–D, lane 1, slow migrating non-acid glycosphingolipids of human embryonic stem cell line SA181 (fraction 181:IV) (40 μg); lane 2, slow migrating non-acid glycosphingolipids of human embryonic stem cell line SA121 (fraction 121:IV) (40 μg); lane 3, reference A type 1 hexaglycosylceramide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 4, reference Lex pentaglycosylceramide (Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 5, reference Ley hexaglycosylceramide (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer) (0.2 μg); lane 6, reference Lea pentaglycosylceramide (Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 7, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); lane 8, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (4 μg). The numbers to the left of the chromatogram in A denote the approximate number of carbohydrate residues in the bands. The chromatograms were eluted with chloroform/methanol/water (60:35:8, v/v/v), and the binding assays were done as described under “Experimental procedures”. Autoradiography was for 12 h.
LC-ESI/MS of the Slow Migrating Non-acid Glycosphingolipids from hESC
LC-ESI/MS of oligosaccharides using graphitized carbon columns gives resolution of isomeric saccharides, and the carbohydrate sequence can be deduced from series of C-type fragment ions obtained by MS2 (19). In addition, diagnostic cross-ring 0,2A-type fragment ions are present in MS2 spectra of oligosaccharides with a Hex or HexNAc substituted at C-4 and thus allow differentiation of linkage positions.
The base peak chromatogram obtained from LC-ESI/MS of the oligosaccharides obtained by hydrolysis of fraction 181:IV with Rhodococcus endoglycoceramidase II is shown in Fig. 6A. Molecular ions corresponding to oligosaccharides ranging from trisaccharides (detected as [M − H+]− ions at m/z 503) to hexasaccharides (were detected as [M − H+]− ions at m/z 1014 and 1055) were found. In addition, a number of minor oligosaccharides were detected by inspection of mass chromatograms of selected ions (see below).
FIGURE 6.
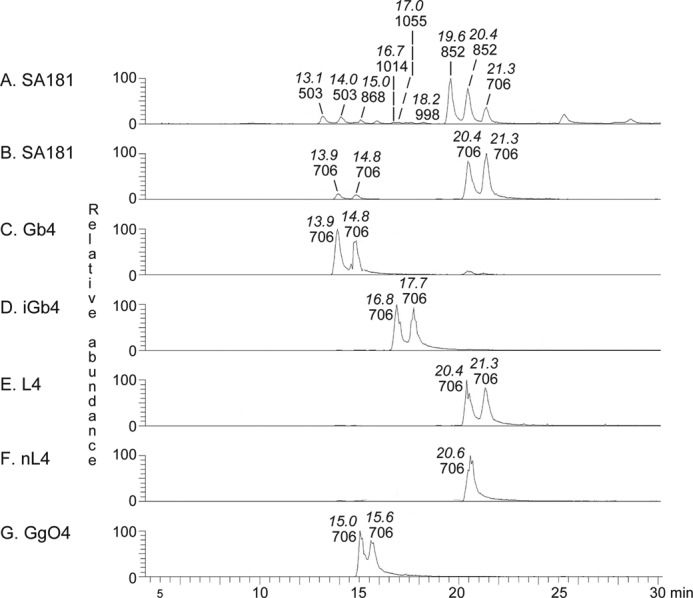
LC-ESI/MS of the oligosaccharides obtained by digestion of fraction 181:IV from human embryonic stem cells and of reference glycosphingolipids with Rhodococcus endoglycoceramidase II. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181. B, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181. C, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from reference globotetraosylceramide (Gb4; GalNAcβ3Galα4Galβ4Glcβ1Cer). D, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from reference isoglobotetraosylceramide (iGb4; GalNAcβ3Galα3Galβ4Glcβ1Cer). E, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from reference lactotetraosylceramide (L4; Galβ3GlcNAcβ3Galβ4Glcβ1Cer). F, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from reference neo-lactotetraosylceramide (nL4; Galβ4GlcNAcβ3Galβ4Glcβ1Cer). G, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from reference gangliotetraosylceramide (GgO4; Galβ3GalNAcβ4Galβ4Glcβ1Cer). The retention times of the molecular ions are given in italic type.
Major Triglycosylceramide of Fraction 181:IV
MS2 of the ion at m/z 503 eluting at 13.1 min gave two C-type fragment ions (C1 at m/z 179 and C2 at m/z 341) identifying a Hex-Hex-Hex sequence (data not shown). There was a prominent 0,2A2 fragment ion at m/z 281 demonstrating a 4-substitution of the internal Hex (19, 26, 27), and thus this trisaccharide was tentatively identified as globotriaose (Galα4Galβ4Glc).
Tetraglycosylceramides of Fraction 181:IV
The mass chromatogram of m/z 706, corresponding to a saccharide with one HexNAc and three Hex (Fig. 6B), had two sets of peaks, eluting at 13.9–14.8 min and 20.4–21.3 min, respectively. The first set of peaks eluted at the same retention time as the saccharide obtained from reference globotetraosylceramide (Fig. 6C), whereas the second set of peaks co-eluted with the saccharides obtained from reference lactotetraosylceramide and neo-lactotetraosylceramide (Fig. 6, E and F).
The MS2 spectrum of the ion at m/z 706 at retention time 13.8 min (Fig. 7A) had a C-type fragment ion series (C1 at m/z 220, C2 at m/z 382, and C3 at m/z 544), demonstrating a HexNAc-Hex-Hex-Hex sequence. Cross-ring 0,2A-type fragments are diagnostic for carbohydrates substituted at C-4 (19, 27, 28), and here the 0,2A3 fragment ion at m/z 484 demonstrated a 4-substituted Hex, whereas the 0,2A4 ion at m/z 646 and the 0,2A4-H2O ion at m/z 628 were derived from cross-ring cleavage of the 4-substituted Glc of the lactose unit at the reducing end. The features of this MS2 spectrum were very similar to the MS2 spectrum of reference globo tetrasaccharide (Fig. 7B), and thus a globo tetrasaccharide (GalNAcβ3Galα4Galβ4Glc) was tentatively identified.
FIGURE 7.
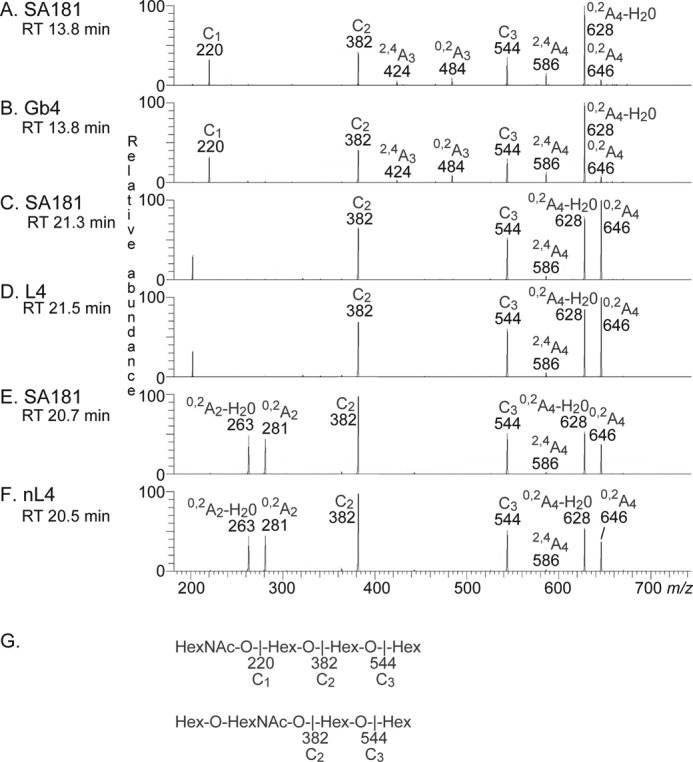
MS2 of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181, and from reference glycosphingolipids. RT, retention time. A, MS2 spectrum of the ion at m/z 706 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 13.8 min). B, MS2 spectrum of the ion at m/z 706 of reference globo tetrasaccharide, Gb4 (retention time 13.8 min). C, MS2 spectrum of the ion at m/z 706 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 21.3 min). D, MS2 spectrum of the ion at m/z 706 of reference lacto tetrasaccharide, L4 (retention time 21.5 min). E, MS2 spectrum of the ion at m/z 706 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 20.7 min). F, MS2 spectrum of the ion at m/z 706 of reference neo-lacto tetrasaccharide, nL4 (retention time 20.5 min). G, interpretation formulas.
MS2 of the ion at m/z 706 at the retention time 21.3 min (Fig. 7C) allowed a preliminary identification of a lacto tetrasaccharide (Galβ3GlcNAcβ3Galβ4Glc). This was concluded from the C-type fragment ions (C2 at m/z 382 and C3 at m/z 544) identifying a Hex-HexNAc-Hex-Hex sequence. Here the MS2 spectrum was very similar to the MS2 spectrum of reference lacto tetrasaccharide (Fig. 7D). Both of these MS2 spectra lacked a 0,2A2 fragment ion at m/z 281, indicating that the HexNAc was substituted at the 3-position.
The MS2 spectrum of the ion at m/z 706 at the retention time 20.7 min (Fig. 7E) was significantly different. This spectrum had a prominent 0,2A2 fragment ion at m/z 281 and a 0,2A2-H2O fragment ion at m/z 263, demonstrating a terminal Hex-HexNAc sequence with a 4-substitution of the HexNAc (i.e. a type 2 chain). In combination with the C2 ion at m/z 382 and the C3 ion at m/z 544, this suggested a neo-lacto tetrasaccharide (Galβ4GlcNAcβ3Galβ4Glc). This suggestion was corroborated by the similarity between this MS2 spectrum and the MS2 spectrum of reference neo-lacto tetrasaccharide (Fig. 7F).
Pentaglycosylceramides of Fraction 181:IV
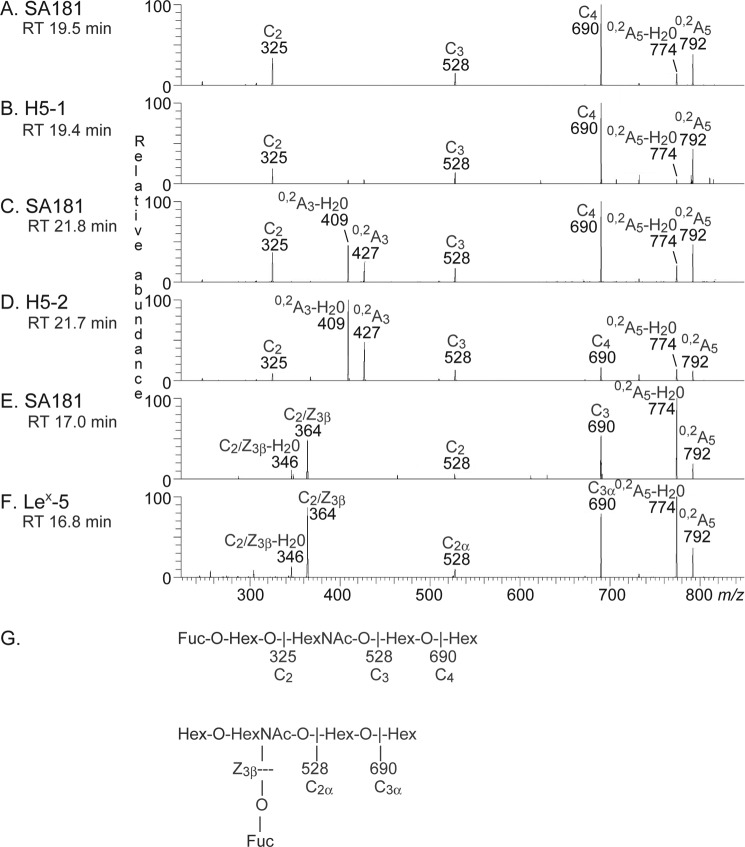
The mass chromatogram of m/z 852, corresponding to a saccharide with one Fuc, one HexNAc, and three Hex (Fig. 8A), had a major set of peaks eluting at 19.6–20.4 min and two minor peaks at 17.0 and 21.8 min, respectively. The major set of peaks co-eluted with the saccharide obtained from reference H type 1 pentaglycosylceramide (Fig. 8B), whereas the minor peaks eluted at the same retention times as the saccharides obtained from reference Lex pentaglycosylceramide (Fig. 8E) and H type 2 pentaglycosylceramide (Fig. 8C), respectively.
FIGURE 8.
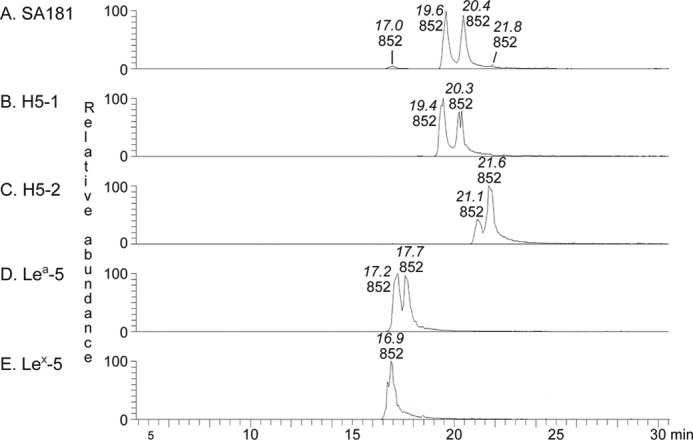
LC-ESI/MS of the oligosaccharides obtained by digestion of fraction 181:IV from human embryonic stem cell line SA181 and of reference glycosphingolipids with Rhodococcus endoglycoceramidase II. A, mass chromatogram of m/z 852 from LC-ESI/MS of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181. B, mass chromatogram of m/z 852 from LC-ESI/MS of the oligosaccharides derived from reference H type 1 pentaglycosylceramide (H5-1; Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer). C, mass chromatogram of m/z 852 from LC-ESI/MS of the oligosaccharides derived from reference H type 2 pentaglycosylceramide (H5-2; Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer). D, mass chromatogram of m/z 852 from LC-ESI/MS of the oligosaccharides derived from reference Lea pentaglycosylceramide (Lea-5; Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer). E, mass chromatogram of m/z 852 from LC-ESI/MS of the oligosaccharides derived from reference Lex pentaglycosylceramide (Lex-5; Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer). The retention times of the molecular ions are given in italic type.
MS2 of the ion at m/z 852 at a retention time of 19.5 min resulted in a series of prominent C-type fragment ions (C2 at m/z 325, C3 at m/z 528, and C4 at m/z 690) identifying a pentasaccharide with Fuc-Hex-HexNAc-Hex-Hex sequence (Fig. 9A). The features of this MS2 spectrum were very similar to the MS2 spectrum of reference H type 1 pentasaccharide (Fig. 9B), which gave a tentative identification of a H type 1 pentasaccharide (Fucα2Galβ3GlcNAcβ3Galβ4Glc).
FIGURE 9.
MS2 of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181 and from reference glycosphingolipids. RT, retention time. A, MS2 spectrum of the ion at m/z 852 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 19.5 min). B, MS2 spectrum of the ion at m/z 852 of reference H type 1 pentasaccharide, H5-1 (retention time 19.4 min). C, MS2 spectrum of the ion at m/z 852 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 21.8 min). D, MS2 spectrum of the ion at m/z 852 of reference H type 2 pentasaccharide, H5-2 (retention time 21.7 min). E, MS2 spectrum of the ion at m/z 852 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 17.0 min). F, MS2 spectrum of the ion at m/z 852 of reference Lex pentasaccharide, Lex-5 (retention time 16.8 min). G, interpretation formulas.
The MS2 spectrum of the ion at m/z 852 at a retention time of 21.8 min (Fig. 9C) suggested a H type 2 pentasaccharide (Fucα2Galβ4GlcNAcβ3Galβ4Glc). This was concluded from the C-type fragment ion series (C2 at m/z 325, C3 at m/z 528, and C4 at m/z 690) identifying a Fuc-Hex-HexNAc-Hex-Hex sequence, and the 0,2A3 fragment ion at m/z 427, and the 0,2A2-H2O fragment ion at m/z 409, demonstrating a substitution of the HexNAc at C-4 (i.e. a type 2 chain). This MS2 spectrum was also very similar to the MS2 spectrum of the reference H type 2 pentasaccharide (Fig. 9D).
A fragment ion at m/z 364 was present in the MS2 spectrum of the ion at m/z 852 at a retention time of 17.0 min (Fig. 9E). This fragment ion is obtained by double glycosidic cleavage of the 3-linked branch at C3 and Z3β and is characteristic for an internal 4-linked GlcNAc substituted with a Fuc at 3-position (23). Taken together with the C2 ion at m/z 528 and the C3 ion at m/z 690 and with the similarity to the MS2 spectrum of the reference Lex pentasaccharide (Fig. 9F), this tentatively identified a Lex pentasaccharide (Galβ4(Fucα3)GlcNAcβ3Galβ4Glc).
Minor Compounds of Fraction 181:IV
A number of minor compounds were also found by further scrutiny of selected ions. First, there was a [M − H+]− ion at m/z 544, eluting at 18.0–18.1 min (data not shown). MS2 of this ion at m/z 544 gave C-type fragment ions at m/z 220 (C1) and at m/z 382 (C2), identifying a HexNAc-Hex-Hex sequence. No 0,2A2 fragment ion at m/z 322 was found, indicating that the internal Hex was 3-substituted. Taken together, this tentatively identified a lactotrisaccharide (GlcNAcβ3Galβ4Glc).
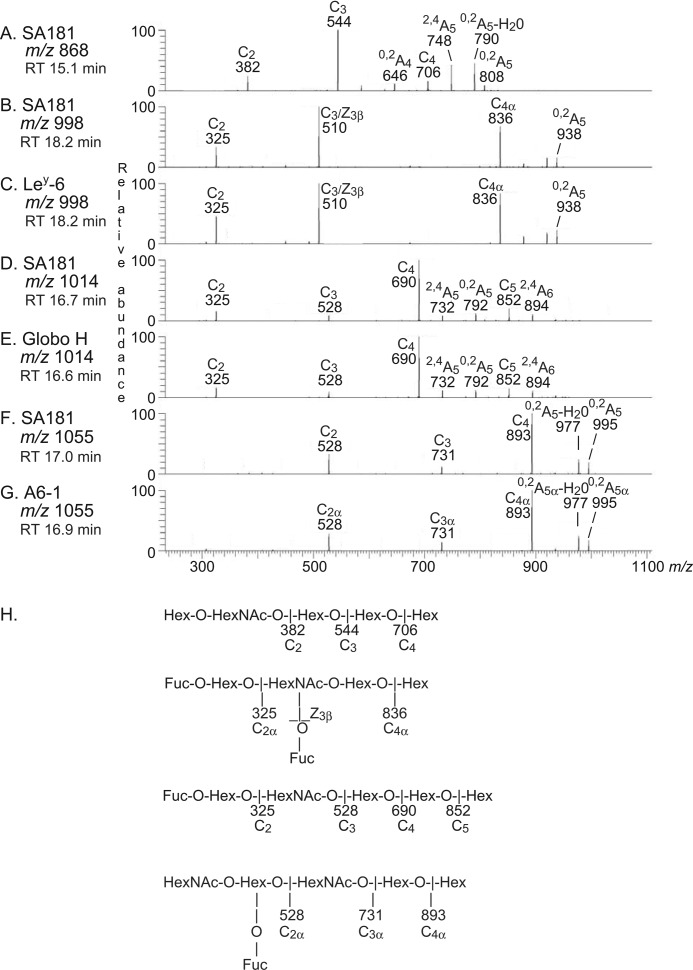
The MS2 spectrum of the ion at m/z 868 at a retention time of 15.1 min is shown in Fig. 10A. A Hex-HexNAc-Hex-Hex-Hex sequence is suggested by the C-type ion series (C2 at m/z 382, C3 at m/z 544, and C4 at m/z 706). The 0,2A4 fragment ion at m/z 646, along with the 0,2A5 fragment ion at m/z 808 and the 0,2A5-H2O fragment ion at m/z 790, demonstrated that the two hexoses at the reducing end were substituted at C-4 (Hex-HexNAc-Hex-4Hex-4Hex), tentatively identifying the Globo pentasaccharide.
FIGURE 10.
MS2 of the oligosaccharides derived from fraction 181:IV from human embryonic stem cell line SA181, and from reference glycosphingolipids. RT, retention time. A, MS2 spectrum of the ion at m/z 868 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 15.1 min). B, MS2 spectrum of the ion at m/z 998 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 18.2 min). C, MS2 spectrum of the ion at m/z 998 of reference Ley hexasaccharide, Ley-6 (retention time 18.2 min). D, MS2 spectrum of the ion at m/z 1014 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 16.7 min). E, MS2 spectrum of the ion at m/z 1014 of reference Globo H hexasaccharide, Globo H (retention time 16.6 min). F, MS2 spectrum of the ion at m/z 1055 of fraction 181:IV from human embryonic stem cell line SA181 (retention time 17.0 min). G, MS2 spectrum of the ion at m/z 1055 of reference A type 1 hexasaccharide, A6-1 (retention time 16.9 min). H, interpretation formulas.
The MS2 spectrum of the ion at m/z 998 at a retention time of 18.2 min had a C2 ion at m/z 325, indicating a terminal Fuc-Hex sequence (Fig. 10B). The prominent ion at m/z 510 is characteristic for an internal 4-linked GlcNAc substituted with a Fuc at 3-position and is obtained by double glycosidic cleavage of the 3-linked branch at C3 and Z3β (27). Taken together with C4α ion at m/z 836, a Ley hexasaccharide (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc) was thus suggested. This suggestion was strengthened by the similarity between this MS2 spectrum and the MS2 spectrum of reference Ley hexasaccharide (Fig. 10C).
MS2 of the ion at m/z 1014 at the retention time 16.7 min (Fig. 10D) gave a spectrum with similar features as obtained by MS2 of reference Globo H hexasaccharide (Fig. 10E). The series of C-type ions (C2 at m/z 325, C3 at m/z 528, C4 at m/z 690, and C5 at m/z 852) indicated a Fuc-Hex-HexNAc-Hex-Hex-Hex sequence. In addition, there was a 0,2A5 fragment ion at m/z 792. Taken together, these spectral features indicated the presence of a Globo H hexasaccharide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc).
MS2 of the ion at m/z 1055 (Fig. 10F) gave rise to a series of C-type fragment ions (C2 at m/z 528, C3 at m/z 731, and C4 at m/z 893) indicating a HexNAc-(Fuc)Hex-HexNAc-Hex-Hex sequence. There was no 0,2A3 fragment ion at m/z 630, suggesting that the internal HexNAc was substituted at C3 (i.e. a type 1 core chain). This MS2 spectrum was very similar to the MS2 spectrum of reference blood group A type 1 hexasaccharide (Fig. 10G). Thus, a hexasaccharide with HexNAc(Fuc)-Hex-HexNAc-Hex-Hex sequence and an internal HexNAc substituted at C-3 was indicated, most likely a blood group A type 1 hexasaccharide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glc).
In summary, LC/MS of the oligosaccharides derived from the glycosphingolipids of fraction 181:IV gave resolution of isomeric compounds and allowed a preliminary identification of a number of compounds ranging from tri- to hexaglycosylceramides (i.e. globotriaosylceramide, lactotriaosylceramide, globotetraosylceramide, lactotetraosylceramide, neo-lactotetraosylceramide, blood group H type 1 pentaosylceramide, blood group H type 2 pentaosylceramide, Lex pentaosylceramide, globopentaosylceramide, Globo H hexaosylceramide, A type 1 hexaosylceramide, and Ley hexaosylceramide were tentatively identified). Identical results were obtained by LC-ESI/MS of the oligosaccharides obtained by hydrolysis of fraction 121:IV (data not shown).
It should here be noted that the intensities of the peaks in the base peak chromatograms do not reflect the relative quantities of the compounds because the oligosaccharides were obtained from the glycosphingolipids by hydrolysis with the Rhodococcus endoglycoceramidase II. The hydrolytic capacity of this enzyme is not universal, and galactosylceramide, globo-series glycosphingolipids, the GD1b ganglioside, and the fucosyl-GM1 ganglioside are relatively resistant (23, 29).
Proton NMR of the Slow Migrating Non-acid Glycosphingolipids from hESC
Both fraction 121:IV and fraction 181:IV were analyzed by proton NMR, and in fraction 181:IV, the amounts of glycosphingolipids were sufficiently high for a DQF-COSY spectrum to be recorded with a signal/noise ratio that allowed unambiguous identification of six different compounds in this fraction, thanks to a wide range of both one- and two-dimensional COSY spectra of pure reference glycosphingolipids. Fig. 11 shows a section of the DQF-COSY spectrum displaying mainly H1/H2 connectivities, and the corresponding part of the one-dimensional spectrum is shown above. The results are summarized in Table 3, and the connectivities in Fig. 11 are labeled accordingly. As an example, the carbohydrate sequence of Globo H (structure 10) was identified from its one-dimensional NMR spectrum (15, 17) and the present DQF-COSY NMR data. Thus, the α-signals at 4.947 and 4.804 ppm originate from Fucα2 (H2 at 3.500 ppm) and Galα4 (H2 at 3.77 ppm overlapping with corresponding H2 signals from globotetraosylceramide and globopentaosylceramide), respectively, whereas the β-signals from Galβ3 and GalNAcβ3 are seen close to one another at 4.459 ppm (H2 at 3.50 ppm) and 4.476 ppm (H2 at 3.87 ppm), respectively. Adding a lactose core with Galβ4 at 4.26 ppm and Glcβ1 at 4.16 ppm, signals that overlap with several other structures, the overall structure can be summed up as Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer. Additional glycosphingolipids identified in fraction 181:IV were globotriaosylceramide (structure 5), globotetraosylceramide (structure 6), lactotetraosylceramide (structure 7), globopentaosylceramide (structure 8), and the H type 1 pentaosylceramide (structure 9), in agreement with the mass spectrometry data described above. Neither Ley, H type 2, nor blood group A structures were discernible by NMR due to the lower sensitivity of this technique compared with mass spectrometry. However, a very minor methyl doublet at 0.99 ppm, with a connectivity to a quartet centered around 4.65 ppm (H5/H6), indicating a Fucα3 residue, suggests that a small amount of Lex pentaosylceramide was present (data not shown) in accordance with the mass spectrometry data.
FIGURE 11.
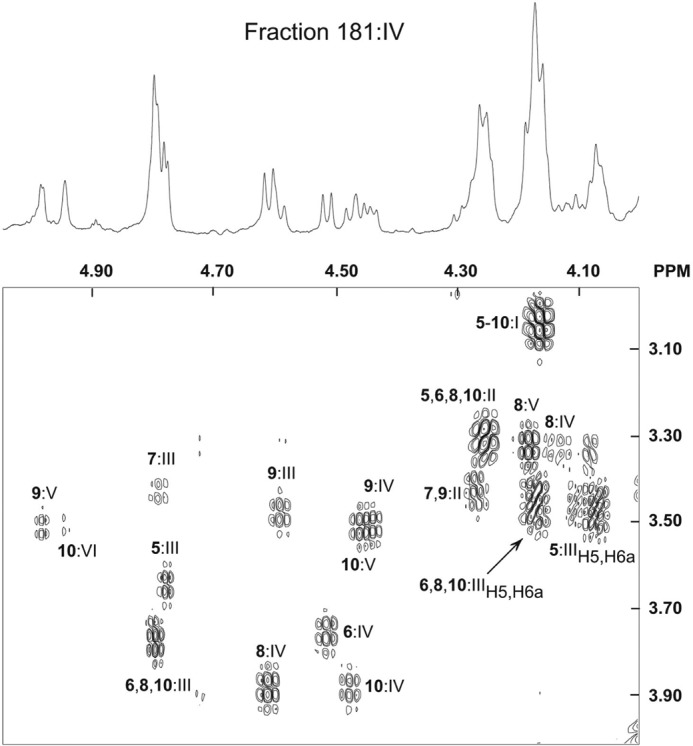
The H1/H2 region of the DQF-COSY proton NMR spectrum of fraction 181:IV from human embryonic stem cell line SA181, recorded at 600 MHz (30 °C). The spectrum at the top is the corresponding one-dimensional spectrum of the anomeric region. The sample (∼0.4 mg) was dissolved in dimethyl sulfoxide-D2O (98:2, v/v) after deuterium exchange. All labeled connectivities represent H1/H2 cross-peaks, except for Galα4 H5/H6a cross-peaks, which are denoted as such. The H1/H2 labels are derived from structures 5–10, and Roman numerals I-VI denote individual sugars, as listed in Table 3.
Further Characterization of the Slow Migrating Glycosphingolipids of Fractions 181:IV and 121:IV
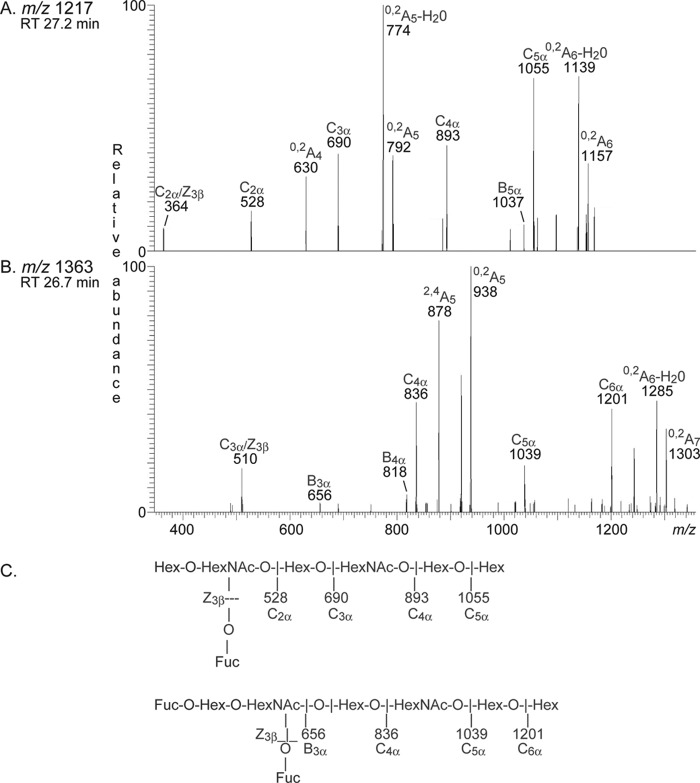
The monoclonal antibodies directed against the Lex and Ley determinants gave an intense staining in the slow migrating regions of fractions 121:IV and 181:IV (Fig. 5, C and D). In an attempt to define the structures of these complex glycosphingolipids, fractions 121:IV and 181:IV (∼0.7 mg each) were further separated by Iatrobeads chromatography. After pooling, four fractions were obtained in each case. The two fractions containing the slow migrating compounds from each separation were denoted fractions 121:IV-C, 121:IV-D, 181:IV-C, and 181:IV-D, respectively. With LC-ESI/MS of the oligosaccharides obtained from these four fractions by endoglycoceramidase digestion and by proton NMR, the same glycosphingolipids as in fractions 121:IV and 181:IV described above were identified. In addition, by inspection of selected ions from LC-ESI/MS, a [M − H+]− ion at m/z 1217, eluting at 27.1–27.4 min, was found in fractions 121:IV-C and 181:IV-C, and a [M − H+]− ion at m/z 1363, eluting at 26.5–27.0.4 min, was found in fractions 121:IV-D and 18:IV-D (data not shown).
The MS2 spectrum of the ion at m/z 1217 (Fig. 12A) had a fragment ion at m/z 364, which is diagnostic of an internal 4-linked GlcNAc substituted with a Fuc at 3-position (27). In addition, there was a C-type ion series (C2 at m/z 528, C3 at m/z 690, C4 at 893, and C5 at 1055), which, together with the prominent 0,2A5 fragment ion at m/z 792 and the 0,2A5-H2O fragment ion at m/z 774, allowed a tentative identification of a heptasaccharide with terminal Lex determinant and internal type 2 chain (Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc).
FIGURE 12.
MS2 of complex oligosaccharides in fractions 181:IV-C and 181:IV-D from human embryonic stem cell line SA181. RT, retention time. A, MS2 spectrum of the ion at m/z 1217 of fraction 181:IV-C from human embryonic stem cell line SA181 (retention time 27.2 min). B, MS2 spectrum of the ion at m/z 1363 of fraction 181:IV-D from human embryonic stem cell line SA181 (retention time 26.7 min). C, interpretation formulas.
MS2 of the ion at m/z 1363 (Fig. 12B) gave a fragment ion at m/z 510, indicating an internal 4-linked GlcNAc substituted with a Fuc at 3-position (27). A B3 ion was seen at m/z 656, and a series of C-type ions at m/z 836 (C4), m/z 1039 (C5), and m/z 1201 (C6) was also present. Furthermore, there was a 2,4A5 ion at m/z 878 and a 0,2A5 ion at m/z 938. Thus, these MS2 spectral features suggested an octasaccharide with terminal Ley determinant and internal type 2 chain (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc).
Binding of Human Serum Antibodies to the Total Non-acid Glycosphingolipids from hESC
Binding of natural mixed human serum antibodies to hESC non-acid glycosphingolipids is shown in Fig. 13. This mixed serum sample contains naturally occurring anti-Gal antibodies binding to the B5 glycosphingolipid (Galα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of rabbit erythrocytes in lane 5. In addition, a distinct binding to a compound migrating in the pentaglycosylceramide region in the non-acid fractions from hESC was obtained (lane 3). The human serum also bound to reference Lex pentaglycosylceramide (lane 2).
FIGURE 13.
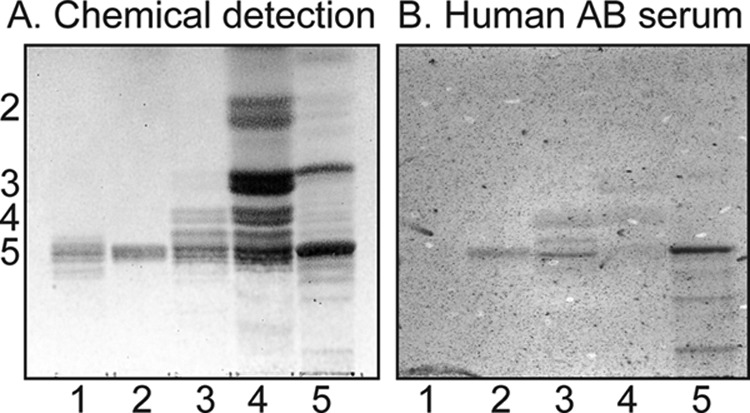
Binding of human serum antibodies to the total non-acid glycosphingolipids from human embryonic stem cells. Shown are a thin-layer chromatogram after detection with anisaldehyde (A) and an autoradiogram obtained by binding of human blood group AB serum (B). Lane 1, reference H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); lane 2, reference Lex pentaglycosylceramide (Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); lane 3, slow migrating non-acid glycosphingolipids of human embryonic stem cell line SA121 (fraction 121:IV-D) (30 μg); lane 4, total non-acid glycosphingolipids of human embryonic stem cell line SA121 (40 μg); lane 5, total non-acid glycosphingolipids of rabbit erythrocytes (40 μg). The numbers to the left of the chromatogram in A denote the approximate number of carbohydrate residues in the bands. The chromatograms were eluted with chloroform/methanol/water (60:35:8, v/v/v), and the binding assays were done as described under “Experimental Procedures.”
DISCUSSION
Carbohydrate antigen expression in embryonic stem cells has so far been studied mainly by biological reagents, such as monoclonal antibodies and lectins, that do not discriminate between protein- and lipid-linked cell surface carbohydrates. A few studies have analyzed the glycosphingolipid composition of murine embryonic stem cells using immunostaining methodology, but the only glycosphingolipid identified by biochemical methods was, until recently, the ganglioside GD3, as reviewed in Ref. 30. In 2011, Liang et al. (9) characterized the glycosphingolipid composition of two commercially available hESC lines (HES5 and H9) using both immune techniques and mass spectrometry of glycolipid extracts. By flow cytometry analysis, globopentaosylceramide/SSEA3, sialyl-globopentaosylceramide/SSEA4, Globo H, and blood group H with type 1 core chain were identified. In addition, the crude glycolipid fraction obtained in the upper phase of the Folch partition was per-methylated and subjected to mass spectrometry analysis. Thereby, the non-acid glycosphingolipids globotetraosylceramide, lactotetraosylceramide, the H type 1 pentaosylceramide, globopentaosylceramide/SSEA3, and Globo H hexaosylceramide and the gangliosides GM3, sialyl-globopentaosylceramide/SSEA4, disialyl-globopentaosylceramide, and GD1a/GD1b were identified.
In this study, we have obtained a higher resolution of the glycosphingolipids present in the SA121 and SA181 hESC lines. This was due to a large amount of cell material (1 × 109 cells), allowing several purification steps and separation of the glycosphingolipids into total non-acid and acid fractions, respectively. The glycosphingolipid material obtained also permitted us to obtain partly purified glycosphingolipid subfractions, which made it possible to identify minor compounds using a combination of mass spectrometry and proton NMR spectroscopy, giving a complete structural assignment, including anomerity and binding positions of the glycosidic linkages.
The short chain glycosphingolipid compounds present in the SA121 and SA181 hESC lines were identified as glucosylceramide, galactosylceramide, lactosylceramide, galabiaosylceramide, lactotriaosylceramide, and globotriaosylceramide. These compounds, along with part of the more polar ones (4–7 carbohydrate residues), are retained in the lower phase after Folch partitioning and are therefore not included in the study of Liang et al. (9). Regarding the complex glycosphingolipids, we identified, in addition to the globo-series and type 1 core glycosphingolipids listed above, a number of type 2 core glycosphingolipids not previously described in human embryonic stem cells (i.e. neo-lactotetraosylceramide, the H type 2 pentaosylceramide, the Lex pentaosylceramide, and the Ley hexaosylceramide). The A type 1 hexaosylceramide was also identified. In addition, a heptaosylceramide with terminal Lex determinant and internal type 2 chain (Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) and an octaosylceramide with terminal Ley determinant and internal type 2 chain (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) were tentatively identified. The identified glycosphingolipids are summarized in Table 4.
TABLE 4.
Summary of non-acid glycosphingolipids characterized in human embryonic stem cells by binding of lectins and monoclonal antibodies in the chromatogram binding assay (CBA), MS, and proton NMR
| Glycosphingolipid | SA121 cell line |
SA181 cell line |
||||
|---|---|---|---|---|---|---|
| CBA | MS | NMR | CBA | MS | NMR | |
| Glucosylceramidea | NDb | +c | + | ND | +c | + |
| Galactosylceramide | ND | +c | + | ND | +c | + |
| Lactosylceramide | ND | +d | + | ND | +d | + |
| Galabiaosylceramide | ND | +d | + | ND | +d | + |
| Globotriaosylceramide | ND | + | + | ND | + | + |
| Globotetraosylceramide | ND | + | + | ND | + | + |
| Globopentaosylceramide/SSEA-3 | + | + | + | + | + | + |
| Globo H hexaosylceramide | + | + | + | + | + | + |
| Lactotriaosylceramide | ND | + | − | ND | + | − |
| Lactotetraosylceramide | ND | + | + | ND | + | + |
| Neolactotetraosylceramide | + | + | − | + | + | − |
| H type 1 pentaosylceramide | + | + | + | + | + | + |
| H type 2 pentaosylceramide | − | + | − | − | + | − |
| A type 1 hexaosylceramide | + | + | − | + | + | − |
| Lex/SSEA-1 pentaosylceramide | + | + | + | + | + | + |
| Ley hexaosylceramide | + | + | − | + | + | − |
| Lex/SSEA-1 heptaosylceramide | + | + | − | + | + | − |
| Ley octaosylceramide | + | + | − | + | + | − |
a Glycosphingolipids not previously characterized in human embryonic stem cells are in italic type.
b ND, not determined.
c Glucosylceramide and galactosylceramide give identical mass spectra.
d Lactosylceramide and galabiaosylceramide give identical mass spectra.
By antibody binding studies and mass spectrometry of the acid glycosphingolipid fractions from the SA121 and SA181 hESC lines, the gangliosides GM3, GD3, and sialyl-globopentaosylceramide/SSEA4 have been tentatively identified, along with some acid glycosphingolipids not described previously in human embryonic stem cells. These studies will be reported separately.
The biosynthesis of glycosphingolipids starts at the cytosolic side of the endoplasmic reticulum with the addition of a Gal or Glc to the ceramide. After transfer to the luminal side and transport to the early Golgi apparatus, a stepwise glycosylation is catalyzed by a number of glycosyltransferases (31). The Galβ4GlcNAc linkage of type 2 chains is formed by several UDP-galactose:β-N-acetylglucosamine β1,4-galactosyl-transferases, designated β4Gal-T1, β4Gal-T2, β4Gal-T3, and β4Gal-T4, which all catalyze the transfer of galactose to form neo-lacto-series glycosphingolipids (32, 33). The occurrence of type 2 chain glycosphingolipids in the human embryonic stem cell lines thus corresponds to the expression of one or several of these β4-galactosyltransferases in addition to the glycosyltransferases investigated by Liang et al. (9).
The future applications for hESC in clinical medicine are yet not defined. If stem cells can be generated from the specific individual in need of treatment, the self/non-self immune barrier will not be an obstacle. However, in all therapeutic settings involving transfer of cells with a different genetic background, these cells will expose the recipient to non-self cell surface antigenic determinants that may evoke an immune response and subsequent graft rejection. The blood group ABO system is a strong immunological barrier in organ transplantation comparable with or even stronger than the HLA system (34). In the hESC analyzed in this work, a blood group A type 1 hexaglycosylceramide was identified. The two hESC lines were genotyped as having a blood group A1 genotype, and blood group A antigens were detected on their cell surface by immunohistochemistry.5 However, selecting a blood group O phenotype hESC will eliminate the A and B antigens as an immune barrier in clinical applications of hESC.
Binding studies with mixed serum from several healthy human blood group AB blood donors demonstrated a reactivity by preformed antibodies to the pentaglycosylceramide region of the non-acid glycosphingolipid fractions of the SA121 and SA181 hESC lines. Testing of this serum against reference glycosphingolipids in the chromatogram binding assay showed binding to the Lex pentaglycosylceramide. No other glycosphingolipids identified in the two cell lines reacted with the AB serum (data not shown), suggesting that the reactivity was due to Lex recognition.
Similar reactivity in the pentaglycosylceramide region was also observed when using serum from a single blood group A individual, whereas serum from a single blood group O individual gave binding to bands in the penta- and heptaglycosylceramide region, most likely due to additional recognition of the blood group A heptaglycosylceramide (data not shown). Taken together, these observations suggest a possible humoral immune response against carbohydrate antigens of hESC. Such reactivities need to be further addressed before these cells can be clinically implemented.
Culture of hESC in the presence of animal-derived compounds may lead to uptake and incorporation of the non-human sialic acid NeuGc (35), which may give an immune response due to the circulating antibodies to NeuGc that are present in most humans (36). It should be noted that the hESC lines (HES5 and H9) studied by Liang et al. (9) were cultured using mouse embryonic fibroblast conditioned media on a Matrigel surface, the latter being a complex mix of unspecified mouse material. The two hESC lines analyzed in this study were grown in a feeder cell and serum-free system, and the glycosphingolipids analyzed are therefore not contaminated with compounds of animal origin. This was confirmed by the absence of binding of antibodies directed against Galα3Gal determinants (data not shown), which are found in all animal species below non-human primates in the evolutionary chain (37).
Another important aspect of comparing different hESC lines propagated in different culturing systems is the level of pluripotency. It is well known that pluripotency is a relative term and that different culturing systems may have different efficacies in keeping the cells undifferentiated. As suggested by Liang et al., the onset of differentiation may start transition of, for example, glycosphingolipids. It would therefore be interesting to expand these studies in the future to comprise both different hESC lines and different culturing techniques.
The complex glycosphingolipids in the SA121 and SA181 cells contained a series of compounds with the Ley determinant. This is of particular interest because it has been shown in mice that the blastocysts express Ley and that intrauterine injection of anti-Ley monoclonal antibodies blocks blastocyst cell adhesion and their continuous development (38). The Ley antigen has also been postulated to be an onco-fetal antigen involved in the carcinogenesis processes (39).
Changes in antigen expression upon differentiation of embryonic stem cells have been documented (40). Regarding carbohydrate antigens, the stage-specific embryonic antigens, SSEA-1 to -4, and the Lex(SSEA-1)/Ley antigens have been studied in mice (41, 42), whereas studies of human embryonic stem cells are more limited. By quantification of the mRNA for the glycosyltransferases involved in the biosynthesis of the glycosphingolipid components (9), a change in glycosphingolipid composition was found when the undifferentiated hESC were changed into embryonic body outgrowth. The compounds with globo and lacto core chains were reduced, and ganglio-series structures were increased. This finding is similar to reports of retinoic acid-induced differentiation of human embryonic carcinoma cells (43, 44) and the hESC line H7 (40), where a switch from globo-series compounds to ganglio-series compounds was found. In the 1980s, it was shown that during the early mouse embryogenesis (up to day 7) a stage-specific transition of carbohydrate epitopes from globo- to lacto- and ganglio-series occurs (reviewed in Ref. 45).
Interestingly, several of the glycosphingolipids of hESC are overexpressed in various types of human cancers (46), and it has been suggested that these carbohydrate profiles may be related to properties common to both cancer cells and embryonic stem cells, such as the capability of eternal cell proliferation (47). A further suggestion is that some of these glycans may serve as markers for human embryonic stem cells and for cancers (9). However, several of the monoclonal antibodies used for detection of the glycosphingolipids were cross-reactive, which is not uncommon for monoclonal antibodies directed against carbohydrate epitopes (48). The most problematic issue is the anti-SSEA-3/Globopenta, antibody MC631, an antibody often used for characterization of human embryonic stem cells (5). In our hands, this antibody also bound to globotetraosylceramide, the Globo H hexaglycosylceramide, and the H type 1 pentaglycosylceramide. Thus, development of more specific monoclonal antibodies against carbohydrate epitopes is urgently needed for further studies of carbohydrate antigens as disease biomarkers or markers for human embryonic stem cells.
In summary, a number of glycosphingolipids not previously described in hESC were identified in this study, demonstrating that the glycosylation of hESC is more complex than previously appreciated. Cell-cell interactions mediated by cell surface glycoconjugates play important roles in various biological events, such as embryogenesis and carcinogenesis (49–51). Thus, our findings enhance our knowledge of hESC and provide a platform for further studies of the functional roles of glycosphingolipids during cellular differentiation and proliferation.
Acknowledgments
The use of the LTQ linear quadrupole ion trap mass spectrometer (obtained by Swedish Research Council Grant 342-2004-4434 to Gunnar Hansson) and the Varian 600-MHz instrument at the Swedish NMR Centre, Hasselblad Laboratory, Göteborg University, is gratefully acknowledged.
This work was supported by Swedish Research Council Grant 12628, the Swedish Cancer Foundation, and governmental grants to the Sahlgrenska University Hospital.
The glycosphingolipid nomenclature follows the recommendations by the IUPAC-IUB Commission on Biochemical Nomenclature (53). It is assumed that Gal, Glc, GlcNAc, GalNAc, NeuAc, and NeuGc are of the d-configuration; that Fuc is of the l-configuration; and that all sugars are present in the pyranose form.
In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length and the number after the colon gives the total number of double bonds in the molecule. Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation (e.g. h16:0). For long chain bases, “d” denotes dihydroxy, and “t” denotes trihydroxy. Thus, d18:1 designates sphingosine (1,3-dihydroxy-2-aminooctadecene), and t18:0 designates phytosphingosine (1,3,4-trihydroxy-2-aminooctadecane).
K. Säljö and L. Rydberg, personal communication.
- hESC
- human embryonic stem cell(s)
- ESI
- electrospray ionization
- SSEA
- stage-specific embryonic antigen
- DQF
- double quantum-filtered.
REFERENCES
- 1. Evans M. J., Kaufman M. H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 [DOI] [PubMed] [Google Scholar]
- 2. Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- 3. Smith A. (1998) Cell therapy: in search of pluripotency. Curr. Biol. 8, R802–R804 [DOI] [PubMed] [Google Scholar]
- 4. Smith A. G. (2001) Embryo-derived stem cells. Of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 [DOI] [PubMed] [Google Scholar]
- 5. Wright A. J., Andrews P. W. (2009) Surface marker antigens in the characterization of human embryonic stem cells. Stem Cell Res. 3, 3–11 [DOI] [PubMed] [Google Scholar]
- 6. Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. (1983) Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 2, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. (1983) New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J. Biol. Chem. 258, 8934–8942 [PubMed] [Google Scholar]
- 8. Suila H., Pitkänen V., Hirvonen T., Heiskanen A., Anderson H., Laitinen A., Natunen S., Miller-Podraza H., Satomaa T., Natunen J., Laitinen S., Valmu L. (2011) Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J. Mol. Cell Biol. 3, 99–107 [DOI] [PubMed] [Google Scholar]
- 9. Liang Y. J., Kuo H. H., Lin C. H., Chen Y. Y., Yang B. C., Cheng Y. Y., Yu A. L., Khoo K. H., Yu J. (2010) Switching of the core structures of glycosphingolipids from globo- and lacto- to ganglio-series upon human embryonic stem cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 107, 22564–22569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang Y. J., Yang B. C., Chen J. M., Lin Y. H., Huang C. L., Cheng Y. Y., Hsu C. Y., Khoo K. H., Shen C. N., Yu J. (2011) Changes in glycosphingolipid composition during differentiation of human embryonic stem cells to ectodermal or endodermal lineages. Stem Cells 29, 1995–2004 [DOI] [PubMed] [Google Scholar]
- 11. Andrews P. D., Becroft M., Aspegren A., Gilmour J., James M. J., McRae S., Kime R., Allcock R. W., Abraham A., Jiang Z., Strehl R., Mountford J. C., Milligan G., Houslay M. D., Adams D. R., Frearson J. A. (2010) High-content screening of feeder-free human embryonic stem cells to identify pro-survival small molecules. Biochem. J. 432, 21–33 [DOI] [PubMed] [Google Scholar]
- 12. Karlsson K.-A. (1987) Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138, 212–220 [DOI] [PubMed] [Google Scholar]
- 13. Holgersson J., Jovall P.-A., Breimer M. E. (1991) Glycosphingolipids of human large intestine. Detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J. Biochem. 110, 120–131 [DOI] [PubMed] [Google Scholar]
- 14. Breimer M. E., Hansson G. C., Karlsson K.-A., Leffler H. (1982) Isolation and partial characterization of blood group A and H active glycosphingolipids of rat small intestine. J. Biol. Chem. 257, 906–912 [PubMed] [Google Scholar]
- 15. Holgersson J., Jovall P.-A., Samuelsson B.E., Breimer M. E. (1990) Structural characterization of non-acid glycosphingolipids in kidneys of single blood group O and A pigs. J. Biochem. 108, 766–777 [DOI] [PubMed] [Google Scholar]
- 16. Teneberg S., Angström J., Jovall P.-A., Karlsson K.-A. (1994) Characterization of binding of Galβ4GlcNAc-specific lectins from Erythrina christagalli and Erythrina corallodendron to glycosphingolipids. Detection, isolation and characteriztion of a novel glycosphingolipid of bovine buttermilk. J. Biol. Chem. 269, 8554–8563 [PubMed] [Google Scholar]
- 17. Benktander J., Ångström J., Breimer M. E., Teneberg S. (2012) Re-definition of the the carbohydrate binding specificity of Helicobacter pylori BabA adhesin. J. Biol. Chem. 287, 31712–31724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuelsson B. E., Pimlott W., Karlsson K.-A. (1990) Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 193, 623–646 [DOI] [PubMed] [Google Scholar]
- 19. Karlsson H., Halim A., Teneberg S. (2010) Differentiation of glycosphingolipid-derived glycan structural isomers by liquid chromatography-mass spectrometry. Glycobiology 20, 1103–1116 [DOI] [PubMed] [Google Scholar]
- 20. Koerner T. A., Jr., Prestegard J. H., Demou P. C., Yu R. K. (1983) High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry 22, 2676–2687 [DOI] [PubMed] [Google Scholar]
- 21. Hansson G. C., Karlsson K.-A., Larson G., McKibbin J. M., Blaszczyk M., Herlyn M., Steplewski Z., Koprowski H. (1983) Mouse monoclonal antibodies against human cancer cell lines with specificities for blood group and related antigens. Characterization by antibody binding to glycosphingolipids in a chromatogram binding assay. J. Biol. Chem. 258, 4091–4097 [PubMed] [Google Scholar]
- 22. Diswall M., Angström J., Schuurman H. J., Dor F. J., Rydberg L., Breimer M. E. (2007) Biochemical studies of Gal antigen expression in small intestine and pancreas from α1,3-galactosyltransferase knock-out miniature swine. Transplantation 84, 1348–1356 [DOI] [PubMed] [Google Scholar]
- 23. Ito M., Yamagata T. (1989) Purification and characterization of glycosphingolipid-specific endoglycosidases (endoglycoceramidases) from a mutant strain of Rhodococcus sp. Evidence for three molecular species of endoglycoceramidase with different specificities. J. Biol. Chem. 264, 9510–9519 [PubMed] [Google Scholar]
- 24. Marion D., Wüthrich K. (1983) Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 113, 967–974 [DOI] [PubMed] [Google Scholar]
- 25. Hallén U., Angström J., Björkner A. E. (2008) Glycolipid binding epitopes involved in adherence of the periodontitis-associated bacterium Porphyromonas gingivalis. Glycoconj. J. 25, 561–572 [DOI] [PubMed] [Google Scholar]
- 26. Dabrowski J., Trauner K., Koike K., Ogawa T. (1988) Complete 1H-NMR spectral assignments for globotriaosyl-Z- and isoglobotriaosyl-E-ceramide. Chem. Phys. Lipids 49, 31–37 [DOI] [PubMed] [Google Scholar]
- 27. Chai W., Piskarev V., Lawson A. M. (2001) Branching pattern and sequence analysis of underivatized oligosaccharides by combined MS/MS of singly and doubly charged molecular ions in negative-ion electrospray mass spectrometry. Anal. Chem. 73, 651–657 [DOI] [PubMed] [Google Scholar]
- 28. Robbe C., Capon C., Coddeville B., Michalski J.-C. (2004) Diagnostic ions for the rapid analysis by nano-electrospray ionization quadrupole time of flight mass spectrometry of O-glycans from human mucins. Rapid Commun. Mass Spectrom. 18, 412–420 [DOI] [PubMed] [Google Scholar]
- 29. Li Y.-T., Chou C.-W., Li S.-C., Kobayashi U., Ishibashi Y.-H., Ito M. (2009) Preparation of homogenous oligosaccharide chains from glycosphingolipids. Glycoconj. J. 26, 929–933 [DOI] [PubMed] [Google Scholar]
- 30. Yanagisawa M. (2011) Stem cell glycolipids. Neurochem. Res. 36, 1623–1635 [DOI] [PubMed] [Google Scholar]
- 31. Schnaar R. L., Suzuki A., Stanley P. (2008) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds) pp. 129–141, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 32. Almeida R., Amado M., David L., Levery S. B., Holmes E. H., Merkx G., van Kessel A. G., Rygaard E., Hassan H., Bennett E., Clausen H. (1997) A family of human β4-galactosyltransferases. Cloning and expression of two novel UDP-galactose:β-N-acetylglucosamine β1,4-galactosyltransferases, β4Gal-T2 and β4Gal-T3. J. Biol. Chem. 272, 31979–31991 [DOI] [PubMed] [Google Scholar]
- 33. Schwientek T., Almeida R., Levery S. B., Holmes E. H., Bennett E., Clausen H. (1998) Cloning of a novel member of the UDP-galactose:β-N-acetylglucosamine β1,4-galactosyltransferase family, β4Gal-T4, involved in glycosphingolipid biosynthesis. J. Biol. Chem. 273, 29331–29340 [DOI] [PubMed] [Google Scholar]
- 34. Breimer M. E. (2011) Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalTKO era. Xenotransplantation 18, 215–228 [DOI] [PubMed] [Google Scholar]
- 35. Martin M. J., Muotri A., Gage F., Varki A. (2005) Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 11, 228–232 [DOI] [PubMed] [Google Scholar]
- 36. Tangvoranuntakul P., Gagneux P., Diaz S., Bardor M., Varki N., Varki A., Muchmore E. (2003) Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. U.S.A. 100, 12045–12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galili U., Clark M. R., Shohet S. B., Buehler J., Macher B. A. (1987) Evolutionary relationship between the natural antibody and the Galα1–3Gal epitope in primates. Proc. Natl. Acad. Sci. U.S.A. 84, 1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu Z. M., Kojima N., Stroud M. R., Hakomori S., Fenderson B. A. (1995) Monoclonal antibody directed to Ley oligosaccharide inhibits implantation in the mouse. Biol. Reprod. 52, 903–912 [DOI] [PubMed] [Google Scholar]
- 39. Hakomori S., Nudelman E., Levery S. B., Kannagi R. (1984) Novel fucolipids accumulating in human adenocarcinoma. I. Glycolipids with di- or trifucosylated type 2 chain. J. Biol. Chem. 259, 4672–4680 [PubMed] [Google Scholar]
- 40. Draper J. S., Pigott C., Thomson J. A., Andrews P. W. (2002) Surface antigens of human embryonic stem cells. Changes upon differentiation in culture. J. Anat. 200, 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williams S. A., Stanley P. (2009) Complex N-glycans or core 1-derived O-glycans are not required for the expression of stage-specific antigens SSEA-1, SSEA-3, SSEA-4 or Ley in preimplantation mouse embryos. Glycoconj. J. 26, 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fenderson B. A., Holmes E. H., Fukushi Y., Hakomori S. (1986) Coordinate expression of X and Y haptens during murine embryogenesis. Dev. Biol. 114, 12–21 [DOI] [PubMed] [Google Scholar]
- 43. Fenderson B. A., Andrews P. W., Nudelman E., Clausen H., Hakomori S. (1987) Glycolipid core structure switching from globo- to lacto- and ganglio-series during retinoic acid-induced differentiation of TERA-2-derived human embryonal carcinoma cells. Dev. Biol. 122, 21–34 [DOI] [PubMed] [Google Scholar]
- 44. Chen C., Fenderson B. A., Andrews P. W., Hakomori S. (1989) Glycolipid glycosyltransferases in human embryonal carcinoma cells during retinoic acid induced differentiation. Biochemistry 28, 2229–2238 [DOI] [PubMed] [Google Scholar]
- 45. Hakomori S. (2008) Structure and function of glycosphingolipids and sphingolipids. Recollections and future trends. Biochim. Biophys. Acta 1780, 325–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heimburg-Molinaro J., Lum M., Vijay G., Jain M., Almogren A., Rittenhouse-Olson K. (2011) Cancer vaccines and carbohydrate epitopes. Vaccine 29, 8802–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tateno H., Toyota M., Saito S., Onuma Y., Ito Y., Hiemori K., Fukumura M., Matsushima A., Nakanishi M., Ohnuma K., Akutsu H., Umezawa A., Horimoto K., Hirabayashi J., Asashima M. (2011) Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J. Biol. Chem. 286, 20345–20353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manimala J. C., Roach T. A., Li Z., Gildersleeve J. C. (2007) High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology 17, 17C–23C [DOI] [PubMed] [Google Scholar]
- 49. Kobata A. (1992) Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 209, 483–501 [DOI] [PubMed] [Google Scholar]
- 50. Varki A. (1993) Biological roles of oligosaccharides. All of the theories are correct. Glycobiology 3, 97–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haltiwanger R. S., Lowe J. B. (2004) Role of glycosylation in development. Annu. Rev. Biochem. 73, 491–537 [DOI] [PubMed] [Google Scholar]
- 52. Angström J., Larsson T., Hansson G. C., Karlsson K.-A., Henry S. (2004) Default biosynthesis pathway for blood group-related glycolipids in human small intestine as defined by structural identification of linear and branched glycosylceramides in a group O Le(a-b-) nonsecretor. Glycobiology 14, 1–12 [DOI] [PubMed] [Google Scholar]
- 53. Chester M. A. (1998) IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids. Recommendations 1997. Eur. J. Biochem. 257, 293–298 [DOI] [PubMed] [Google Scholar]