Background: The molecular mechanism involved in sperm maturation is still not completely elucidated.
Results: We identified an epididymis-specific gene, Spink13, that is essential for the acrosomal integrity and sperm maturation.
Conclusion: Our findings provided direct evidence of a modulatory effect of SPINK13 on fertilization.
Significance: We expect further study of SPINK13 will provide a new putative target for post-testicular male contraceptives.
Keywords: Epididymis, Fertilization, Serpin, siRNA, Spermatozoa, Kazal-type Serine Protease Inhibitor, Sperm Maturation
Abstract
Sperm maturation involves numerous surface modifications by a variety of secreted proteins from epididymal epithelia. The sperm surface architecture depends on correct localization of its components and highlights the importance of the sequence of the proteolytic processing of the sperm surface in the epididymal duct. The presence of several protease inhibitors from different families is consistent with the hypothesis that correctly timed epididymal protein processing is essential for proper sperm maturation. Here we show that the rat (Rattus norvegicus) epididymis-specific gene Spink13, an androgen-responsive serine protease inhibitor, could bind to the sperm acrosome region. Furthermore, knockdown of Spink13 in vivo dramatically enhanced the acrosomal exocytosis during the process of capacitation and thus led to a significant reduction in male fertility, indicating that Spink13 was essential for sperm maturation. We conclude that blockade of SPINK13 may provide a new putative target for post-testicular male contraceptives.
Introduction
The mammalian spermatozoa produced in the testis do not have the ability to fertilize oocytes; they become fertilization-competent during passage through the epididymis, where they undergo sequential membrane modifications collectively known as sperm maturation (1–5). During this process, the male gamete will undergo a sequence of well orchestrated biochemical modifications that are modulated by the epididymal intraluminal composition, in particular secreted epididymal proteins (6, 7). The molecular mechanism involved in the process that transforms an immature gamete into a sperm with full fertilizing capability is still not completely elucidated. During their journey, sperm plasma membrane domains are remodeled by proteolytic degradation/release of testicular factors: adsorption of epididymal proteins on their surfaces and enzymatic modifications such as glycosylation/deglycosylation of specific proteins (8–10). For example, epididymal angiotensin-converting enzyme has been reported to mediate the translocation of ADAM3 (a disintegrin and a metalloprotease 3) in the sperm membrane (11) and to minimize sperm motility during epididymal storage, and knock-out animals for the angiotensin-converting enzyme gene are subfertile (12), suggesting that this enzyme is required for normal fertility. Another example is fertilin β, which is a member of a molecular family known as ADAMs, participates in sperm-egg membrane binding, and undergoes a cascade of proteolytic cleavages by proprotein convertase subtilisin/kexin type 4 to their acrosome-bound forms during sperm maturation in the epididymis (13). This suggests that the sperm surface architecture depends on correct localization of its components and highlights the importance of the sequential proteolytic processing of the sperm surface proteins in the epididymal duct. Proteolysis in the epididymal lumen thus needs to be well controlled, and several protease inhibitors expressed in the certain epididymal regions are expected to be responsible for this control (3, 8).
In previous studies (14) in Macaca mulatta, we identified an epididymis-specific gene, ESC6 (epididymis-specific clone 6), now renamed as SPINK13, belonging to the Kazal-type serine protease inhibitor (SPINK) family. The SPINK family has a characteristic signature that contains at least one conserved Kazal domain with six consensus cysteines forming a 1–5/2–4/3–6 disulfide bond pattern (15). The SPINKs are members of an ancient gene family and exist in animals as diverse as insects, birds, and mammals (16–18). All SPINK members are responsible for the regulation of serine protease activities, preventing uncontrolled proteolysis (19). This retention over species and evolutionary time has led to the belief that these moieties play an essential role(s) in biology. However, the role(s) that have allowed for this conservation and evolution have not been defined, and the biology of SPINKs in normal homeostasis and disease pathogenesis is poorly understood. To date, there are at least 10 gene members in the human genome reported as SPINK1 (20), SPINK2 (21), SPINK4 (22), SPINK5 (23), SPINK6 (24), SPINK7 (25), SPINK8 (26), SPINK9 (27), SPINK13, and SPINK14 (28). Imbalance between SPINK proteins and proteases may cause severe diseases, such as pancreatitis, celiac disease, Netherton syndrome, skin barrier defects, and cancer (20, 27, 29–33). Among them, several SPINKs are expressed in the epithelial cells of the male reproductive tract, where some of them are involved in the process of fertilization. SPINK2 functions as a trypsin/acrosin inhibitor and is expressed mainly in the testis, epididymis, and seminal vesicle (34). Gene expression profiling of infertile men diagnosed with azoospermia revealed that they had a 4-fold decrease in SPINK2 expression compared with fertile men (29). Recent studies have found that mutant male mice with reduced Spink2 levels exhibit impaired fertility (35). Spink3 (orthologue gene of human SPINK1) knock-out mice have no pancreatic acinar cells because of autophagic cell death and impaired regeneration (36). The rat SPINK3 protein, also called caltrin (calcium transport inhibitor), is secreted by the seminal vesicles and binds specifically to the acrosomal region of the sperm head and inhibits the extracellular Ca2+ uptake as well as the acrosin activity in vitro (37). Other four novel Kazal protease inhibitors, Spink8, Spink10, Spink1, and Spink12, which are highly expressed in the mouse epididymis, have been identified recently (26). Such inhibitors are likely important for maintaining equilibrium of protease activity for essential processes that preserve sperm and tissue integrity.
We hypothesized that SPINK has been retained over species and time because it plays a major role in the protection against proteolytic degradation. Specifically, we hypothesized that SPINK13 contributes to the ability of the epididymis in sperm maturation. To test this hypothesis, we investigated the effect of SPINK13 on male fertility. Studies using RNAi knockdown systems in vivo indicate a modulatory effect of SPINK13 on sperm function, assuring the successful acrosome reaction in proper time and place during fertilization.
EXPERIMENTAL PROCEDURES
Animals
Healthy Sprague-Dawley rats and BALB/c mice, supplied by the Animal Center of the Chinese Academy of Sciences (Shanghai, China) and housed under standard laboratory conditions, were used in the present study. The experiments were conducted according to a protocol approved by the Institute Animal Care Committee. The protocol conformed to internationally accepted guidelines for the care and use of laboratory animals. To study the developmental profile and tissue distribution of the protein, we followed our previously described methods (38). To investigate the androgen effects, male rats were castrated and administered testosterone as previously described (39).
DNA and Protein Sequence Analysis
The rat orthologue of human SPINK13 was obtained by homology, searching the protein database using the human SPINK13 protein sequence as a query using Blastp (National Center for Biotechnology Information). HomoloGene was used for automated detection of homologues among the eukaryotic genomes. SignalP 4.0 was used to identify the putative signal peptide cleavage sites. The peptide sequences and putative functions of the novel genes were deduced using the ExPASy Translate tool. Big-PI Predictor was used to predict the potential glycosylphosphatidylinositol (GPI)3 modification sites of the protein. The phylogenetic trees of the protein families were generated using MEGA 3.1. The sequences were aligned by ClustalW, and the tree was constructed using the neighbor joining method.
RNA Isolation and Northern Blot Analysis
Total RNA was extracted from tissue homogenates with TRIzol (Invitrogen) following the manufacturer's recommendations. Northern blot analysis was performed according to the procedure described previously (39). Twelve micrograms of total RNA from each sample were subjected to 1.0% (w/v) agarose-formaldehyde gel electrophoresis, blotted onto nylon membranes by capillary transfer, and hybridized with a probe as previously described. PCR-amplified fragments of Spink13 cDNA (NM_001109539.1) (from 168 to 493 bp in cDNA; total of 326 bp) were used as the templates to prepare the 32P-labeled cDNA probe using the prime-α-gene labeling kit (Promega, Madison, WI). An 18 S rRNA hybridization signal was used as a loading control.
Protein Expression and Purification
To raise the specific antibody to SPINK13, we adopted the double-copy protein expression scheme described previously (40). Briefly, two SPINK13 fragments corresponding to the mature SPINK13 peptide of 71 amino acids were inserted into pET-28(a) vector (Novagen, Darmstadt, Germany). The recombinant protein in the inclusion bodies was induced by 1 mm isopropyl thiogalactoside from the strain Escherichia coli BL21 Codon Plus RP (Novagen). The purification of the recombinant protein from inclusion bodies was performed using a Novagen pET expression systems kit.
Generation of mAbs
Four 8-week-old BALB/c female mice were immunized intraperitoneally with 100 μg (per mouse) double-copy rSPINK13 (drSPINK13) recombinant protein mixed with Freund's complete adjuvant, followed by three booster injections every 2 weeks in incomplete Freund's adjuvant. Protocols used for the preparation of mAbs and ascetic fluids referred to a laboratory manual (41). All of the hybridomas were cloned by at least three rounds of limiting dilution. The class and subclass of each monoclonal antibody from the individual hybridoma clone was determined using the mouse monoclonal antibody isotyping kit (Sigma-Aldrich). They were purified by affinity chromatography in a protein A-Sepharose column (GE Healthcare Bio-Sciences Corp.) following the procedure, as we previously reported (38).
Sequential Extraction of Sperm-associated Proteins
Sequential extraction of epididymal spermatozoa protein was performed as described (42). Rat sperm were collected from the different epididymal regions and diluted into PBS-PI (PBS, pH 7.4, containing the protease inhibitor, 0.5% aprotinin, and 0.1% PMSF). Similar numbers of epididymal spermatozoa were sequentially extracted with low salt (PBS-PI with 5 mm EDTA, pH 7), high salt (PBS-PI with 5 mm EDTA, 0.5 m NaCl, pH 7), 0.1% Triton X-100 (PBS-PI with 0.1% Triton X-100, 5 mm EDTA, pH 7), 2% SDS, and Laemmli buffer containing 5% SDS and 1% β-mercaptoethanol. The samples were separated by 16.5% Tricine-SDS-PAGE followed by Western blot analysis.
Immunoblotting and Immunocytochemistry of SPINK13
Western blot analysis, immunohistochemical staining of tissues, and indirect immunofluorescence detection of proteins associated with spermatozoa were conducted following a previously described protocol (39). Lectin peanut agglutinin conjugated with Alexa Fluor 488 (diluted 1:500; Molecular Probes) was used to monitor the acrosomes of the spermatozoa. All pictures were taken using a BX51 fluorescence microscope (Olympus).
Treatment of Sperm with Phosphatidylinositol-Specific Phospholipase C
2 × 106 sperm were incubated with 1 unit/ml of PI-PLC (Molecular Probes) for 30 min at 22 °C, with untreated sperm as control. Sperm pellet and its supernatants were then collected by centrifugation at 1000 × g for 30 min at 4 °C. The proteins were reconstituted in Laemmli buffer containing 5% SDS and 1% β-mercaptoethanol for Western blot analysis.
Knockdown of Spink13 Expression by Lentivirus-mediated RNA Interference
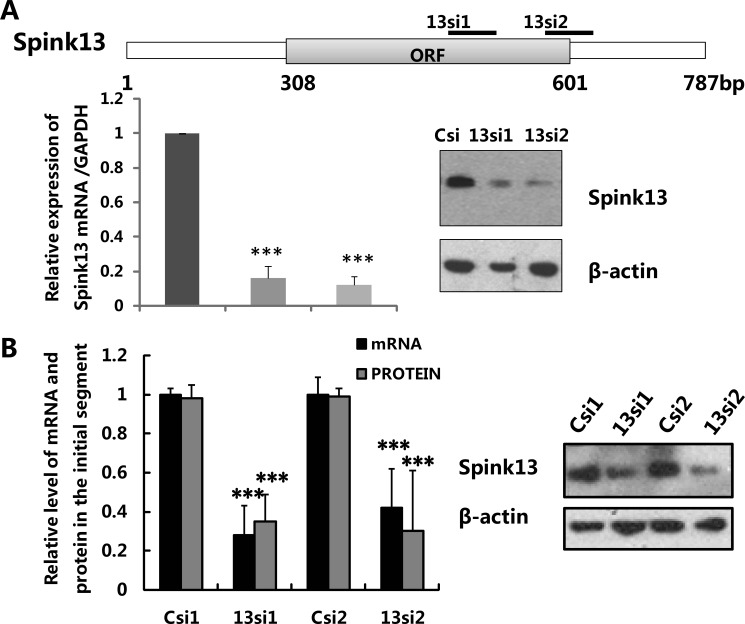
Two potential siRNAs of 19 nucleotides specific to Spink13 mRNA (13si1 and 13si2) were selected empirically with a design tool incorporating standard parameters (43), and siRNA (WI siRNA Selection Program) targeting enhanced green fluorescent protein (EGFP) (GenBankTM accession number YP_003162718) was used as control for sequence specificity (Csi). The sense strands of siRNAs were: 13si1 (sequence 1), beginning at nucleotide 515, 5′-CTACAAGAACGAGTGCTTC-3′; 13si2 (sequence 2), beginning at nucleotide 597, 5′-TAGCGATTAACCGAACACC-3′; and Csi, beginning at nucleotide 106, 5′-GGCGATGCCACCTACGGCAAG-3′.
Protocols used for examination of the knockdown efficiency and production of lentiviral particles were as previously described (44). Briefly, the psiRNA constructs and a pcDNA 3.0 vector expressing Spink13 were co-transfected into the mouse epididymal epithelial cells (PC1) using Lipofectamine 2000 (Invitrogen). The knockdown efficiency was determined by real time PCR and Western blot of extracts from transfected cultures 48 h after transfection. Lentiviral particles were produced by transient co-transfection of 293T cells using pRNAi/Lenti vectors (HaiGene, Harbin, China), an encapsulation plasmid (p8.9), and a vesicular stomatitis virus expression plasmid. The supernatants were subjected to ultracentrifugation. The resulting pellet was resuspended in PBS, aliquoted, and frozen at −80 °C until use. The surgical procedures were performed according to a previously described method with modification. (44) Briefly, the skin covering the epididymis of a male Sprague-Dawley rat was cut open under anesthesia. The proximal epididymis was then gently squeezed out and fastened with tweezers, and 1 × 106 transducing units of shRNA lentivirus was injected into the interstitial space of the initial segment of epididymis. The Spink13-shRNAs lentivirus (13si1 or 13si2) was delivered into the left epididymis, and the right epididymis was treated with Csi-shRNA lentivirus (Csi). The total RNA and protein of the caput epididymis and the spermatozoa were collected for assessment 7 days after injection.
In Vitro Sperm Function Evaluation
Analysis of epididymal sperm in vitro is concerned with the following four aspects: (a) Analysis of sperm motility (44): The computer-assisted sperm analysis was performed to determine the sperm motility characteristics using the HTM-TOXIVOS (Rat Head Toxicology, version 12.3A; Hamilton-Thorn Research, Beverly, MA). (b) Analysis of acrosin proteolytic activity: The proteolytic activity of individual spermatozoa was determined using a gelatin substrate film assay following the procedure described by Liu and Baker (45). Gelatin substrate film slides were prepared on one side of glass slides with a 5% gelatin solution. Epididymal spermatozoa were spread on the surface of gelatin-coated slides and incubated at 37 °C to allow proteolysis of the gelatin by acrosin. At intervals, the slides were observed by phase contrast microscopy. Within 4 h, formation of a clear halo (diameter > 10 um) around the sperm head indicated acrosin proteolytic activity. The percentage of sperm showing a halo was assessed by counting 200 sperm. (c) Detection of tyrosine phosphorylation: sperm collected at different capacitation times were treated as previously reported. Western blot analysis was performed by using anti-phosphotyrosine monoclonal antibody as previously described (44). (d) Evaluation of sperm capacitation and acrosome reaction: Chlortetracycline fluorescence staining method, as used in previous studies (46), was conducted to morphologically assess sperm capacitation. The status of the acrosome was assessed using Coomassie Brilliant Blue G-250 staining as previously described (47). For analyzing the sperm acrosome reaction, the capacitated sperm described above was treated with 10 μmol/liter A23187 in Me2SO (0.2%) at 37 °C for 30 min. At the end of the incubations, the samples were stained with chlortetracycline to assess sperm capacitation or acrosome reaction rate and Coomassie Brilliant Blue G-250 to evaluate acrosome reaction rate. For every experiment, 200 spermatozoa were scored in duplicate, and the percentage of spermatozoa that had undergone the acrosome reaction was calculated.
In Vitro Fertilization Assay
We carried out in vitro fertilization assay following a standard protocol (48). Oocytes were collected from superovulated rats 16 h after human chorionic gonadotrophin (Sigma-Aldrich) injection and placed in human tube fluid medium (Irvine Scientific, Santa Ana, CA). The oocytes were treated with 0.1% hyaluronidase for 10 min at room temperature and washed with human tube fluid medium. Capacitated sperm at a concentration of 1 × 105 sperm/ml were co-incubated with ∼30 oocytes in a 50-μl drop of human tube fluid covered by embryo-tested mineral oil (Sigma-Aldrich) at 37 °C and 5% CO2. After 6 h of insemination, the eggs were removed and transferred serially through several drops of fresh fertilization medium to remove sperm loosely associated with the egg surface. After 48 h of incubation, the embryos were observed under light microscopy. The presence of development to the two-cell stage was considered as an indication of successful fertilization.
Assessment of the Fertility of Spink13 Knockdown Rat
The mating experiments were performed following our previous protocol (46). After injection of the same lentivirus into the bilateral epididymis (the treated group were injected with Spink13-shRNA lentivirus, and the control group were injected with Csi-shRNA lentivirus), each male rat was mated with two normal females in succession for 7 days. The mated male rats were sampled for determining the knockdown efficiency. At 18 days, the pregnant females were subjected to hysterectomy to determine the number of embryos or fetuses. After birth, the number, viability, and size of the offspring in a litter were recorded.
Statistical Analysis
Statistical analysis was performed using the SigmaPlot 10.0. The data are presented as the mean values ± S.E. (n ≥ 3). The p values were calculated using Student's t test, and differences with a p value of <0.05 were considered statistically significant.
RESULTS
SPINK13 Is a Member of the SPINK Family
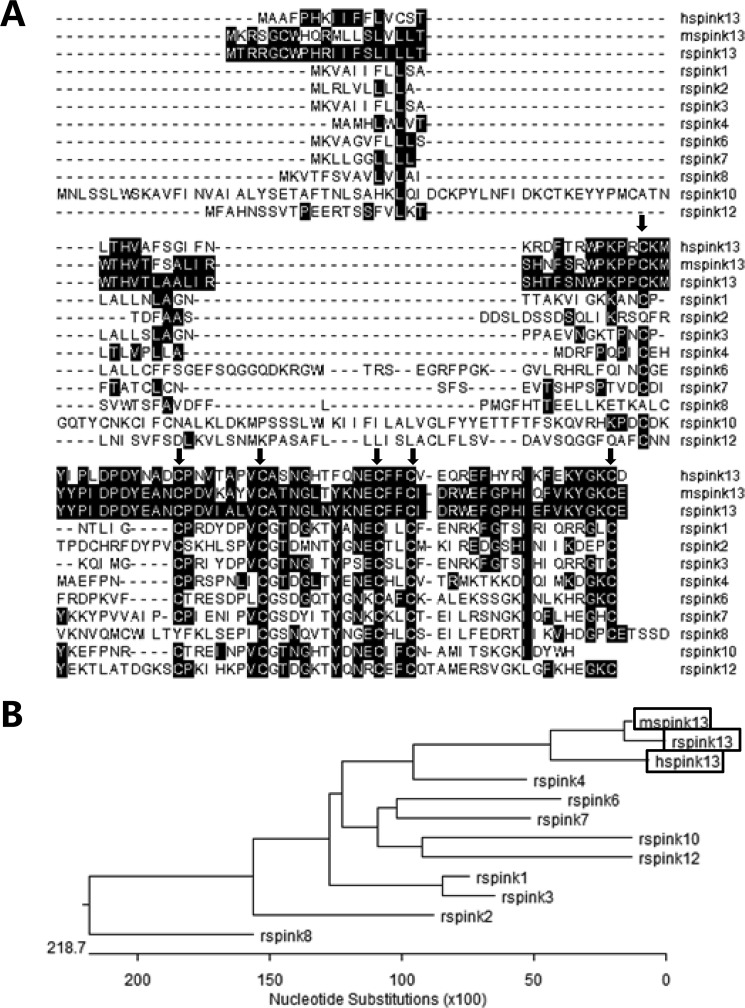
To identify the novel epididymis-specific gene in the rat, the human ESC6/SPINK13 protein sequence (NP_001035218.1) was used as query to search the protein database using Blastp. The amino acid sequence of rat SPINK13 (NP_001103009.1) is 53% identical to the human SPINK13 (NP_001035218.1) and 84% identical to the mouse SPINK13 (NP_001161895.1) (Fig. 1A). The search for identification of putative homologues at HomoloGene (87519) revealed that all of the SPINK13 has a common characteristic that contains one conserved Kazal domain. The gene of rat Spink13, which consists of five exons, is located on rat chromosome 18q12.1 in close proximity to other Kazal protease inhibitors (supplemental Fig. S1). The Spink13 cDNA sequence corresponds to the predicted rat UniGene Rn.218699, under which no tissue or developmental expression data are yet available. The predicted protein contains 97 amino acids with an estimated size of 11 kDa. The N-terminal 26 amino acids probably form a signal peptide, as predicted with the SignalP 3.0 server. Cleavage of this peptide would lead to a mature protein of 71 amino acids with an estimated size of 8.3 kDa and a calculated isoelectric point (pI) of 5.61. Based on the alignment of the Kazal domain of SPINK13 with other reported SPINK members with identified functions in rat, they appear to have typical Kazal domains composed of six consensus cysteine residues with the primary structural pattern of C1XnC2X7C3X10C4X2/3C5XmC6. The phylogenetic relations of the SPINK13 to the closely related members of the Kazal family are shown in Fig. 1B. Collectively, these results indicated that SPINK13 belongs to the Kazal-type serine protease inhibitor family.
FIGURE 1.
The structural features classified Spink13 into the SPINK family. A, multiple protein sequence alignment of SPINK family proteins: hSPINK13, NP_001035218.1; mSpink13, NP_001161895.1; rSpink1, NP_690919.1; rSpink2, NP_001008870.1; rSpink3, NP_036806.1; rSpink4, NP_001008871.1; rSpink6, NP_001008874.1; rSpink7, NP_001002816.2; rSpink8, NP_001100330.1; rSpink10, XP_001063288.2; rSpink12, XP_001061351.1. Residues that match rSpink13 are shaded in black. The arrows indicate matching residues. C1–C6 stand for the six conserved cysteine residues of the SPINK protein family. B, phylogenetic tree analysis of SPINKs showing homology of SPINK13 with the other members of the family.
Characteristics of Spink13 mRNA Expression
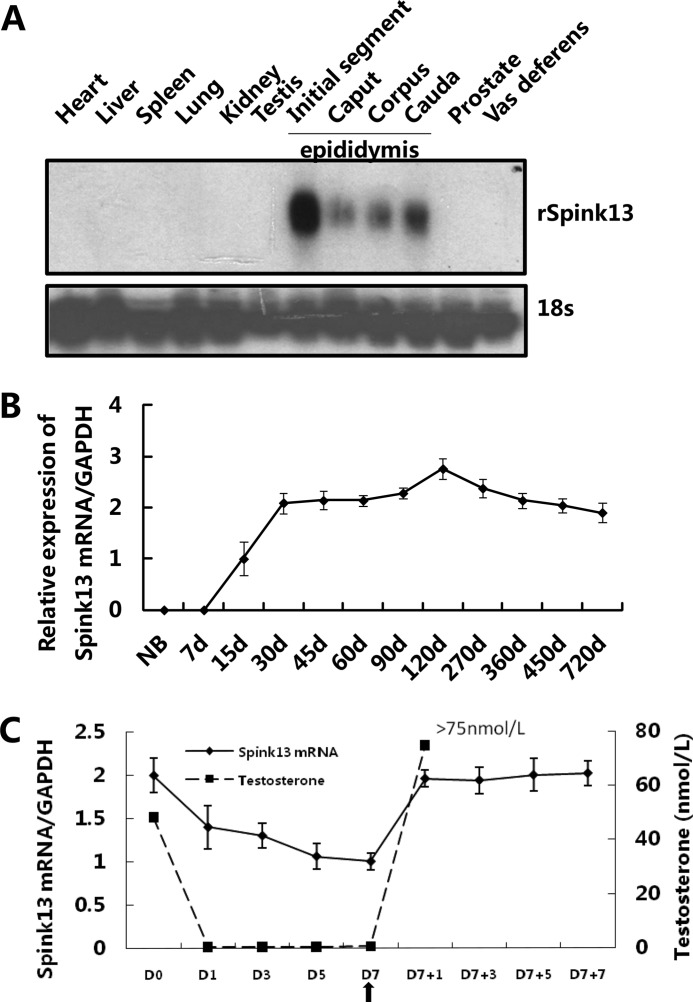
To elucidate the expression pattern of Spink13, a Northern blot analysis was applied to a panel of rat tissues. This result showed that its expression was restricted to the epididymis with highest expression in the initial segment (Fig. 2A). We examined Spink13 expression in the epididymis of rats at different ages and found that it first appeared at a low level in 15-day-old rats, increased from 30 days onward, reached its highest level in 120-day-old rats, and remained at a stable level in mature animals (Fig. 2B). Moreover, to evaluate the effect of testosterone on Spink13 gene expression in the epididymis, we conducted the testosterone replacement in castrated rats. In castrated animals, the abundance of Spink13 mRNA in the epididymis decreased as serum testosterone levels declined rapidly. Testosterone replacement in the animals 7 days after castration restored to the original an increase in Spink13 mRNA levels (Fig. 2C). These results suggest that Spink13 gene expression in the epididymis is up-regulated by androgen.
FIGURE 2.
The expression of Spink13 mRNA is restricted to epididymis and regulated by androgen. A, tissue distribution of Spink13 mRNA in adult rats was analyzed by Northern blotting analysis. 18 S rRNA was used as the internal control. B, relative amounts of Spink13 mRNA (real time PCR) in rat epididymis at different developmental stages (n = 3). GAPDH was used as internal control. d, days. C, relative expression levels of Spink13 mRNA (real time PCR) in the rat epididymis and the serum testosterone levels (expressed as nanomoles per liter) during androgen manipulation (n = 4–7). The arrow indicates the time of androgen supplementation. GAPDH was used as an internal control.
Native Status and Localization of SPINK13 Proteins in Rat Epididymis
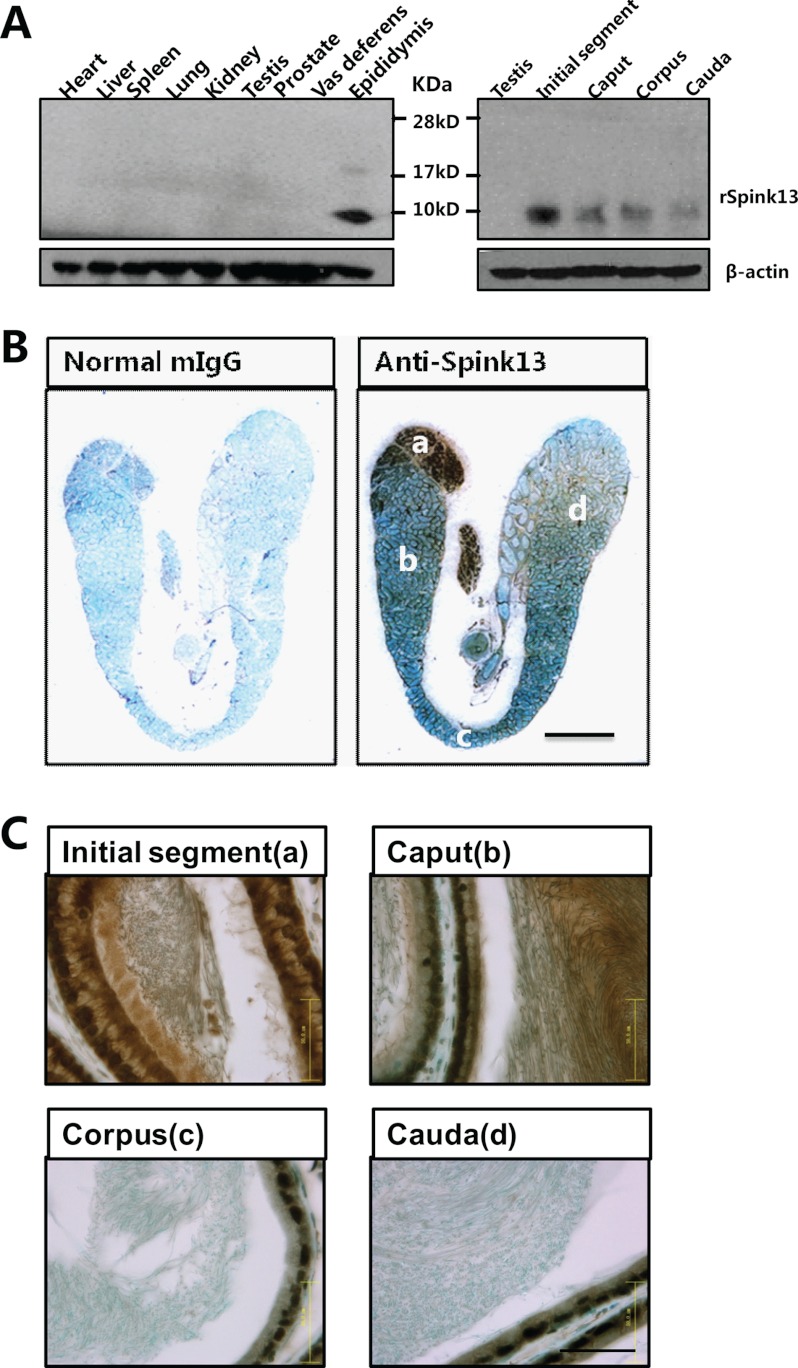
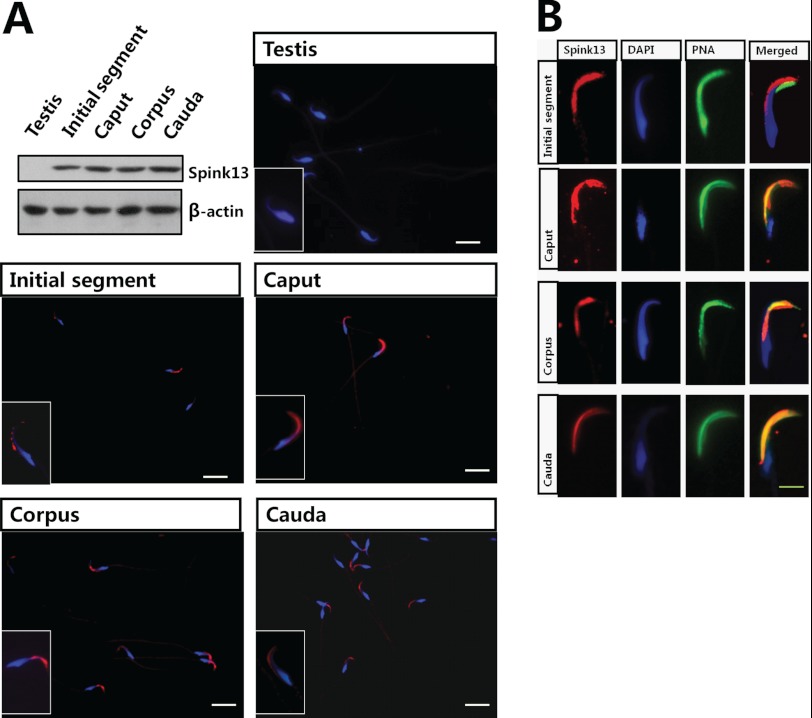
For further characterization and functional studies, the mAb against SPINK13 was generated, and a single specific band of ∼9 kDa was detected in the epididymis but not in other tissues tested (Fig. 3A). The subclass of the mAb was IgG2b. Epitope mapping studies using multiple peptide synthesis strategy revealed that this mAb recognized the N-terminal 24 amino acids of the mature protein (supplemental Fig. S2A). Specificity of the monoclonal antibody was assessed by preincubation with an excess of the epitope peptide, and no immunoreactivity of the tissues was found using the antibody absorbed with peptide (data not shown). Immunohistochemistry further confirmed that the positive signal was mainly located in the initial segment of epididymis (Fig. 3B), which is consistent with the tissue distribution pattern of the Spink13 mRNA as seen in Fig. 2A. Strong immunoreactivity was present in the initial segment (Fig. 3C), including the epithelial cells, the lumen, and the sperm. As shown in Fig. 4A, a fluorescent signal representing SPINK13 protein was localized to the sperm heads and appeared to be restricted to the acrosomal region in epididymal spermatozoa, but not in testicular spermatozoa. These results further suggested that the SPINK13 protein was secreted into the lumen of the initial segment of the epididymis and was bound to sperm. To determine whether the binding pattern of SPINK13 protein with spermatozoa is altered during epididymal transit, indirect immunofluorescence staining was performed on the sperm from different epididymal regions. In the initial segment SPINK13 proteins were localized on the dorsal surface of the acrosomal region of sperm, whereas in cauda epididymis, SPINK13 proteins were primarily restricted to the acrosomal region (Fig. 4B). It is obvious that the distribution of SPINK13 gradually became more restricted to the acrosomal region in spermatozoa during epididymal transit, which suggested that SPINK13 proteins might regulate acrosomal exocytosis.
FIGURE 3.
Predominant Spink13 expression in the luminal epithelium of epididymis. A, protein extracts from various tissues were analyzed by Western blot analysis. β-Actin was used as the internal control. B, immunolocalization of rat SPINK13 in a 120-day-old rat epididymis. Bar, 4 mm. C, magnified photographs of individual fields of B. Panel a, initial segment; panel b, caput; panel c, corpus; panel d, cauda. Bars, 50 μm.
FIGURE 4.
Localization of rat SPINK13 protein on sperm. A, localization of SPINK13 protein in rat epididymal spermatozoa by Western blot and indirect immunofluorescence assays. β-Actin was used as the internal control. Bars, 10 μm. B, refined localization of SPINK13 (TRITC-labeled) on the epididymis sperm surface. The acrosome of the sperm was stained by Alexa Fluor 488-conjugated lectin peanut agglutinin (PNA), and sperm DNA was stained with DAPI (bars 5 μm).
Generation of Spink13 Knockdown Rats
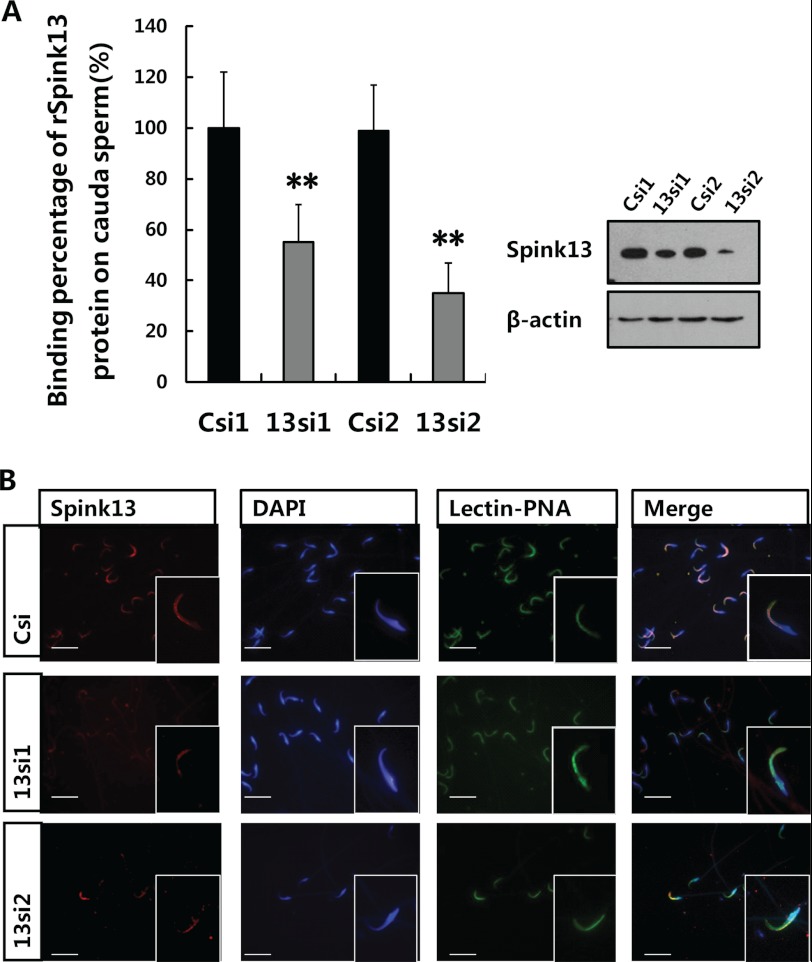
Recently, it was demonstrated that mice with genetic deletion of the SPINK gene family display postnatal (49) or neonatal (50) lethal phenotype, which made subsequent analysis difficult. RNAi is a powerful technique for gene silencing in functional studies. The advantage is that the timing and extent of the gene silencing can be controlled, so that genes that are essential will only be silenced at chosen stages or in chosen tissues. We applied lentiviral vector-mediated RNAi to knock down Spink13 gene expression in rat epididymis to confirm its role in the sperm maturation process. According to the RNAi mechanism-based rules, two potential siRNAs (13si1 and 13si2) targeting the different site of Spink13 gene and a siRNA (Csi) of EGFP as control for silencing specificity were designed. Both 13si1 and 13si2 were able to significantly reduce Spink13 expression at the mRNA and protein levels in the mouse epididymal epithelial cell line (PC1) (Fig. 5A). Lentiviral particles that generated the effective shRNAs were produced and locally injected into the initial segment of epididymis. As shown in Fig. 5B, expression of the Spink13 gene in the initial segment was suppressed by ∼40–60% at the protein levels after 7 days by specific RNAi treatment. When Spink13 was notably down-regulated, there were no significant decreases in the mRNA level of six Spinks expressed predominantly in the epididymis (supplemental Fig. S6 and Table S1), which demonstrated the specificity of the selected siRNAs. Moreover, Western blot analysis indicated a nearly 45% decrease in the amount of SPINK13 proteins bound to the cauda sperm in the Spink13 RNAi-treated male rats (Fig. 6A). Indirect immunofluorescence staining also confirmed the reduction of SPINK13 signals in the cauda spermatozoa of RNAi-treated animals (Fig. 6B).
FIGURE 5.
Knockdown of Spink13 expression by RNAi in rat epididymis. A, evaluation of the selected siRNAs for rat SPINK13. Real time PCR and Western blot analysis show reduced expression of Spink13 mRNA (left panel) and protein (right panel) in vitro 48 h after co-transfection of the psiRNA constructs and a pcDNA 3.0 vector expressing rat SPINK13 into mouse PC1 cells. The data are expressed as the means ± S.E. (n = 3). ***, p < 0.001 versus control. GAPDH and β-actin were used as the internal controls for RNA and protein loading. B, expression of Spink13 was significantly suppressed by 13si1 and 13si2 in the initial segment of the rat epididymis at the mRNA and protein levels. The data are expressed as the means ± S.E. (n = 12). ***, p < 0.001; **, p < 0.01 versus control. GAPDH and β-actin were used as loading controls. In A and B, Csi1 and Csi2, EGFP siRNA control; 13si1 and 13si2, two siRNAs specifically targeting the different sites of Spink13 sequence.
FIGURE 6.
Knockdown of Spink13 in vivo led to a significant reduction in the binding of SPINK13 onto sperm. A, corresponding cauda sperm-binding percentages obtained by Western blot analysis. The data are expressed as the means ± S.E. (n = 12). **, p < 0.01 versus control. β-Actin was used as the internal control. B, immunofluorescence staining of sperm from the cauda epididymis treated with control siRNA and two specific siRNAs. Bars, 10 μm. In A and B, Csi1 and Csi2, EGFP siRNA control; 13si1 and 13si2, two siRNAs specifically targeting the different sites of Spink13 sequence.
SPINK13 Inhibits Sperm Acrosome Reaction in Vivo
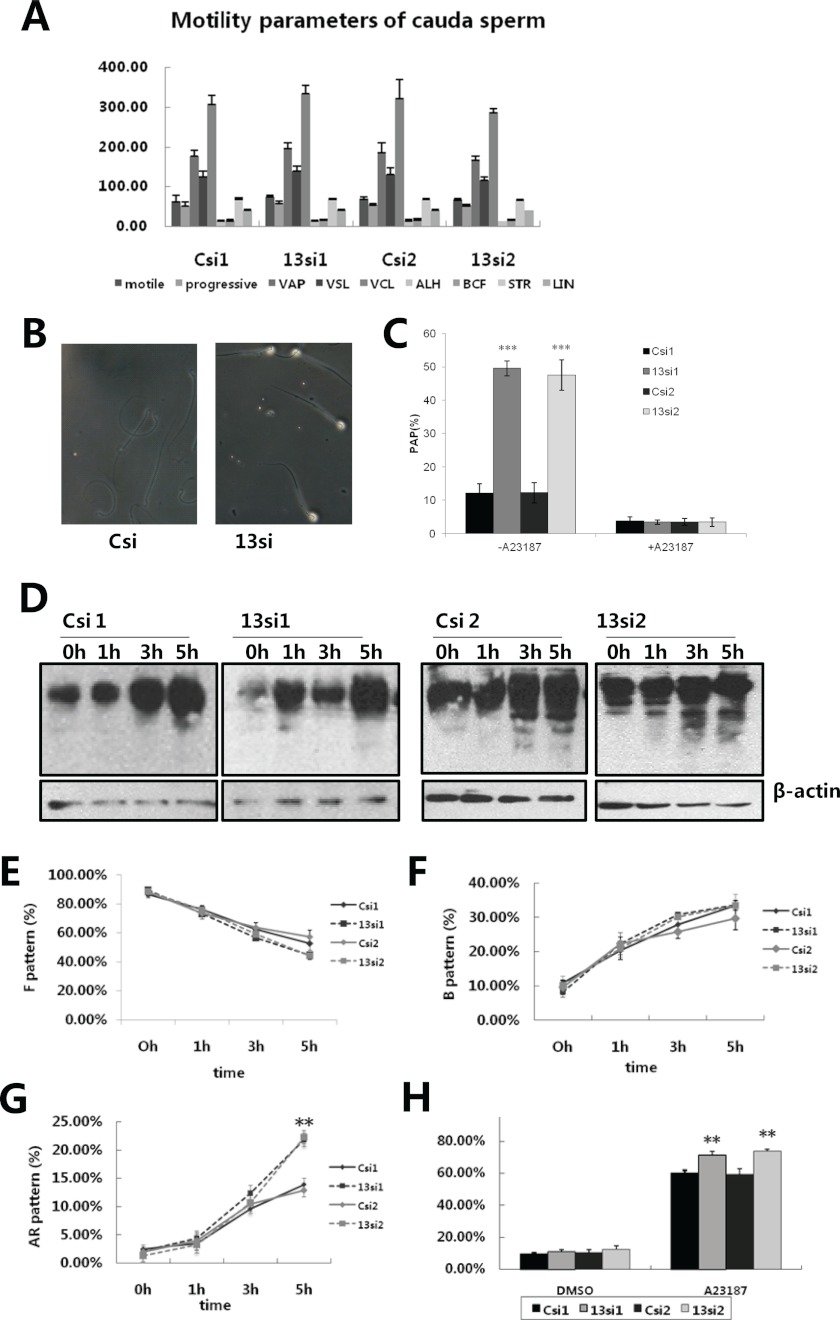
We next characterized the phenotype of Spink13 down-regulated sperm from caudal epididymis in vitro. The motility of cauda epididymal sperm seemed unchanged in spink13 knockdown rats (Fig. 7A). Gelatin substrate film assay was conducted to assess acrosin proteolytic activity of individual sperm. The heads of ∼50% of Spink13 down-regulated sperm had protein-digested halos generated by acrosin activity on the gelatin substrate films. By contrast, only small refringent areas were observed in sperm from control rats after 3 h of incubation (Fig. 7, B and C). After A23187-induced acrosome reactions, the absence of gelatin digestion indicated that acrosomal reaction liberates more proteases, meaning that proteolytic activity is inhibited by SPINK13 during capacitation.
FIGURE 7.
Sperm analysis in Spink13 knockdown rats. A, motility parameters of spermatozoa in the cauda region after Spink13 expression had been down-regulated. VAP, average path velocity; VSL, straight line (rectilinear) velocity; VCL, curvilinear velocity; BCF, beat cross frequency; STR, straightness; LIN, linearity. The data are expressed as the means ± S.E. (n = 6). B, acrosin activity of Spink13 down-regulated sperm visualized by 3 h of incubation on a gelatin substrate slide. Representative cells are shown. C, effects of Spink13 siRNA on acrosin proteolytic activity. The data are expressed as the means ± S.E. (n = 6). D, the pattern of protein-tyrosine phosphorylation on caudal sperm after Spink13 expression is inhibited. β-Actin was used as loading control. A representative experiment is shown (n = 6). E–H, the changes in the state of incapacitated sperm (F pattern, E), capacitated sperm (B pattern, F), spontaneous acrosome-reacted sperm (AR pattern, G), and the acrosome reaction induced by calcium ionophore A23187 (H) in the Spink13 RNAi-treated and control groups. The data are expressed as the means ± S.E. (n = 6–12). **, p < 0.01 versus control). In A–F, Csi1 and Csi2, EGFP siRNA control; 13si1 and 13si2, two siRNAs specifically targeting the different sites of Spink13 sequence.
The protein-tyrosine phosphorylation levels of cauda sperm were similar between Spink13 RNAi-treated and control rats. (Fig. 7D). This is consistent with the observation with chlortetracycline staining (Fig. 7, E–G), another indicator for evaluating the acquisition of capacitation state. It is conceivable that Spink13 down-regulated sperm may have the ability to become capacitated normally. However, knockdown of spink13 significantly increased the percentage of acrosome-reacted sperm at the later stage of capacitation (Fig. 7G). Remarkably, the reduction in Spink13 expression and sperm binding led to significant acceleration in the A23187-induced acrosome reactions (Fig. 7H). In parallel, the sperm acrosomal status was also enhanced in Spink13 down-regulated sperm by staining with Coomassie Brilliant Blue G-250 (data not shown). These results suggested that SPINK13 plays a crucial role in protecting sperm from premature acrosome reaction.
Reduced Fertility in Vitro and in Vivo in Spink13 Down-regulated Rats
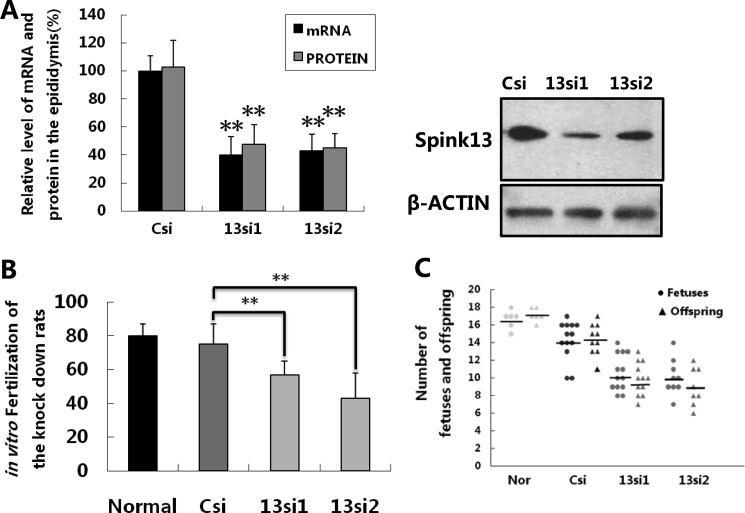
Mammalian sperm must undergo the acrosomal reaction, which liberates more serine proteases necessary to bind and penetrate Zona pellucida and finally fuse with the oocyte. We performed in vitro fertilization assays to evaluate whether the down-regulation of Spink13 affected the sperm fertilizing ability. The in vitro fertilization assay showed that the fertilization rate of the cauda sperm in Spink13 RNAi-treated rats was much lower than that of the control groups (Fig. 8B). Finally, we performed mating experiments to evaluate the fertility of Spink13 down-regulated male rats. As shown in Fig. 8C, the numbers of live offspring and normal fetuses were significantly reduced in the litters from receptive female rats that had mated with specific RNAi-treated male rats, compared with the numbers in the normal and control groups. These data confirmed that SPINK13 is essential in the sperm maturation process, and the sperm lacking SPINK13 seems the cause of fertility reduction.
FIGURE 8.
Reduced fertility in vitro and in vivo in Spink13 knockdown male rats. A, relative expression levels of Spink13 including mRNA and protein in the epididymis from all successfully mated rats after RNAi treatment. The data are expressed as the means ± S.E. (n = 8–13). **, p < 0.01 versus corresponding control). B, in vitro fertilization assay of the sperm in Spink13 knockdown rats. The data are expressed as the means ± S.E. (n = 8–13). **, p < 0.01 versus corresponding control). C, numbers of normal fetuses (circles) and live offspring (triangles) from normal female rats mated with normal, control (Csi), and knockdown (13si1 and 13si2) male rats; horizontal lines indicate the averages. In A–C, Csi, EGFP siRNA control; 13si1 and 13si2, two siRNAs specifically targeting the different sites of Spink13 sequence.
DISCUSSION
In mammals, the epididymis is responsible for sperm transport, concentration, storage, and maturation. Sperm maturation involves the acquisition of forward motility and fertilizing ability. Identification and characterization of differentially expressed genes in epididymis have provided us additional insight into the mechanisms of sperm maturation. Our previous study demonstrated that several epididymal proteins, such as Bin1b (5), HongrES1 (46), and Defb15 (44), are essential in sperm motility, capacitation, and male fertility, respectively. In the present study, we identified an epididymis-specific gene, Spink13, and demonstrated its physiological function in regulation of acrosome reaction. The biological role of SPINK13 in the sperm acrosome and how its down-regulation causes the fertility reduction remains to be elucidated. One possibility is that SPINK13 functions as a protease inhibitor necessary for the regulation of critical proteases involved in early signaling events during fertilization, which is consistent with the hypothesis that the regulated serine protease activity might be the key of sperm maturation (8). Furthermore, the fact that SPINK13 is evolutionarily conserved from rodent to human and has no expression in testis indicates that it is a putative target for post-testicular male contraception.
The SPINK13 protein has the characteristic signature of serine proteases inhibitors consisting of an N-terminal signal peptide followed by a Kazal domain, but its serine protease inhibitory activity is still unclear. According to Fig. 7 (B and C), rat SPINK13 showed potent inhibitory effect on acrosin proteolytic activity using the gelatin substrate film method. It is very likely that other target proteases might exist in addition to acrosin. The active SPINK13 recombinant protein after heterologous expression with proper folding will be a key step in further studies. However, the inhibitory target specificity by SPINK13 is achieved primarily through temporal and spatial restrictions, such as the site of expression, membrane anchorage, and the microenvironment of epididymis, thus complicating the identification of in vivo target proteases of SPINK13 using conventional protease inhibitor assay.
An alignment of the SPINK13 protein sequence of humans, mice, and rats reveals a remarkable conservation of its sequence, especially in the Kazal signature. The mAb reacted specifically against recombination human and mouse SPINK13 protein (supplemental Fig. S2), confirming a high homology of rat SPINK13 to its human and mouse homologue, as predicted by their protein sequences. This high conservation suggests that SPINK13 gene was positively selected during evolution because of its biological importance.
It is generally known that gene expression in the epididymis is driven by androgens signaling through the androgen receptor; many gene expressions in the epididymis have been demonstrated to be regulated by androgen (51, 52). This study shows that androgen also regulates the Spink13 gene. Androgen and its receptor form a complex that interacts with androgen response elements in androgen-responsive genes and regulates their expression. Considering the high homology of rat Spink13 to its mouse homologue, we referred to the results of ChIP-seq in mouse caput epididymis (53) to predict 12 potential androgen receptor-binding sites in intergenic and intronic regions (supplemental Fig. S3). Most of these androgen receptor-binding sites harbored conserved androgen response element motifs across rat and mouse based on whole genome alignment. Thus, it is possible that androgen-androgen receptor acts directly on Spink13 gene to regulate its gene expression. It is noteworthy that the decline of Spink13 mRNA is not very sharp in response to the rapid decrease of androgen level. We speculate other regulation mechanisms are also involved, such as mRNA stability.
We noted in Fig. 3C that the immunopositive signals for SPINK13 were more intense in the lumen of the initial segment than in other regions of the epididymis, whereas the staining signals were also observed in the nucleus of the epithelial cells in the whole tissue. This result is consistent with the tissue distribution pattern of the Spink13 mRNA, so we can infer that there must be dual localization of SPINK13 in rat epididymis. To date, there have been numerous reports of nuclear proteins that possess a signal sequence for extra cellular localization but lack an nuclear localization signal for nuclear localization, such as extracellular hormones, growth factors, and cytokines (54). However, the dual localization of SPINK13 must be carefully controlled, and the function of SPINK13 in the nucleus needs further investigation.
SPINK13 is secreted by the epithelium of the initial segment and associated with the sperm surface. Sequential extraction of epididymal spermatozoa from different regions was performed to confirm these observations (supplemental Fig. S4). Although low levels of the proteins were present in the high salt extractions, Triton X-100 was required to extract the majority of SPINK13 protein from the initial segment spermatozoa, but 2% SDS rather than Triton X-100 was required to extract the majority of the SPINK13 protein from cauda spermatozoa. Previous studies have demonstrated that sperm maturation involves the sequence of modifications of the sperm plasma membrane during epididymal transit. These epididymal proteins binding to sperm plasma membrane through electrostatic interactions are usually referred to as coating proteins, which can be washed from the sperm surface with a high ionic strength solution. It is not clear how SPINK13 is transferred from epididymal epithelium to sperm membrane. Bioinformatics analysis revealed that all of the SPINK13 orthologous protein contains a potential C-terminal GPI modification site. In mammals, more than 200 glycoproteins have been identified that are linked to the plasma membrane via a GPI anchor. Many epididymal proteins behave like integral membrane proteins and have GPI anchors, for example HE5 (CD52) in humans (55), SPAM1 and hyaluronidase in mice (56, 57), and P26h and P25b in hamsters and bulls, respectively (58, 59). Epididymal angiotensin-converting enzyme has been reported to plays a crucial role in fertilization through the GPI-anchored protein releasing activity (12). To investigate whether SPINK13 is linked to the plasma membrane via a GPI anchor, we test its susceptibility to PI-PLC cleavage. SPINK13 was weakly detected in the supernatant of sperm treated with PI-PLC (supplemental Fig. S5), indicating that it might be anchored to sperm membrane via a GPI-lipid linkage.
The localization of Spink13 at the sperm surface overlying the acrosome during epididymal transit suggested that this protein might be implicated in cellular events associated with sperm acrosomal exocytosis, as well as fertilization. Moreover, when Spink13 was notably down-regulated by using lentivirus-mediated RNAi in vivo, the rate of spontaneous acrosomal exocytosis significantly increased during capacitation. Relative to wild type sperm, Spink13 down-regulated sperm are more sensitive to Ca2+ ionophore-induced acrosome reactions throughout capacitation. By contrast, the kinetics of protein-tyrosine phosphorylation and sperm motility are unaltered in down-regulated sperm relative to wild type. The impaired fertilizing capacity in Spink13 down-regulated sperm could also be evidenced by their reduced fertilization rate in vitro or through natural mating. These results are not unique; Ou et al. (60) also found that the Spink3-spermatozoa interaction resulted in a decrease of intracellular calcium concentration of the cell head and suppressed both the acrosome reaction induced by Ca2+-ionophore A23187 and the cell fertility.
Thus, it is concluded that SPINK13 may facilitate fertilization by protecting spermatozoa from undergoing premature acrosome reaction. Although acrosome-reacted spermatozoa can fertilize eggs (61, 62), the low fertilizing efficiency for the acrosome-reacted spermatozoa recovered from the perivitelline space suggests that the time for acrosome reaction of sperm is important and that premature acrosome reaction might be detrimental to sperm fertilizing ability (62). The molecular mechanism involved in the process that SPINK13 modulates the acrosome reaction is not completely elucidated. Lack of SPINK13 might result in the impaired processing of its physiologic target and functional alterations in the signal transduction machinery of sperm. However, much remains to be elucidated about other changes in sperm quality in SPINK13 knockdown rats that might account for reduced fertility.
At present, a number of serine proteases have been identified on sperm acrosome, and some of them are embedded in the plasma membrane or the outer acrosomal membrane, composing a lytic consortium on the apical surface of the sperm (63–66). Protease activation and inhibition must be precise in time and place. Several protease inhibitors expressed in certain regions are expected to be responsible for this control. They are thought to play a role in capacitation by stabilizing ZP binding sites during uterine passage, followed by dissociation to allow sperm-ZP interaction (8). Hence, our future work will concentrate on the molecular mechanism for this regulation and clarify its related signal networks. Although the acrosome reaction has been known for many years, the identity and mechanism of extracellular and intracellular signaling molecules regulating this process are still unclear. The present study provides evidence that epididymal specific SPINK family member SPINK13 has a regulatory effect on the acrosome reaction without affecting capacitation. We expect further study of SPINK13 will not only increase our understanding of this physiologically complex process but will also provide a new putative target for post-testicular male contraceptives.
Acknowledgments
We thank Aihua Liu and Gail Grossman for tissue section preparation and technical assistance in examining vaginal smears of female rats and Drs. Yuchan Zhou and Wuzi Dong for advice concerning RNAi.
This work was supported by Chinese Academy of Sciences Knowledge Innovation Program Grant KSCX2-EW-R-07, and National Natural Science Foundation Grants 30930053, 31271600, and 30770815.

This article contains supplemental Table S1 and Figs. S1–S6.
- GPI
- glycosylphosphatidylinositol
- EGFP
- enhanced green fluorescent protein
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- PI-PLC
- Phosphatidylinositol-Specific Phospholipase C.
REFERENCES
- 1. Orgebin-Crist M. C. (1967) Sperm maturation in rabbit epididymis. Nature 216, 816–818 [DOI] [PubMed] [Google Scholar]
- 2. Ikawa M., Inoue N., Benham A. M., Okabe M. (2010) Fertilization. A sperm's journey to and interaction with the oocyte. J. Clin. Invest. 120, 984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sipilä P., Jalkanen J., Huhtaniemi I. T., Poutanen M. (2009) Novel epididymal proteins as targets for the development of post-testicular male contraception. Reproduction 137, 379–389 [DOI] [PubMed] [Google Scholar]
- 4. Cornwall G. A. (2009) New insights into epididymal biology and function. Hum. Reprod. Update 15, 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou C. X., Zhang Y. L., Xiao L., Zheng M., Leung K. M., Chan M. Y., Lo P. S., Tsang L. L., Wong H. Y., Ho L. S., Chung Y. W., Chan H. C. (2004) An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 6, 458–464 [DOI] [PubMed] [Google Scholar]
- 6. Jones R. (1998) Plasma membrane structure and remodelling during sperm maturation in the epididymis. J. Reprod. Fertil. Suppl. 53, 73–84 [PubMed] [Google Scholar]
- 7. Dacheux J. L., Gatti J. L., Dacheux F. (2003) Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc. Res. Tech. 61, 7–17 [DOI] [PubMed] [Google Scholar]
- 8. Cesari A., Monclus Mde L., Tejón G. P., Clementi M., Fornes M. W. (2010) Regulated serine proteinase lytic system on mammalian sperm surface. There must be a role. Theriogenology 74, 699–711.e1–5 [DOI] [PubMed] [Google Scholar]
- 9. Tulsiani D. R. (2003) Glycan modifying enzymes in luminal fluid of rat epididymis. Are they involved in altering sperm surface glycoproteins during maturation? Microsc. Res. Tech. 61, 18–27 [DOI] [PubMed] [Google Scholar]
- 10. Jones R., Ma A., Hou S. T., Shalgi R., Hall L. (1996) Testicular biosynthesis and epididymal endoproteolytic processing of rat sperm surface antigen 2B1. J. Cell Sci. 109, 2561–2570 [DOI] [PubMed] [Google Scholar]
- 11. Yamaguchi R., Yamagata K., Ikawa M., Moss S. B., Okabe M. (2006) Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol. Reprod. 75, 760–766 [DOI] [PubMed] [Google Scholar]
- 12. Kondoh G., Tojo H., Nakatani Y., Komazawa N., Murata C., Yamagata K., Maeda Y., Kinoshita T., Okabe M., Taguchi R., Takeda J. (2005) Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 11, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gyamera-Acheampong C., Mbikay M. (2009) Proprotein convertase subtilisin/kexin type 4 in mammalian fertility. A review. Hum. Reprod. Update 15, 237–247 [DOI] [PubMed] [Google Scholar]
- 14. Liu Q., Hamil K. G., Sivashanmugam P., Grossman G., Soundararajan R., Rao A. J., Richardson R. T., Zhang Y. L., O'Rand M. G., Petrusz P., French F. S., Hall S. H. (2001) Primate epididymis-specific proteins. Characterization of ESC42, a novel protein containing a trefoil-like motif in monkey and human. Endocrinology 142, 4529–4539 [DOI] [PubMed] [Google Scholar]
- 15. Wapenaar M. C., Monsuur A. J., Poell J., van 't Slot R., Meijer J. W., Meijer G. A., Mulder C. J., Mearin M. L., Wijmenga C. (2007) The SPINK gene family and celiac disease susceptibility. Immunogenetics 59, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kazal L. A., Spicer D. S., Brahinsky R. A. (1948) Isolation of a crystalline trypsin inhibitor-anticoagulant protein from pancreas. J. Am. Chem. Soc. 70, 3034–3040 [DOI] [PubMed] [Google Scholar]
- 17. van Hoef V., Breugelmans B., Spit J., Simonet G., Zels S., Vanden Broeck J. (2012) Phylogenetic distribution of protease inhibitors of the Kazal-family within the Arthropoda. Peptides, in press [DOI] [PubMed] [Google Scholar]
- 18. Kato I., Schrode J., Kohr W. J., Laskowski M., Jr. (1987) Chicken ovomucoid. Determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry 26, 193–201 [DOI] [PubMed] [Google Scholar]
- 19. Lu S. M., Lu W., Qasim M. A., Anderson S., Apostol I., Ardelt W., Bigler T., Chiang Y. W., Cook J., James M. N., Kato I., Kelly C., Kohr W., Komiyama T., Lin T. Y., Ogawa M., Otlewski J., Park S. J., Qasim S., Ranjbar M., Tashiro M., Warne N., Whatley H., Wieczorek A., Wieczorek M., Wilusz T., Wynn R., Zhang W., Laskowski M., Jr. (2001) Predicting the reactivity of proteins from their sequence alone. Kazal family of protein inhibitors of serine proteinases. Proc. Natl. Acad. Sci. U.S.A. 98, 1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Witt H., Luck W., Hennies H. C., Classen M., Kage A., Lass U., Landt O., Becker M. (2000) Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat. Genet. 25, 213–216 [DOI] [PubMed] [Google Scholar]
- 21. Fink E., Hehlein-Fink C., Eulitz M. (1990) Amino acid sequence elucidation of human acrosin-trypsin inhibitor (HUSI-II) reveals that Kazal-type proteinase inhibitors are structurally related to β-subunits of glycoprotein hormones. FEBS Lett. 270, 222–224 [DOI] [PubMed] [Google Scholar]
- 22. Agerberth B., Söderling-Barros J., Jörnvall H., Chen Z. W., Ostenson C. G., Efendić S., Mutt V. (1989) Isolation and characterization of a 60-residue intestinal peptide structurally related to the pancreatic secretory type of trypsin inhibitor. Influence on insulin secretion. Proc. Natl. Acad. Sci. U.S.A. 86, 8590–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mägert H. J., Ständker L., Kreutzmann P., Zucht H. D., Reinecke M., Sommerhoff C. P., Fritz H., Forssmann W. G. (1999) LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J. Biol. Chem. 274, 21499–21502 [DOI] [PubMed] [Google Scholar]
- 24. Meyer-Hoffert U., Wu Z., Kantyka T., Fischer J., Latendorf T., Hansmann B., Bartels J., He Y., Gläser R., Schröder J. M. (2010) Isolation of SPINK6 in human skin. Selective inhibitor of kallikrein-related peptidases. J. Biol. Chem. 285, 32174–32181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cui Y., Bi M., Su T., Liu H., Lu S. H. (2010) Molecular cloning and characterization of a novel esophageal cancer related gene. Int. J. Oncol. 37, 1521–1528 [DOI] [PubMed] [Google Scholar]
- 26. Jalkanen J., Kotimäki M., Huhtaniemi I., Poutanen M. (2006) Novel epididymal protease inhibitors with Kazal or WAP family domain. Biochem. Biophys. Res. Commun. 349, 245–254 [DOI] [PubMed] [Google Scholar]
- 27. Brattsand M., Stefansson K., Hubiche T., Nilsson S. K., Egelrud T. (2009) SPINK9. A selective, skin-specific Kazal-type serine protease inhibitor. J. Invest. Dermatol. 129, 1656–1665 [DOI] [PubMed] [Google Scholar]
- 28. Puente X. S., López-Otín C. (2004) A genomic analysis of rat proteases and protease inhibitors. Genome Res. 14, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rockett J. C., Patrizio P., Schmid J. E., Hecht N. B., Dix D. J. (2004) Gene expression patterns associated with infertility in humans and rodent models. Mutat. Res. 549, 225–240 [DOI] [PubMed] [Google Scholar]
- 30. Hoefnagel J. J., Dijkman R., Basso K., Jansen P. M., Hallermann C., Willemze R., Tensen C. P., Vermeer M. H. (2005) Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood 105, 3671–3678 [DOI] [PubMed] [Google Scholar]
- 31. Tüysüz B., Ojalvo D., Mat C., Zambruno G., Covaciu C., Castiglia D., D'Alessio M. (2010) A new SPINK5 donor splice site mutation in siblings with Netherton syndrome. Acta Derm. Venereol. 90, 95–96 [DOI] [PubMed] [Google Scholar]
- 32. Gasiorowska A., Talar-Wojnarowska R., Czupryniak L., Smolarz B., Romanowicz-Makowska H., Kulig A., Malecka-Panas E. (2011) The prevalence of cationic trypsinogen (PRSS1) and serine protease inhibitor, Kazal type 1 (SPINK1) gene mutations in Polish patients with alcoholic and idiopathic chronic pancreatitis. Dig. Dis. Sci. 56, 894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stenman U. H. (2011) Role of the tumor-associated trypsin inhibitor SPINK1 in cancer development. Asian J. Androl. 13, 628–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Möritz A., Lilja H., Fink E. (1991) Molecular cloning and sequence analysis of the cDNA encoding the human acrosin-trypsin inhibitor (HUSI-II). FEBS Lett. 278, 127–130 [DOI] [PubMed] [Google Scholar]
- 35. Lee B., Park I., Jin S., Choi H., Kwon J. T., Kim J., Jeong J., Cho B. N., Eddy E. M., Cho C. (2011) Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J. Biol. Chem. 286, 29108–29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J., Ohmuraya M., Hirota M., Baba H., Zhao G., Takeya M., Araki K., Yamamura K. (2008) Expression pattern of serine protease inhibitor Kazal type 3 (Spink3) during mouse embryonic development. Histochem. Cell Biol. 130, 387–397 [DOI] [PubMed] [Google Scholar]
- 37. Dematteis A., Miranda S. D., Novella M. L., Maldonado C., Ponce R. H., Maldera J. A., Cuasnicu P. S., Coronel C. E. (2008) Rat caltrin protein modulates the acrosomal exocytosis during sperm capacitation. Biol. Reprod. 79, 493–500 [DOI] [PubMed] [Google Scholar]
- 38. Zhen W., Li P., He B., Guo J., Zhang Y. L. (2009) The novel epididymis-specific β-galactosidase-like gene Glb1l4 is essential in epididymal development and sperm maturation in rats. Biol. Reprod. 80, 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu C. F., Liu Q., Zhang L., Yuan H. X., Zhen W., Zhang J. S., Chen Z. J., Hall S. H., French F. S., Zhang Y. L. (2007) RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol. Reprod. 76, 63–73 [DOI] [PubMed] [Google Scholar]
- 40. Xiao L. Q., Liu A. H., Zhang Y. L. (2004) An effective method for raising antisera against β-defensins. Double-copy protein expression of mBin1b in E. coli. Acta Biochim. Biophys. Sin. (Shanghai) 36, 571–576 [DOI] [PubMed] [Google Scholar]
- 41. Harlow E., Lane D. P. (1988) Antibodies: A Laboratory Manual, pp. 139–242, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 42. Syntin P., Cornwall G. A. (1999) Immunolocalization of CRES (cystatin-related epididymal spermatogenic) protein in the acrosomes of mouse spermatozoa. Biol. Reprod. 60, 1542–1552 [DOI] [PubMed] [Google Scholar]
- 43. Birmingham A., Anderson E., Sullivan K., Reynolds A., Boese Q., Leake D., Karpilow J., Khvorova A. (2007) A protocol for designing siRNAs with high functionality and specificity. Nat. Protoc. 2, 2068–2078 [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y., Diao H., Ni Z., Hu S., Yu H., Zhang Y. (2011) The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol. Life Sci. 68, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu D. Y., Baker H. W. (1993) Inhibition of acrosin activity with a trypsin inhibitor blocks human sperm penetration of the zona pellucida. Biol. Reprod. 48, 340–348 [DOI] [PubMed] [Google Scholar]
- 46. Zhou Y., Zheng M., Shi Q., Zhang L., Zhen W., Chen W., Zhang Y. (2008) An epididymis-specific secretory protein HongrES1 critically regulates sperm capacitation and male fertility. PLoS One 3, e4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bendahmane M., Zeng H. T., Tulsiani D. R. (2002) Assessment of acrosomal status in rat spermatozoa. Studies on carbohydrate and non-carbohydrate agonists. Arch. Biochem. Biophys. 404, 38–47 [DOI] [PubMed] [Google Scholar]
- 48. Berger T., Miller M. G., Horner C. M. (2000) In vitro fertilization after in vivo treatment of rats with three reproductive toxicants. Reprod. Toxicol. 14, 45–53 [DOI] [PubMed] [Google Scholar]
- 49. Ohmuraya M., Hirota M., Araki M., Mizushima N., Matsui M., Mizumoto T., Haruna K., Kume S., Takeya M., Ogawa M., Araki K., Yamamura K. (2005) Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology 129, 696–705 [DOI] [PubMed] [Google Scholar]
- 50. Hewett D. R., Simons A. L., Mangan N. E., Jolin H. E., Green S. M., Fallon P. G., McKenzie A. N. (2005) Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum. Mol. Genet. 14, 335–346 [DOI] [PubMed] [Google Scholar]
- 51. Courty Y. (1991) Testosterone and corticosterone co-regulate messenger RNA coding for secretory proteins in the epididymis of the lizard (Lacerta vivipara). J. Reprod. Fertil. 91, 292–300 [DOI] [PubMed] [Google Scholar]
- 52. Moore H. D., Curry M. R., Penfold L. M., Pryor J. P. (1992) The culture of human epididymal epithelium and in vitro maturation of epididymal spermatozoa. Fertil. Steril. 58, 776–783 [PubMed] [Google Scholar]
- 53. Hu S., Yao G., Guan X., Ni Z., Ma W., Wilson E. M., French F. S., Liu Q., Zhang Y. (2010) Research resource. Genome-wide mapping of in vivo androgen receptor binding sites in mouse epididymis. Mol. Endocrinol. 24, 2392–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arnoys E. J., Wang J. L. (2007) Dual localization. Proteins in extracellular and intracellular compartments. Acta Histochem. 109, 89–110 [DOI] [PubMed] [Google Scholar]
- 55. Yeung C. H., Pérez-Sánchez F., Schröter S., Kirchhoff C., Cooper T. G. (2001) Changes of the major sperm maturation-associated epididymal protein HE5 (CD52) on human ejaculated spermatozoa during incubation. Mol. Hum. Reprod. 7, 617–624 [DOI] [PubMed] [Google Scholar]
- 56. Chen H., Griffiths G., Galileo D. S., Martin-DeLeon P. A. (2006) Epididymal SPAM1 is a marker for sperm maturation in the mouse. Biol. Reprod. 74, 923–930 [DOI] [PubMed] [Google Scholar]
- 57. Martin-DeLeon P. A. (2006) Epididymal SPAM1 and its impact on sperm function. Mol. Cell. Endocrinol. 250, 114–121 [DOI] [PubMed] [Google Scholar]
- 58. Légaré C., Bérubé B., Boué F., Lefièvre L., Morales C. R., El-Alfy M., Sullivan R. (1999) Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol. Reprod. Dev. 52, 225–233 [DOI] [PubMed] [Google Scholar]
- 59. Frenette G., Sullivan R. (2001) Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 59, 115–121 [DOI] [PubMed] [Google Scholar]
- 60. Ou C. M., Tang J. B., Huang M. S., Sudhakar Gandhi P. S., Geetha S., Li S. H., Chen Y. H. (2012) The mode of reproductive-derived Spink (serine protease inhibitor Kazal-type) action in the modulation of mammalian sperm activity. Int. J. Androl. 35, 52–62 [DOI] [PubMed] [Google Scholar]
- 61. Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S. A., Chiba K., Hirohashi N. (2011) Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. U.S.A. 108, 4892–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Inoue N., Satouh Y., Ikawa M., Okabe M., Yanagimachi R. (2011) Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. U.S.A. 108, 20008–20011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hobson J. P., Netzel-Arnett S., Szabo R., Réhault S. M., Church F. C., Strickland D. K., Lawrence D. A., Antalis T. M., Bugge T. H. (2004) Mouse DESC1 is located within a cluster of seven DESC1-like genes and encodes a type II transmembrane serine protease that forms serpin inhibitory complexes. J. Biol. Chem. 279, 46981–46994 [DOI] [PubMed] [Google Scholar]
- 64. Nakamura Y., Inoue M., Okumura Y., Shiota M., Nishikawa M., Arase S., Kido H. (2003) Cloning, expression analysis, and tissue distribution of esp-1/testisin, a membrane-type serine protease from the rat. J. Med. Invest 50, 78–86 [PubMed] [Google Scholar]
- 65. Honda A., Yamagata K., Sugiura S., Watanabe K., Baba T. (2002) A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 277, 16976–16984 [DOI] [PubMed] [Google Scholar]
- 66. Takano N., Matsui H., Takahashi T. (2005) TESSP-1. A novel serine protease gene expressed in the spermatogonia and spermatocytes of adult mouse testes. Mol. Reprod. Dev. 70, 1–10 [DOI] [PubMed] [Google Scholar]