Abstract
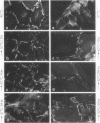
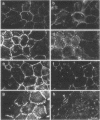
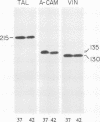
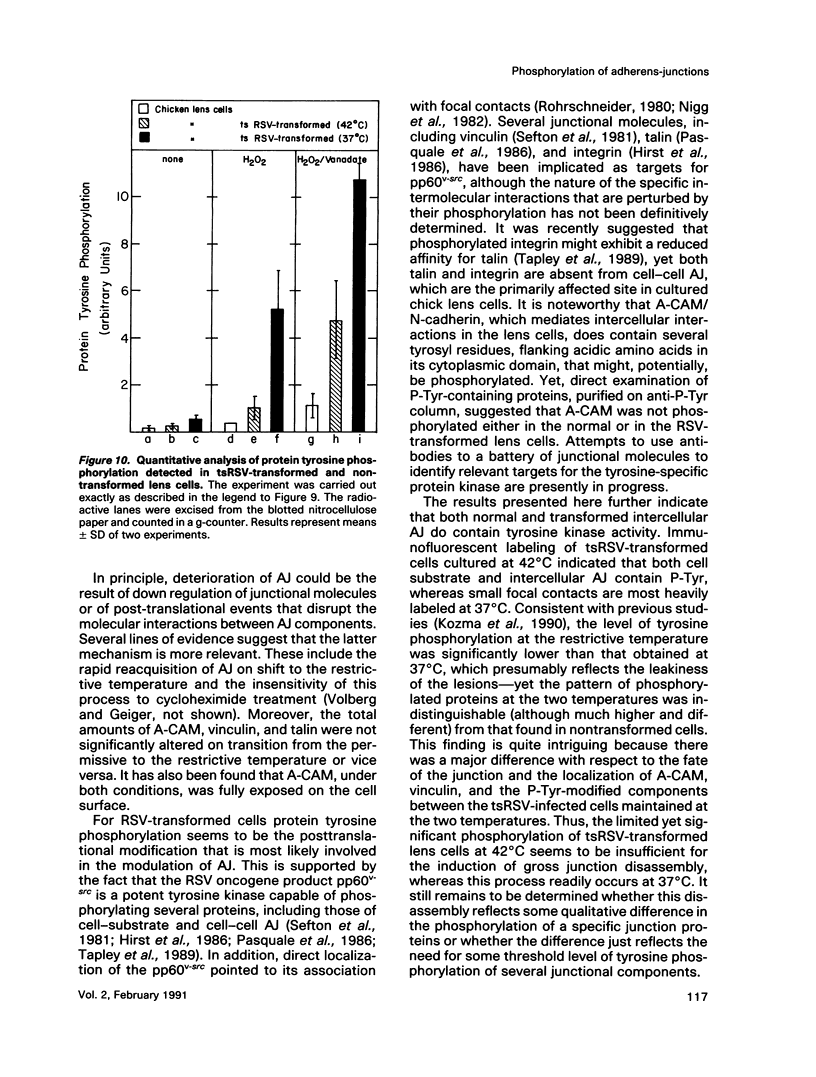
Transformation of cultured chick lens epithelial cells with a temperature-sensitive mutant of Rous sarcoma virus (tsRSV) leads to radical changes in cell shape and interactions. When cultured at the restrictive temperature (42 degrees C), the transformed cells largely retained epithelial morphology and intercellular adherens junctions (AJ), whereas on switch to the permissive temperature (37 degrees C) they rapidly became fibroblastoid, their AJ deteriorated, and cell adhesion molecules (A-CAM) (N-cadherin) largely disappeared from intercellular contact sites. The microfilament system that was primarily associated with these junctions was markedly rearranged on shift to 37 degrees C and remained associated mainly with cell-substrate focal contacts. These apparent changes in intercellular AJ were not accompanied by significant alterations in the cellular content of several junction-associated molecules, including A-CAM, vinculin, and talin. Immunolabeling with phosphotyrosine-specific antibodies indicated that both cell-substrate and intercellular AJ were the major cellular targets for the pp60v-src tyrosine-specific protein kinase. It was further shown that intercellular AJ components serve as substrates to tyrosine kinases also in nontransformed lens cells, because the addition of a combination of vanadate and H2O2--which are potent inhibitors of protein tyrosine phosphatases--leads to a remarkable accumulation of immunoreactive phosphotyrosine-containing proteins in these junctions. This finding suggests that intercellular junctions are major sites of action of protein tyrosine kinases and that protein tyrosine phosphatases play a major role in the regulation of phosphotyrosine levels in AJ of both normal and RSV-transformed cells.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. The role of changes in cell shape and contacts in the regulation of cytoskeleton expression during differentiation. J Cell Sci Suppl. 1987;8:293–312. doi: 10.1242/jcs.1987.supplement_8.16. [DOI] [PubMed] [Google Scholar]
- Bendori R., Salomon D., Geiger B. Contact-dependent regulation of vinculin expression in cultured fibroblasts: a study with vinculin-specific cDNA probes. EMBO J. 1987 Oct;6(10):2897–2905. doi: 10.1002/j.1460-2075.1987.tb02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Boschek C. B., Jockusch B. M., Friis R. R., Back R., Grundmann E., Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981 Apr;24(1):175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chan C. P., McNall S. J., Krebs E. G., Fischer E. H. Stimulation of protein phosphatase activity by insulin and growth factors in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6257–6261. doi: 10.1073/pnas.85.17.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P. M., Di Renzo M. F., Tarone G., Giancotti F. G., Naldini L., Marchisio P. C. Detection of phosphotyrosine-containing proteins in the detergent-insoluble fraction of RSV-transformed fibroblasts by azobenzene phosphonate antibodies. EMBO J. 1984 Mar;3(3):483–489. doi: 10.1002/j.1460-2075.1984.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. Cytoskeleton-associated cell contacts. Curr Opin Cell Biol. 1989 Feb;1(1):103–109. doi: 10.1016/s0955-0674(89)80045-6. [DOI] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Singer S. J. Immunocytochemical localization of alpha-actinin in intestinal epithelial cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2833–2837. doi: 10.1073/pnas.76.6.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Volk T., Volberg T., Bendori R. Molecular interactions in adherens-type contacts. J Cell Sci Suppl. 1987;8:251–272. doi: 10.1242/jcs.1987.supplement_8.14. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Matsuda M., Hanafusa H. A glycoprotein in the plasma membrane matrix as a major potential substrate of p60v-src. Mol Cell Biol. 1990 Feb;10(2):830–836. doi: 10.1128/mcb.10.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Heffetz D., Zick Y. H2O2 potentiates phosphorylation of novel putative substrates for the insulin receptor kinase in intact Fao cells. J Biol Chem. 1989 Jun 15;264(17):10126–10132. [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kapprell H. P., Cowin P., Franke W. W. Biochemical characterization of the soluble form of the junctional plaque protein, plakoglobin, from different cell types. Eur J Biochem. 1987 Aug 3;166(3):505–517. doi: 10.1111/j.1432-1033.1987.tb13543.x. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Schmid E., Franke W. W., Geiger B. Different modes of internalization of proteins associated with adhaerens junctions and desmosomes: experimental separation of lateral contacts induces endocytosis of desmosomal plaque material. EMBO J. 1982;1(6):725–732. doi: 10.1002/j.1460-2075.1982.tb01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kozma L. M., Reynolds A. B., Weber M. J. Glycoprotein tyrosine phosphorylation in Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1990 Feb;10(2):837–841. doi: 10.1128/mcb.10.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl protein phosphatases. Biochem J. 1989 Jan 1;257(1):23–36. doi: 10.1042/bj2570023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A., Pasquale E. B. Tyrosine phosphorylated proteins in different tissues during chick embryo development. J Cell Biol. 1988 May;106(5):1747–1755. doi: 10.1083/jcb.106.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A., Pasquale E. B., Wang J. Y., Singer S. J. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., D'Urso N., Comoglio P. M., Giancotti F. G., Tarone G. Vanadate-treated baby hamster kidney fibroblasts show cytoskeleton and adhesion patterns similar to their Rous sarcoma virus-transformed counterparts. J Cell Biochem. 1988 Jun;37(2):151–159. doi: 10.1002/jcb.240370203. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Inhibition of chicken embryo lens differentiation and lens junction formation in culture by pp60v-src. Mol Cell Biol. 1988 Apr;8(4):1414–1420. doi: 10.1128/mcb.8.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Swarup G., Speeg K. V., Jr, Cohen S., Garbers D. L. Phosphotyrosyl-protein phosphatase of TCRC-2 cells. J Biol Chem. 1982 Jul 10;257(13):7298–7301. [PubMed] [Google Scholar]
- Takata K., Singer S. J. Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J Cell Biol. 1988 May;106(5):1757–1764. doi: 10.1083/jcb.106.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985 Jul;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Ungar F., Geiger B., Ben-Ze'ev A. Cell contact- and shape-dependent regulation of vinculin synthesis in cultured fibroblasts. 1986 Feb 27-Mar 5Nature. 319(6056):787–791. doi: 10.1038/319787a0. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A 135-kd membrane protein of intercellular adherens junctions. EMBO J. 1984 Oct;3(10):2249–2260. doi: 10.1002/j.1460-2075.1984.tb02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. II. Antibody-mediated modulation of junction formation. J Cell Biol. 1986 Oct;103(4):1451–1464. doi: 10.1083/jcb.103.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]