Background: The etiology and neurophysiology of generalized pruritus associated with liver disease remain unknown.
Results: Rats with bile duct ligation displayed enhanced scratching and thermal hyperalgesia dependent on PAR2 activation and TRPV1 potentiation.
Conclusion: Pruritus and hyperalgesia in BDL-rats are associated with neuroinflammation and involve PAR2-induced TRPV1 sensitization.
Significance: Pharmacological modulation of PAR2 and/or TRPV1 emerges as a potential therapeutic approach for liver pruritus refractory to conventional treatments.
Keywords: Liver, Mast Cell, Neuroinflammation, Pharmacology, TRP Channels, Capsaicin Receptor, Peripheral Sensitization, Protease-activated Receptor
Abstract
Persistent pruritus is a common disabling dermatologic symptom associated with different etiologic factors. These include primary skin conditions, as well as neuropathic, psychogenic, or systemic disorders like chronic liver disease. Defective clearance of potential pruritogenic substances that activate itch-specific neurons innervating the skin is thought to contribute to cholestatic pruritus. However, because the underlying disease-specific pruritogens and itch-specific neuronal pathways and mechanism(s) are unknown, symptomatic therapeutic intervention often leads to no or only limited success. In the current study, we aimed to first validate rats with bile duct ligation (BDL) as a model for hepatic pruritus and then to evaluate the contribution of inflammation, peripheral neuronal sensitization, and specific signaling pathways and subpopulations of itch-responsive neurons to scratching behavior and thermal hypersensitivity. Chronic BDL rats displayed enhanced scratching behavior and thermal hyperalgesia indicative of peripheral neuroinflammation. BDL-induced itch and hypersensitivity involved a minor contribution of histaminergic/serotonergic receptors, but significant activation of protein-activated receptor 2 (PAR2) receptors, prostaglandin PGE2 formation, and potentiation of transient receptor potential vanilloid 1 (TRPV1) channel activity. The sensitization of dorsal root ganglion nociceptors in BDL rats was associated with increased surface expression of PAR2 and TRPV1 proteins and an increase in the number of PAR2- and TRPV1-expressing peptidergic neurons together with a shift of TRPV1 receptor expression to medium sized dorsal root ganglion neurons. These results suggest that pruritus and hyperalgesia in chronic cholestatic BDL rats are associated with neuroinflammation and involve PAR2-induced TRPV1 sensitization. Thus, pharmacological modulation of PAR2 and/or TRPV1 may be a valuable therapeutic approach for patients with chronic liver pruritus refractory to conventional treatments.
Introduction
Chronic pruritus is a common, seriously debilitating symptom that can arise from dermatologic disorders, but often also appears in other disease states as a secondary symptom (1). For instance, chronic itch has been associated with a wide variety of pathologies such as malignancy, or hematologic, infectious, neuropsychiatric, metabolic, and systemic disorders. Patients with end-stage renal and hepatic diseases often present with pruritus that might result from diminished renal or hepatic clearance of pruritogenic substances.
The origin of hepatic pruritus remains poorly understood. The pathological accumulation of specific pruritogens is thought to directly or indirectly activate unmyelinated C-fibers that innervate the skin (2, 3). However, both the identity of disease-specific pruritogen(s) and the neuronal pathways and mechanism(s) involved in pruritus are highly controversial. For instance, although bile salts and other cholephiles are traditionally assumed to be major pruritogens, no correlation exists between pruritus intensity and either serum biomarkers for cholestasis or with the course of the disease (1, 4). It is widely accepted that other substances like the well known pruritogens histamine and serotonin (5-HT),2 which are released from mast cells, or endogenous opioids, which are liberated from immune cells, may accumulate in hepatic pruritus and activate sensory neurons (5, 6). Whereas 5-HT and opioid receptor antagonists have therapeutic potential in a subset of cholestasis patients, antihistaminics have proven ineffective (7). Recently, increased serum levels of autotaxin and lysophosphatidic acid (LPA) were proposed as mediators in human cholestatic pruritus (8–11). In addition to inducing histamine release from mast cells, LPA impinges on proinflammatory transcriptional factors, promotes cytokine production and platelet activation (12), and enhances cytokine-induced COX-2 expression (13). Indeed, high serum levels of proinflammatory cytokines like IL-31 (14) or tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), which are both associated with liver fibrosis (15, 16), might also contribute to itch.
TRPV1 (transient receptor potential vanilloid 1) is a polymodal receptor channel that conducts cations (preferentially Ca2+) in response to either the irritant compound capsaicin or to extracellular acidic pH and to noxiously high temperatures (≥43 °C). Pharmacological and genetic abrogation of TRPV1 function in rodents significantly reduces pain in acute and chronic inflammatory animal models (17, 18). Whereas TRPV1 channel activity is low under normal conditions, mediators released from injured cells and activated immune cells during inflammation potentiate TRPV1 activity through activation of G protein-coupled receptors, thereby leading to sensitization of nociceptive neurons (19).
Besides being a pain sensor, TRPV1 may also be an itch integrator of both histaminergic (2, 20) and some nonhistaminergic pruritogens like serotonin or imiquimod (2, 21). Binding of pruritogens to their receptors, whose identity is often unclear, can trigger distinct and specific intracellular signaling pathways that can activate and potentiate TRPV1 function (2, 22). Hence, TRPV1 may be a common downstream effector in the itch signaling pathway. Indeed, most noxious stimuli can produce pain and/or itch, depending on how and where they are applied (23). Recently, certain subsets of TRPV1-expressing C-fibers with specific sensory modalities have been reported to respond distinctly to different pruritogens like histamine, 5-HT, or chloroquine (2, 21, 24). Nevertheless, our understanding of the mechanisms involved in pruritus signaling by TRPV1-expressing neurons remains limited.
To our knowledge, no previous study has addressed the neuronal mechanism(s) underlying pruritus associated with cholestatic liver disease, a dynamic and complex condition leading to inflammation, liver fibrosis, and eventually cirrhosis. We hypothesized that a Wistar rat model with chronic bile duct ligation (BDL) (16) may display increased scratching bouts and provide a model of cholestatic pruritus. We investigated the involvement of specific pathways and subpopulations of itch-responsive neurons by pharmacological intervention with selective antagonists and by immunohistochemistry. In particular, because neurogenic inflammation in chronic BDL rats (16, 25) may contribute to pruritus through TRPV1 sensitization (26, 27), we investigated the role of TRPV1 as a final integrator in the itch signaling pathway.
Here, we show that Wistar BDL rats exhibit augmented scratching accompanied by peripheral sensitization of primary afferents as revealed by thermal hyperalgesia. Both symptoms showed an early onset that correlated with an increase in both serum biomarkers for cholestasis and proinflammatory cytokines. Whereas histamine/serotonin pathways only play a minor role in the scratching and thermal hyperalgesia phenotype of our rat model, our results demonstrate an involvement of protease-activated receptor 2 (PAR2) activation, peripheral inflammation, and TRPV1 potentiation. Hence targeting the recruitment or modulation of PAR2 or TRPV1 may be useful therapeutic strategies to treat chronic itch and neuroinflammation in liver disease patients.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar rats (200–250 g) were obtained from Charles River, France. Experimental procedures were approved by the Ethics Committee and met European Union guidelines for care and management of experimental animals. BDL rats were operated on as described (16). Control rats were sham-operated. Behavioral studies started 48 h after surgery and only when no signs of pain or distress were apparent. Pharmacological treatments began 3 weeks after surgery.
Drugs and Reagents
The contribution of different signaling pathways was assessed by subcutaneous administration of cyproheptadine (20 mg/kg) (28), intraperitoneal injection of naloxone HCl (20 mg/kg), gabexate mesylate (10 mg/kg, subcutaneously), FSLLRY-NH2 (200 μg, subcutaneously), ibuprofen (20 mg/kg, intraperitoneally), meloxicam (1 mg/kg, subcutaneously), capsazepine (2 mg/kg, subcutaneously) (29), DD04107 or a lipopeptide containing the scrambled (Scr) sequence of DD04107 (3 mg/kg, subcutaneously). Drugs were prepared immediately before use in 0.9% NaCl from a stock solution. Control groups received the corresponding vehicle. Cyproheptadine, protease, and opioid antagonists were administered 30 min, and other drugs 60 min, before behavioral tests.
All drugs were from Sigma unless otherwise stated. PAR2 agonist (PAR2-AP), a negative retropeptide control (PAR2-RP), and FSLLRY-NH2 (PAR2-Antg) were from Bachem. Meloxicam (Metacam®) was from Roche Applied Science, gabexate mesylate was from Tocris (Bristol, UK), and palmitoylated DD04107 and the scrambled control lipopeptide were synthesized by DiverDrugs (Gavà, Spain).
Primary antibodies used were anti-α-CGRP (Novus, Littleton, CO), anti-PAR2 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-TRPV1 (Alomone, Jerusalem, Israel), and NF200 and monoclonal anti-β-actin (Sigma). Secondary antibodies were from Jackson (West Grove, PA).
Behavioral Analysis
Rats were acclimatized in a measuring cage for 30 min, followed by videotaping of scratching behavior for 30 min or 1 h. Spontaneous scratching was quantified by counting the number of scratches of any region of the body performed by forepaws or hindpaws.
For Hargreaves' Plantar Test a standard apparatus (Ugo Basile, Italy) was used that automatically measured the paw withdrawal latency (PWL) to a thermal radiant stimulus (30). To avoid tissue injury in refractory animals, stimulation was automatically terminated after 32 s. PWL was determined before and after drug or vehicle treatment in BDL and sham control rats. Data are presented as mean ± S.E. with a minimum of six animals/group.
Determination of Cholestasis and Inflammation
Serum bilirubin, alkaline phosphatase (AP), glutamic oxaloacetic transaminase and glutamic pyruvic transaminase were determined by standard techniques and prostaglandin PGE2 by ELISA (GE Healthcare).
Ca2+ Imaging in Primary Cultures of Dorsal Root Ganglion (DRG) Neurons
Primary adult sham and BDL DRG cultures were established as described (24). Fluorescence measurements were performed after ∼12 h in vitro in paired BDL and sham DRG cultures as in Ref. 31. To investigate PAR2 modulation of TRPV1 activity, cells were exposed for 5 min to PAR2 agonist (AP), antagonist (Antg), or vehicle, followed by a 10-s pulse with 100 nm capsaicin (32).
Biotin Labeling of Surface TRPV1 and PAR2 Proteins
Dissociated DRG neurons from sham- and BDL-operated rats were surface biotinylated with sulfo-NHS-SS-Biotin (Pierce) and processed as described (33). Biotinylated proteins were isolated with streptavidin-agarose, resolved by SDS-PAGE, and detected with the following antibodies and dilutions: TRPV1 (1:1000), PAR2 (1:100), and β-actin (1:200). Immunoblots were digitized and quantified. The absence of contaminating intracellular proteins in membrane fractions was verified by labeling of actin.
Immunofluorescent Staining of DRG Neurons and Measurement of Size of Neuronal Somata
Rats were overanesthetized and then transcardially perfused with saline and then with 4% paraformaldehyde (pH 7.4). DRGs were quickly removed, postfixed, and then placed in sucrose solution. 8-μm sections were co-incubated with anti-TRPV1 (1:1000) and FITC-isolectin B4 (IB4) (1:50) or one of the following antibodies: anti-NF200 (1:1000), anti-α-CGRP (1:300), anti-GRP (1:500), or anti-PAR2 (1:100) overnight at 4 °C. Cy3- or Alexa Fluor 488-tagged secondary antibodies were used in 1:200 dilutions for 1 h at 22 °C. Sections were then washed three times for 10 min in PBS and mounted. Controls for double-labeling experiments included imaging with both green and red filters in the presence of only one primary antibody and/or by omission of all primary antibodies. Settings for each channel were initially adjusted to avoid bleeding through. Images were taken on a Leica DM5000B epifluorescence microscope fitted with a digital camera. When comparing different samples, identical settings were used.
The areas of TRPV1-positive (TRPV1+) neurons and DRG neuronal markers were analyzed by tracing the soma perimeter on a computer screen in a calibrated image and using ImageJ software (National Institutes of Health, Bethesda, MD). Calculated areas were plotted against the percentage of total TRPV1+ neurons. More than five sections per animal, three pairs of rats, and >1000 total neurons per phenotype were analyzed.
Statistical Analysis
Results are expressed as mean ± S.E. The nonparametric Mann-Whitney U-test was used for paired comparisons. Between groups, one-way ANOVA was followed by Tukey's test. Differences were considered significant when *p ≤ 0.1, **p < 0.01, and ***p < 0.001.
Additional methods and materials are described in supplemental Experimental Procedures.
RESULTS
Chronic BDL Rats Displayed Increased Scratching Mediated by PAR2 Signaling and Neuroinflammation
We evaluated whether spontaneous scratching behavior was increased in Wistar BDL rats, an animal model that leads to jaundice, inflammation, and cirrhosis (16). Cumulative spontaneous scratching bouts (SSBs) during 1-h observation periods were recorded in BDL- and sham-operated animals at different time points. As shown in Fig. 1A, BDL rats exhibited a significant (≈2-fold) elevation in the number of SSBs (SSB = 59 ± 4, n = 12, in BDL compared with 39 ± 4, n = 8, in sham rats) 48 h after surgery, a behavior that persisted for at least 3 months. Cholestasis was evident from increased serum bilirubin and AP levels, as well as from elevated levels of hepatic injury markers glutamic oxaloacetic transaminase and glutamic pyruvic transaminase (Table 1). Similarly, proinflammatory IL-6 was elevated in BDL sera, as shown previously (16).
FIGURE 1.
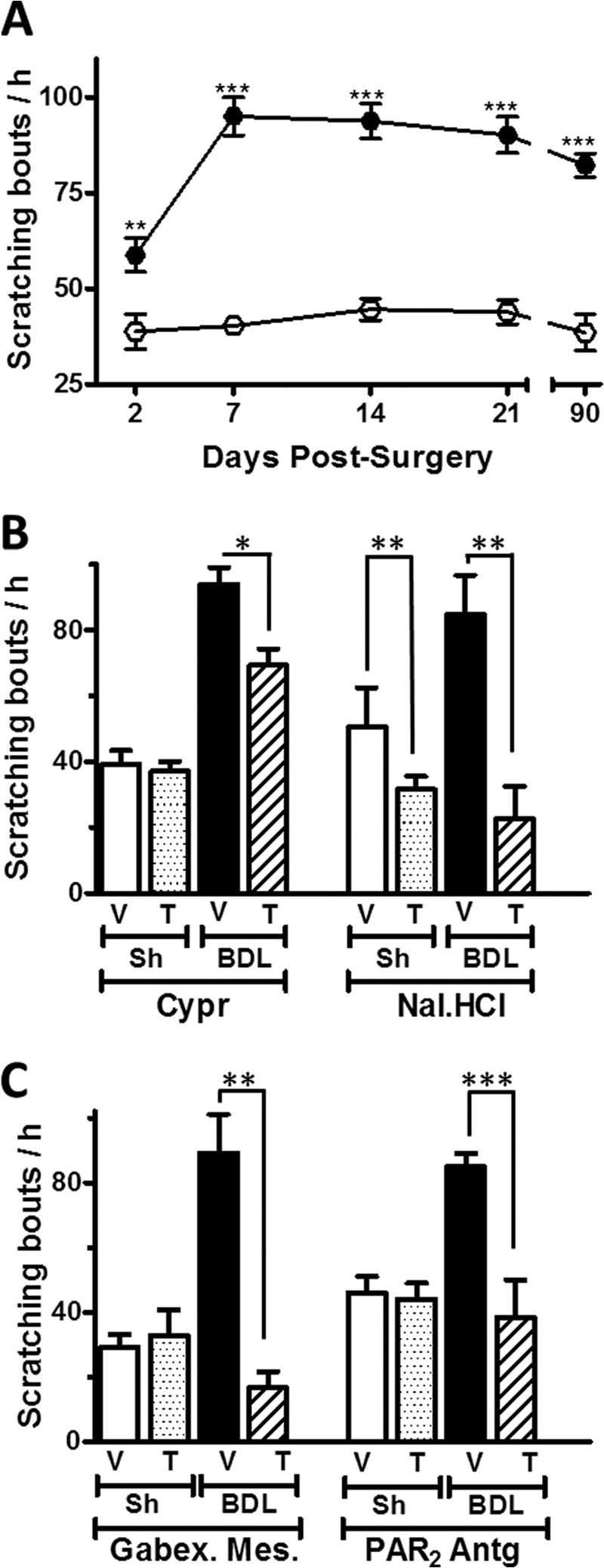
Effect of the blockade of histamine/serotonin-, opioid-, protease-, and PAR2-activated pathways in the enhanced scratching behavior of a rat model with BDL. A, time course of cumulative spontaneous scratching bouts/h in BDL (●) and sham-operated (○) control rats after surgery (n = 8–12). B, effect of the mixed His/5-HT antagonist cyproheptadine (Cypr) and the systemic opioid antagonist naloxone HCl (Nal·HCl) on the spontaneous scratching bouts/h. Sh, sham. C, spontaneous scratching bouts after administration of the protease inhibitor gabexate mesylate (Gabex·Mes) and PAR2 antagonist (PAR2-Antg) recorded during the first 30 min. V, vehicle; T, treatment. n = 6–9 animals. Each data point represents mean ± S.E. (error bars). *, p < 0.0; **, p < 0.01; ***, p < 0.001, Mann-Whitney U-test).
TABLE 1.
Serum biomarkers of bilirubin (BR), alkaline phosphatase (AP), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) obtained 7 days after surgery
Results are expressed as mean ± S.E. with (**p < 0.01, n = 5, Mann-Whitney U-test).
| Groups | BR | AP | GOT | GPT |
|---|---|---|---|---|
| mg/dl | units/liter | units/liter | units/liter | |
| Sham | 0.14 ± 0.01 | 601.5 ± 59.5 | 84.8 ± 4.4 | 57.4 ± 1.2 |
| BDL | 6.43 ± 1.35** | 1018 ± 46.6** | 210.7 ± 27.2** | 109.5 ± 14.3** |
To determine the involvement of different molecular pathways in enhanced scratching, we first administered the mixed histaminergic/serotonergic antagonist cyproheptadine. This procedure slightly decreased SSBs in BDL rats (SSBs = 66 ± 4 compared with 94 ± 5 with vehicle, n = 7) (Fig. 1B). In contrast, scratching bouts were markedly reduced when BDL rats were treated with the systemic opioid receptor antagonist naloxone HCl (Nal·HCl) (SSB = 22 ± 5 versus 84 ± 6 with saline, n = 7) (Fig. 1B).
Besides histamine and endogenous opioids (33), also tryptase, chimase, and other proteases are released during inflammation by immune cells (34). To assess the involvement of proteases in the scratching behavior, we administered the specific lima bean trypsin inhibitor, the serine protease inhibitor gabexate mesylate, and the PAR2 receptor antagonist, FSLLRY-NH2 into BDL- and sham-operated rats. Importantly, all three antagonists produced a marked decrease in scratching behavior. Thus, for instance, the lima bean trypsin inhibitor reduced the SSBs in BDL rats to 38 ± 7 (compared with 86 ± 10 with saline (n = 7) (data not shown)). Similarly, as illustrated in Fig. 1C, the serine protease inhibitor gabexate mesylate reduced SSBs to 16 ± 1 compared with 89 ± 2 with saline injection (n = 7), and the PAR2 antagonist FSLLRY-NH2 diminished SSBs to 38 ± 11 versus 85 ± 4 with saline treatment (n = 7). Because all three inhibitors are predicted to prevent activation of PAR2, the scratching behavior in BDL rats may be mediated, at least in part, by PAR2 activation.
Chronic BDL Rats Display Increased Thermal Hyperalgesia
Primary afferents responding to serotonin and PAR2 activation are polymodal C-fibers that also respond to noxious stimuli like mustard oil and capsaicin (34). Moreover, PAR2 has been implicated in both pruritus (3, 28, 34–36) and hyperalgesia (37, 38). We therefore asked whether hepatic pruritus in Wistar BDL rats might be accompanied by altered nociceptive thermal responses. Indeed, the PWL from a hot source was significantly shorter in BDL rats than in control animals. For instance, 3 weeks after surgery, the PWL in BDL rats was reduced to 9.0 ± 0.4 s (n = 21) compared with 15.6 ± 0.5 s in controls (n = 19). The thermal hypersensitivity in BDL rats remained constantly increased over the time of our analysis (Fig. 2A).
FIGURE 2.
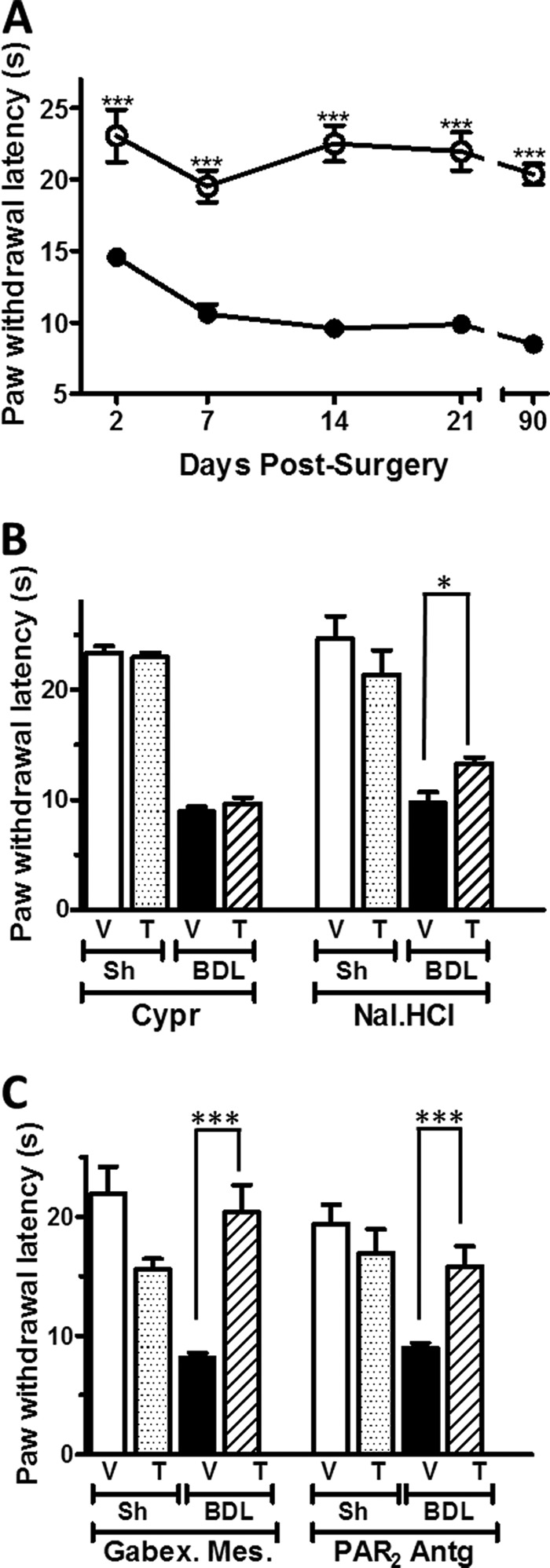
Effect of the blockade of histamine/serotonin-, opioid-, protease-, and PAR2-activated pathways on the increased thermal hyperalgesia in a rat model with BDL. A, time course of paw withdrawal latency from a heat source in BDL (●) and sham (Sh)-operated (○) control rats after surgery (n = 8–12). B and C, effect of the mixed His/5-HT antagonist cyproheptadine (Cypr) and the systemic opioid antagonist naloxone HCl (Nal·HCl) (B) and of the protease inhibitor gabexate mesylate (Gabex·Mes) and PAR2 antagonist (PAR2-Antg) on thermal sensitivity. V, vehicle; T, treatment. n = 6–9 animals. Each data point represents mean ± S.E. (error bars). *, p < 0.1; ***, p < 0.001, Mann-Whitney U-test).
We next evaluated the role of histamine/serotonin, endogenous opioids, and protease pathways on thermal hypersensitivity. Whereas the histaminergic/serotonergic antagonist cyproheptadine did not interfere with the withdrawal latency in Wistar chronic BDL rats, a single dose of Nal·HCl or of the protease inhibitors gabexate methylate or of PAR2-Antg significantly attenuated BDL thermal hyperalgesia (Fig. 2, B and C). Interestingly, administration of either gabexate mesylate or of PAR2-Antg reverted PWL values close to those of control rats.
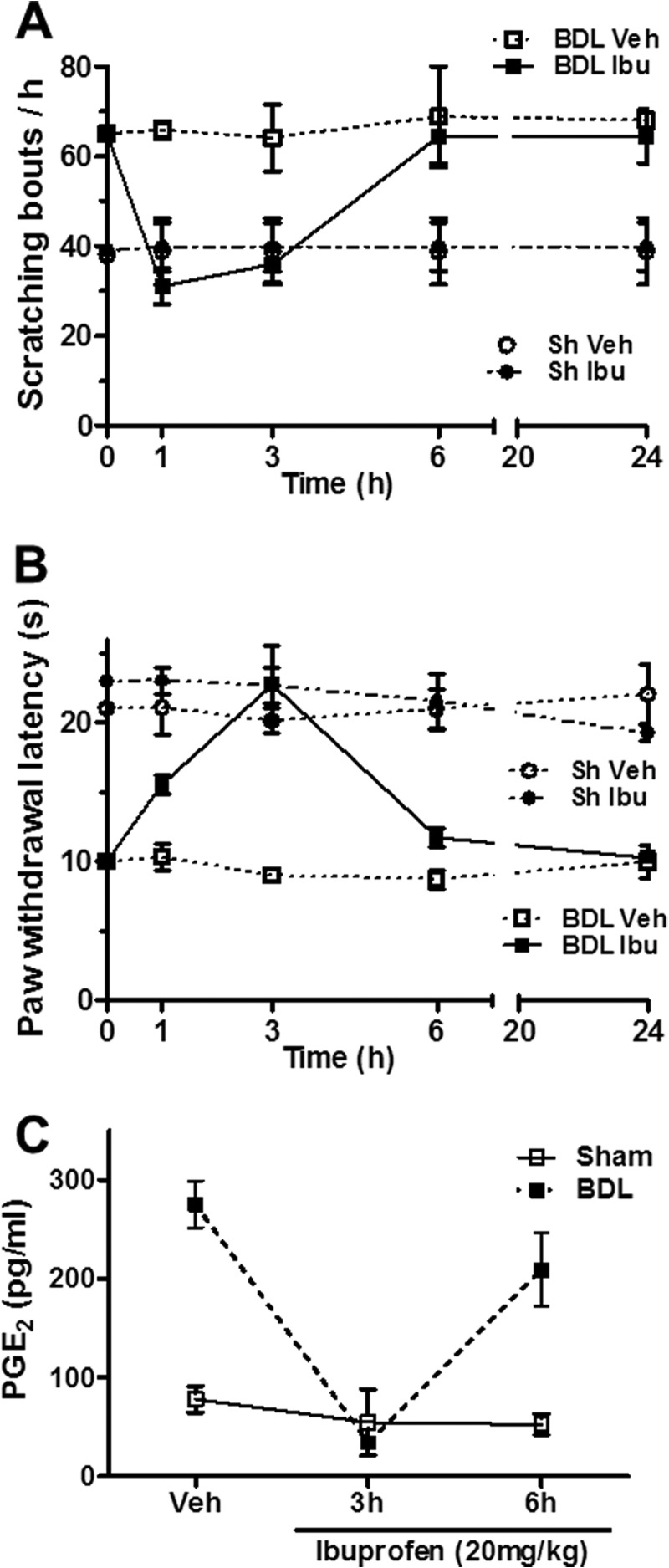
PAR2 activation triggers inflammatory responses through PLA2 activation and arachidonic acid release (39). We therefore used ibuprofen, a COX-1/COX-2 inhibitor, and the preferential COX-2 inhibitor meloxicam (data not shown) to analyze the role of eicosanoids in the itch response. Ibuprofen administration reduced scratching bouts by approximately 50% (Fig. 3A). Furthermore, as expected, heat hypersensitivity in BDL rats was reversed both by ibuprofen (Fig. 3B) and by the selective COX-2 antagonist meloxicam (data not shown). Importantly, the ibuprofen-induced change in serum levels of PGE2 correlated remarkably well with the observed behavioral index (Fig. 3, A and C). These experiments therefore suggest that prostanoids are involved in liver disease-associated pruritus and that thermal hyperalgesia in chronic BDL rats is mostly mediated by a PAR2 signaling pathway.
FIGURE 3.
Role of COX-1/COX-2 in spontaneous scratching and thermal hyperalgesia in BDL rats. A and B, time course of scratching bouts/h (A) and of the paw withdrawal latency from a heat source of BDL and sham-operated rats (B). Vehicle controls and animals treated with a single dose (20 mg/kg, intraperitoneally) of the COX-1/COX-2 inhibitor ibuprofen (Ibu) are compared. C, serum PGE2 levels after the single ibuprofen administration in both BDL- and sham-operated rats. Each point represents the mean ± S.E. (error bars) of n = 6.
TRPV1 Contributes to Itch and Thermal Hyperalgesia in BDL Rats
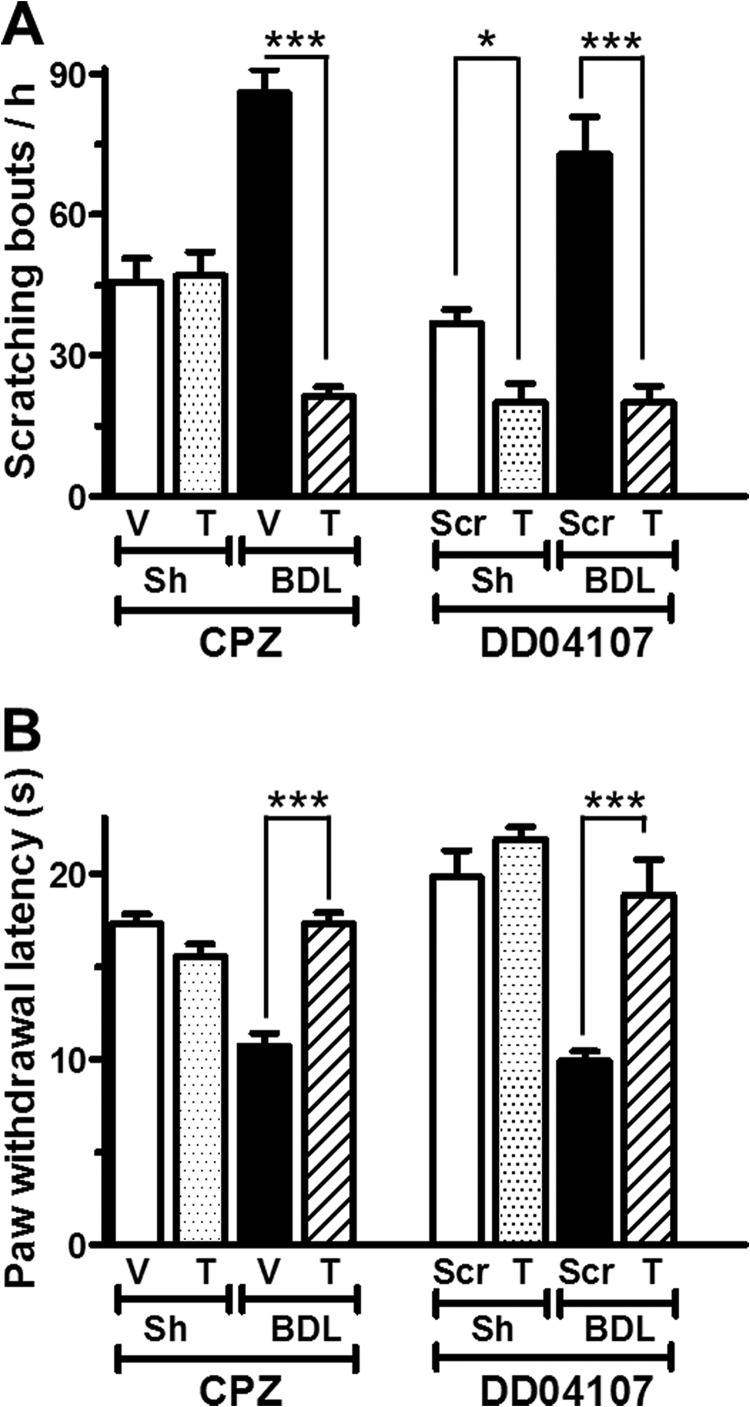
Because activated PAR2 may modulate neurogenic inflammation and increase nociceptor excitability by potentiating TRPV1 activity (40), we determined the contribution of TRPV1 sensitization to pruritus. Systemic administration of the TRPV1 antagonist capsazepine to BDL- and sham-operated rats produced a significant attenuation in SSBs in BDL, but not in control rats (BDL: SSB = 21 ± 2 compared with 86 ± 5 in vehicle, n = 6; sham-operated: SSB = 47 ± 5 compared with 46 ± 5 in vehicle, n = 6) (Fig. 4A). Likewise, capsazepine blocked thermal hyperalgesia in chronic BDL rats (PWL = 17.3 ± 0.6 s compared with 10.7 ± 0.7 s in vehicle-treated BDL rats, n = 9) (Fig. 4B).
FIGURE 4.
Enhanced pruritus and thermal hyperalgesia in BDL rats is attenuated by TRPV1 inhibition and by blockade of TRPV1 membrane translocation. A and B, effect on spontaneous scratching bouts/h (A) and on paw withdrawal latency from a heat source (B) of the selective TRPV1-inhibitor capsazepin (CPZ) or the exocytosis blocker DD04107. V, vehicle; Scr, scrambled sequence of peptide DD04107; T, treatment. n = 9. Each data point represents mean ± S.E. (error bars). *, p < 0.1; ***, p < 0.001, Mann-Whitney U-test).
Enhanced TRPV1 activity may arise either from increased intrinsic channel activity and/or from enhanced plasma membrane expression (19, 40). To assess whether regulated exocytotic plasma membrane insertion of TRPV1 contributed to the inflammatory sensitization of TRPV1 in BDL rats, we evaluated the antinociceptive and antipruritic activities of DD04107, a lipopeptide known to interfere with vesicle fusion in nociceptors (40). It is based on SNAP25 peptide sequence and is thought to compete with SNAP25 for its incorporation into a SNARE complex that is needed for exocytosis in neurons. As illustrated in Fig. 4A, systemic administration of DD04107 significantly decreased the scratching bouts in BDL rats compared with the administration of a lipopeptide containing the scrambled DD04107 sequence (Scr) (SSBs = 20 ± 4 versus 73 ± 8, n = 6, respectively). Similarly, DD04107 selectively attenuated the thermal hypersensitivity in chronic BDL rats (Fig. 4B). Hence, recruitment of TRPV1 to the membrane of nociceptor terminals likely contributes importantly to enhanced scratching behavior in BDL rats.
Acutely Dissociated DRG Neurons from BDL Rats Display PAR2 Up-regulation and Increased TRPV1 Activity
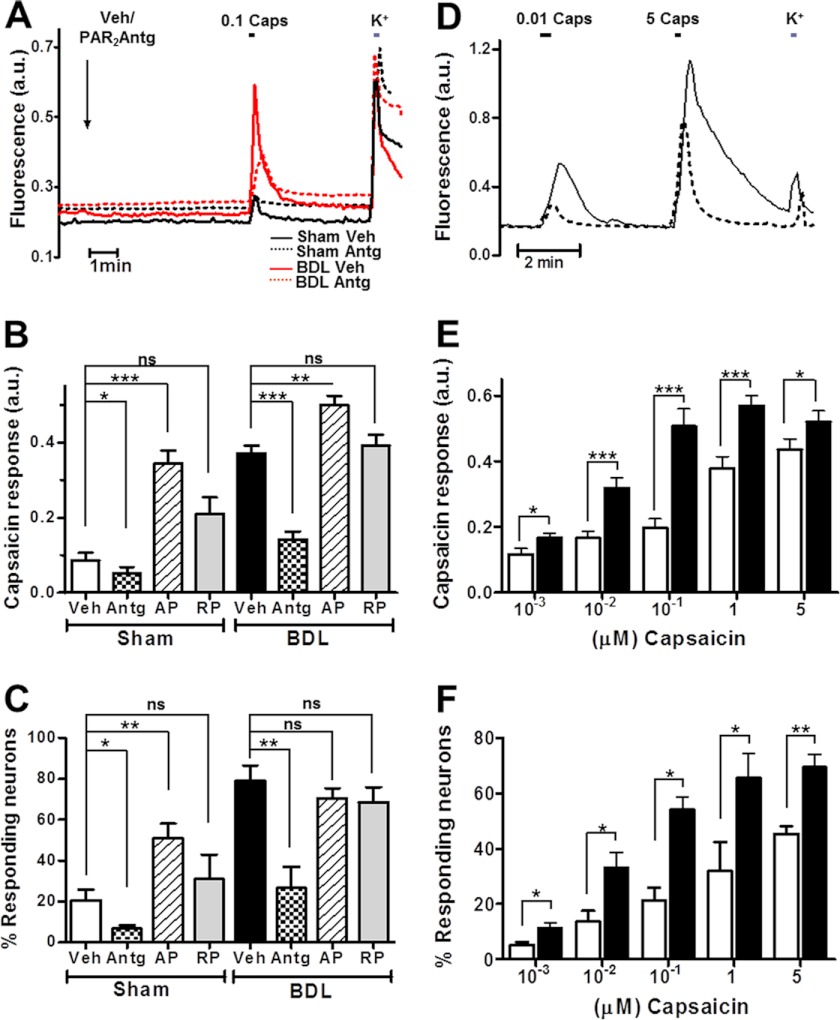
Because pharmacological inhibition of PAR2 and TRPV1 significantly reduced scratching and thermal hyperalgesia in BDL rats, we asked whether altered PAR2 and/or TRPV1 function might be detected in acutely dissociated DRG neurons from BDL rats. To prevent serial dilution of proinflammatory substances, cells were only cultured for approximately 12 h, with no change in culture medium. Paired nociceptor cultures from BDL- and sham-operated rats were tested by exposing them for 5 min to either vehicle (Veh), 100 μm PAR2 agonist (AP), or antagonist (Antg), always followed by a test pulse of the TRPV1 agonist capsaicin (100 nm). Because the TRPV1 channel is highly permeable to Ca2+, we used the ratiometric Ca2+-sensitive indicator dye Fura-2 to assess capsaicin responses. As shown in Fig. 5, under control (Veh) conditions, BDL DRG neurons displayed both significantly increased magnitudes of individual responses (Fig. 5, A and B) and a larger percentage of responding neurons (Fig. 5C). Interestingly, previous activation of PAR2 with PAR2-AP produced a >2-fold increase in the percentage of capsaicin-responsive neurons in sham cultures, reaching a level that was close to that observed with vehicle in BDL neurons (Fig. 5C). Hence, even when inflammatory agents might be partially or completely diluted, BDL DRG nociceptors appeared submaximally activated, suggesting a long lasting modification of TRPV1 activity. As negative control, PAR2-RP had no effect. Moreover, PAR2 receptor silencing by transfection of paired BDL and control neurons with a specific PAR2 small interfering RNA (siRNA), previously shown to strongly reduce PAR2 protein levels in nociceptor cultures, significantly produced concomitant reduced capsaicin responses in both types of cultures (supplemental Fig. 1, A–D). The augmented PAR2 activity in BDL DRGs was associated with an increase in PAR2 receptor immunoreactivity in DRGs (Fig. 6A) and an ∼2-fold enhanced recruitment of PAR2 receptors to the plasma membrane as revealed by immunodetection of surface biotinylated fractions (Fig. 6B). Altogether, these results indicate that nociceptors of BDL rats are sensitized by increased PAR2 signaling.
FIGURE 5.
PAR2 sensitizes TRPV1 in BDL nociceptors. A, representative traces of changes in Fura-2 fluorescence indicative of Ca2+ influx upon a 0.1 μm capsaicin (0.1 Caps) pulse, expressed in arbitrary units (a.u.), in sham (black) or BDL (red) nociceptors exposed to PAR2-Antg (dashed line) or vehicle (solid trace). All neurons show a typical Ca2+ rise in response to raising extracellular [K+] to 40 mm. Neurons from sham-operated controls produced smaller capsaicin responses than those from BDL rats. PAR2 inhibition (PAR2-Antg) suppressed capsaicin responses in BDL neurons more strongly than in control neurons. B, mean Fura-2 fluorescence changes induced by 0.1 capsaicin obtained as in A following different PAR2 treatments in BDL and sham nociceptors. Veh, vehicle; Antg, PAR2-Antg; AP, PAR2-activating peptide; and RP, a retroactive peptide as a negative control. C, percentage of K+-responsive neurons that also react to capsaicin at 0.1 μm. n (paired cultures) = 3. D, representative traces of capsaicin-induced Ca2+ responses as reported by Fura-2 fluorescence at different agonist concentrations in BDL neurons (solid trace) compared with sham neurons (dashed line). E, mean peak Fura-2 fluorescence magnitude obtained as in D with different capsaicin concentrations in BDL and sham nociceptor cultures (black and white columns, respectively). F, proportion of capsaicin-responsive sham and BDL neurons in culture at different capsaicin concentrations. Each data point represents mean ± S.E. (error bars); n.s., nonsignificance; *, p < 0.1; **, p < 0.01; ***, p < 0.001, Mann-Whitney U-test).
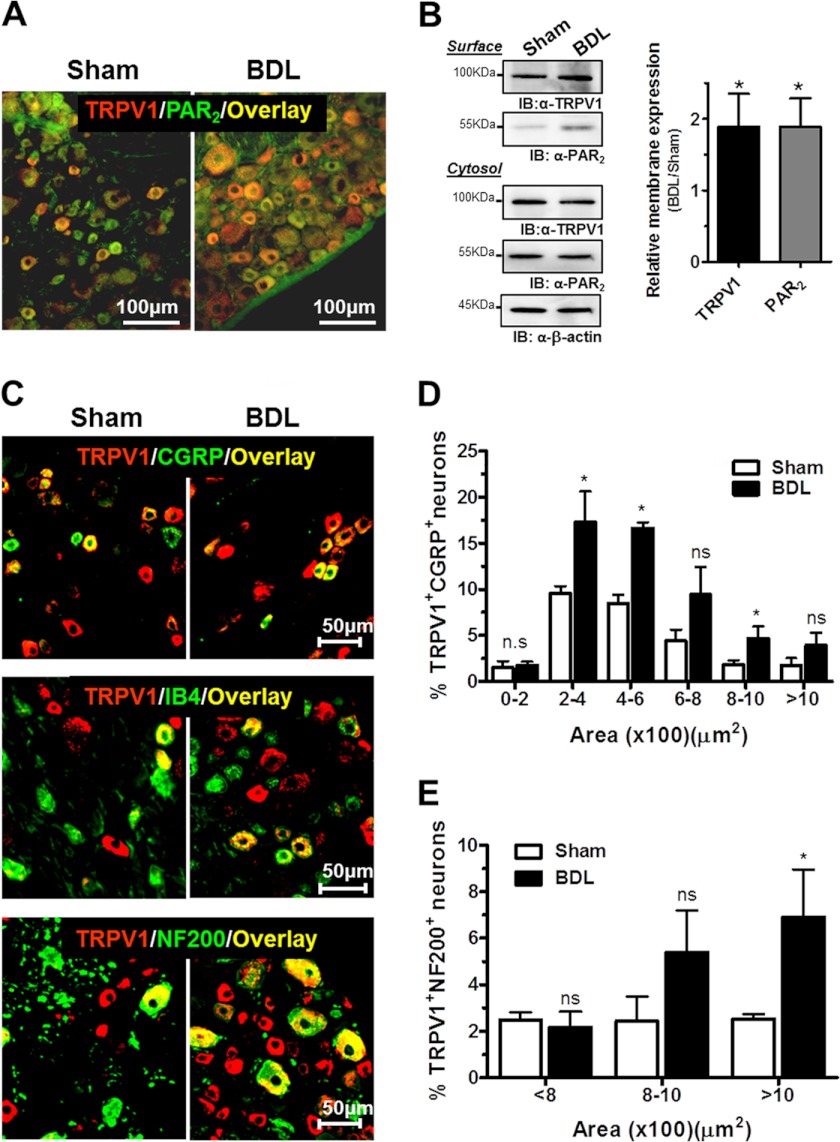
FIGURE 6.
Changes in PAR2 and TRPV1 protein expression induced in BDL rats. A, representative merged images from immunohistochemical detection of TRPV1 (red), PAR2 (green), and overlay (yellow) in sham (left) and BDL DRG sections (right). The number of cells expressing both TRPV1 and PAR2 was robustly increased in BDL rats. B, representative Western blots of TRPV1 and PAR2 protein in plasma membrane and cytosolic fractions from sham and BDL DRG cultures obtained by cell surface biotinylation. Optical band densities were analyzed and normalized to cytosolic β-actin (right). Averages from four independent experiments are shown. C, merged images showing red-labeled TRPV1 together with CGRP (top), IB4 (middle), or NF200 (bottom) labeled in green. Co-localization results are in yellow. Scale bars, 50 μm. D and E, area frequency distribution of TRPV1+CGRP+ and TRPV1+NF200+ neurons, respectively. N (pairs) = 3. Each data point represents mean ± S.E. (error bars; n.s., nonsignificance; *, p < 0.1, Mann-Whitney U-test).
To better characterize TRPV1 potentiation in BDL rats, we evaluated the average magnitude of Ca2+ influx and the percentage of responding nociceptors from paired BDL and sham DRG cultures at different agonist concentrations (from 1 nm to 5 μm capsaicin). Over the entire concentration range, TRPV1-mediated Ca2+ influx into BDL neurons was increased approximately 2-fold compared with controls (Fig. 5, D and E). The half-maximal effective capsaicin concentration (EC50) for evoking intracellular [Ca2+] increases was 12 ± 2 nm in BDL neurons versus 360 ± 30 nm for sham cultures. A transcription-independent, ≈2-fold increase in TRPV1 protein levels was observed in DRG homogenates from BDL rats compared with controls (supplemental Fig. 2). Importantly, similar to enhanced PAR2 surface expression in BDL nociceptors, a significant translocation of TRPV1 receptor channels to the plasma membrane was found by surface protein biotinylation (Fig. 6B). These data were further confirmed by immunocytochemistry of nonpermeabilized live BDL and sham neurons using a TRPV1 antibody that recognizes an extracellular epitope (supplemental Fig. 3). The percentage of capsaicin-responsive neurons was also notably higher in BDL DRG cultures (Fig. 5F). Thus, whereas ≈40% of sham neurons were activated by 1 μm capsaicin, this percentage increased up to ≈80% in BDL cultures. These results were bolstered by immunohistochemistry that revealed a robust increase in the number of TRPV1-expressing neurons in BDL DRG sections (Fig. 6A).
We next examined whether the increased percentage of capsaicin-responsive neurons correlated with a shift of TRPV1 expression to a specific DRG subpopulation. DRG cryosections were co-labeled for TRPV1 and CGRP or IB4, two markers commonly used to identify peptidergic and nonpeptidergic C-fibers, respectively, and for neurofilament NF200 to identify medium and large size myelinated A-fibers. Fig. 6C displays examples of DRG sections from control and BDL rats (left and right panels, respectively) labeled for TRPV1 (red) and different DRG markers (green), and double-labeled neurons (yellow). Remarkably, whereas the percentage of cells immunoreactive to TRPV1 and IB4 remained unaltered, the proportion of peptidergic neurons co-expressing TRPV1 and CGRP (TRPV1+CGRP+) was significantly increased in BDL rats (36.7 ± 4.5% compared with 25.4 ± 3.9% in sham control, *p < 0.1).
Agreeing with previous work (37), area histograms showed that TRPV1 was expressed mainly in small diameter neurons. TRPV1+CGRP+ neurons clustered in the smaller group (areas <600 μm2). Notably, the number of TRPV1+CGRP+ was significantly increased in BDL rats (Fig. 6, C and D). A second major difference was noticed within medium sized neurons (800–1200 μm2) (Fig. 6E), which probably correspond to somata of A-fibers. The percentage of TRPV1+NF200+ neurons was increased approximately 3-fold in BDL rats compared with sham-operated rats (20 ± 5 and 6 ± 2%, respectively). Taken together, these findings indicate that both an up-regulation of PAR2 and TRPV1 protein expression in nociceptors as well as a potentiation of their activity underlie the scratching behavior observed in this model of chronic liver disease.
DISCUSSION
In this study, we have established an animal model for pruritus associated with cholestasis. Our work suggests that histamine/serotonin pathways play only a minor role in hepatic pruritogenesis. By contrast, stimulation of PAR2 receptors, which might be activated by mast cell tryptase released in inflammatory processes, leads to the potentiation of the capsaicin TRPV1 receptor channel activity. This activation, which also leads to thermal hyperalgesia, involves several mechanisms, including increased cellular expression, plasma membrane residence, and intrinsic channel activity.
We used a model of cholestatic liver fibrosis based on bile duct ligation in Wistar rats, a procedure that eventually leads to cirrhosis and to hepatic encephalopathy (16, 25, 41). We found that BDL rats display increased spontaneous scratching indicative of itch, a common dermatologic symptom in chronic liver disease. Increased scratching emerged shortly after surgery and lasted for up to 3 months, the latest time point investigated. Unlike previous models of acute cholestasis by bile duct resection in rodents that display decreased nociception (5, 42), our model resulted in increased thermal hyperalgesia. This difference cannot be accounted for by differences in the duration of cholestasis, as we observed thermal hyperalgesia already 48 h after surgery. However, differences between rat strains in nociception thresholds (43, 44) could well account for these differences. Importantly, our observation of thermal hyperalgesia in BDL rats perfectly fits to the up-regulation and sensitization of the heat-sensitive TRPV1 channel which we have demonstrated in this work.
Patients with cholestasis and itch display signs of peripheral neuroinflammation as reflected by increased numbers of dermal mast cells (45). Furthermore, increased mast cell degranulation has been reported in animals with chronic biliary obstruction (46). However, despite intensive investigation, the mediators and neuronal mechanisms involved in cholestatic pruritus remain still unresolved. Recently, autaxin and LPA levels have been correlated with itch intensity in cholestatic patients (47). LPA induces histamine release from mast cells (48). However, histamine seems to play only a minor role in cholestatic pruritus both in patients (49) and in our rat model.
On the other hand, autaxin is highly expressed in submucosal mast cells also containing chimase and tryptase (50). Tryptase and other proteases, released during mast cell degranulation, not only serve to cleave many substrates involved in inflammation and wound repair, but also potently activate protease-activated PAR2 receptors (51). Indeed, we found a significant activation of PAR2 receptors, enhanced downstream PGE2 formation, and potentiation of TRPV1 channel activity. DRG nociceptors in BDL rats displayed increased overall expression of PAR2 and TRPV1 proteins, an increased number of PAR2- and TRPV1-expressing peptidergic neurons, and a shift of TRPV1 receptor expression to medium sized DRG neurons. This changed pattern of TRPV1 expression has been found in animal models of hyperalgesia associated with acute and chronic inflammation (52, 53), supporting our conclusion that inflammation plays an important role in cholestatic pruritus.
Protease- and PAR2-mediated signaling pathways have been implicated in neurogenic inflammation, in certain types of generalized pruritus (28, 54), and also in hyperalgesia (37, 38). Notably, patients refer cholestatic pruritus as an irritation that cannot be relieved by scratching (4), a sensation that could be well accounted for by peripheral neuronal sensitization. An activation of the Mas-related G protein-coupled receptor MgrprC11, rather than of PAR2, was recently proposed as underlying the scratching behavior elicited by PAR2-AP agonist or trypsin administration (24). On the other hand, mucunain, the active ingredient of cowhage, is known to induce itch and a burning sensation through activation of PAR2 and PAR4 (55). Moreover, other reports stressed a pivotal role of PAR2 in skin conditions such as in dry-skin itch (35) and in atopic dermatitis (56, 57). The importance of PAR2 activation in our model was confirmed not only pharmacologically by antagonist administration, but also by specific silencing of the PAR2 receptor in primary BDL nociceptor cultures.
PAR2 activation has been reported to modulate the expression and ion channel activity of TRPV1 (37, 38). We therefore evaluated the magnitude of TRPV1 activity and its modulation by PAR2 in nociceptor cultures. Under basal conditions, both the percentage of capsaicin-responding neurons and the individual cellular response to the TRPV1 agonist capsaicin were ≈4-fold higher in BDL nociceptors. TRPV1 sensitization by PAR2 activation appeared to reach near maximal levels in BDL rats, because the percentage of TRPV1+ neurons derived from those rats was not further increased when challenged with PAR2 agonist. These results suggest an up-regulation of PAR2 signaling in BDL nociceptors which was corroborated by Western blotting and immunohistochemistry.
Although TRPV1 protein levels in BDL nociceptors were only increased 2-fold, the TRPV1 channel activity elicited by the agonist capsaicin was increased approximately 30-fold. In addition to increasing transcription and/or translation, proinflammatory stimuli can sensitize TRPV1 by several additional mechanisms that either lead to a fast, acute decrease of the activation threshold by receptor phosphorylation or to an increased residence of the protein at the cell surface. The functional coupling of PAR2 to TRPV1 through PKCϵ- and PKA-mediated phosphorylation of TRPV1 has been well documented (38). The effect of an in vivo application of an inhibitor previously demonstrated to block regulated exocytosis of TRPV1 in DRG nociceptor cultures (19, 40) now suggested an important contribution of increased plasma membrane insertion of TRPV1 in the genesis of both pruritus and thermal hyperalgesia.
In summary, our work indicates that activation of PAR2 signaling, presumably by inflammatory release of proteases from cutaneous mast cells found in close proximity to nerve terminals, sensitizes nociceptors by augmenting the expression and activity of neuronal TRPV1 channels. The presence of additional proinflammatory mediators like autaxin, LPA, IL-6, PGE2, and TNFα may further enhance nociceptor sensitization and/or mast cell degranulation, thus contributing to the persistence of a pruritogenic state. PAR2 receptors and TRPV1 channels may be interesting therapeutic targets for developing compounds that attenuate chronic systemic pruritus arising from liver disease or possibly from other systemic disorders.
Acknowledgments
We thank Drs. F. J. Rodríguez-Jiménez and V. Moreno-Manzano (Centro de Investigación Principe Felipe) for help with quantitative RT-PCR experiments.
This work was supported by the Ministry of Science and Innovation Grants BFU2009-08346 (to A. F.-M.), SAF2008-00062 (to V. F.), and SAF2007-63193 (to R. P.-C.); Consolider-Ingenio 2010 Program Grant CSD2008-00005 (to A. F.-M., V. F., and R. P.-C.); and la Generalitat Valenciana Grants Prometeo-2010-046 (to A. F.-M.), AP-092/09, Prometeo-2009-027, and ACOMP2010-220 (to V. F.).

This article contains supplemental Figs. 1–3 and Experimental Procedures.
- 5-HT
- serotonin
- AP
- alkaline phosphatase
- BDL
- bile duct ligation
- CGRP
- calcitonin gene-related peptide
- DRG
- dorsal root ganglion
- IB4
- isolectin B4
- NF200
- neurofilament 200 kDa
- LPA
- lysophosphatidic acid
- PAR2
- protease-activated receptor 2
- PGE2
- prostaglandin E2
- PWL
- paw withdrawal latency
- SSB
- spontaneous scratching bout
- TRPV1
- transient receptor potential vanilloid 1.
REFERENCES
- 1. Weisshaar E., Kucenic M. J., Fleischer A. B., Jr. (2003) Pruritus: a review. Acta Derm. Venereol. Suppl. 213, 5–32 [PubMed] [Google Scholar]
- 2. Imamachi N., Park G. H., Lee H., Anderson D. J., Simon M. I., Basbaum A. I., Han S. K. (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. U.S.A. 106, 11330–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steinhoff M., Neisius U., Ikoma A., Fartasch M., Heyer G., Skov P. S., Luger T. A., Schmelz M. (2003) Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci. 23, 6176–6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergasa N. V., Mehlman J. K., Jones E. A. (2000) Pruritus and fatigue in primary biliary cirrhosis. Baillieres Best Pract. Res. Clin. Gastroenterol. 14, 643–655 [DOI] [PubMed] [Google Scholar]
- 5. Nelson L., Vergnolle N., D'Mello C., Chapman K., Le T., Swain M. G. (2006) Endogenous opioid-mediated antinociception in cholestatic mice is peripherally, not centrally, mediated. J. Hepatol. 44, 1141–1149 [DOI] [PubMed] [Google Scholar]
- 6. Weisshaar E., Ziethen B., Röhl F. W., Gollnick H. (1999) The antipruritic effect of a 5-HT3 receptor antagonist (tropisetron) is dependent on mast cell depletion: an experimental study. Exp. Dermatol. 8, 254–260 [DOI] [PubMed] [Google Scholar]
- 7. Glasova H., Beuers U. (2002) Extrahepatic manifestations of cholestasis. J. Gastroenterol. Hepatol. 17, 938–948 [DOI] [PubMed] [Google Scholar]
- 8. Kremer A. E., Martens J. J., Kulik W., Ruëff F., Kuiper E. M., van Buuren H. R., van Erpecum K. J., Kondrackiene J., Prieto J., Rust C., Geenes V. L., Williamson C., Moolenaar W. H., Beuers U., Oude Elferink R. P. (2010) Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 139, 1008–1018 [DOI] [PubMed] [Google Scholar]
- 9. Kremer A. E., Martens J. J., Kulik W., Williamson C., Moolenaar W. H., Kondrackiene J., Beuers U., Elferink R. P. J. O. (2009) Identification of lysophosphatidic acid as neural activator in blood of pruritic cholestatic patients. Acta Derm-Venereol. 89, 702 [Google Scholar]
- 10. Kremer A. E., Martens J. J., Kulik W., Williamson C., Moolenaar W. H., Kondrackiene J., Beuers U., Elferink R. P. J. O. (2010) Autotaxin but not bile salts correlate with itch intensity in cholestasis. J. Hepatol. 52, S1 [Google Scholar]
- 11. Kremer A. E., van Dijk R., Leckie P., Schaap F. G., Kuiper E. M., Mettang T., Reiners K. S., Raap U., van Buuren H. R., van Erpecum K. J., Davies N. A., Rust C., Engert A., Jalan R., Oude Elferink R. P., Beuers U. (2012) Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 56, 1391–1400 [DOI] [PubMed] [Google Scholar]
- 12. van Meeteren L. A., Moolenaar W. H. (2007) Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res. 46, 145–160 [DOI] [PubMed] [Google Scholar]
- 13. Nochi H., Tomura H., Tobo M., Tanaka N., Sato K., Shinozaki T., Kobayashi T., Takagishi K., Ohta H., Okajima F., Tamoto K. (2008) Stimulatory role of lysophosphatidic acid in cyclooxygenase-2 induction by synovial fluid of patients with rheumatoid arthritis in fibroblast-like synovial cells. J. Immunol. 181, 5111–5119 [DOI] [PubMed] [Google Scholar]
- 14. Takaoka A., Arai I., Sugimoto M., Honma Y., Futaki N., Nakamura A., Nakaike S. (2006) Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp. Dermatol. 15, 161–167 [DOI] [PubMed] [Google Scholar]
- 15. Barak V., Selmi C., Schlesinger M., Blank M., Agmon-Levin N., Kalickman I., Gershwin M. E., Shoenfeld Y. (2009) Serum inflammatory cytokines, complement components, and soluble interleukin 2 receptor in primary biliary cirrhosis. J. Autoimmun. 33, 178–182 [DOI] [PubMed] [Google Scholar]
- 16. Jover R., Rodrigo R., Felipo V., Insausti R., Sáez-Valero J., García-Ayllón M. S., Suárez I., Candela A., Compañ A., Esteban A., Cauli O., Ausó E., Rodríguez E., Gutiérrez A., Girona E., Erceg S., Berbel P., Pérez-Mateo M. (2006) Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology 43, 1257–1266 [DOI] [PubMed] [Google Scholar]
- 17. García-Martinez C., Humet M., Planells-Cases R., Gomis A., Caprini M., Viana F., De La Pena E., Sanchez-Baeza F., Carbonell T., De Felipe C., Pérez-Paya E., Belmonte C., Messeguer A., Ferrer-Montiel A. (2002) Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc. Natl. Acad. Sci. U.S.A. 99, 2374–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caterina M. J., Julius D. (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517 [DOI] [PubMed] [Google Scholar]
- 19. Planells-Cases R., Garcìa-Sanz N., Morenilla-Palao C., Ferrer-Montiel A. (2005) Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 451, 151–159 [DOI] [PubMed] [Google Scholar]
- 20. Shim W. S., Tak M. H., Lee M. H., Kim M., Kim M., Koo J. Y., Lee C. H., Kim M., Oh U. (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 27, 2331–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim S. J., Park G. H., Kim D., Lee J., Min H., Wall E., Lee C. J., Simon M. I., Lee S. J., Han S. K. (2011) Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. U.S.A. 108, 3371–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bíró T., Tóth B. I., Marincsák R., Dobrosi N., Géczy T., Paus R. (2007) TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta 1772, 1004–1021 [DOI] [PubMed] [Google Scholar]
- 23. Ross S. E. (2011) Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr. Opin. Neurobiol. 21, 880–887 [DOI] [PubMed] [Google Scholar]
- 24. Liu Q., Weng H. J., Patel K. N., Tang Z., Bai H., Steinhoff M., Dong X. (2011) The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 4, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montoliu C., Piedrafita B., Serra M. A., del Olmo J. A., Urios A., Rodrigo J. M., Felipo V. (2009) IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 43, 272–279 [DOI] [PubMed] [Google Scholar]
- 26. Seeliger S., Derian C. K., Vergnolle N., Bunnett N. W., Nawroth R., Schmelz M., Von Der Weid P. Y., Buddenkotte J., Sunderkötter C., Metze D., Andrade-Gordon P., Harms E., Vestweber D., Luger T. A., Steinhoff M. (2003) Proinflammatory role of proteinase-activated receptor 2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 17, 1871–1885 [DOI] [PubMed] [Google Scholar]
- 27. Namer B., Carr R., Johanek L. M., Schmelz M., Handwerker H. O., Ringkamp M. (2008) Separate peripheral pathways for pruritus in man. J. Neurophysiol. 100, 2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa R., Marotta D. M., Manjavachi M. N., Fernandes E. S., Lima-Garcia J. F., Paszcuk A. F., Quintão N. L., Juliano L., Brain S. D., Calixto J. B. (2008) Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br. J. Pharmacol. 154, 1094–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perkins M. N., Campbell E. A. (1992) Capsazepine reversal of the antinociceptive action of capsaicin in vivo. Br J. Pharmacol. 107, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hargreaves K., Dubner R., Brown F., Flores C., Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 31. Lainez S., Valente P., Ontoria-Oviedo I., Estévez-Herrera J., Camprubí-Robles M., Ferrer-Montiel A., Planells-Cases R. (2010) GABAA receptor-associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 24, 1958–1970 [DOI] [PubMed] [Google Scholar]
- 32. Amadesi S., Cottrell G. S., Divino L., Chapman K., Grady E. F., Bautista F., Karanjia R., Barajas-Lopez C., Vanner S., Vergnolle N., Bunnett N. W. (2006) Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cϵ- and A-dependent mechanisms in rats and mice. J. Physiol. 575, 555–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanz-Salvador L., Andrés-Borderia A., Ferrer-Montiel A., Planells-Cases R. (2012) Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 287, 19462–19471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akiyama T., Merrill A. W., Carstens M. I., Carstens E. (2009) Activation of superficial dorsal horn neurons in the mouse by a PAR2 agonist and 5-HT: potential role in itch. J. Neurosci. 29, 6691–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akiyama T., Merrill A. W., Zanotto K., Carstens M. I., Carstens E. (2009) Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J. Pharmacol. Exp. Ther. 329, 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsujii K., Andoh T., Ui H., Lee J. B., Kuraishi Y. (2009) Involvement of tryptase and proteinase-activated receptor 2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J. Pharmacol. Sci. 109, 388–395 [DOI] [PubMed] [Google Scholar]
- 37. Amadesi S., Nie J., Vergnolle N., Cottrell G. S., Grady E. F., Trevisani M., Manni C., Geppetti P., McRoberts J. A., Ennes H., Davis J. B., Mayer E. A., Bunnett N. W. (2004) Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 24, 4300–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai Y., Moriyama T., Higashi T., Togashi K., Kobayashi K., Yamanaka H., Tominaga M., Noguchi K. (2004) Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J. Neurosci. 24, 4293–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Merwe J. Q., Ohland C. L., Hirota C. L., MacNaughton W. K. (2009) Prostaglandin E2 derived from cyclooxygenases 1 and 2 mediates intestinal epithelial ion transport stimulated by the activation of protease-activated receptor 2. J. Pharmacol. Exp. Ther. 329, 747–752 [DOI] [PubMed] [Google Scholar]
- 40. Camprubí-Robles M., Planells-Cases R., Ferrer-Montiel A. (2009) Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 23, 3722–3733 [DOI] [PubMed] [Google Scholar]
- 41. Rodrigo R., Cauli O., Gomez-Pinedo U., Agusti A., Hernandez-Rabaza V., Garcia-Verdugo J. M., Felipo V. (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139, 675–684 [DOI] [PubMed] [Google Scholar]
- 42. Hasanein P., Parviz M., Keshavarz M., Javanmardi K., Allahtavakoli M., Ghaseminejad M. (2007) Modulation of cholestasis-induced antinociception in rats by two NMDA receptor antagonists: MK-801 and magnesium sulfate. Eur. J. Pharmacol. 554, 123–127 [DOI] [PubMed] [Google Scholar]
- 43. Miura T., Okazaki R., Yoshida H., Namba H., Okai H., Kawamura M. (2005) Mechanisms of analgesic action of neurotropin on chronic pain in adjuvant-induced arthritic rat: roles of descending noradrenergic and serotonergic systems. J. Pharmacol. Sci. 97, 429–436 [DOI] [PubMed] [Google Scholar]
- 44. Banik R. K., Sato J., Yajima H., Mizumura K. (2001) Differences between the Lewis and Sprague-Dawley rats in chronic inflammation induced norepinephrine sensitivity of cutaneous C-fiber nociceptors. Neurosci. Lett. 299, 21–24 [DOI] [PubMed] [Google Scholar]
- 45. Twycross R., Greaves M. W., Handwerker H., Jones E. A., Libretto S. E., Szepietowski J. C., Zylicz Z. (2003) Itch: scratching more than the surface. Qjm 96, 7–26 [DOI] [PubMed] [Google Scholar]
- 46. Clements W. D., O'Rourke D. M., Rowlands B. J., Ennis M. (1994) The role of mast cell activation in cholestatic pruritus. Agents Actions 41, C30–31 [DOI] [PubMed] [Google Scholar]
- 47. Kremer A. E., van Dijk R., Leckie P., Schaap F. G., Kuiper E. M., Mettang T., Reiners K. S., Raap U., van Buuren H. R., van Erpecum K. J., Davies N. A., Rust C., Engert A., Jalan R., Oude Elferink R. P., Beuers U. (2012) Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 56, 1391–1400 [DOI] [PubMed] [Google Scholar]
- 48. Hashimoto T., Ohata H., Momose K., Honda K. (2005) Lysophosphatidic acid induces histamine release from mast cells and skin fragments. Pharmacology 75, 13–20 [DOI] [PubMed] [Google Scholar]
- 49. Mela M., Mancuso A., Burroughs A. K. (2003) Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol. Ther. 17, 857–870 [DOI] [PubMed] [Google Scholar]
- 50. Mori K., Kitayama J., Aoki J., Kishi Y., Shida D., Yamashita H., Arai H., Nagawa H. (2007) Submucosal connective tissue-type mast cells contribute to the production of lysophosphatidic acid (LPA) in the gastrointestinal tract through the secretion of autotaxin (ATX)/lysophospholipase D (lysoPLD). Virchows Archiv. 451, 47–56 [DOI] [PubMed] [Google Scholar]
- 51. Steinhoff M., Buddenkotte J., Shpacovitch V., Rattenholl A., Moormann C., Vergnolle N., Luger T. A., Hollenberg M. D. (2005) Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocrine Rev. 26, 1–43 [DOI] [PubMed] [Google Scholar]
- 52. Amaya F., Oh-hashi K., Naruse Y., Iijima N., Ueda M., Shimosato G., Tominaga M., Tanaka Y., Tanaka M. (2003) Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 963, 190–196 [DOI] [PubMed] [Google Scholar]
- 53. Yu L., Yang F., Luo H., Liu F. Y., Han J. S., Xing G. G., Wan Y. (2008) The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund's adjuvant. Mol. Pain 4, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ui H., Andoh T., Lee J. B., Nojima H., Kuraishi Y. (2006) Potent pruritogenic action of tryptase mediated by PAR2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 530, 172–178 [DOI] [PubMed] [Google Scholar]
- 55. Reddy V. B., Iuga A. O., Shimada S. G., LaMotte R. H., Lerner E. A. (2008) Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J. Neurosci. 28, 4331–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yosipovitch G., Papoiu A. D. (2008) What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep. 8, 306–311 [DOI] [PubMed] [Google Scholar]
- 57. Briot A., Deraison C., Lacroix M., Bonnart C., Robin A., Besson C., Dubus P., Hovnanian A. (2009) Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 206, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]