Background: Stimulation of cells by bacterial lipoproteins involves formation of a ternary TLR1·TLR2·lipoprotein complex.
Results: Cell stimulation is enhanced by either serum LBP or sCD14, which act by catalytically delivering lipopeptides directly to TLR1-TLR2.
Conclusion: LBP and sCD14 have redundant roles in driving TLR1·TLR2·lipopeptide ternary complex formation.
Significance: Improved understanding of innate immune sensing of bacteria may lead to better therapeutics for treating inflammation.
Keywords: Innate Immunity, Lipid-binding Protein, Lipoprotein, Lipoprotein Receptor, Toll-like Receptors (TLR), CD14, LBP, Lipopeptide Recognition, Ternary Complex Formation
Abstract
Bacterial lipoproteins are the most potent microbial agonists for the Toll-like receptor 2 (TLR2) subfamily, and this pattern recognition event induces cellular activation, leading to host immune responses. Triacylated bacterial lipoproteins coordinately bind TLR1 and TLR2, resulting in a stable ternary complex that drives intracellular signaling. The sensitivity of TLR-expressing cells to lipoproteins is greatly enhanced by two lipid-binding serum proteins known as lipopolysaccharide-binding protein (LBP) and soluble CD14 (sCD14); however, the physical mechanism that underlies this increased sensitivity is not known. To address this, we measured the ability of LBP and sCD14 to drive ternary complex formation between soluble extracellular domains of TLR1 and TLR2 and a synthetic triacylated lipopeptide agonist. Importantly, addition of substoichiometric amounts of either LBP or sCD14 significantly enhanced formation of a TLR1·TLR2 lipopeptide ternary complex as measured by size exclusion chromatography. However, neither LBP nor sCD14 was physically associated with the final ternary complex. Similar results were obtained using outer surface protein A (OspA), a naturally occurring triacylated lipoprotein agonist from Borrelia burgdorferi. Activation studies revealed that either LBP or sCD14 sensitized TLR-expressing cells to nanogram levels of either the synthetic lipopeptide or OspA lipoprotein agonist. Together, our results show that either LBP or sCD14 can drive ternary complex formation and TLR activation by acting as mobile carriers of triacylated lipopeptides or lipoproteins.
Introduction
As central elements of the innate immune system, Toll-like receptors provide a first line of immune defense against infectious agents. Through direct sensing of bacterial, fungal, or viral components, TLRs2 activate intracellular signaling events that drive the cellular expression and release of immune mediators (1, 2). These activation events not only induce inflammatory processes but also initiate and orchestrate the long lasting protective responses of the adaptive immune system (3). Humans possess 10 TLR family members, numbered 1 through 10, subsets of which are expressed in leukocytes and the epithelial cells of mucosal surfaces (4, 5). TLRs 1, 2, 4, and 6 are expressed on the plasma membrane, sense microbial and fungal cell wall components, and stimulate the production of classic proinflammatory molecules. TLRs are type 1 transmembrane receptors comprising an extracellular leucine-rich repeat domain and an intracellular Toll-interleukin-1 receptor homology (TIR) signaling domain. TLRs signal via ligand-induced receptor dimerization, which results in the juxtaposition of two TIR domains that act as a scaffold for the recruitment of proximal signaling adaptor molecules (2, 6).
The potent proinflammatory activity of Gram-negative bacterial lipopolysaccharide (LPS; endotoxin) can be largely ascribed to activation of the cell surface TLR4 complex (7). MD-2, a small secreted protein associated with TLR4, is largely responsible for the direct binding of LPS, an event that results in TLR4 homodimerization and proinflammatory gene expression (8, 9). LPS is a highly amphipathic molecule that naturally exists in solution as large aggregates. LPS-binding protein (LBP) and CD14 are two proteins whose coordinate actions result in the disaggregation and delivery of LPS monomers to the TLR4·MD-2 complex. LBP is a 60-kDa glycoprotein and a member of the fatty acid-binding protein superfamily that is expressed in the liver and released into the bloodstream (10, 11). CD14 is a 55-kDa glycosylphosphatidylinositol membrane-anchored glycoprotein on myeloid cells (12–15) and exists as a soluble protein found in a variety of body fluids (16, 17). Numerous biophysical studies have revealed that CD14 delivers LPS monomers to MD-2 (7–9, 18–23). Because CD14 binds LPS aggregates poorly, the efficiency of TLR4-mediated cell activation is greatly enhanced by LBP, which quickly disaggregates LPS and then catalytically delivers LPS monomers to CD14 (11, 12). In the presence of both LBP and CD14, the sensitivity of TLR4-expressing cells to LPS is enhanced more than 100-fold (11, 24, 25).
TLR2 mediates inflammatory responses to a wide variety of lipidated microbial components, including bacterial lipoproteins, atypical lipopolysaccharides, and lipomannans (26–28). Among these microbial agonists, bacterial lipoproteins are by far the most potent (26, 29–31). Outer surface protein A (OspA) of the Lyme disease-causing bacterium Borrelia burgdorferi is a widely studied bacterial lipoprotein with potent TLR2 stimulatory activity (32–34). The immunogenic activity of OspA requires the N-terminal acyl chains of the lipoprotein (35, 36). (S)-[2,3-bis(palmitoyloxy)-(2-R,S)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH3HCl (Pam3CSK4) is a triacylated N-terminal analog of OspA and is a widely used synthetic lipopeptide agonist for TLR2 (37). TLR2-mediated cellular responses are the result of microbial agonist-induced TLR2 heterodimerization with either TLR1, TLR6, or TLR10 (38–40). The crystal structure of human TLR1 and TLR2 bound to Pam3CSK4 reveals that the lipopeptide coordinately binds to both receptors to form a stable TLR1·TLR2·Pam3CSK4 ternary signaling complex (41). In this coordinate binding, two acyl chains of Pam3CSK4 accommodate a hydrophobic pocket of TLR2, and the third acyl chain accommodates a narrow hydrophobic channel of TLR1 (41). These lipopeptide binding interactions drive TLR1 and TLR2 dimerization, enabling the two intracellular TIR domains to form a scaffold that subsequently recruits adaptor molecules necessary for signaling (42).
Similar to LPS, LBP and CD14 have been shown to sensitize cells to lipopeptides and lipoproteins (43, 44). Because LPS, lipopeptides, and lipoproteins are all amphipathic, it has generally been assumed that, similar to their interaction with LPS, LBP functions to disaggregate lipopeptide for delivery to CD14, which subsequently delivers monomeric lipopeptide agonist to the corresponding TLR. However, the mechanism that underlies LBP- and CD14-mediated lipoprotein sensitization has not been formally explored. In this study, we examined the role of LBP and CD14 in the physical generation of the ternary TLR1·TLR2·lipopeptide complex by performing biophysical measurements with functional soluble forms of the TLR1 and TLR2 extracellular domains. We found that either LBP or soluble CD14 (sCD14) was able to independently enhance ternary TLR1·TLR2·Pam3CSK4 complex formation even at substoichiometric concentrations and that neither protein is associated with the final ternary complex. We also found, in cell-based assays, that the sensitivity of cells to minute amounts of agonist was enhanced by the addition of either LBP or sCD14.
EXPERIMENTAL PROCEDURES
Reagents
The synthetic bacterial lipopeptide (S)-[2,3-bis(palmitoyloxy)-(2-R,S)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH·3HCl (Pam3CSK4) was purchased from Enzo Life Sciences (formerly Alexis Biochemicals, Plymouth Meeting, PA). The non-acylated synthetic peptide (S)-[2,3-bis(acetyloxy)-(2-R,S)-propyl]-(R)-cysteinyl-(S)-seryl-(S)-lysyl-(S)-lysyl-(S)-lysyl-(S)-lysine × 3 CF3COOH (Ac2CSK4) was purchased from EMC Microcollections (Tuebingen, Germany). The recombinant OspA purified from B. burgdorferi bacterial extract (Recombitek Lyme) was purchased from Merial Inc. (Athens, GA).
HRP-conjugated anti-FLAG (clone M2) monoclonal antibody was purchased from Sigma-Aldrich, and the HRP-conjugated anti-hemagglutinin (HA) monoclonal antibody was purchased from Miltenyi Biotec Inc. (Auburn, CA). Unconjugated polyclonal anti-human LBP and anti-human CD14 goat IgG antibodies were kind gifts from Dr. Peter Tobias (The Scripps Research Institute, La Jolla, CA). Anti-OspA rabbit IgG polyclonal antibody was purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA). The secondary antibodies HRP-conjugated rabbit anti-goat IgG and HRP-conjugated goat anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The unconjugated anti-human TLR1 mAb (clone GD2.F4, CD281) and anti-human TLR2 mAb (clone T2.5, CD282) were obtained from eBioscience (San Diego, CA).
Human LBP from Xoma Corp. (Berkeley, CA) was generously provided by Dr. Theresa L. Gioannini and Dr. Jerrold P. Weiss (University of Iowa, Iowa City, IA). Low endotoxin albumin from bovine serum was purchased from Sigma-Aldrich, and human serum albumin (25%) was obtained from Octopharma (Hoboken, NJ). Human soluble CD14 used in HEK 293F cell-based assays was purchased from Peprotech Inc. (Rocky Hill, NJ).
Construction and Expression of Soluble TLR-Fc Fusion Proteins
Soluble extracellular domains of FLAG-tagged TLR2, HA-tagged TLR1, and HA-tagged TLR1P315L were produced using the hybrid leucine-rich repeat technique described by Jin et al. (41). The soluble TLR-Fc fusion expression vectors were constructed by overlap extension PCR using primers and methods described previously (40). Briefly, coding regions for the extracellular domains of TLR1 or TLR2 (amino acids 22–476 and 17–508, respectively) were fused to the highly conserved leucine-rich repeat C-terminal capping module of a hagfish variable lymphocyte receptor (VLRB.61) by overlap extension PCR and subsequently cloned as BglII/NheI fragments into a modified pDisplay vector (kindly provided by Dr. David M. Kranz, Department of Biochemistry, University of Illinois at Urbana-Champaign). This vector contains the Fc domain of human IgG1 downstream of the NheI site and the sequence for either the FLAG or HA tag upstream of the BglII site. A thrombin cleavage site (LVPRGS) was added at the 3′-end of the TLRvlr hybrid to allow cleavage of the soluble TLR from the Fc fusion protein. Recombinant DNA plasmids were verified by DNA sequencing (University of Illinois at Urbana-Champaign Core Sequencing Facility).
Freestyle HEK 293F cells (Invitrogen) were adapted to grow in Freestyle serum-free expression medium (Invitrogen). Adherent cells were cultured at 37 °C in a humidified environment containing 5% CO2. Stable HEK 293F cell lines expressing soluble TLR2, TLR1, and TLR1P315L were generated by transfection followed by G418 selection and limiting dilution as described previously (40). Stable cell lines were grown in Freestyle serum-free expression medium containing 0.25 mg/ml G418 and cultured in suspension at 37 °C with continuous shaking at 125 rpm in a humidified environment containing 8% CO2.
Purification of Soluble TLRs
Soluble TLR-Fc fusion proteins were purified from stable HEK 293F cell supernatant by affinity chromatography using protein G-Sepharose for fast flow (GE Healthcare) on an ÄKTA prime purification system (GE Healthcare) as described previously (40). The Fc tag was removed after the first round of purification by adding restriction grade thrombin protease (Novagen, Madison, WI) at a concentration of 1 unit of thrombin/0.25 mg of TLR extracellular domain-Fc protein. After 18 h of incubation at room temperature, the TLR extracellular domain was separated from the Fc fragments by another round of affinity chromatography in the ÄKTA prime system using a 1-ml prepacked protein A column (Pierce) and PBS, pH 7.4 running buffer. The TLRs were concentrated from the flow-through using an Amicon Ultra-4 centrifugal device (Millipore) and centrifuged at 2500 × g for 15–25 min at 4 °C to a volume of 0.5 ml. The concentrated protein was then loaded on a Superdex 200 10/300GL gel filtration column (GE Healthcare) in PBS, pH 7.4 running buffer at a flow rate of 0.5 ml/min. The eluted fractions containing monomeric TLR extracellular domains were pooled and concentrated using size exclusion centrifugation (Amicon). The final protein concentration after three rounds of purification was measured using the Pierce BCA protein assay kit. The protein yields for recombinant soluble TLR1, TLR1P315L, and TLR2 were 0.5, 0.3, and 0.25 mg/liter of medium, respectively. Protein purity was determined by mass spectrometry (Mass Spectrometry Laboratory, School of Chemical Sciences, University of Illinois at Urbana-Champaign) using MALDI as the ionization technique and sinapinic acid as a calibration matrix.
Soluble TLR ELISAs
Briefly, 0.5 μg/ml concentrations of commercially available anti-TLR 1 (clone GD2F4) and anti-TLR2 (clone T2.5) monoclonal antibodies were coated onto 96-well microtiter plates at 4 °C overnight. Nonspecific binding was blocked by 5% bovine serum albumin (BSA) in PBS. Diluted samples of purified soluble TLR (sTLR) proteins (1.0 μg/ml) were added to the wells and incubated at room temperature for 2 h. Binding of soluble TLR1 and TLR2 to their respective antibodies was detected using HRP-conjugated anti-HA antibody and HRP-conjugated anti-FLAG antibody, respectively, followed by the addition of o-phenylenediamine substrate. The colorimetric detection was quantified by measuring absorbance at 490 nm.
Soluble TLR Competition Assays
SW620 cells (a human colonic epithelial cell line; ATCC CCL-227) were seeded in 24-well plates overnight at a density of 1 × 105 cells/ml in RPMI 1640 medium supplemented with 10% (v/v) FBS and 2 mm l-glutamine. Cells were co-transfected with full-length genes for membrane-bound TLR2 and TLR1 together with a firefly luciferase gene driven by the IL-8 promoter and a Renilla luciferase gene driven by a basal promoter (pRL-null) as a transfection control (Promega, Madison, WI). Transfections were performed using a cationic lipid agent, FuGENE 6 (Roche Applied Science), at a 4:1 lipid:DNA ratio. Forty-eight hours post-transfection, the medium was replaced with Invitrogen Opti-MEM (Invitrogen), and the cells were stimulated for 6 h by the addition of the triacylated agonist Pam3CSK4 (10 ng/ml) with or without sTLR2 or sTLR1 (1 μg/ml). Following the manufacturer's protocol for the Dual-Luciferase assay (Promega), cell lysates were collected 6 h poststimulation and analyzed for firefly and Renilla luciferase activity using a BioTek Synergy HT plate reader (BioTek, Winooski, VT). The transfection efficiency across different wells was normalized by dividing the firefly luciferase activity by the Renilla luciferase control.
Generation of Bioactive Soluble CD14
Human soluble CD14 protein was cloned, expressed, and purified as described previously (45) with the following exceptions. Briefly, human CD14 (amino acids 1–337) was amplified from genomic DNA and cloned into a modified pDisplay vector (a kind gift from Dr. David Kranz, University of Illinois Urbana-Champaign) preceding a thrombin cleavage site (LVPRGS) and the Fc domain of human IgG1. The final construct was sequenced (University of Illinois Urbana-Champaign Sequencing Center) following site-directed mutagenesis (C306S) via primer extension to avoid unnatural disulfide bonding resulting from the truncated coding region of our construct. Following transfection and stable selection of human HEK 293F cells (Invitrogen), human soluble CD14 was purified from cell supernatant in three chromatographic steps, including protein G affinity chromatography, thrombin cleavage, protein A affinity chromatography, and size exclusion chromatography. Finally, fractions containing CD14 were pooled and concentrated to 10 mg/ml using an Amicon Ultra-4 unit (Millipore) as measured by Pierce BCA assay. Human soluble CD14 (amino acids 1–337; C306S) was stored at 4 °C for up to 6 months and is bioactive as measured by LPS binding activity and the ability to facilitate LPS-induced IL-8 production from human epithelial SW620 cells (45).
Size Exclusion Chromatography Assays
The TLR1·TLR2·lipopeptide ternary complex was formed by preincubating 0.25 μm TLR2, 0.25 μm TLR1, and 2.5 μm Pam3CSK4 (with or without 0.05 μm LBP and/or 0.25 μm sCD14) in PBS, pH 7.4 buffer to a final volume of 0.5 ml. The mixture was incubated in a 37 °C water bath for 2 h and injected into a Superdex 200 10/300GL gel filtration column (GE Healthcare) at a flow rate of 0.5 ml/min in PBS, pH 7.4 running buffer. Twenty minutes after injection of the sample, 0.5-ml fractions were collected covering 1 column bed volume (about 24 ml; 48 min). The chromatogram was recorded using a manual UV recorder. The data were then replotted using the xyExtract v5.1 graph digitizer software (Wilton and Cleide Pereira da Silva, Campina Grande, Paraíba, Brazil).
Eluted fractions were separated by 7.5% SDS-PAGE and transferred to an Immobilon-P membrane (Millipore). The membranes were blocked with 5% nonfat dry milk in TBS buffer containing 0.05% Tween 20. Western blotting was performed to detect TLR1 and TLR2 using HRP-conjugated anti-HA and anti-FLAG antibodies (both diluted at 1:1000 in 5% nonfat dry milk), respectively. LBP and/or CD14 was detected using either a polyclonal goat anti-LBP or goat anti-CD14 (diluted 1:500 in 5% nonfat dry milk) followed by a HRP-conjugated rabbit anti-goat IgG polyclonal antibody (diluted 1:5000 in 5% nonfat dry milk). Chemiluminescence was detected using the Pierce ECL Western blotting substrate. Membranes were then exposed to a HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ) and developed.
Cell Activation Assays
HEK 293F cells were seeded in a 48-well tissue culture plate overnight at a density of 1.6 × 105 cells/ml (50,000 cells/well) in Freestyle serum-free expression medium (Invitrogen). Cells were co-transfected with 50 ng each of TLR1 and TLR2 together with 75 ng of a firefly luciferase reporter gene driven by an NF-κB promoter and 25 ng of a Renilla luciferase (pRL-null) transfection control (Promega). Transfections were performed using a cationic lipid agent, 293fectin (Invitrogen), at a 3:1 lipid:DNA ratio. Forty-eight hours post-transfection, LBP, sCD14 (Peprotech), or a combination of both was added to the wells to a final concentration of 0.1 μg/ml. Cells were then stimulated with 1 ng/ml agonist for 6 h. Alternatively, LBP and/or sCD14 were preincubated with the agonists for 1 h at 37 °C prior to addition to cells. Following the manufacturer's protocol for the Dual-Luciferase assay (Promega), cell lysates were collected and analyzed for NF-κB-luciferase and Renilla activity using a BioTek Synergy HT plate reader (BioTek). The transfection efficiency across different wells was normalized by dividing the IL-8 luciferase activity by the Renilla activity.
RESULTS
Purified Soluble TLRs Are Monomeric and Biologically Functional
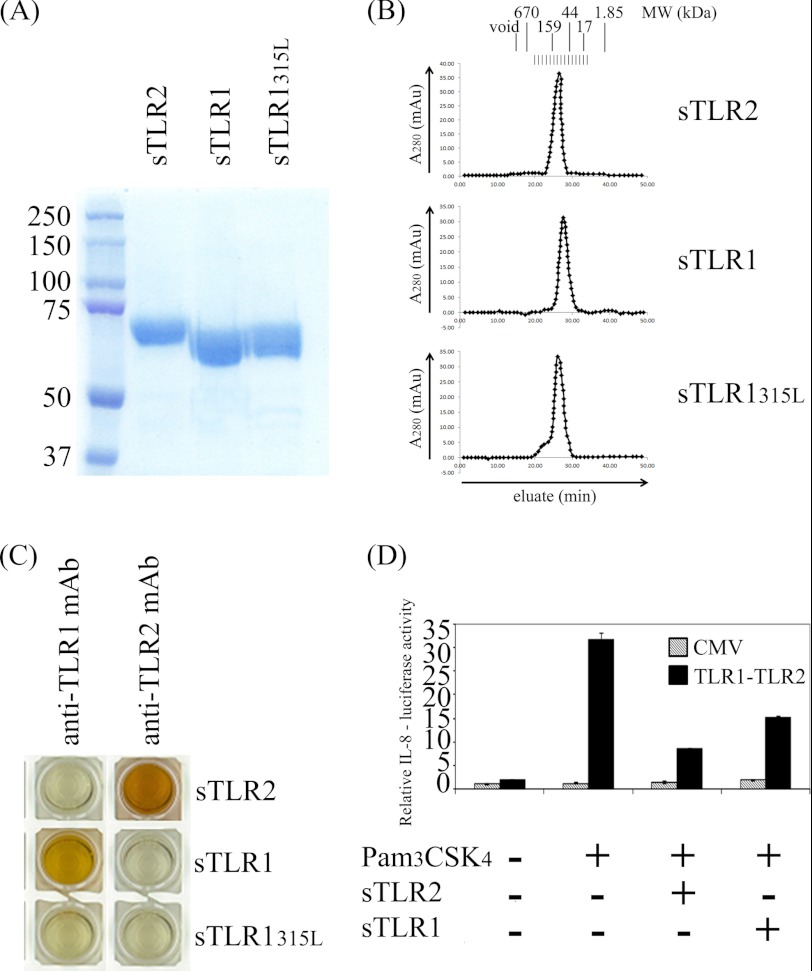
To define the role of LBP and sCD14 in driving the formation of a TLR1·TLR2·lipoprotein ternary complex, recombinant soluble extracellular domains of TLR1 and TLR2 were isolated and purified from HEK 293F cells by affinity chromatography (see “Experimental Procedures”). As expected, both proteins were isolated and verified as monomeric proteins by gel filtration chromatography (Fig. 1, A and B). The sizes of the protein monomers were estimated using gel filtration standard markers that are composed of globular proteins. Based on these standard proteins, TLR1 and TLR2 were estimated to have a relative molecular mass of about 65 and 83 kDa, respectively. This is slightly different from the molecular mass calculated by mass spectrometry (70.65 kDa for TLR1 and 71.85 kDa for TLR2) because TLRs are extended proteins and not globular in nature. TLR1 and TLR2 were recognized by their respective monoclonal antibodies and did not exhibit any cross-reaction, indicating that each protein is properly folded (Fig. 1C).
FIGURE 1.
Recombinant sTLR proteins are monomeric, properly folded, and biologically functional. A, 100 μg of each purified sTLR protein as indicated was loaded for 7.5% SDS-PAGE and stained with Coomassie blue dye. B, each purified sTLR protein as indicated was analyzed by size exclusion chromatography using a Superdex 200 column. C, soluble TLRs were incubated in microtiter plate wells coated with either the anti-TLR1 mAb (clone GD2F4) or the anti-TLR2 mAb (T2.5) as indicated. Binding of soluble TLR1, TLR1P315L, or TLR2 was detected using HRP-conjugated anti-HA and anti-FLAG mAbs. D, SW620 cells were co-transfected with full-length TLR1, TLR2, an IL-8 promoter-driven luciferase reporter gene, and a Renilla luciferase transfection control. 48 h post-transfection, the cells were stimulated with 10 ng/ml Pam3CSK4 with or without 1 μg/ml soluble TLR1 or TLR2 as indicated. Firefly luciferase activities were normalized to that of the Renilla luciferase control. These values were normalized to that of empty CMV vector whose value was taken as 1. Error bars represent the S.D. of three independent events. mAu, milliabsorbance units.
To assess the biological activity of the purified proteins, we performed TLR reconstitution experiments in human epithelial cells (SW620) and measured the relative expression of a luciferase reporter gene driven by the human IL-8 promoter. As expected, luciferase expression was induced in cells transfected with TLR1 and TLR2 upon stimulation with 10 ng/ml Pam3CSK4. However, upon addition of either soluble TLR1 or soluble TLR2, SW620 cells showed diminished responses to Pam3CSK4, suggesting that each soluble receptor has bioactivity presumably either by actively competing for the agonist or by forming a signaling-deficient complex with the transmembrane TLR partner (Fig. 1D).
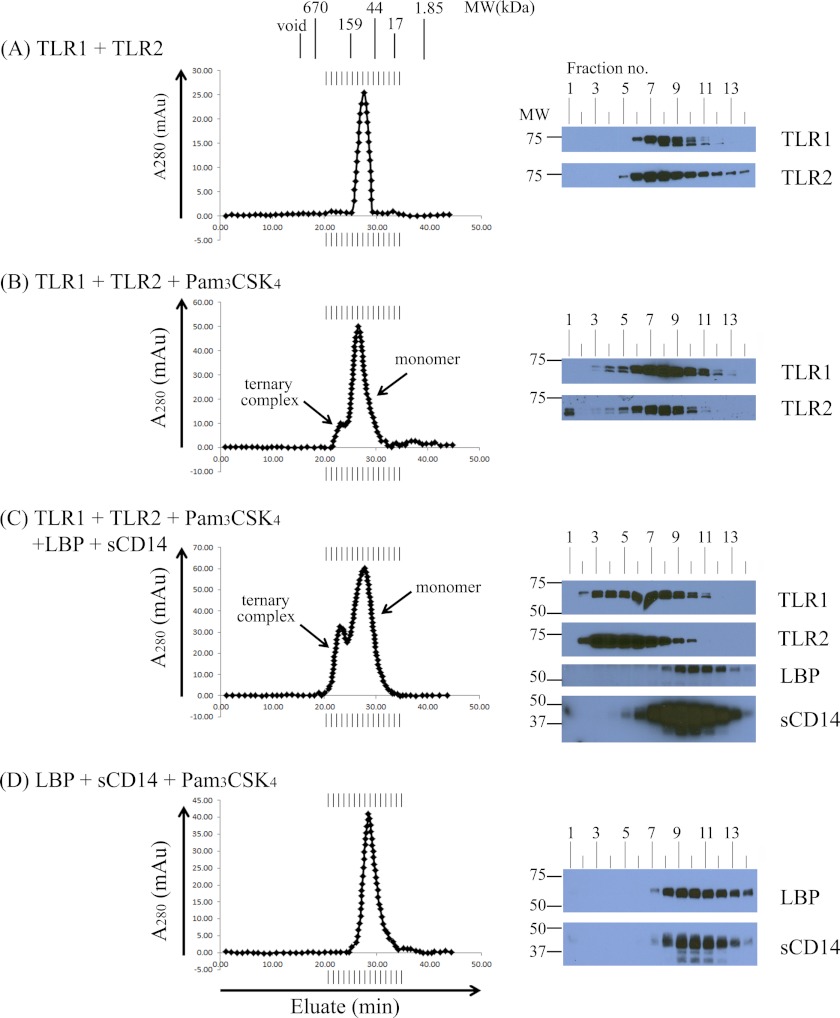
LBP and sCD14 Enhance TLR1·TLR2·Pam3CSK4 Formation but Are Not Part of the Stable Ternary Complex
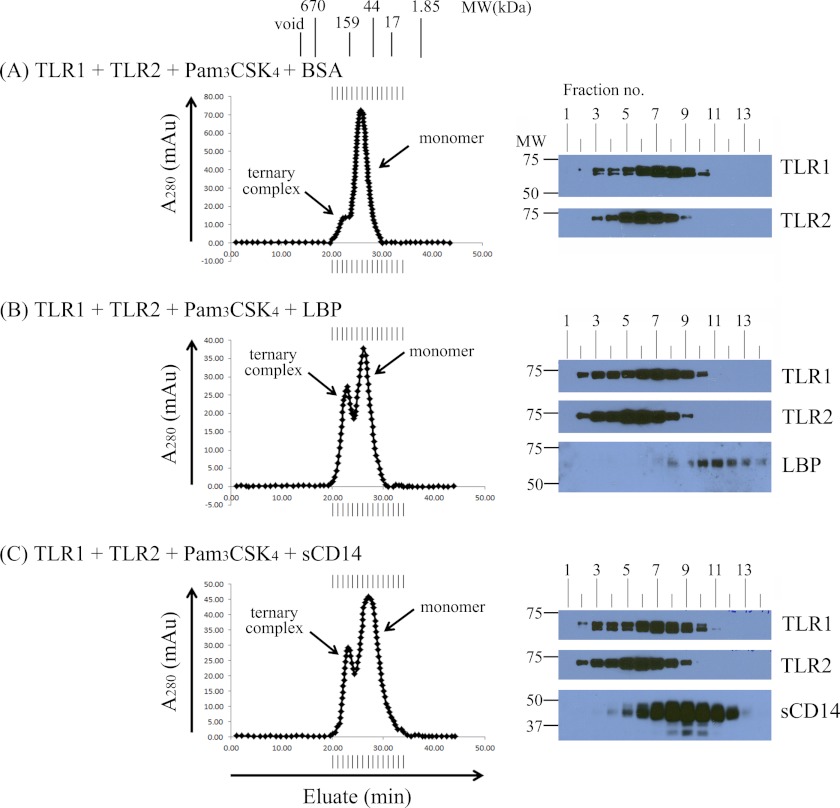
To measure receptor complex formation, we performed size exclusion chromatography using a Superdex 200 column (GE Healthcare) to distinguish monomeric TLRs from larger TLR complexes. First, we preincubated equimolar amounts of soluble TLR1 and TLR2 in the absence of agonist for 2 h at 37 °C but did not observe the formation of larger TLR complexes by size exclusion chromatography (Fig. 2A). When soluble TLR1 and TLR2 were incubated for 2 h at 37 °C with a 10-fold molar excess of Pam3CSK4, a novel peak eluted at an earlier time compared with the monomeric proteins that corresponds to the expected size of a ternary TLR1·TLR2·Pam3CSK4 complex. Western blot analysis revealed that both TLR1 and TLR2 were present in the fractions that constitute the small peak with a calculated relative molecular mass of ∼183 kDa (Fig. 2B). The modest peak size indicates that only a small amount of stable ternary complex is formed from the incubation of purified soluble TLR monomers with lipopeptide agonist.
FIGURE 2.
LBP and soluble CD14 enhance soluble TLR1·TLR2·Pam3CSK4 ternary complex formation but are not part of the final ternary complex. In A–D, various combinations of 0.25 μm TLR1, 0.05 μm TLR2, 2.5 μm Pam3CSK4, 0.05 μm LBP, and/or 0.25 μm sCD14 were incubated for 2 h at 37 °C in a 500-μl volume of PBS buffer, pH 7.4 as indicated. Protein complexes were separated by size exclusion chromatography. The expected molecular weight of the TLR monomers and dimers was estimated by column calibration using known molecular weight standards. Proteins in eluted fractions were separated by 7.5% SDS-PAGE and transferred by Western blotting, and TLR1, TLR2, LBP, and sCD14 were detected using suitable antibodies and HRP conjugates (see “Experimental Procedures”). The results shown are representative of at least three independent experiments. mAu, milliabsorbance units.
Because LBP and CD14 are known to sensitize TLR1- and TLR2-mediated inflammatory responses to lipopeptides and lipoproteins, we assessed their ability to drive ternary complex formation. To this end, purified LBP and sCD14 along with TLR1, TLR2, and Pam3CSK4 were incubated for 2 h at 37 °C followed by size exclusion chromatography. This incubation resulted in a more robust peak corresponding to the ternary complex compared with that observed in the absence of LBP and sCD14 (Fig. 2C). Western blot analysis revealed that LBP and sCD14 continued to elute in fractions expected of monomers, suggesting that they are not part of the final ternary complex (Fig. 2C). Additionally, a 2-h incubation of LBP and sCD14 with Pam3CSK4 alone did not induce any higher order protein complexes (Fig. 2D). Ternary complexes were not formed when protein mixtures were loaded directly onto the gel filtration column, suggesting that incubation at 37 °C is required to form stable complexes in solution (data not shown). Taken together, these results demonstrate that LBP and sCD14 can enhance TLR1·TLR2·Pam3CSK4 ternary complex formation without becoming part of the stable complex.
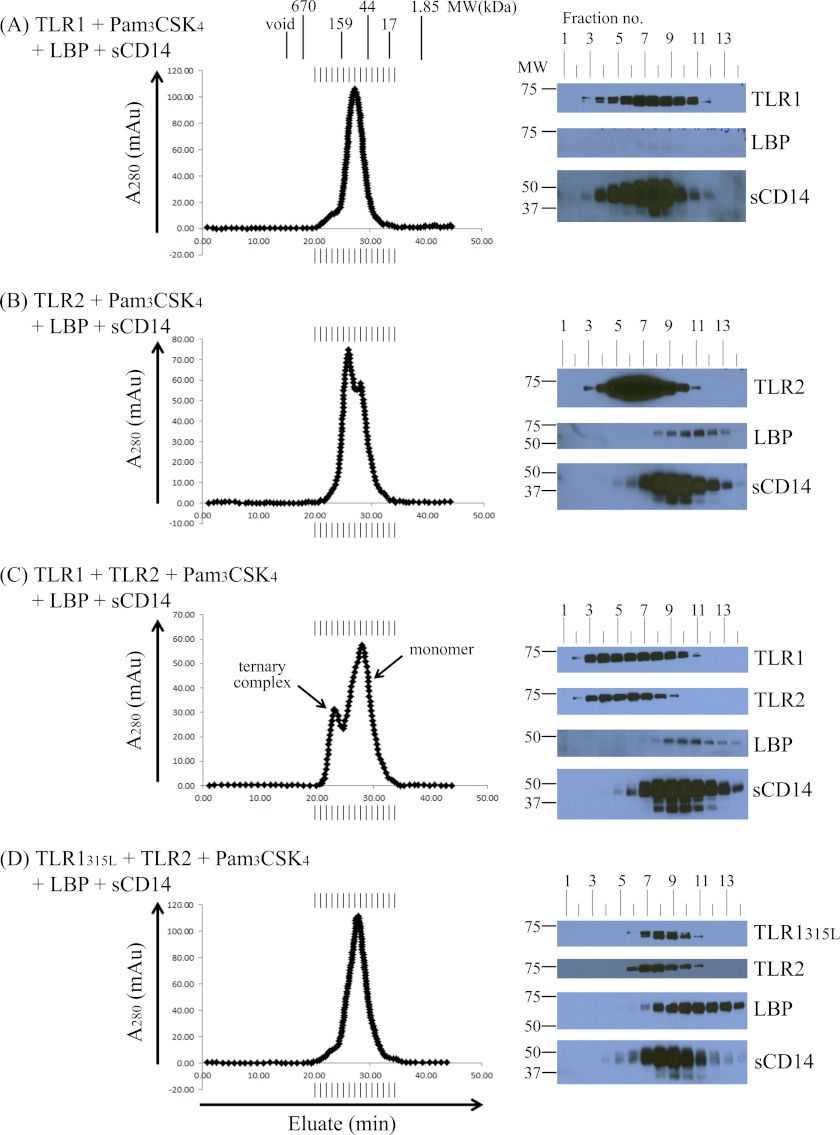
Lipopeptide Induces TLR1 and TLR2 Heterodimers but Not Homodimers
To demonstrate that lipopeptides induce heterodimers, but not homodimers, of TLR1 and TLR2, we incubated LBP, sCD14, and Pam3CSK4 together with either TLR1 alone or TLR2 alone. Size exclusion chromatography revealed that neither TLR1 nor TLR2 form homodimers even in the presence of LBP, sCD14, and excess agonist (Fig. 3, A and B). The two peaks observed in Fig. 3B correspond to the size discrepancy between the soluble TLR2 monomer (relative molecular mass of ∼87.3 kDa) and the sCD14 monomer (relative molecular mass of ∼48.3 kDa). Western blot analysis revealed that although TLR1 and TLR2 did not form stable homodimers sCD14 behaved differently in the two conditions (Fig. 3, A and B, right panel). In the presence of TLR1 alone (Fig. 3A, right panel), sCD14 eluted in earlier fractions, whereas in the presence of TLR2, sCD14 eluted in later fractions as a monomeric protein (Fig. 3B, right panel). This suggests that sCD14 and TLR1 interact in a Pam3CSK4-dependent fashion. Because sCD14 behaves as a monomeric protein in the presence of all five components, Fig. 3C suggests that the binding of TLR2 to the sCD14·TLR1·Pam3CSK4 complex displaces sCD14, leading to formation of the final ternary TLR1·TLR2·Pam3CSK4 ternary complex. To our knowledge, this is the first experiment to suggest a defined order of events for ternary complex formation.
FIGURE 3.
Soluble TLRs do not form homodimers when incubated with Pam3CSK4. 0.5 μm TLR1 (A), 0.5 μm TLR2 (B), a 0.25 μm concentration each of TLR1 and TLR2 (C), or a 0.25 μm concentration each of TLR1P315L and TLR2 (D) were preincubated with a 5-fold molar excess of Pam3CSK4 (2.5 μm) together with 0.05 μm LBP and 0.25 μm sCD14 in PBS, pH 7.4 buffer for 2 h at 37 °C in a 500-μl reaction volume. Proteins and protein complexes were separated and analyzed as described in the legend of Fig. 2. The results shown are representative of at least three independent experiments. mAu, milliabsorbance units.
P315L is a naturally occurring TLR1 polymorphism that has been shown previously to greatly attenuate cellular responses to synthetic lipopeptides as well as a variety of other microbial TLR1 agonists (46). Subsequent protein crystallography work has shown that Pro-315 is physically located at the entrance of the hydrophobic channel of TLR1 and forms part of TLR1-TLR2 dimer interface in the ternary complex (41). We generated the soluble form of the TLR1P315L variant that, similar to wild-type TLR1, purified as a monomeric protein with a relative molecular mass of 77.5 kDa as calculated by size exclusion chromatography and a molecular mass of 70.84 kDa as measured by mass spectrometry (Fig. 1, A and B). We have demonstrated previously that the P315L mutation destroys the epitope of GD2F4, a monoclonal antibody against TLR1 that inhibits lipopeptide-induced cell activation (46). As expected, GD2F4 did not bind soluble TLR1P315L in our ELISA (Fig. 1C). To directly assess the effect of the P315L mutation on complex formation, we incubated TLR1P315L with TLR2, Pam3CSK4, LBP, and sCD14 for 2 h at 37 °C and analyzed the reaction products by size exclusion chromatography. In contrast to wild-type TLR1, TLR1P315L did not form a stable ternary complex with TLR2 and Pam3CSK4 (Fig. 3D). The fact that TLR1 Pro-315 is critical for ternary complex formation explains the highly attenuated responses of this naturally occurring TLR1 variant to triacylated lipopeptides.
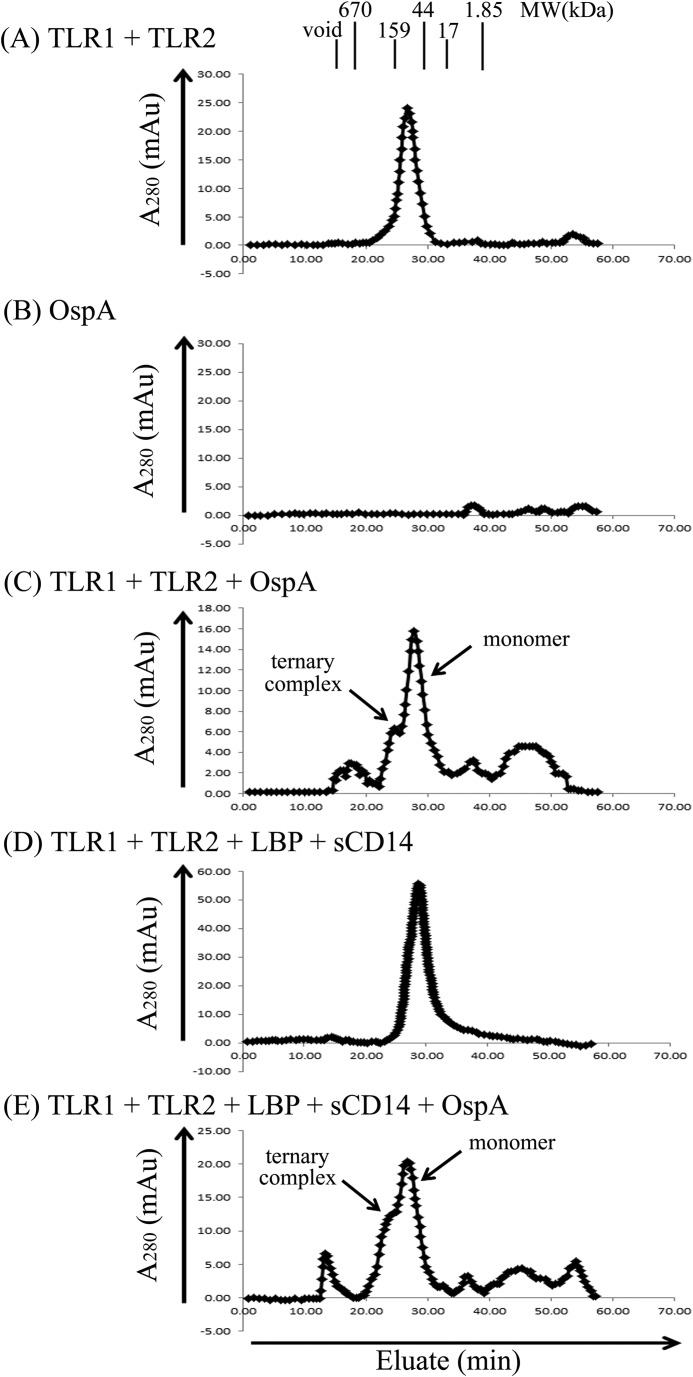
TLR1 and TLR2 Form a Ternary Complex with the OspA Lipoprotein of B. burgdorferi
We next tested our system using a naturally occurring lipoprotein, OspA from B. burgdorferi. OspA is a 30-kDa membrane-associated lipoprotein with a typical tripalmitoyl-S-glycerylcysteine (Pam3Cys) moiety covalently attached to the N terminus of the protein (32, 47). Although OspA has been shown to activate cells through TLR1 and TLR2, to date there has been no direct physical evidence demonstrating that OspA induces formation of a stable ternary complex. On the gel filtration column, OspA did not elute as a single peak at 30 kDa, perhaps reflecting the amphipathic nature of the molecule that may drive aggregation and/or nonspecific binding to the column matrix (Fig. 4B). However, when TLR1 and TLR2 were incubated with a 5-fold molar excess of OspA prior to column loading, a small peak was observed in the region consistent with formation of a ternary TLR1·TLR2·OspA complex (Fig. 4C). When LBP, sCD14, OspA, TLR1, and TLR2 were incubated together, we observed a more robust peak corresponding to the ternary complex, suggesting that addition of LBP and sCD14 enhances TLR1·TLR2·OspA ternary complex formation (Fig. 4E). In the absence of OspA, TLR1 and TLR2 eluted as monomers (Fig. 4A) as did LBP and sCD14 (Fig. 4D). Taken together, our data demonstrate that, similar to Pam3CSK4, the naturally occurring OspA lipoprotein from B. burgdorferi induces ternary complex formation, which is enhanced by the addition of LBP and sCD14.
FIGURE 4.
LBP and soluble CD14 enhance complex formation between TLR1, TLR2, and the OspA lipoprotein of B. burgdorferi. In A–E, various combinations of 0.25 μm TLR1, 0.25 μm TLR2, 1.25 μm OspA, 0.05 μm LBP, and/or 0.25 μm sCD14 were incubated for 2 h at 37 °C in a 500-μl volume of PBS buffer, pH 7.4 as indicated. Proteins and protein complexes were separated and analyzed as described in the legend of Fig. 2. The results shown are representative of at least three independent experiments. mAu, milliabsorbance units.
Either LBP or sCD14 Can Independently Enhance TLR1·TLR2·Pam3CSK4 Ternary Complex Formation
In the TLR4 system, it is known that LPS is sequentially delivered in monomeric form first by LBP to CD14 and then by CD14 to either soluble MD-2 or the MD-2·TLR4 complex. To validate whether both LBP and sCD14 are required to enhance TLR recognition of lipopeptide, we assessed the ability of either protein to independently enhance TLR1·TLR2·Pam3CSK4 ternary complex formation. Surprisingly, we observed that addition of either LBP or sCD14 to the 2-h incubation independently enhanced TLR1·TLR2·Pam3CSK4 ternary complex formation (Fig. 5, B and C). Consistent with our earlier Western blot data, neither LBP nor sCD14 was part of the final stable complex (Fig. 5, B and C). BSA, a well established lipid carrier protein, did not enhance ternary complex formation (compare Fig. 5A with Fig. 2B). To our knowledge, this is the first report showing that LBP can independently and directly deliver a triacylated lipopeptide to TLR1 and TLR2.
FIGURE 5.
Either LBP or soluble CD14 can independently enhance TLR1·TLR2·Pam3CSK4 ternary complex formation. 0.25 μm TLR1, 0.25 μm TLR2, and 2.5 μm Pam3CSK4 were incubated with 0.25 μm BSA (A), 0.05 μm LBP (B), and 0.25 μm sCD14 (C) for 2 h at 37 °C in a 500-μl volume of PBS, pH 7.4 buffer. Proteins and protein complexes were separated and analyzed as described in the legend of Fig. 2. The results shown are representative of at least three independent experiments. mAu, milliabsorbance units.
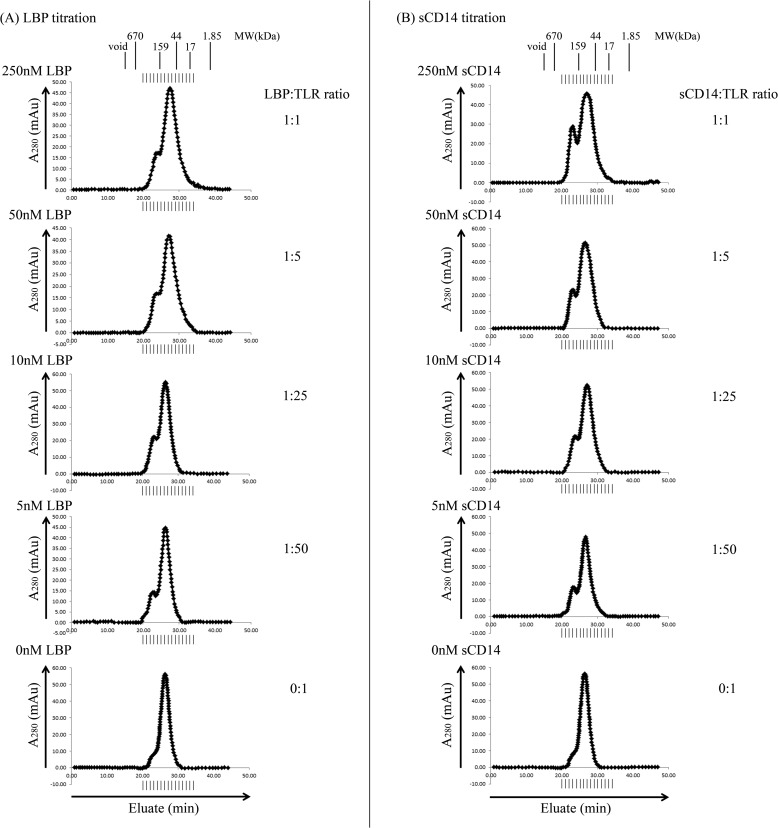
To determine whether enhanced ternary complex formation is mediated by substoichiometric amounts of either LBP or sCD14, varying concentrations of these proteins were independently incubated with a constant amount of TLR1, TLR2, and Pam3CSK4 for 2 h at 37 °C followed by size exclusion chromatography analysis. Substoichiometric amounts of either LBP or sCD14 enhanced TLR1·TLR2·Pam3CSK4 ternary complex formation even at 5 nm, a concentration that is 50 times lower than that of each TLR and 500 times lower than that of the Pam3CSK4 agonist in the reaction (Fig. 6, A and B). This demonstrates that neither LBP nor sCD14 is consumed in the reaction and suggests that either protein can catalytically deliver lipopeptide agonist to the TLRs. Compared with CD14, ternary complex formation was far less dependent on the concentration of LBP, suggesting that LBP is a more robust catalyst than sCD14 (Fig. 6, A and B).
FIGURE 6.
Substoichiometric concentrations of either LBP or soluble CD14 are sufficient to enhance TLR1·TLR2·Pam3CSK4 ternary complex formation. 0.25 μm TLR1, 0.25 μm TLR2, and 2.5 μm Pam3CSK4 were incubated with various concentrations (250, 50, 10, 5, and 0 nm) of either LBP (left panel) or sCD14 (right panel) for 2 h in a 500-μl volume at 37 °C in PBS buffer, pH 7.4. Proteins and protein complexes were separated and analyzed as described in the legend of Fig. 2. The results shown are representative of at least three independent experiments. mAu, milliabsorbance units.
Cellular Responses to Microbial Lipoproteins Are Enhanced by Either LBP or sCD14
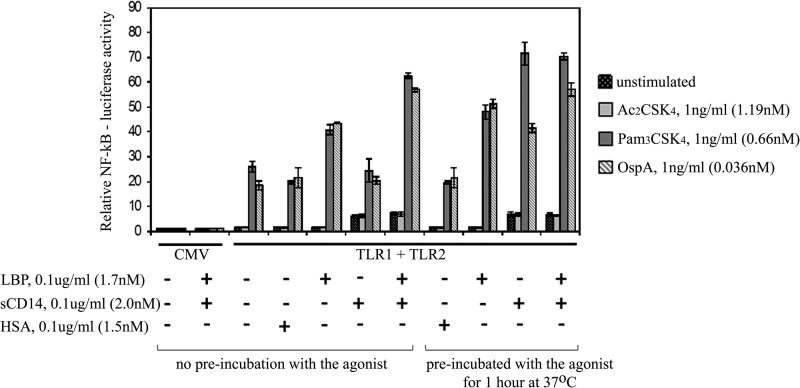
To assess the biological significance of our biophysical measurements, we measured the effects of LBP and sCD14 on cellular responses to synthetic lipopeptide and natural microbial lipoprotein agonists. To this end, HEK 293F cells were transfected with full-length TLR1 and TLR2 together with a luciferase reporter gene driven by NF-κB and then stimulated with either Pam3CSK4 or OspA. To ensure that the cell activation assay completely lacked endogenous sources of either LBP or CD14, the HEK 293F cells were grown in serum-free medium and maintained in this medium throughout the assay. In the absence of LBP or sCD14, a 1 ng/ml concentration of either Pam3CSK4 or OspA elicited a 20-fold induction of NF-κB-driven luciferase activity that was dependent on prior transfection of the HEK 293F cells with TLR1 and TLR2 (Fig. 7). Nanogram levels of LBP, but not sCD14 or human serum albumin, enhanced the sensitivity of TLR1- and TLR2-expressing HEK 293F cells to either Pam3CSK4 or OspA when compared with cells stimulated with agonists in the absence of any lipid carrier. We modified the same experiment by preincubating either LBP or sCD14 with agonist for 1 h at 37 °C prior to addition to the HEK 293F cells. This preincubation had little effect on LBP-mediated cell stimulation but enabled sCD14 to significantly enhance the stimulation of cellular NF-κB by either Pam3CSK4 or OspA (Fig. 7). Taken together, these results demonstrate that either LBP or sCD14 can enhance cellular responses to lipopeptide or lipoprotein. This finding is entirely consistent with our biophysical studies showing that TLR1·TLR2·lipopeptide ternary complex formation was enhanced by either protein. The fact that sCD14, but not LBP, requires preincubation with the agonist to increase cellular responses is consistent with our biophysical data, suggesting that sCD14 is a poor catalyst compared with LBP in the delivery of agonist to TLR1 and TLR2 (Fig. 6). The addition of both LBP and sCD14 further enhanced cellular responses to Pam3CSK4 and OspA over that of either sCD14 or LBP alone, suggesting that LBP and sCD14 may act in a cooperative manner to efficiently deliver the agonists to the TLRs (Fig. 7).
FIGURE 7.
Either LBP or soluble CD14 enhances cellular responses to Pam3CSK4 and OspA. HEK 293F cells were co-transfected with vectors expressing full-length TLR1 and TLR2 or empty CMV control vector as indicated together with an NF-κB-promoter driven luciferase reporter gene and a Renilla luciferase reporter gene. About 48 h post-transfection, cells were stimulated with 1 ng/ml Pam3CSK4, OspA, or the non-acylated Ac2CSK4 control in the presence of 0.1 μg/ml LBP, sCD14, or human serum albumin (HSA) as indicated (left side). In one set of experiments, agonists were preincubated with proteins for 1 h at 37 °C prior to addition to transfected cells (right side). Cell values on the y axis represent the level of constitutive reporter activation normalized to the empty CMV vector control (value of 1). Error bars represent the S.D. of three independent values.
DISCUSSION
LBP and CD14 have been shown previously to directly bind diacylated and triacylated lipopeptides (43), suggesting a role in delivery of these potent agonists to the TLR2 system. Despite the abundance of evidence demonstrating direct binding of lipopeptides to LBP and CD14, only a few studies have carefully examined the functional roles of these two proteins in the activation of TLR2. sCD14 has been shown to mediate the transfer of lipopeptides to TLR2 on the cell surface of HEK 293 cells, CHO cells, and primary monocytes in vitro (48–50). Pulldown assays performed with HEK cells overexpressing TLR1 and TLR2 have shown that sCD14 does not stably bind to the TLR1·TLR2·Pam3CSK4 complex (50). Additionally, immobilized TLR2 incubated with preformed lipopeptide·sCD14 complexes has been shown to bind lipopeptide but not sCD14 (48). The results of our size exclusion chromatography experiments using entirely soluble components are consistent with the above findings in that LBP and sCD14 drive formation of TLR1·TLR2·Pam3CSK4 ternary complexes but are not themselves part of the final complex. Interestingly, in the absence of TLR2, we observed that both CD14 and TLR1 eluted in earlier fractions (Fig. 3A). Although not proven, this observation supports a model where, upon binding lipopeptide, CD14 stably interacts with TLR1 but is then displaced by TLR2 during formation of the final ternary complex.
To date, we are unaware of any studies that have formally assessed whether LBP and sCD14 function in a coordinated or sequential fashion similar to that established for the TLR4 system. Cell-based in vitro studies suggest that lipopeptides are sequentially delivered from LBP to CD14 and then to TLR1-TLR2 (43, 51). Here we provide physical evidence that either LBP or sCD14 can independently catalyze the formation of a TLR1·TLR2·Pam3CSK4 ternary complex. Thus, unlike their non-redundant and sequential role in the delivery of LPS to the TLR4 complex, our data suggest that LBP and sCD14 are functionally redundant in the direct presentation of lipopeptides to TLR1 and TLR2. To our knowledge, this is the first report demonstrating that LBP can deliver agonists directly to the TLRs in a CD14-independent manner.
In addition to existing as a soluble protein found in body fluids (52), CD14 also exists as a glycosylphosphatidylinositol-anchored membrane receptor (membrane-bound CD14 or mCD14) predominantly expressed on the surface of myeloid cells (13). Flow cytometry experiments have demonstrated that LBP transfers lipopeptides to mCD14 on peripheral blood monocytes (43). Importantly, in two independent studies, anti-CD14 antibodies have been shown to block the responses of mCD14-expressing myeloid cells to lipopeptide, suggesting that in membrane form CD14 is absolutely required for delivery of lipopeptides to TLR2 (43, 44). Although this may seem to contradict the data presented here that show a redundant function for LBP and sCD14, the mechanism of agonist delivery to TLRs may be different for membrane-anchored versus soluble forms of CD14. Indeed, confocal microscopy and fluorescence resonance energy transfer studies have shown that upon lipopeptide binding mCD14 stably physically associates with TLR1 and TLR2 (49). This situation is quite different from that observed for sCD14, which does not form part of the ternary complex.
Pam3CSK4 is a 1.5-kDa amphipathic molecule that forms aggregates or micelles in solution and exhibits nonspecific hydrophobic interactions. On the other hand, the TLRs recognize monomeric forms of this triacylated lipidated agonist (41). Thus, the aggregated state of Pam3CSK4 in PBS buffer may explain why the formation of ternary complex in solution is inefficient and could be enhanced by the disaggregating activities of LBP or sCD14. It is well established that human LBP and CD14 directly bind to a variety of TLR2 agonists, which largely comprise acylated microbial components. The agonists include bacterial lipoproteins (44, 53, 54), Gram-positive bacterial lipoteichoic acids (51, 55), mycobacterial lipomannans and lipoarabinomannans (56), pneumococcal peptidoglycans (57), and Treponema-derived glycolipids (58). Thus, LBP and CD14 may function to disaggregate and deliver a variety of acylated microbial agonists to the TLR2 system.
LBP-deficient mice are hyporesponsive to LPS as well as to Gram-negative bacteria as evidenced by lower levels of serum inflammatory cytokines and lower survival rates following infection with Salmonella or Escherichia coli (59–61). In contrast, the responses of LBP-deficient mice are indistinguishable from that of wild-type mice following infection with the Gram-positive organisms Staphylococcus aureus (62) and Streptococcus pneumoniae (63). Additionally, LBP deficiency has no effect on murine responses to intranasal infection with mycobacterial pathogens (64). Similar to LBP knockouts, CD14-deficient mice are resistant to septicemic shock mediated by either LPS injection or by Gram-negative bacterial infection (65). However, following exposure to either live or killed Staphylococcus aureus, CD14-deficient and control wild-type mice exhibit similar symptoms of shock and proinflammatory cytokine production (66). Additionally, following intravenous infection with Mycobacterium avium, CD14-deficient mice and wild-type control mice have indistinguishable levels of serum tumor necrosis factor (TNF)-α production, macrophage inducible nitric-oxide synthase expression, and bacterial loads (67). The phenotypes of both LBP and CD14 knock-out mice support the idea that LBP and CD14 have a non-redundant function in the delivery of Gram-negative bacterial LPS to the TLR4 complex but a redundant function in the delivery of Gram-positive bacterial or mycobacterial components to the TLR1-TLR2 system.
Taken altogether, we have demonstrated that recognition of lipoproteins by TLR1 and TLR2 involves multiple players that assist in the delivery of agonists to the final receptor complex. Understanding this fluid and dynamic process of TLR sensing of agonists mediated by LBP and CD14 has a profound impact on therapeutic strategies designed to treat chronic inflammatory conditions, sepsis, and infection. The use of neutralizing antibodies or inhibitory molecules against human CD14 has been suggested as a possible therapeutic approach against sepsis (68, 69). Although this approach may be effective for treating Gram-negative bacterial sepsis, it may not be as effective in the treatment of sepsis caused by other bacteria due to the redundancy of function between LBP and CD14 for the direct delivery of lipoproteins or other bacterial agonists to the TLR1-TLR2 system.
LBP is an acute phase protein, and clinical studies have shown that serum concentrations increase about 10–50-fold in human patients with either Gram-negative or Gram-positive bacteremia (70). Although it has been shown that high concentrations of LBP in acute phase serum may confer protection to the host by inhibiting the LPS response in human monocytes (71), the opposite has been observed for lipoproteins. Human monocytes stimulated with either triacylated or diacylated lipopeptides exhibit an increase in TNF expression that is proportional to the amount of added LBP (43). This may lead to an overwhelming systemic inflammatory response and consequently a profound deleterious effect on the host. Thus, understanding the role of LBP in the TLR2 system may be essential for treating bacterially induced septicemia.
Acknowledgments
We thank Dr. Jerrold P. Weiss (Department of Microbiology, University of Iowa) and Dr. Theresa M. Gioannini (Department of Internal Medicine, University of Iowa) for generously providing the recombinant LBP used in this study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI052344 (to R. I. T.) from the NIAID. This work was also supported by the University of Illinois Department of Microbiology.
- TLR
- Toll-like receptor
- sTLR
- soluble TLR
- TIR
- Toll-interleukin-1 receptor homology
- LBP
- lipopolysaccharide-binding protein
- CD14
- cluster of differentiation 14
- sCD14
- soluble cluster of differentiation 14
- Pam3CSK4
- (S)-[2,3-bis(palmitoyloxy)-(2-R,S)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH·3HCl
- OspA
- outer surface protein A
- Ac2CSK4
- (S)-[2,3-bis(acetyloxy)-(2-R,S)-propyl]-(R)-cysteinyl-(S)-seryl-(S)-lysyl-(S)-lysyl-(S)-lysyl-(S)-lysine × 3 CF3COOH
- Fc
- constant fragment of immunoglobulin G
- mCD14
- membrane-bound CD14.
REFERENCES
- 1. Medzhitov R. (2009) Approaching the asymptote: 20 years later. Immunity 30, 766–775 [DOI] [PubMed] [Google Scholar]
- 2. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 3. Iwasaki A., Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 [DOI] [PubMed] [Google Scholar]
- 4. Muzio M., Polentarutti N., Bosisio D., Prahladan M. K., Mantovani A. (2000) Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J. Leukoc. Biol. 67, 450–456 [DOI] [PubMed] [Google Scholar]
- 5. Zarember K. A., Godowski P. J. (2002) Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168, 554–561 [DOI] [PubMed] [Google Scholar]
- 6. O'Neill L. A. (2008) When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 [DOI] [PubMed] [Google Scholar]
- 7. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 8. Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672 [DOI] [PubMed] [Google Scholar]
- 10. Tobias P. S., Soldau K., Ulevitch R. J. (1986) Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J. Exp. Med. 164, 777–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. (1990) Structure and function of lipopolysaccharide binding protein. Science 249, 1429–1431 [DOI] [PubMed] [Google Scholar]
- 12. Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433 [DOI] [PubMed] [Google Scholar]
- 13. Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. (1988) The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 141, 547–552 [PubMed] [Google Scholar]
- 14. Setoguchi M., Nasu N., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. (1989) Mouse and human CD14 (myeloid cell-specific leucine-rich glycoprotein) primary structure deduced from cDNA clones. Biochim. Biophys. Acta 1008, 213–222 [DOI] [PubMed] [Google Scholar]
- 15. Antal-Szalmas P., Strijp J. A., Weersink A. J., Verhoef J., Van Kessel K. P. (1997) Quantitation of surface CD14 on human monocytes and neutrophils. J. Leukoc. Biol. 61, 721–728 [DOI] [PubMed] [Google Scholar]
- 16. Haziot A., Rong G. W., Silver J., Goyert S. M. (1993) Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J. Immunol. 151, 1500–1507 [PubMed] [Google Scholar]
- 17. Funda D. P., Tucková L., Farré M. A., Iwase T., Moro I., Tlaskalová-Hogenová H. (2001) CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect. Immun. 69, 3772–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. da Silva Correia J., Soldau K., Christen U., Tobias P. S., Ulevitch R. J. (2001) Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. J. Biol. Chem. 276, 21129–21135 [DOI] [PubMed] [Google Scholar]
- 19. Gioannini T. L., Teghanemt A., Zhang D., Levis E. N., Weiss J. P. (2005) Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J. Endotoxin Res. 11, 117–123 [DOI] [PubMed] [Google Scholar]
- 20. Teghanemt A., Widstrom R. L., Gioannini T. L., Weiss J. P. (2008) Isolation of monomeric and dimeric secreted MD-2. J. Biol. Chem. 283, 21881–21889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., Lee J. O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist eritoran. Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 22. Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 23. Ulevitch R. J., Tobias P. S. (1995) Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13, 437–457 [DOI] [PubMed] [Google Scholar]
- 24. Martin T. R., Mathison J. C., Tobias P. S., Letúrcq D. J., Moriarty A. M., Maunder R. J., Ulevitch R. J. (1992) Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J. Clin. Investig. 90, 2209–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ulevitch R. J., Tobias P. S. (1999) Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11, 19–22 [DOI] [PubMed] [Google Scholar]
- 26. Lien E., Sellati T. J., Yoshimura A., Flo T. H., Rawadi G., Finberg R. W., Carroll J. D., Espevik T., Ingalls R. R., Radolf J. D. (1999) Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274, 33419–33425 [DOI] [PubMed] [Google Scholar]
- 27. Hirschfeld M., Weis J. J., Toshchakov V., Salkowski C. A., Cody M. J., Ward D. C., Qureshi N., Michalek S. M., Vogel S. N. (2001) Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69, 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vignal C., Guérardel Y., Kremer L., Masson M., Legrand D., Mazurier J., Elass E. (2003) Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-α and IL-8 secretion by a CD14-Toll-like receptor 2-dependent mechanism. J. Immunol. 171, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 29. Hirschfeld M., Kirschning C. J., Schwandner R., Wesche H., Weis J. H., Wooten R. M., Weis J. J. (1999) Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163, 2382–2386 [PubMed] [Google Scholar]
- 30. Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. (1999) Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11, 443–451 [DOI] [PubMed] [Google Scholar]
- 31. Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. (1990) Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect. Immun. 58, 983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morrison T. B., Weis J. H., Weis J. J. (1997) Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158, 4838–4845 [PubMed] [Google Scholar]
- 34. Erdile L. F., Guy B. (1997) OspA lipoprotein of Borrelia burgdorferi is a mucosal immunogen and adjuvant. Vaccine 15, 988–996 [DOI] [PubMed] [Google Scholar]
- 35. Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G, Mays J. P. (1993) Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weis J. J., Ma Y., Erdile L. F. (1994) Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 62, 4632–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffmann P., Heinle S., Schade U. F., Loppnow H., Ulmer A. J., Flad H. D., Jung G., Bessler W. G. (1988) Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology 177, 158–170 [DOI] [PubMed] [Google Scholar]
- 38. Takeuchi O., Kaufmann A., Grote K., Kawai T., Hoshino K., Morr M., Mühlradt P. F., Akira S. (2000) Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2-and MyD88-dependent signaling pathway. J. Immunol. 164, 554–557 [DOI] [PubMed] [Google Scholar]
- 39. Takeuchi O., Kawai T., Mühlradt P. F., Morr M., Radolf J. D., Zychlinsky A., Takeda K., Akira S. (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13, 933–940 [DOI] [PubMed] [Google Scholar]
- 40. Guan Y., Ranoa D. R., Jiang S., Mutha S. K., Li X., Baudry J., Tapping R. I. (2010) Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J. Immunol. 184, 5094–5103 [DOI] [PubMed] [Google Scholar]
- 41. Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., Lee J. O. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 [DOI] [PubMed] [Google Scholar]
- 42. Jin M. S., Lee J. (2008) Structures of the Toll-like receptor family and its ligand complexes. Immunity 29, 182–191 [DOI] [PubMed] [Google Scholar]
- 43. Schröder N. W., Heine H., Alexander C., Manukyan M., Eckert J., Hamann L., Göbel U. B., Schumann R. R. (2004) Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 173, 2683–2691 [DOI] [PubMed] [Google Scholar]
- 44. Sellati T. J., Bouis D. A., Kitchens R. L., Darveau R. P., Pugin J., Ulevitch R. J., Gangloff S. C., Goyert S. M., Norgard M. V., Radolf J. D. (1998) Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160, 5455–5464 [PubMed] [Google Scholar]
- 45. Kelley S. L., Lukk T., Nair S. K., Tapping R. I. (2013) The crystal structure of human soluble CD14 reveals a bent solenoid with a hydrophobic amino-terminal pocket. J. Immunol. 190, 1304–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omueti K. O., Mazur D. J., Thompson K. S., Lyle E. A., Tapping R. I. (2007) The polymorphism P315L of human Toll-like receptor 1 impairs innate immune sensing of microbial cell wall components. J. Immunol. 178, 6387–6394 [DOI] [PubMed] [Google Scholar]
- 47. Belisle J. T., Brandt M. E., Radolf J. D., Norgard M. V. (1994) Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J. Bacteriol. 176, 2151–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vasselon T., Detmers P. A., Charron D., Haziot A. (2004) TLR2 recognizes a bacterial lipopeptide through direct binding. J. Immunol. 173, 7401–7405 [DOI] [PubMed] [Google Scholar]
- 49. Manukyan M., Triantafilou K., Triantafilou M., Mackie A., Nilsen N., Espevik T., Wiesmüller K. H., Ulmer A. J., Heine H. (2005) Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur. J. Immunol. 35, 911–921 [DOI] [PubMed] [Google Scholar]
- 50. Nakata T., Yasuda M., Fujita M., Kataoka H., Kiura K., Sano H., Shibata K. (2006) CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell. Microbiol. 8, 1899–1909 [DOI] [PubMed] [Google Scholar]
- 51. Schröder N. W., Morath S., Alexander C., Hamann L., Hartung T., Zähringer U., Göbel U. B., Weber J. R., Schumann R. R. (2003) Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278, 15587–15594 [DOI] [PubMed] [Google Scholar]
- 52. Krüger C., Schütt C., Obertacke U., Joka T., Müller F. E., Knöller J., Köller M., König W., Schönfeld W. (1991) Serum CD14 levels in poly traumatized and severely burned patients. Clin. Exp. Immunol. 85, 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wooten R. M., Morrison T. B., Weis J. H., Wright S. D., Thieringer R., Weis J. J. (1998) The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160, 5485–5492 [PubMed] [Google Scholar]
- 54. Sellati T. J., Bouis D. A., Caimano M. J., Feulner J. A., Ayers C., Lien E., Radolf J. D. (1999) Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163, 2049–2056 [PubMed] [Google Scholar]
- 55. Fan X., Stelter F., Menzel R., Jack R., Spreitzer I., Hartung T., Schütt C. (1999) Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect. Immun. 67, 2964–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elass E., Coddeville B., Guérardel Y., Kremer L., Maes E., Mazurier J., Legrand D. (2007) Identification by surface plasmon resonance of the mycobacterial lipomannan and lipoarabinomannan domains involved in binding to CD14 and LPS-binding protein. FEBS Lett. 581, 1383–1390 [DOI] [PubMed] [Google Scholar]
- 57. Weber J. R., Freyer D., Alexander C., Schröder N. W., Reiss A., Küster C., Pfeil D., Tuomanen E. I., Schumann R. R. (2003) Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity 19, 269–279 [DOI] [PubMed] [Google Scholar]
- 58. Opitz B., Schröder N. W., Spreitzer I., Michelsen K. S., Kirschning C. J., Hallatschek W., Zähringer U., Hartung T., Göbel U. B., Schumann R. R. (2001) Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-κB translocation. J. Biol. Chem. 276, 22041–22047 [DOI] [PubMed] [Google Scholar]
- 59. Jack R. S., Fan X., Bernheiden M., Rune G., Ehlers M., Weber A., Kirsch G., Mentel R., Fürll B., Freudenberg M., Schmitz G., Stelter F., Schütt C. (1997) Lipopolysaccharide-binding protein is required to combat a murine Gram-negative bacterial infection. Nature 389, 742–745 [DOI] [PubMed] [Google Scholar]
- 60. Wurfel M. M., Monks B. G., Ingalls R. R., Dedrick R. L., Delude R., Zhou D., Lamping N., Schumann R. R., Thieringer R., Fenton M. J., Wright S. D., Golenbock D. (1997) Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. J. Exp. Med. 186, 2051–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Knapp S., de Vos A. F., Florquin S., Golenbock D. T., van der Poll T. (2003) Lipopolysaccharide binding protein is an essential component of the innate immune response to Escherichia coli peritonitis in mice. Infect. Immun. 71, 6747–6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fierer J., Swancutt M. A., Heumann D., Golenbock D. (2002) The role of lipopolysaccharide binding protein in resistance to Salmonella infections in mice. J. Immunol. 168, 6396–6403 [DOI] [PubMed] [Google Scholar]
- 63. Branger J., Florquin S., Knapp S., Leemans J. C., Pater J. M., Speelman P., Golenbock D. T., van der Poll T. (2004) LPS-binding protein-deficient mice have an impaired defense against Gram-negative but not Gram-positive pneumonia. Int. Immunol. 16, 1605–1611 [DOI] [PubMed] [Google Scholar]
- 64. Branger J., Leemans J. C., Florquin S., Speelman P., Golenbock D. T., van der Poll T. (2005) Lipopolysaccharide binding protein-deficient mice have a normal defense against pulmonary mycobacterial infection. Clin. Immunol. 116, 174–181 [DOI] [PubMed] [Google Scholar]
- 65. Haziot A., Ferrero E., Köntgen F., Hijiya N., Yamamoto S., Silver J., Stewart C. L., Goyert S. M. (1996) Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 [DOI] [PubMed] [Google Scholar]
- 66. Haziot A., Hijiya N., Schultz K., Zhang F., Gangloff S. C., Goyert S. M. (1999) CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-α production. J. Immunol. 162, 4801–4805 [PubMed] [Google Scholar]
- 67. Ehlers S., Reiling N., Gangloff S., Woltmann A., Goyert S. (2001) Mycobacterium avium infection in CD14-deficient mice fails to substantiate a significant role for CD14 in antimycobacterial protection or granulomatous inflammation. Immunology 103, 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schimke J., Mathison J., Morgiewicz J., Ulevitch R. J. (1998) Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc. Natl. Acad. Sci. U.S.A. 95, 13875–13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Piazza M., Calabrese V., Baruffa C., Gioannini T., Weiss J., Peri F. (2010) The cationic amphiphile 3,4-bis(tetradecyloxy)benzylamine inhibits LPS signaling by competing with endotoxin for CD14 binding. Biochem. Pharmacol. 80, 2050–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Froon A. H., Dentener M. A., Greve J. W., Ramsay G., Buurman W. A. (1995) Lipopolysaccharide toxicity-regulating proteins in bacteremia. J. Infect. Dis. 171, 1250–1257 [DOI] [PubMed] [Google Scholar]
- 71. Zweigner J., Gramm H. J., Singer O. C., Wegscheider K., Schumann R. R. (2001) High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98, 3800–3808 [DOI] [PubMed] [Google Scholar]