Background: CB1 is activated by agonist CP55940 in a G protein-dependent manner.
Results: β-Arrestin 2 plays a role in CB1 internalization, whereas β-arrestin 1 is critical for ORG27569-induced ERK1/2, MEK1/2, and c-Src phosphorylation.
Conclusion: Allosteric modulator ORG27569 endows CB1 with downstream signaling selectivity.
Significance: This work discusses the first case of β-arrestin involvement in CB1-biased signaling.
Keywords: 7-Helix Receptor, Allosteric Regulation, Arrestin, G Protein-coupled Receptors (GPCR), Signal Transduction, CB1, Biased Signaling, Receptor Internalization
Abstract
The cannabinoid receptor 1 (CB1) is a G protein-coupled receptor primarily expressed in brain tissue that has been implicated in several disease states. CB1 allosteric compounds, such as ORG27569, offer enormous potential as drugs over orthosteric ligands, but their mechanistic, structural, and downstream effects upon receptor binding have not been established. Previously, we showed that ORG27569 enhances agonist binding affinity to CB1 but inhibits G protein-dependent agonist signaling efficacy in HEK293 cells and rat brain expressing the CB1 receptor (Ahn, K. H., Mahmoud, M. M., and Kendall, D. A. (2012) J. Biol. Chem. 287, 12070–12082). Here, we identify the mediators of CB1 receptor internalization and ORG27569-induced G protein-independent signaling. Using siRNA technology, we elucidate an ORG27569-induced signaling mechanism for CB1 wherein β-arrestin 1 mediates short term signaling to ERK1/2 with a peak at 5 min and other upstream kinase components including MEK1/2 and c-Src. Consistent with these findings, we demonstrate co-localization of CB1-GFP with red fluorescent protein-β-arrestin 1 upon ORG27569 treatment using confocal microscopy. In contrast, we show the critical role of β-arrestin 2 in CB1 receptor internalization upon treatment with CP55940 (agonist) or treatment with ORG27569. These results demonstrate for the first time the involvement of β-arrestin in CB1-biased signaling by a CB1 allosteric modulator and also define the differential role of the two β-arrestin isoforms in CB1 signaling and internalization.
Introduction
The cannabinoid receptor 1 (CB1)2 is a member of the class A rhodopsin-like G protein-coupled receptor (GPCR) family. It is localized on presynaptic nerve terminals and is thought to play a direct role in the inhibition of neurotransmitter release. It binds the main psychoactive component of Cannabis sativa (marijuana), Δ9-tetrahydrocannabinol, and has been implicated in several disease states. These include drug addiction, anxiety, depression, obesity, and chronic pain. The abundance of CB1 in the central nervous system makes it a valuable therapeutic target, including for treatment of anorexia in patients who suffer from AIDS wasting syndrome, reducing the nausea and vomiting associated with chemotherapy treatment, and relief of neuropathic pain in multiple sclerosis.
A few allosteric modulators of the CB1 receptor have been identified including ORG27569, ORG29647, ORG27759, and PSNCBAM-1 (1, 2). Interestingly, these compounds were found to be allosteric enhancers of agonist binding affinity but allosteric inhibitors of agonist signaling efficacy in HEK293 cells and rat brain expressing the CB1 receptor. More recently, an inhibitor of the dopamine transporter, RTI-371, was shown to increase the intrinsic activity of the CB1 agonist CP55940 in RD-HGA16 cells as a positive allosteric modulator (3). This suggests that allosteric modulatory activity of the CB1 receptor may play a role in the modulation of dopamine neurotransmission. Although the mechanistic and structural basis of receptor binding of these compounds and the consequent physiological effects have not been established, they offer enormous potential as drugs with advantages over orthosteric ligands. For instance, they can inhibit or potentiate orthosteric ligand binding affinity and/or modulate their signaling efficacy, whereas the orthosteric ligands only bind and act competitively. In addition, allosteric modulators can be designed to achieve high subtype selectivity by binding a highly sequence divergent domain. Furthermore, there is growing evidence showing that some allosteric modulators mediate receptor activation in their own right in addition to modulating orthosteric ligand pharmacology (4, 5). For instance, McN-A-343 and AC-42 inhibited the binding of N-methylscopolamine to rat M2 and human M1 muscarinic acetylcholine receptors and played a partial agonist role for the receptors in the absence of orthosteric ligand (6, 7).
The potential signaling diversity of GPCRs suggests the possible existence of multiple discrete active conformations (8–10). This implies that specific ligands might direct distinct signaling responses by preferentially stabilizing one or more of these active conformations. Thus, it is now widely believed that ligand efficacy is not a linear event but rather collateral (11). For example, carvedilol was found to act as an inverse agonist for cAMP production while displaying positive agonism for β-arrestin-mediated activation of ERK1/2 phosphorylation via the β2-adrenergic receptor (β2AR) (12). Allosteric modulators also may stabilize distinct receptor conformations, relative to those induced by orthosteric ligands, and therefore trigger a distinct repertoire of receptor signaling and receptor regulatory properties (4, 13).
It is well established that for most GPCRs, receptor phosphorylation by G protein-coupled receptor kinases (GRKs) and the subsequent recruitment of β-arrestin provide an important mechanism for GPCR desensitization, internalization, and trafficking (14–17). In addition to these classical functions of β-arrestins, accumulating evidence over the last decade demonstrates a novel function of β-arrestins, as signal transducers that scaffold various signaling molecules upon activation of GPCRs. By binding to both the nonreceptor tyrosine kinase c-Src and agonist-occupied β2-adrenergic receptor, β-arrestin 1 can confer tyrosine kinase activity upon the receptor (18, 19). A wide variety of extracellular signals transduced via numerous cell surface receptors and integrins activate MAPKs, including ERK1/2, which in turn play a major role in the integration of multiple biological responses such as cell proliferation, differentiation, and survival (20–22). Thus, regulation of MAPK activation is crucial for generating the proper physiological outcomes from a particular stimulus and consequently the impact on modulation of the activity of the transcription factor (23, 24). Two isoforms of β-arrestin, β-arrestin 1 and β-arrestin 2, have 78% sequence identity and are known to differentially regulate rhodopsin-like GPCR desensitization, internalization, and signaling. However, the detailed mechanisms by which they impact these events are largely unknown. Developing a better understanding of the molecular basis of β-arrestin selectivity, its multifaceted regulation, and delineation of the physiological consequences of arrestin signaling is extremely important.
In previous studies, we showed that the CB1 allosteric modulator ORG27569 promoted G protein-independent ERK1/2 signaling (25). By taking advantage of the constitutively active and inactive CB1 mutant receptors T210I and T210A along with the wild-type receptor, we elucidated the impact of ORG27569 on CB1 allostery. We established that ORG27569 induces a CB1 receptor state that is characterized by enhanced agonist affinity and decreased inverse agonist affinity consistent with an active form. Although ORG27569 antagonizes CP55940-induced GTPγS binding indicative of G protein coupling inhibition in a concentration-dependent manner, the ORG27569-induced conformational change of the CB1 receptor leads to cellular internalization and activation of downstream ERK1/2. Thus, our data provided the first case of allosteric ligand-biased signaling via CB1.
The goal of the present study is to identify the mediator of ORG27569-induced G protein-independent signaling and of receptor internalization using a siRNA strategy. We demonstrate that β-arrestin 2 plays a key role in CB1 internalization, whereas β-arrestin 1 is critical for ORG27569-induced ERK1/2 signaling. Our results also reveal other components involved in ORG27569-specific signaling. These signaling patterns were observed in HEK293 and neuronal cells. These results demonstrate for the first time the involvement of β-arrestin in CB1-biased signaling by a CB1 allosteric modulator and also define the differential role of the two β-arrestin isoforms in CB1 signaling and internalization.
EXPERIMENTAL PROCEDURES
CB1 Expression and siRNA Transfection
The plasmid DNA encoding the CB1 receptor and the corresponding C-terminally GFP-tagged receptor were described previously (26). HEK293 cells were maintained as described (27). Transfection was carried out using Lipofectamine (Invitrogen) according to the manufacturer's instructions. 24 h post-transfection, the cells were washed and incubated for an additional 24 h in serum-free growth medium. siRNA (Qiagen) transfection was carried out as described (28, 29). The siRNA sequences targeting β-arrestin 1 and β-arrestin 2 are 5′-CTCGACGTTCTGCAAGGTCTA-3′ and 5′-CTCGAACAAGATGACCAGGTA-3′, respectively, and nonsilencing RNA duplex was used as control. HEK293 cells that were 40–50% confluent in a 6-well plate were transfected with 2.6 μg of siRNA. Silencing of β-arrestin 1 and β-arrestin 2 expression was assessed by immunoblotting using anti-β-arrestin 1 (1: 2000; EMD Millipore, Billerica, MA) and anti-β-arrestin 2 (1:1000; Novus Biologicals, Littleton, CO) antibodies, respectively. Hippocampal cultures were prepared as described previously (30). In brief, dissociated hippocampal neurons from embryonic day 18 rats were plated on 15-mm-diameter coverslips coated with poly-d-lysine (Sigma-Aldrich) and laminin (ATCC, Manassas, VA) in Neurobasal medium supplemented with B-27, 25 μm glutamate, 500 μm glutamine (Invitrogen), and 2-mercaptoethanol at a density of ∼150/mm2 and maintained at 37 °C in a humidified 5% CO2 incubator. The cells were fed 5 days after plating and weekly thereafter with plating medium without glutamate and 2-mercaptoethanol.
Confocal Microscopy
HEK293 cells expressing the CB1 receptor C-terminally fused to GFP were seeded onto 35-mm glass-bottomed dishes (Matek, Ashland, MA) precoated with poly-d-lysine. The cells were treated with different compounds for various lengths of time and then washed three times with PBS, followed by fixation with 4% paraformaldehyde for 10 min at room temperature. For co-localization studies with a subcellular marker, the cells were permeabilized by 0.1% Triton X-100 in DMEM containing 5% normal goat serum, pH 7.6. After incubating with blocking solution (5% normal goat serum in DMEM) for 30 min at room temperature, the cells were incubated with the lysosomal-associated membrane protein 1 (LAMP-1) (H4A3) antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), diluted 1:200 in DMEM containing 5% normal goat serum. After washing with PBS, cells were incubated with Cy3-labeled donkey anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories) diluted 1:200 for 30 min at room temperature. For co-localization of CB1 and β-arrestins, HEK293 cells were co-transfected with plasmids encoding CB1 and β-arrestin 1 (Addgene, Cambridge, MA) or β-arrestin 2 (generous gift from Dr. Kenneth Mackie). 48 h post-transfection, the cells were treated with different compounds for various lengths of time as indicated and then washed three times with PBS, followed by fixation as described above. The cells were mounted in Vectashield mounting medium (Vector Laboratories) and visualized using a Leica TCS SP2 confocal microscope (Leica Microsystems, Wetzler, Germany). Images were collected from at least three independently transfected cell dishes and processed for presentation in figures using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Quantification of co-localization was performed using ImageJ software (National Institutes of Health) with the JACoP plugin (31) as described previously (31, 32). For the analysis, images of ∼9–15 cells were taken. Images for the receptor-GFP (green) and LAMP-1 or β-arrestins (red) were processed in parallel, and the background was subtracted and converted into 8-bit format for analysis. The extent of co-localization was quantified using the intensity correlation analysis. The Pearson's correlation coefficient (PCC) r, which provides an estimate of the goodness of co-localization, was determined (−1 = a negative correlation, 0 = no correlation, and +1 = a positive correlation). PCC (r) was presented as the mean ± S.E.
Immunoblotting Studies
Cells expressing CB1 receptors were exposed to the different compounds for the times indicated. The cells were washed with ice-cold PBS, and cell lysates were obtained by resuspending the cells with ice-cold lysis buffer consisting of 150 mm NaCl, 1.0% Igepal® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris, pH 7.5, and a protease inhibitor mixture 4-(2-aminoethyl)benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin (Sigma). The samples were heated at 95 °C for 5 min, resolved by SDS-PAGE gel electrophoresis in 10% gels, and transferred onto PVDF membrane. After blocking with blocking buffer (Fisher), the membrane was incubated for 1 h with the corresponding antibody (1:3000 phospho-p44/42 and p44/42 antibodies; 1:3000 phospho-MEK1/2 and MEK1/2 antibodies; 1:3000 phospho-Src and Src antibodies; 1:5000 phospho-AKT and AKT antibodies; Cell Signaling Technology, Danvers, MA). The membranes were washed with PBS and then incubated with anti-rabbit or anti-mouse peroxidase-conjugated secondary antibody (1:6000; Cell Signaling Technology) for 1 h at room temperature. The specific immunoreactive proteins were detected using the SuperSignal West Femto chemiluminescent substrate system (Thermo Fisher Scientific) following the manufacturer's protocol. Immunoreactive bands of phospho-ERK1/2, phospho-MEK1/2, phospho-Src, and phospho-Akt were quantified by densitometric analysis using the ImageJ program and normalized to the intensity of total-ERK1/2, MEK1/2, Src, and Akt, respectively. The data are expressed as a fold increase above the basal level of phosphorylation.
Data Analysis
Statistical analyses were calculated using GraphPad Prism 5 (GraphPad Software). The data are expressed as the means ± S.E. Statistical significances were assessed by one-way analysis of variance followed by Bonferroni's post hoc test when required.
RESULTS
Effect of β-Arrestin siRNA on CB1 Internalization
ORG27569 represents a novel class of CB1 ligands with unique properties (25). As a positive allosteric modulator, ORG27569 impacts agonist and inverse agonist affinity but inhibits G protein coupling (1, 25). It induces robust internalization of CB1 when co-treated with CP55940 and increases ERK1/2 signaling on its own via CB1, suggesting that this compound promotes an “active” conformation. Interestingly, unlike CP55940-induced ERK1/2 phosphorylation, ORG27569-induced ERK1/2 phosphorylation is Gi protein-independent. Thus, it possesses some agonistic properties as an “ago-allosteric modulator” (33–38).
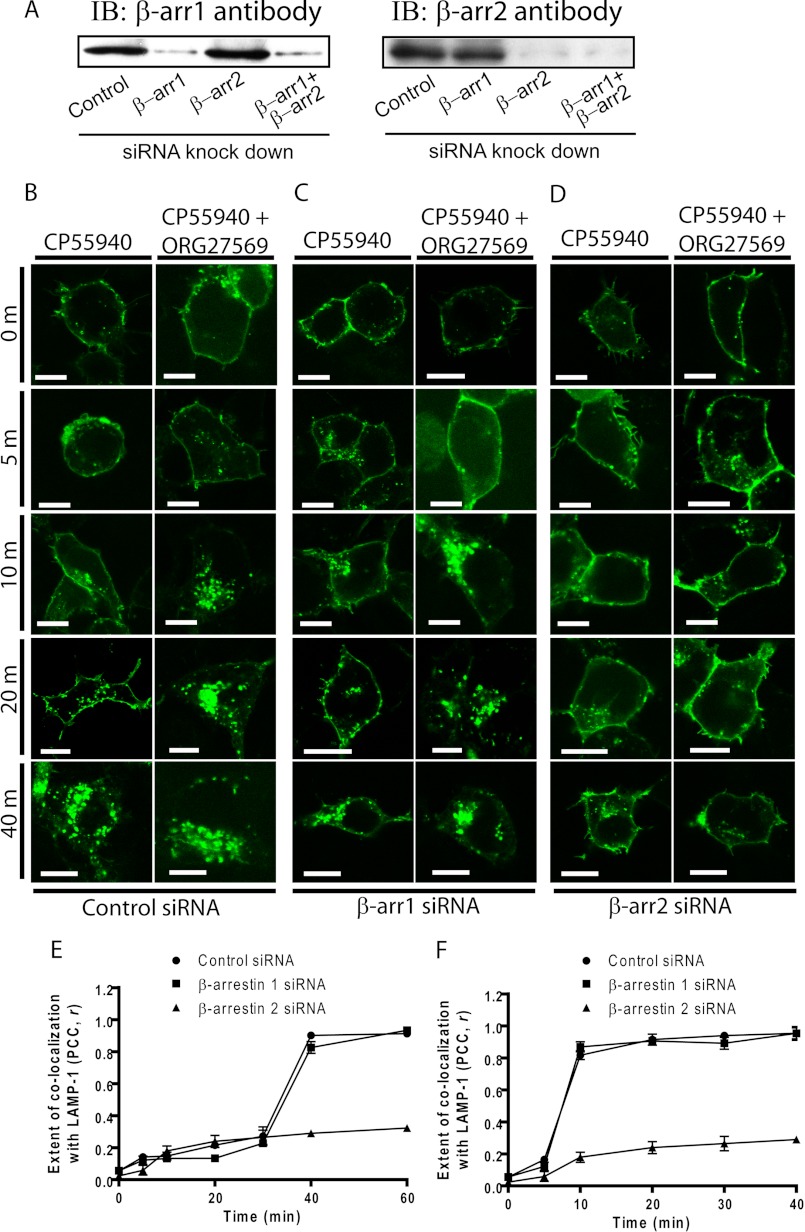
To evaluate the potential role of β-arrestins and determine which β-arrestin is involved in the robust receptor internalization induced by ORG27569, we knocked down the cellular expression of β-arrestin 1 and separately, β-arrestin 2, by transfecting cells with siRNA specifically directed against each isoform. Fig. 1A shows effective isoform-specific silencing of endogenous β-arrestin 1 and β-arrestin 2 (over 90%) by using the siRNAs targeting β-arrestin 1 and β-arrestin 2, respectively. Because the wild-type receptor localized mainly to intracellular vesicles in various cell lines in the absence of ligand (32, 39) consistent with its partial constitutive activity, we used the previously characterized inactive T210A mutant receptor, which is exclusively expressed at the cell surface (25, 26). The rate of receptor internalization after co-treatment with CP55940+ORG27569 was more rapid than that of CP55940-induced internalization (Fig. 1, B, E, and F). Maximum levels of receptor internalization were observed at 40 and 10 min for CP55940 treatment and ORG27569+CP55940 co-treatment, respectively. We used 0.5 μm CP55940 and 10 μm ORG27569 in this study, because our previous study (25) and unpublished pilot experimental data indicate that concentrations at and over 0.1 and 10 μm for CP55940 and ORG27569, respectively, produced maximum inhibition of GTPγS binding. Moreover, we observed a maximum effect on ERK1/2 signaling with over 0.1 and 10 μm concentrations for CP55940 and ORG27569, respectively (25). At the same time, to minimize possible secondary effects of those compounds, we tried to keep the compound concentrations as low as possible. The extent of internalization of the receptors from the cell surface was assessed by co-localization of the receptors with the late endosome marker, LAMP-1, as previously described (25, 32). Although suppressing β-arrestin 1 expression levels had no effect on CB1 internalization (Fig. 1C), both CP55940-induced and ORG27569+CP55940-induced receptor internalization was dramatically attenuated in β-arrestin 2 siRNA-transfected cells (Fig. 1D). These results clearly demonstrate that the CB1 receptor undergoes β-arrestin 2-mediated internalization following both CP55940 treatment and co-treatment of ORG27569+CP55940. Next, we examined the effect of siRNA-suppressed β-arrestin expression on CB1 internalization induced by ORG27569 alone because prolonged treatment with ORG27569 also induced slow receptor internalization after 3 h (25). Our results indicate that β-arrestin 2 is critical for ORG27569-induced receptor internalization (Fig. 2). Thus, β-arrestin 2, but not β-arrestin 1, appears to play a critical role in receptor internalization regardless of the rate of internalization induced by different compounds.
FIGURE 1.
Effect of siRNA-mediated suppression of β-arrestin levels on cellular internalization of the CB1 T210A-GFP receptor in the absence or presence of ORG27569. A, the representative immunoblot (IB) depicts isoform-specific silencing of endogenous β-arrestin 1 (β-arr1) or β-arrestin 2 (β-arr2) expression by siRNAs. B–D, HEK293 cells were co-transfected with either control (B), β-arrestin 1 (C), or β-arrestin 2 (D) siRNAs and plasmid encoding the T210A-GFP receptor. The HEK293 cells were treated with 0.5 μm CP55940 in the presence of vehicle alone (0.03% Me2SO; left columns) or ORG27569 (10 μm; right columns) for the times indicated before fixation. Scale bars, 15 μm. E and F, the extent of co-localization of CB1 and LAMP-1 (the marker for the late endosome/lysosome) is quantified using the intensity correlation analysis for CP55940 treatment (n = 9) (E) and co-treatment of CP55940+ORG27569 (n = 9) (F). The PCC r was calculated at each time point (−1 = a negative correlation, 0 = no correlation, and +1 = a positive correlation). PCC (r) is presented as the means ± S.E.
FIGURE 2.
Knockdown of β-arrestin 2 impairs the ORG27569-induced internalization of CB1 T210A-GFP. A–C, HEK293 cells were co-transfected with either control (A), β-arrestin 1 (B, β-arr1), or β-arrestin 2 (C, β-arr2) siRNAs and plasmid encoding the T210A-GFP receptor. The cells were incubated with 10 μm ORG27569 for 0, 3, and 4 h as indicated. After incubation, the cells were washed and fixed as described under “Experimental Procedures.” Localization of GFP-tagged receptor (green, left columns), the late endosome/lysosome marker, LAMP-1 (red, middle columns), and an overlay of the fluorescence images (yellow, right columns) are shown. The images are representative of at least three independent transfections that produced similar results. Scale bars, 15 μm (see A). D, the extent of co-localization quantified using the intensity correlation analysis is shown as described in the Fig. 1 legend. Nine images of each condition were analyzed (n = 9).
β-Arrestin 1, but Not β-Arrestin 2 Is Required for ORG27569-induced ERK1/2 Phosphorylation
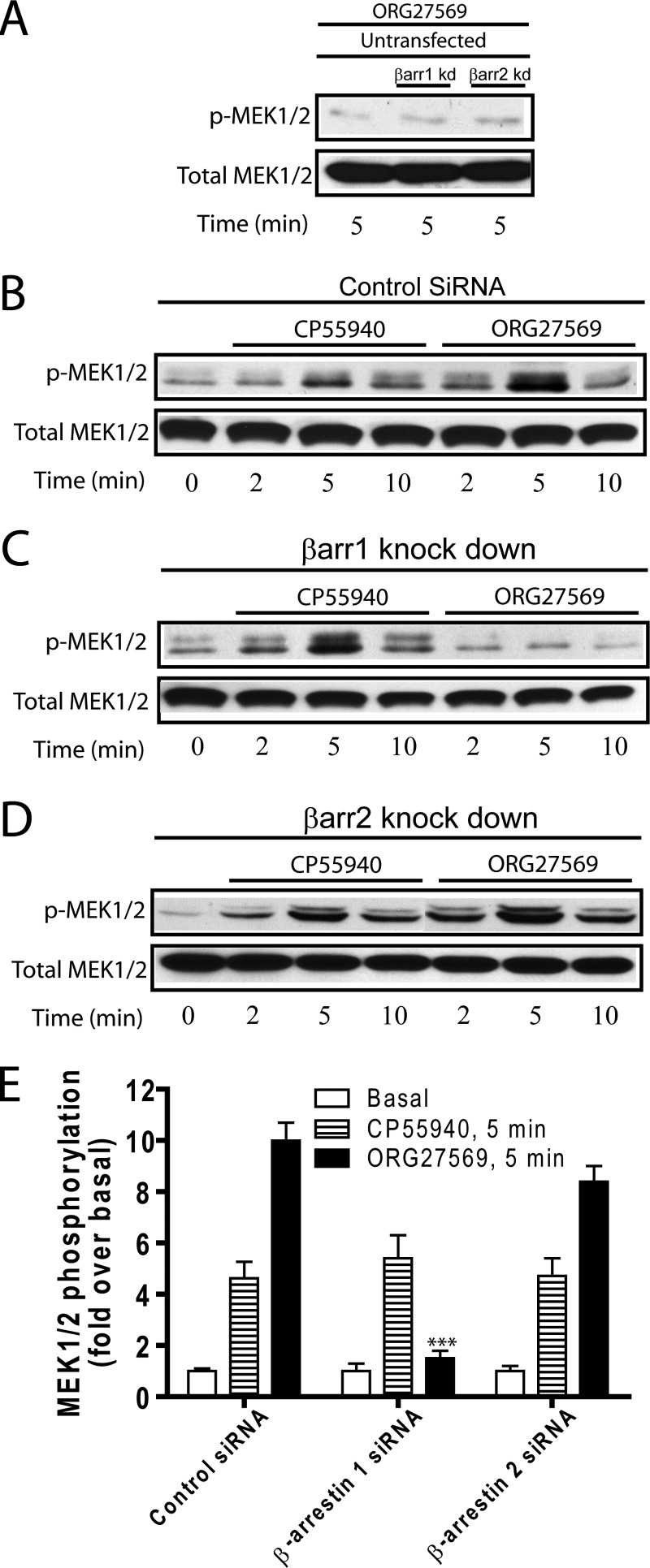
To determine whether β-arrestins contribute to the G protein-independent ERK1/2 activation induced by ORG27569, we again used siRNA transfection to silence the expression of endogenous β-arrestin 1 or β-arrestin 2. Because ORG27569 functions in the absence and presence of CP55940, treatment by these compounds alone was compared for ease of interpretation. In mock transfected cells as control, suppression of β-arrestin expression showed no effect on ORG27569-induced ERK1/2 phosphorylation (Fig. 3A), suggesting that the ORG27569 does not induce non-CB1-mediated ERK1/2 phosphorylation in HEK293 cells. Consistent with previous results, ERK1/2 phosphorylation reached maximal levels at 5 min of treatment with 0.5 μm CP55940 or 10 μm ORG27569 and then rapidly declined by 10 min. Western blot analysis of each sample lysate was performed with phospho-ERK1/2 (Fig. 3, A–D, upper boxes) and total (Fig. 3, A–D, lower boxes) ERK1/2 antibodies for comparison. Interestingly, although ORG27569 treatment did not significantly alter the time course of ERK1/2 phosphorylation, it produced a significantly higher increase in ERK1/2 phosphorylation compared with the stimulation by CP55940 in HEK293 cells expressing CB1 (Fig. 3B). Most strikingly, Fig. 3C shows that the reduced expression of β-arrestin 1 nearly abolished ORG27569-induced ERK1/2 phosphorylation, whereas co-transfection with β-arrestin 2 siRNA did not alter patterns of ERK1/2 phosphorylation compared with those shown by control siRNA transfection (Fig. 3D). In contrast, treatment with β-arrestin 1 or β-arrestin 2 siRNA did not change the CP55940-induced response consistent with agonist-induced G protein coupling and signaling (Fig. 3E).
FIGURE 3.
Inhibition of ORG27569-stimulated ERK1/2 phosphorylation by β-arrestin 1 siRNA using CB1 wild-type receptor. A, mock transfected HEK293 cells were used as control. B–D, HEK293 cells co-expressing CB1 wild type and either control (B), β-arrestin 1 (C, βarr1), or β-arrestin 2 (D, βarr2) siRNAs were exposed to CP55940 (0.5 μm) or ORG27569 (10 μm) for 0, 2, 5, or 10 min as indicated. Cell lysates were separated on SDS-PAGE and analyzed by Western blots probed with phospho-ERK1/2 (p-ERK1/2) followed by an HRP-conjugated anti-rabbit secondary antibody. The total level of ERK1/2 was detected for comparison. Immunoreactive signals were visualized by a chemiluminescent substrate system as described under “Experimental Procedures.” The levels of phosphorylation for the untransfected HEK293 cells are shown as a control. Representative blots of phosphorylated and total ERK1/2 of at least three separate experiments are shown for each condition. Note that the two bands correspond to the predominant isoforms, p42 (ERK2) and p44 (ERK1), for ERK signaling. E, graphs provide the quantified ERK1/2 phosphorylation levels for 5 and 10 min deduced from at least three experiments. The data are expressed as fold increases above the basal level of phosphorylation. The data represent the means ± S.E. of at least three independent experiments. Each treatment under the β-arrestin knockdown conditions was compared with its corresponding treatment under the control siRNA condition to determine the statistical significance of the differences using one-way analysis of variance and Bonferroni's post hoc test. *, p < 0.05; **, p < 0.01; ***, p < 0.005. F, β-arrestin 1 recruitment in HEK293 cells expressing wild-type CB1 receptor. HEK293 cells co-expressing the wild-type CB1-GFP and red fluorescent protein-β-arrestin 1 were exposed to ORG27569 for 0, 5, 15, 30, and 60 min. Cellular distribution of CB1-GFP and red fluorescent protein-β-arrestin 1 and the extent of co-localization between the two proteins were determined by confocal fluorescence microscopy as described in the legends to Figs. 1 and 2.
Because our data show that β-arrestin 1 plays a critical role in ORG27569-induced ERK1/2 phosphorylation, we hypothesized that β-arrestin 1 might interact with ORG27569-activated CB1 to form a signaling complex. We therefore co-expressed CB1-GFP and red fluorescent protein-β-arrestin 1 in HEK293 cells. In the absence of ligand, the wild-type receptor mainly resided in the endosome, whereas β-arrestin 1 was found to be distributed evenly throughout the cytoplasm. The addition of 10 μm ORG27569 for 5 min resulted in strong co-localization of the receptor and β-arrestin 1, suggesting that they become spatially sufficiently close to form a complex (Fig. 3F). The extent of co-localization then rapidly declined by 10 min, concomitant with ERK1/2 phosphorylation (Fig. 3B). No significant co-localization with β-arrestin 1 was observed with CP55940 treatment.
β-Arrestin 1 Is Required for ERK1/2 Phosphorylation in ORG27569-dependant Signaling
To elucidate the ORG27569-induced biased signaling mechanisms, we investigated the activation of upstream kinases resulting from the ORG-induced CB1-β-arrestin 1 interaction. These include the MEK1/2, Src, and Akt kinases. First, phosphorylation of MEK1/2, an upstream kinase (MAPKK) of MAPK in the MEK-ERK signaling pathway, was assessed to evaluate the effect of β-arrestin on its signaling. Not surprisingly, the overall pattern of MEK1/2 phosphorylation was comparable to that of ERK1/2 phosphorylation (Fig. 4). In control siRNA transfection, both CP55940 and ORG27569 produced a peak at 5 min and then diminished after 10 min (Fig. 4B). The silencing of β-arrestin 1 resulted in abolishing MEK1/2 phosphorylation in treatment with ORG27569 but had no significant effect on CP55940-induced phosphorylation (Fig. 4C). Silencing of β-arrestin 2 had no effect on either (Fig. 4, D and E). Thus, we demonstrated that the MEK1/2-ERK1/2 pathway is β-arrestin 1-dependent upon ORG27569 treatment of CB1.
FIGURE 4.
Inhibition of ORG27569-stimulated MEK1/2 phosphorylation by β-arrestin 1 siRNA using CB1 wild type. A, mock transfected HEK293 cells were used as control. B–D, HEK293 cells co-transfected with CB1 wild type and either control (B), β-arrestin 1 (C, βarr1), or β-arrestin 2 (D, βarr2) siRNAs were exposed to CP55940 (0.5 μm) or ORG27569 (10 μm) for 0, 2, 5, or 10 min as indicated. The cell lysates were analyzed by Western blots probed with phospho-MEK1/2 (p-MEK1/2) followed by an HRP-conjugated anti-rabbit secondary antibody. The total level of MEK1/2 was also detected for comparison using MEK1/2 antibody. The levels of phosphorylation for the mock transfected HEK293 cells are shown as a control. Representative blots of phosphorylated and total MEK1/2 of at least three separate experiments that produced similar results are shown for each condition. E, graphs provide the quantified MEK1/2 phosphorylation levels for 5 and 10 min deduced from at least three experiments. The data are expressed as fold increases above the basal level of phosphorylation. The data represent the means ± S.E. of at least three independent experiments. Each treatment under the β-arrestin knockdown conditions was compared with its corresponding treatment under the control siRNA condition to determine the statistical significance of the difference using one-way analysis of variance and Bonferroni's post hoc test. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
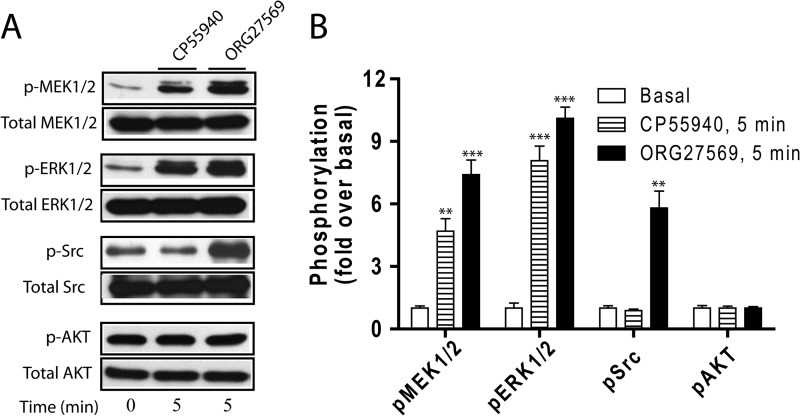
Second, we assessed the activation of further upstream kinases, Src and Akt, to better understand the ORG27569-biased signaling pathway. Src phosphorylation is a necessary step in the activation of various mitogenic signaling pathways activated by GPCRs. It has been shown that nonreceptor tyrosine kinases from the Src family are recruited to GPCRs via β-arrestin interactions (40, 41). Akt is also known to play a role in the β-arrestin-mediated ERK1/2 signaling pathway (42, 43). Interestingly, unlike phosphorylation of ERK1/2 or MEK1/2, CP55940 failed to induce Src phosphorylation, whereas ORG27569 produced a peak of Src phosphorylation at 5 min (Fig. 5A). Fig. 5 (B and C) show that Src phosphorylation only required β-arrestin 1 but not β-arrestin 2. Collectively, these data suggest that the Src pathway is only activated by ORG27569 and does so via β-arrestin 1 interaction (Fig. 5D). We also tested the effect of ORG27569 on Akt phosphorylation. Both CP55940 and ORG27569 failed to alter the levels of Akt phosphorylation compared with the basal level of phospho-Akt (data not shown). Therefore, no effect of β-arrestin siRNAs on ORG27569-induced Akt phosphorylation was observed.
FIGURE 5.
Effect of siRNA-mediated suppression of β-arrestin levels on Src phosphorylation using CB1 wild type. A–C, HEK293 cells co-transfected with CB1 wild type and either control (A), β-arrestin 1 (B, βarr1), or β-arrestin 2 (C, βarr2) siRNAs were treated with CP55940 (0.5 μm) or ORG27569 (10 μm) for 0, 2, 5, or 10 min as indicated. The cell lysates were analyzed as described in Fig. 3 using phospho-Src and total Src antibodies. Representative blots of phosphorylated and total kinases of at least three separate experiments are shown for each condition. D, graphs provide the quantified phosphorylation levels for 5 and 10 min deduced from at least three experiments. The data are expressed as fold increases above the basal level of phosphorylation. The data represent the means ± S.E. of at least three independent experiments. Each treatment under the β-arrestin knockdown conditions was compared with its corresponding treatment under the control siRNA condition to determine the statistical significance of the differences using one-way analysis of variance and Bonferroni's post hoc test. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
To ensure that the effect of ORG27569 on the Src-MEK1/2-ERK1/2 signaling cascade is not limited to HEK293 cells, we tested the effect of ORG27569 on the activation of the signaling components using rat hippocampal neurons endogenously expressing the CB1 receptor. Consistent with the phosphorylation patterns in HEK293 cells, ORG27569 treatment resulted in increased phosphorylation of ERK1/2, MEK1/2, and Src compared with basal levels of each component, whereas Akt phosphorylation remained unaffected by CP55940 or ORG27569 (Fig. 6).
FIGURE 6.
CP55940- and ORG27569-induced ERK1/2, MEK1/2, Src, and Akt signaling in hippocampal neuronal cells. A, rat hippocampal neurons endogenously expressing CB1 were exposed to CP55940 (0.5 μm) or ORG27569 (10 μm) for 5 min. Cell lysates were resolved by SDS-PAGE, and ERK1/2, MEK1/2, Src, and Akt phosphorylation was detected as described in the legend to Fig. 4. Representative blots of phosphorylated and total kinases are depicted. B, graphs show the quantified phosphorylation level of each kinase deduced from three experiments. The data are expressed as fold increases above basal levels of phosphorylation. The data represent the means ± S.E. of at least three independent experiments. Statistical significance of the differences was assessed using one-way analysis of variance and Bonferroni's post hoc test., *, p < 0.05; **, p < 0.01; ***, p < 0.005.
DISCUSSION
A majority of the Food and Drug Administration-approved drugs that act on GPCRs bind at the primary orthosteric site of the receptor and regulate receptor function by directly stimulating receptor response, blocking constitutive receptor activity, or blocking the binding of the native agonist. Despite the abundance of such drugs, efforts have failed to produce highly selective compounds that produce little to no side effects. In recent years, however, much progress has been made in the discovery and pharmacological optimization of allosteric modulators that bind a site distinct from the primary orthosteric ligand sites. Allosteric modulators have great potential for selectivity because they target unique regions on the receptor. These allosteric modulators promote a novel conformation of the GPCR that affects orthosteric ligand affinity and/or efficacy and results in the activation of any number of downstream signaling cascades. Some allosteric modulators have also been shown to act as agonists that are able to engender receptor activation in their own right. This class of compounds is referred to as “allosteric agonists.” We have determined that the CB1 allosteric modulator ORG27569 is functionally selective. Although ORG27569 inhibited G protein coupling, it biases signaling toward β-arrestin-dependent ERK1/2 pathways mediated by CB1.
Since the first evidence that β-arrestins can scaffold the tyrosine kinase c-Src to activate ERK1/2 (44, 45), other components for β-arrestin-dependent signaling have been identified including MAPK, PI3K-Akt, and NF-κB pathways (reviewed in Ref. 46). This finding changed the widely held notion of the linear response of GPCR activation and uncovered the multidimensional signaling modes of receptors. However, the specificity of recruitment of these signaling components and the molecular basis underlying specific pathway selectivity are largely unknown. More recently, structural studies using x-ray crystallography and NMR shed light on the structural basis of biased signaling via β1AR and β2AR (47, 48). The biased β-blockers bucindolol and carvedilol stimulate G protein-independent arrestin-mediated signaling while acting as inverse agonists of the G protein pathway. Interestingly, they require additional residues to interact with, which include transmembrane helices 2, 3, and 7 and the extracellular loop 2, compared with other structurally characterized inverse agonists for the β1ARs (47). The major structural changes that result in binding and activation of G protein by GPCRs include large outward movements of transmembrane helices 5 and 6 (49). In contrast, using site-specific 19F-NMR, Liu et al. (48) recently demonstrated that the β-arrestin-biased ligands predominantly impact the conformational states of transmembrane helix 7 of the β2AR, suggesting that the biased ligand promotes distinct conformational changes upon binding, which leads to different effector activation (e.g., β-arrestin).
The localization of the binding site(s) of ORG27569 on the receptor is unknown. Although most GPCR allosteric binding sites have been identified on the extracellular surface of GPCRs, some sites have been proposed to reside on the intracellular face. Mutational analysis identified Lys-320 at the N terminus of helix 8 of CXCR2 as a critical intracellular allosteric antagonist binding site (50). More recently Dowal et al. (51) demonstrated the importance of helix 8 in the activity of the proteinase-activated receptor 1 allosteric modulator. Although the ORG27569 binding site in CB1 is unidentified, it follows that ORG27569 is positioned to bind CB1 and be effective in altering the conformation of the receptor. Thus, the resulting CB1 receptor is structurally sufficiently distinct to preferentially couple to the β-arrestin signaling pathway over the G protein signaling pathway. Because the candidate contact site of the CB1 receptor with G protein is the intracellular surface of the receptor, particularly intracellular loops 2 and 3 and helix 8, as suggested from the x-ray structure of the β2AR-Gs protein complex (49), it is expected that these G protein interfacial regions are structurally altered by ORG27569 binding and become poorly fitted for G protein coupling. Moreover, our observation that β-arrestin 1 is critical for ORG27569-induced ERK1/2 signaling indicates that the ORG27569-induced CB1 receptor conformational change not only prefers to bind β-arrestin over G protein but also becomes specific enough to selectively bind β-arrestin 1 over β-arrestin 2.
An important finding of the current study is that two different β-arrestin isoforms regulate distinct cellular events upon ORG27569 binding. Our results indicate that ORG27569 induces ERK1/2 phosphorylation and robust receptor internalization. Those signaling and internalization events are mediated by β-arrestin 1 and β-arrestin 2, respectively. Conformational changes of GPCRs upon activation are recognized by immediate downstream effectors such as GRKs to initiate specific sets of functional responses. It has been shown that although the role of GRK subtypes is receptor- and expression system-dependent, GRK2 and 3 are usually responsible for agonist-dependent desensitization and internalization, whereas GRK5 and 6 are often required for β-arrestin-mediated ERK activation (29, 52, 53). This suggests that different subtypes of GRKs phosphorylate distinct Ser/Thr residues on the receptor, which in turn recruit different β-arrestin subtypes to regulate specific functions. Given that β-arrestins recognize specific phosphorylated conformations of the receptor by GRKs, ORG27569 may promote a conformational change that facilitates phosphorylation by different GRK subtypes than CP55940.
The experiments here establish that ERK and MEK can be activated via G protein or β-arrestin 1 coupling to CB1 as has been observed for the angiotensin II type 1a receptors (45), the proteinase-activated receptor 2 (44), and the muscarinic acetylcholine receptor M2 (54). We also establish that Src can only be activated via β-arrestin 1 coupling as was shown for the β2AR (19, 55) and the neurokinin-1 receptor (56) (Fig. 7). Our results, for the first time, outline the specific roles of β-arrestin 1 in ORG27569-induced biased signaling and β-arrestin 2 in robust receptor internalization. Although we cannot rule out the possibility of ligand-specific differences, it is important to note that β-arrestin 2 was required for internalization via both the CP55940 agonist and the allosteric modulator ORG27569 regardless of the rate of internalization induced by the different compounds. The inactive T210A receptor was used for internalization studies because it is exclusively expressed at the cell surface (25, 26), whereas the wild-type receptor is localized mainly to intracellular vesicles in the absence of ligand (32, 39). Although some populations of the wild-type receptors can localize to the cell surface upon SR141716A treatment (data not shown), it is extremely difficult to thoroughly wash out the SR141716A before CP55940 or ORG27569 treatment. To avoid any residual effects the SR141716A may have on CP55940 and ORG27569 effective concentrations and the kinetics of internalization, we opted to use the T210A receptor. However, we cannot rule out completely the possibility that the wild-type and T210A receptors may have subtle differences in their internalization rates upon treatment with compounds.
FIGURE 7.
Proposed model of the ORG27569-induced β-arrestin 1-mediated ERK1/2 signaling. ORG27569 binding to CB1 promotes β-arrestin 1 recruitment which in turn recruits Src, MEK1/2, and ERK1/2 kinases to form a signaling complex.
Consistent with our previous findings (25), Baillie et al. (57) recently reported that although ORG27569 inhibited CP55940-induced and basal levels of GTPγS binding, it induced ERK1/2 phosphorylation in both the absence and presence of CB1 agonist. However, in contrast to the data presented here, they reported that ORG27569 alone has no effect on β-arrestin recruitment and that it instead acts as an agonist of Gs signaling and a weak partial agonist of Gi-mediated ERK signaling. A few differences in the assays used may account for the discrepancies. Although the β-arrestin recruitment assays that were used by Baillie et al. measured the levels of recruitment of β-arrestin following at least a 150-min incubation, we directly assessed the effect of both β-arrestin 1 and 2 separately using isoform-specific siRNA techniques on ERK1/2 phosphorylation between 0 and 30 min of compound treatment in which ORG27569-induced ERK1/2 phosphorylation occurs. Moreover, whereas we used HEK293 cells and neuronal cells, Chinese hamster ovary cells were used in all of the functional assays by Baillie et al. Thus, cell type-specific downstream signaling pathways induced by CP55940 and ORG27569 are another possible explanation for this difference.
Although the amino acid sequences of the two β-arrestin isoforms are 78% identical with most of the coding differences in the C termini, our results do not support redundant functional roles for the β-arrestin isoforms in CB1 signaling and internalization. Our results suggest that ORG27569 induces a novel receptor form that has distinct β-arrestin requirements for internalization and signaling. This difference in receptor selectivity provides an avenue by which the CB1 functional response can be regulated.
Acknowledgment
We thank Dr. R. S. Walikonis (Department of Physiology and Neurobiology, University of Connecticut) for providing hippocampal neuronal cells.
This work was supported, in whole or in part, by National Institutes of Health Grant DA020763 (to D. A. K.).
- CB1
- cannabinoid receptor 1
- GPCR
- G protein-coupled receptor
- ORG27569
- 5-chloro-3-ethyl-1H-indole-2-carboxylic acid [2-(4-piperidin-1-yl-phenyl)-ethyl]-amide
- CP55940
- (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)-phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- HEK293
- human embryonic kidney cell
- LAMP-1
- lysosomal associated membrane protein 1
- GRK
- G protein-coupled receptor kinase
- AR
- adrenergic receptor
- PCC
- Pearson's correlation coefficient
- GTPγS
- guanosine 5′-O-(thiotriphosphate).
REFERENCES
- 1. Price M. R., Baillie G. L., Thomas A., Stevenson L. A., Easson M., Goodwin R., McLean A., McIntosh L., Goodwin G., Walker G., Westwood P., Marrs J., Thomson F., Cowley P., Christopoulos A., Pertwee R. G., Ross R. A. (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 68, 1484–1495 [DOI] [PubMed] [Google Scholar]
- 2. Horswill J. G., Bali U., Shaaban S., Keily J. F., Jeevaratnam P., Babbs A. J., Reynet C., Wong Kai In P. (2007) PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 152, 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Navarro H. A., Howard J. L., Pollard G. T., Carroll F. I. (2009) Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br. J. Pharmacol. 156, 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y., Rodriguez A. L., Conn P. J. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 315, 1212–1219 [DOI] [PubMed] [Google Scholar]
- 5. Knudsen L. B., Kiel D., Teng M., Behrens C., Bhumralkar D., Kodra J. T., Holst J. J., Jeppesen C. B., Johnson M. D., de Jong J. C., Jorgensen A. S., Kercher T., Kostrowicki J., Madsen P., Olesen P. H., Petersen J. S., Poulsen F., Sidelmann U. G., Sturis J., Truesdale L., May J., Lau J. (2007) Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc. Natl. Acad. Sci. U.S.A. 104, 937–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langmead C. J., Fry V. A., Forbes I. T., Branch C. L., Christopoulos A., Wood M. D., Herdon H. J. (2006) Probing the molecular mechanism of interaction between 4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the muscarinic M(1) receptor. Direct pharmacological evidence that AC-42 is an allosteric agonist. Mol. Pharmacol. 69, 236–246 [DOI] [PubMed] [Google Scholar]
- 7. Valant C., Gregory K. J., Hall N. E., Scammells P. J., Lew M. J., Sexton P. M., Christopoulos A. (2008) A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 283, 29312–29321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez D. M., Hwa J., Gaivin R., Mathur M., Brown F., Graham R. M. (1996) Constitutive activation of a single effector pathway. Evidence for multiple activation states of a G protein-coupled receptor. Mol. Pharmacol. 49, 112–122 [PubMed] [Google Scholar]
- 9. Palanche T., Ilien B., Zoffmann S., Reck M. P., Bucher B., Edelstein S. J., Galzi J. L. (2001) The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J. Biol. Chem. 276, 34853–34861 [DOI] [PubMed] [Google Scholar]
- 10. Galandrin S., Bouvier M. (2006) Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 70, 1575–1584 [DOI] [PubMed] [Google Scholar]
- 11. Kenakin T. (2007) Collateral efficacy in drug discovery. Taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol. Sci. 28, 407–415 [DOI] [PubMed] [Google Scholar]
- 12. Wisler J. W., DeWire S. M., Whalen E. J., Violin J. D., Drake M. T., Ahn S., Shenoy S. K., Lefkowitz R. J. (2007) A unique mechanism of β-blocker action. Carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L., Martin B., Brenneman R., Luttrell L. M., Maudsley S. (2009) Allosteric modulators of G protein-coupled receptors. Future therapeutics for complex physiological disorders. J. Pharmacol. Exp. Ther. 331, 340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferguson S. S. (2001) Evolving concepts in G protein-coupled receptor endocytosis. The role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 15. Howlett A. C., Mukhopadhyay S. (2000) Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem. Phys. Lipids 108, 53–70 [DOI] [PubMed] [Google Scholar]
- 16. Hsieh C., Brown S., Derleth C., Mackie K. (1999) Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 73, 493–501 [DOI] [PubMed] [Google Scholar]
- 17. Martini L., Waldhoer M., Pusch M., Kharazia V., Fong J., Lee J. H., Freissmuth C., Whistler J. L. (2007) Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 21, 802–811 [DOI] [PubMed] [Google Scholar]
- 18. Tohgo A., Choy E. W., Gesty-Palmer D., Pierce K. L., Laporte S., Oakley R. H., Caron M. G., Lefkowitz R. J., Luttrell L. M. (2003) The stability of the G protein-coupled receptor-β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 278, 6258–6267 [DOI] [PubMed] [Google Scholar]
- 19. Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 [DOI] [PubMed] [Google Scholar]
- 20. Schaeffer H. J., Weber M. J. (1999) Mitogen-activated protein kinases. Specific messages from ubiquitous messengers. Mol. Cell Biol. 19, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis T. S., Shapiro P. S., Ahn N. G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74, 49–139 [DOI] [PubMed] [Google Scholar]
- 22. Ahn S., Wei H., Garrison T. R., Lefkowitz R. J. (2004) Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by β-arrestins 1 and 2. J. Biol. Chem. 279, 7807–7811 [DOI] [PubMed] [Google Scholar]
- 23. Witherow D. S., Garrison T. R., Miller W. E., Lefkowitz R. J. (2004) β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc. Natl. Acad. Sci. U.S.A. 101, 8603–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vibhuti A., Gupta K., Subramanian H., Guo Q., Ali H. (2011) Distinct and shared roles of β-arrestin-1 and β-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One 6, e19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahn K. H., Mahmoud M. M., Kendall D. A. (2012) Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 287, 12070–12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'Antona A. M., Ahn K. H., Kendall D. A. (2006) Mutations of CB1 T210 produce active and inactive receptor forms. Correlations with ligand affinity, receptor stability, and cellular localization. Biochemistry 45, 5606–5617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn K. H., Bertalovitz A. C., Mierke D. F., Kendall D. A. (2009) Dual role of the second extracellular loop of the cannabinoid receptor 1. Ligand binding and receptor localization. Mol. Pharmacol. 76, 833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahn S., Nelson C. D., Garrison T. R., Miller W. E., Lefkowitz R. J. (2003) Desensitization, internalization, and signaling functions of β-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 100, 1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shenoy S. K., Drake M. T., Nelson C. D., Houtz D. A., Xiao K., Madabushi S., Reiter E., Premont R. T., Lichtarge O., Lefkowitz R. J. (2006) β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 30. Lim C. S., Walikonis R. S. (2008) Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell Signal. 20, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 32. Ahn K. H., Nishiyama A., Mierke D. F., Kendall D. A. (2010) Hydrophobic residues in helix 8 of cannabinoid receptor 1 are critical for structural and functional properties. Biochemistry 49, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis A. A., Heilman C. J., Brady A. E., Miller N. R., Fuerstenau-Sharp M., Hanson B. J., Lindsley C. W., Conn P. J., Lah J. J., Levey A. I. (2010) Differential effects of allosteric M(1) muscarinic acetylcholine receptor agonists on receptor activation, arrestin 3 recruitment, and receptor downregulation. ACS Chem. Neurosci. 1, 542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gregory K. J., Sexton P. M., Christopoulos A. (2007) Allosteric modulation of muscarinic acetylcholine receptors. Curr. Neuropharmacol. 5, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klaasse E. C., van den Hout G., Roerink S. F., de Grip W. J., Ijzerman A. P., Beukers M. W. (2005) Allosteric modulators affect the internalization of human adenosine A1 receptors. Eur. J. Pharmacol. 522, 1–8 [DOI] [PubMed] [Google Scholar]
- 36. Langmead C. J., Christopoulos A. (2006) Allosteric agonists of 7TM receptors. Expanding the pharmacological toolbox. Trends Pharmacol. Sci. 27, 475–481 [DOI] [PubMed] [Google Scholar]
- 37. Pelkey K. A., Yuan X., Lavezzari G., Roche K. W., McBain C. J. (2007) mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology 52, 108–117 [DOI] [PubMed] [Google Scholar]
- 38. Thomas R. L., Langmead C. J., Wood M. D., Challiss R. A. (2009) Contrasting effects of allosteric and orthosteric agonists on m1 muscarinic acetylcholine receptor internalization and down-regulation. J. Pharmacol. Exp. Ther. 331, 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leterrier C., Bonnard D., Carrel D., Rossier J., Lenkei Z. (2004) Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 279, 36013–36021 [DOI] [PubMed] [Google Scholar]
- 40. Barlic J., Andrews J. D., Kelvin A. A., Bosinger S. E., DeVries M. E., Xu L., Dobransky T., Feldman R. D., Ferguson S. S., Kelvin D. J. (2000) Regulation of tyrosine kinase activation and granule release through β-arrestin by CXCRI. Nat. Immunol. 1, 227–233 [DOI] [PubMed] [Google Scholar]
- 41. Imamura T., Huang J., Dalle S., Ugi S., Usui I., Luttrell L. M., Miller W. E., Lefkowitz R. J., Olefsky J. M. (2001) β-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J. Biol. Chem. 276, 43663–43667 [DOI] [PubMed] [Google Scholar]
- 42. Povsic T. J., Kohout T. A., Lefkowitz R. J. (2003) β-Arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J. Biol. Chem. 278, 51334–51339 [DOI] [PubMed] [Google Scholar]
- 43. Goel R., Phillips-Mason P. J., Raben D. M., Baldassare J. J. (2002) α-Thrombin induces rapid and sustained Akt phosphorylation by β-arrestin 1-dependent and -independent mechanisms, and only the sustained Akt phosphorylation is essential for G1 phase progression. J. Biol. Chem. 277, 18640–18648 [DOI] [PubMed] [Google Scholar]
- 44. DeFea K. A., Zalevsky J., Thoma M. S., Déry O., Mullins R. D., Bunnett N. W. (2000) β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) β-Arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 47. Warne T., Edwards P. C., Leslie A. G., Tate C. G. (2012) Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure 20, 841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu J. J., Horst R., Katritch V., Stevens R. C., Wüthrich K. (2012) Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science 335, 1106–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicholls D. J., Tomkinson N. P., Wiley K. E., Brammall A., Bowers L., Grahames C., Gaw A., Meghani P., Shelton P., Wright T. J., Mallinder P. R. (2008) Identification of a putative intracellular allosteric antagonist binding-site in the CXC chemokine receptors 1 and 2. Mol. Pharmacol. 74, 1193–1202 [DOI] [PubMed] [Google Scholar]
- 51. Dowal L., Sim D. S., Dilks J. R., Blair P., Beaudry S., Denker B. M., Koukos G., Kuliopulos A., Flaumenhaft R. (2011) Identification of an antithrombotic allosteric modulator that acts through helix 8 of PAR1. Proc. Natl. Acad. Sci. U.S.A. 108, 2951–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim J., Ahn S., Ren X. R., Whalen E. J., Reiter E., Wei H., Lefkowitz R. J. (2005) Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 102, 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren X. R., Reiter E., Ahn S., Kim J., Chen W., Lefkowitz R. J. (2005) Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. U.S.A. 102, 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lopez-Ilasaca M., Crespo P., Pellici P. G., Gutkind J. S., Wetzker R. (1997) Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science 275, 394–397 [DOI] [PubMed] [Google Scholar]
- 55. Miller W. E., Maudsley S., Ahn S., Khan K. D., Luttrell L. M., Lefkowitz R. J. (2000) β-Arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of β-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J. Biol. Chem. 275, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 56. DeFea K. A., Vaughn Z. D., O'Bryan E. M., Nishijima D., Déry O., Bunnett N. W. (2000) The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. U.S.A. 97, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baillie G. L., Horswill J. G., Anavi-Goffer S., Reggio P. H., Bolognini D., Abood M. E., McAllister S., Strange P. G., Stephens G. J., Pertwee R. G., Ross R. A. (2013) CB1 receptor allosteric modulators display both agonist and signaling pathway specificity. Mol. Pharmacol. 83, 322–338 [DOI] [PMC free article] [PubMed] [Google Scholar]