Background: b12 is a broadly neutralizing human antibody that targets the conserved receptor binding site on HIV-1 gp120.
Results: Designed gp120 fragment immunogens (b121a/b122a) targeting the b12 binding site were tested in rabbit immunization studies.
Conclusion: Priming with b122a and boosting with gp120 elicited broadly neutralizing sera.
Significance: gp120 fragment immunogens can elicit broadly neutralizing sera in small animals.
Keywords: Antibodies, HIV-1, Humoral Response, Protein Design, Vaccine Development, Competition Binding, Immune Maturation, Prime-Boost
Abstract
b12, one of the few broadly neutralizing antibodies against HIV-1, binds to the CD4 binding site (CD4bs) on the gp120 subunit of HIV-1 Env. Two small fragments of HIV-1 gp120, b121a and b122a, which display about 70% of the b12 epitope and include solubility-enhancing mutations, were designed. Bacterially expressed b121a/b122a were partially folded and could bind b12 but not the CD4bs-directed non-neutralizing antibody b6. Sera from rabbits primed with b121a or b122a protein fragments and boosted with full-length gp120 showed broad neutralizing activity in a TZM-bl assay against a 16-virus panel that included nine Tier 2 and 3 viruses as well as in a five-virus panel previously designed to screen for broad neutralization. Using a mean IC50 cut-off of 50, sera from control rabbits immunized with gp120 alone neutralized only one virus of the 14 non-Tier 1 viruses tested (7%), whereas sera from b121a- and b122a-immunized rabbits neutralized seven (50%) and twelve (86%) viruses, respectively. Serum depletion studies confirmed that neutralization was gp120-directed and that sera from animals immunized with gp120 contained lower amounts of CD4bs-directed antibodies than corresponding sera from animals immunized with b121a/b122a. Competition binding assays with b12 also showed that b121a/2a sera contained significantly higher amounts of antibodies directed toward the CD4 binding site than the gp120 sera. The data demonstrate that it is possible to elicit broadly neutralizing sera against HIV-1 in small animals.
Introduction
The envelope glycoprotein (Env) of HIV-1 is critical for the entry of the virus into the cell. HIV-1-infected people contain substantial quantities of Env-directed antibodies. However, these typically show poor neutralization against the viral quasispecies present in the individual at that point of time, and many react with epitopes that are exposed only in unfolded or misfolded Env protein. Conformational flexibility, masking of conserved epitopes (1) by various strategies, including cryptic epitopes and glycosylation, high mutability (2, 3), and the lack of high resolution structural information for the functional Env trimer are the main factors that have confounded efforts to produce an Env-derived immunogen capable of eliciting broadly neutralizing antibodies against HIV-1.
Env is a trimer of gp120-gp41 dimers. gp120 contains the binding site for the receptors CD4 and a chemokine receptor, typically CCR5 or CXCR4, whereas gp41 is responsible for fusion of the viral and cellular membranes (4–6). The CD4 receptor binding site on gp120 (CD4bs)5 is well conserved among different HIV-1 isolates. b12 is a well characterized broadly neutralizing antibody known to target CD4bs (7, 8). More recently, several other broadly neutralizing CD4bs-directed mAbs have been isolated, such as HJ16 (9), VRC01 (10, 11), and NIH45-46 (12, 13). There have been prior efforts to target CD4bs epitopes using alanine substitutions, cavity filling mutations, and hyperglycosylation, so as to reduce binding to non-neutralizing antibodies and still retain b12 binding (14–18). However, these and other immunogens failed to elicit broadly neutralizing antibodies in animal immunization studies (19). gp120 and gp140 are relatively flexible molecules. Hence, when used as immunogens, it is possible that the resultant antibodies are directed against immunodominant, linear epitopes that are enriched in denatured or unstructured forms of the immunogen. Additionally, gp120 and gp140 are large and complex molecules that are difficult to produce and structurally characterize. Because they display many potential epitopes, it is difficult to map the resulting antibody response. We hypothesized that, besides presenting appropriate epitopes in the right conformation, it may also be important to minimize the total size of the antigen to focus the immune response to the desired epitope. We have therefore recently characterized a bacterially expressed outer domain of gp120 and shown that it could bind CD4 (20). We now report the design of smaller fragments of gp120 that display the b12 epitope. These fragments, hereafter referred to as b121a and b122a, consist largely of a part of the outer domain (Fig. 1). The designed protein fragments were expressed in E. coli to prevent glycosylation and consequent epitope masking that might occur if expressed in a eukaryotic expression system. Proteins were characterized biophysically, found to be partially folded, and could bind b12 with micromolar affinity. Because the designed fragments are originally part of a large protein, it is likely that a fraction of the molecules will not adopt the same conformation as the corresponding regions in the whole molecule. Therefore, a prime-boost rabbit immunization study was designed, which involved priming with the b121a/b122a protein fragments and boosting with full-length gp120. The hypothesis was that this regimen might elicit gp120 cross-reactive antibodies targeted to the b12 epitope that was present in the priming immunogen. A control group was primed with core gp120 and boosted with full-length gp120. Sera obtained following four primes with the b122a fragment protein and two boosts with full-length gp120 showed broad neutralization of a panel of 21 viruses, which included various Tier 1, 2, and 3 viruses across different clades. The difficulty of neutralization increases going from Tier 1 to Tier 3. The majority of immunogens studied to date elicit sera that neutralize a subset of Tier 1 viruses but fail to neutralize most Tier 2 and 3 viruses. Consistent with earlier studies (18, 19), sera from the control group largely neutralized Tier-1 neutralization-sensitive viruses. Depletion studies and competition binding assays with b12 showed that the antibodies in the broadly neutralizing sera are gp120-directed, and an appreciable fraction of antibodies in group 3 sera is directed toward the CD4 binding site.
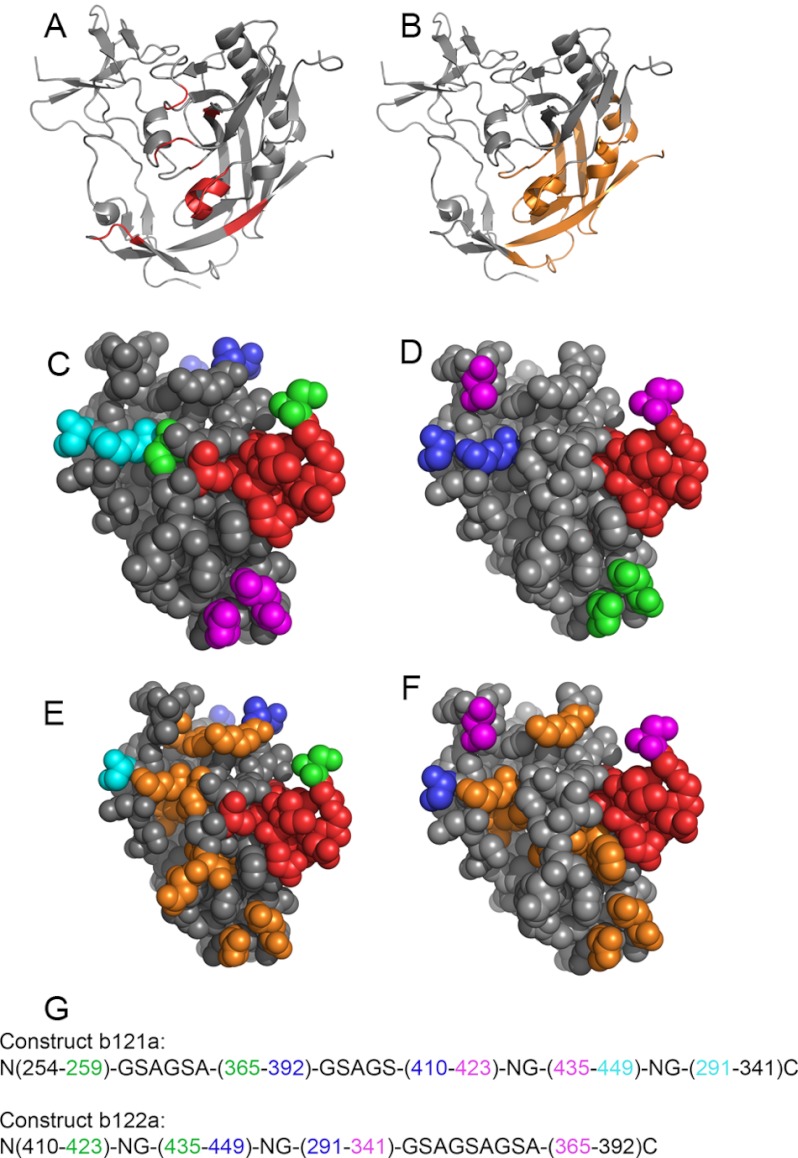
FIGURE 1.
Structure of core gp120 when complexed to the broadly neutralizing antibody b12. The coordinates are from Protein Data Bank entry 2NY7. A, residues involved in binding to b12 are shown in red; B, regions in gp120 included in b121a and b122a are shown in orange and include ∼70% of the binding site. Space-filled structures of the regions included in b121a and b122a are shown in C and D, respectively. The b12 binding site is colored in red. Residues connected by linkers are in the same color. Similar space-filled models of b121a and b122a are shown in E and F, where shown in orange are exposed hydrophobic residues that have been mutated to suitable polar residues based on Rosetta calculations and visual inspection. G, connectivities for b121a (top) and b122a (bottom). Colors are identical to those used in C and D, respectively.
MATERIALS AND METHODS
Ethics Statement
All animal studies were carried out in the SUNY Downstate Medical Center, which is a Category I facility. The Institutional Animal Care and Use Program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care international, and an approved assurance is on file with United States Department of Agriculture Office for Laboratory Animal Welfare (A3260-01). The study adhered to the recommendations of Association for Assessment and Accreditation of Laboratory Animal Care International Guidelines. The SUNY Institutional Animal Care and Use Committee reviewed and approved all animal study designs and protocols reported in this work. The approved Institutional Animal Care and Use Committee protocol that this study was run under at SUNY was 11-283-06.
Purification of Protein
Escherichia coli codon-optimized versions of the b121a and b122a genes were synthesized and cloned into the pET15b(+) vector (Novagen) between the NdeI and BamHI sites and contained an N-terminal His tag. The b122a-19iC construct contains a single cysteine codon inserted N-terminal to the NdeI site. All three constructs could be expressed as soluble proteins in E. coli BL21DE3 cells with a typical yield of 20 mg/liter.
Labeling of Protein for FRET Studies
100 μm b122a-19iC protein (containing a single free cysteine close to the N terminus) was incubated with 5 mm IAEDANS at room temperature for 2 h with gentle rocking. The total reaction volume was 500 μl. The mixture was then desalted on a PD minitrap column filled with G-25 resin (GE Healthcare). Mass spectrometry showed that protein was labeled at a single site. The absorbance of the labeled protein was measured at 322 nm, and using the extinction coefficient of IAEDANS-DTT conjugate at the same wavelength, the amount of fluorophore bound to the protein was calculated. For fluorescence measurements, samples were excited at 280 nm, and emission spectra were recorded from 300 to 550 nm.
Immunization Studies
Three groups, each having three rabbits (New Zealand White, female, 2 months old) were used for immunization studies. In group 1, core JRFL gp120 was used for priming, whereas full-length JRFL gp120 was used for boosting. For groups 2 and 3, b121a and b122a were, respectively, used for priming, whereas full-length JRFL gp120 was used for boosting. For both primes and boosts, all rabbits were injected intramuscularly with 20 μg of the immunogen in AdjuplexTM (Advanced Bioadjuvants LLC, Omaha, NE). Priming was done at weeks 0, 4, 8, and 12, and boosts were given at weeks 16 and 51. Four weeks following the last boost, the rabbits were terminated. Serum samples were collected at 0 and 2 weeks after each immunization, heat-inactivated, and stored in aliquots for further analysis.
Coupling of Full-length gp120 to Dynabeads and Its Antigenic Characterization
12.5 mg of Dynabeads (Invitrogen) were coupled to 0.5 mg of WT or mutant gp120. The antigenic integrity of the bound protein was characterized using FACS as reported previously (21).
Serum Depletion Studies
500 μl of pooled sera (diluted 1:5 in DMEM, 10% FBS to a final volume of 2.5 ml) were incubated with 12.5 mg of gp120-coupled magnetic beads for 2 h at room temperature with gentle rocking. The flow-through was incubated with another 12.5 mg of gp120-coupled magnetic beads. The flow-through collected after the second round of binding is the depleted serum. The same protocol was used for depletion on magnetic beads coupled to the gp120-D368R mutant. Sera before and after depletions were used in neutralization assays.
Neutralization Assay
Neutralizing antibodies were measured in a standardized in vitro assay using luciferase reporter gene expression after a single round of HIV-1 pseudovirus infection in TZM-bl cells (22). Various dilutions of sera were incubated with the virus, and the serum dilution that caused a 50% reduction in relative light units compared with virus control wells was considered as the ID50 of neutralization. Additional experimental details are provided in the supplemental material.
Competition Experiments with Purified IgG
The IgG was purified from 10 ml of pooled terminal bleed serum from each of the three groups using the protocol described (20). All competition experiments were carried out on a Bio-Rad ProteOn instrument. Approximately 6000 resonance units of monoclonal antibodies b12, b6, or PGT128 were immobilized on the chip surface, and 100 nm JRFL gp120 alone or after incubation with a 1:200 dilution of the purified IgGs was passed over the surface.
RESULTS
Construct Design
The crystal structure of HxBc2 gp120 in complex with the broadly neutralizing antibody b12 has been solved previously (Protein Data Bank entry 2NY7) (7). We attempted to design small structured gp120 fragments that retain a large part of the b12 epitope. The b12 binding site in gp120 comprises the following 23 residues: 257, 280–281, 365–373, 386, 417–419, 430–432, 455, and 472–474 (Fig. 1A). Approximately 70% of the b12 epitope (in terms of buried surface area upon complex formation of gp120 with b12) is contained within a relatively compact β-barrel structure on the lower part of the outer domain (Fig. 1B). This is composed of six β-strands, two small helices, and a part of the long helix A2. The first designed construct (b121a) includes residues 254–259, 291–341, 365–392, 410–423, and 435–449 and hence includes 12 of the 23 residues and 66% of the interface area (Fig. 1C). The second construct (b122a) excludes the 254–259 region and hence includes 11 of the 23 residues and 64% of the interface area (Fig. 1D). Because the fragments making up the construct are not contiguous stretches in the original molecule, we have used four linkers to connect them (Fig. 1G). Two of these are β-turns made up of two residues only, and the other two are loops composed of repeats of the sequence GSA. Linker length was based on the Cα–Cα distance between the residues to be connected as described previously (23). Both constructs have three disulfides, between residues 296 and 331, 378 and 445, and 385 and 418.
In order to determine which residues in this fragment were interacting with the rest of the molecule in the context of intact gp120, the in-house software PREDBURASA was used as described previously (24). Hydrophobic residues that were originally buried and interacting with other residues of gp120 but become newly exposed in the fragments were allowed to vary in identity to all polar residues, and the best substitution was selected on the basis of energy values as well as visual inspection of the modeled structure. Modeler (version 9.4) and Rosetta Design (version 2.0) were used for modeling of these mutations and subsequent energy calculations as described previously (20, 25). The final mutations incorporated were V254D, V255S, L259N, I294E, W338H, F376H, F383H, F391K, I423N, Y435N, and I449Q for b121a and I423N, Y435K, I449N, I294E, W375D, F376H, F383K, and F391K for b122a, and these residues are highlighted in orange in Fig. 1, E and F. Except for the above mutations, the sequences are identical to that of regions 254–259, 291–341, 365–392, 410–423, and 435–449 in Protein Data Bank entry 2NY7. None of the variable loop regions are present in the constructs. The V3 loop has been substituted by a GAG linker as present in Protein Data Bank entry 2NY7. The final sequences of the two constructs are shown in supplemental Fig. S1 along with their corresponding alignments with HxBc2 gp120.
Protein Purification and Biophysical Characterization
The proteins were overexpressed in BL21DE3 cells and purified by nickel-nitrilotriacetic acid chromatography from the soluble fraction. The typical protein yield was around 20 mg/liter of cell culture. The proteins were more than 90% pure as determined from SDS-PAGE analysis, and their identities were confirmed by electrospray ionization-MS.
To determine whether the mutations introduced at the interface of the fragments with the rest of gp120 had any role to play in increasing the solubility of the protein, a wild-type version of b122a that did not contain any mutation was synthesized (WT-b122a). For both WT-b122a and b122a, following induction, cells were sonicated, and the supernatant and pellet were loaded on gel. Whereas b122a was entirely in the supernatant, WT-b122a was almost entirely in the pellet fraction, indicating that the introduced mutations helped to increase the solubility of the protein, as intended.
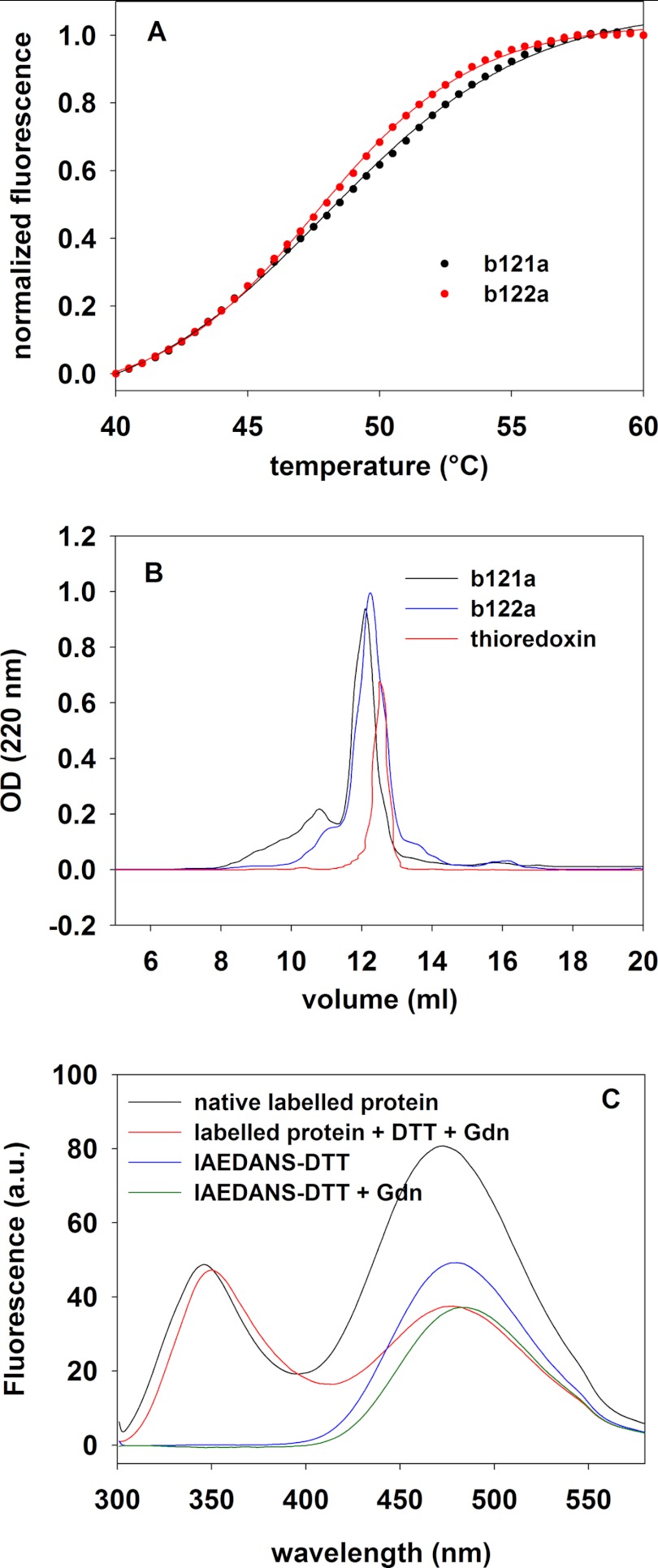
To monitor the stability of the designed proteins, a thermal melt assay was carried out with unfolding monitored by fluorescence of the Sypro orange dye (26, 27). Both b121a and b122a showed an unfolding transition with an apparent Tm around 50 °C (Fig. 2A), indicating the presence of compactness in the native state. For both of the proteins, anti-gp120 sera recognized the native proteins better than a reduced and carboxymethylated (RCAM) version of the same molecule (titer of 1600 with native protein compared with 700 with RCAM), thereby showing that the disulfide bonds are important for its native structure, which is lost upon reduction.
FIGURE 2.
Biophysical characterization of b121a and b122a. A, thermal denaturation of b121a and b122a in a thermal shift assay with Sypro orange dye in PBS, pH 7.4. The fluorescence of the dye in the presence of protein was recorded from 25 to 110 °C, and it was corrected for the corresponding fluorescence of the dye without protein. Both of the proteins show an apparent Tm close to 50 °C. As the protein exposes increased hydrophobic surface with increase in temperature, the dye binds and shows enhanced fluorescence. The data show that b121a and b122a are partially folded and undergo denaturational transitions with temperature. B, analytical gel filtration analysis of b121a (black line) and b122a (blue line) on a Superdex 75 column in PBS buffer at room temperature. For comparison, the elution profile of an equal amount of thioredoxin protein, having a monomer mass of 11.6 kDa is also shown (red line). The absorbance at 220 nm is shown as a function of the elution volume. Both b121a and b122a elute at a similar position as thioredoxin and at the expected position for the monomer. C, FRET experiments demonstrate that b122a is partially structured. A b122a mutant containing a free cysteine proximal to its N terminus was labeled with IAEDANS. Unlabeled protein, labeled protein, and IAEDANS-DTT conjugate were excited at 280 nm, and the emission spectrum was recorded from 300 to 550 nm. The concentrations of proteins (both unlabeled and labeled) used were 20 μm. The excitation and emission slit widths were 2.5 and 5 nm, respectively. Upon the addition of denaturant (guanidine hydrochloride (Gdn)), there is quenching of fluorescence for IAEDANS-DTT conjugate. However, the decrease in fluorescence upon denaturation and reduction is much larger for the labeled protein than for IAEDANS-DTT conjugate. This shows that the energy transfer taking place in the oxidized protein disappears upon denaturation and reduction of the protein disulfides. a.u., arbitrary units.
Both b121a and b122a proteins eluted from an analytical gel filtration column as monomers with elution volumes similar to that of the thioredoxin marker (Fig. 2B). To confirm the presence of disulfides, the denatured proteins were incubated with an excess of iodoacetamide in the dark under non-reducing conditions. Following quenching, the mixture was analyzed by LC electrospray ionization-MS. Both b121a and b122a did not show any increase in mass, indicating that all of the disulfides were formed. However, when the same experiment was performed under reducing conditions, both of the proteins showed incorporation of six iodoacetamide molecules, indicating the presence of six cysteine sulfhydryl groups.
To further elucidate the disulfide connectivity in b122a, tryptic digestion was performed under non-reducing conditions. The peptides were separated on a C18 RP-HPLC column and fragmented by collision-induced dissociation and electron transfer dissociation on a mass spectrometer. The ion masses were analyzed using the MS2DB+ software (28), which showed the native disulfide connectivity as the only true positive connectivity in the molecule (supplemental Fig. S2). The observation of a unique set of three disulfides of the 15 possible disulfide pairings indicates that the b122a immunogen has the correct topology.
To probe whether the three disulfides in the protein enabled it to take up a near native conformation similar to that present in gp120, FRET experiments were carried out. A b122a variant, which incorporated a single free cysteine near its N terminus, was designed, hereafter referred to as b122a-19iC. If residues in b122a have adopted the same conformation as in native gp120, then the distance between this single cysteine at the N terminus and the single tryptophan (Trp-338) in b122a should be ∼15–20 Å, which is close to the R0 (20 Å) for the Trp-IAEDANS FRET pair. Upon excitation of the tryptophan at 295 nm, the IAEDANS-labeled b122a-19iC protein showed a peak at 470 nm, corresponding to the emission maximum of IAEDANS, the intensity of which was reduced to half upon denaturation and reduction of disulfide bonds in the protein (Fig. 2C). In comparison, the addition of denaturant (guanidine hydrochloride) to an IAEDANS-DTT conjugate resulted in a much smaller change in fluorescence of about 15%. These data further confirm the structured nature of the b122a fragment.
Binding Studies Using SPR
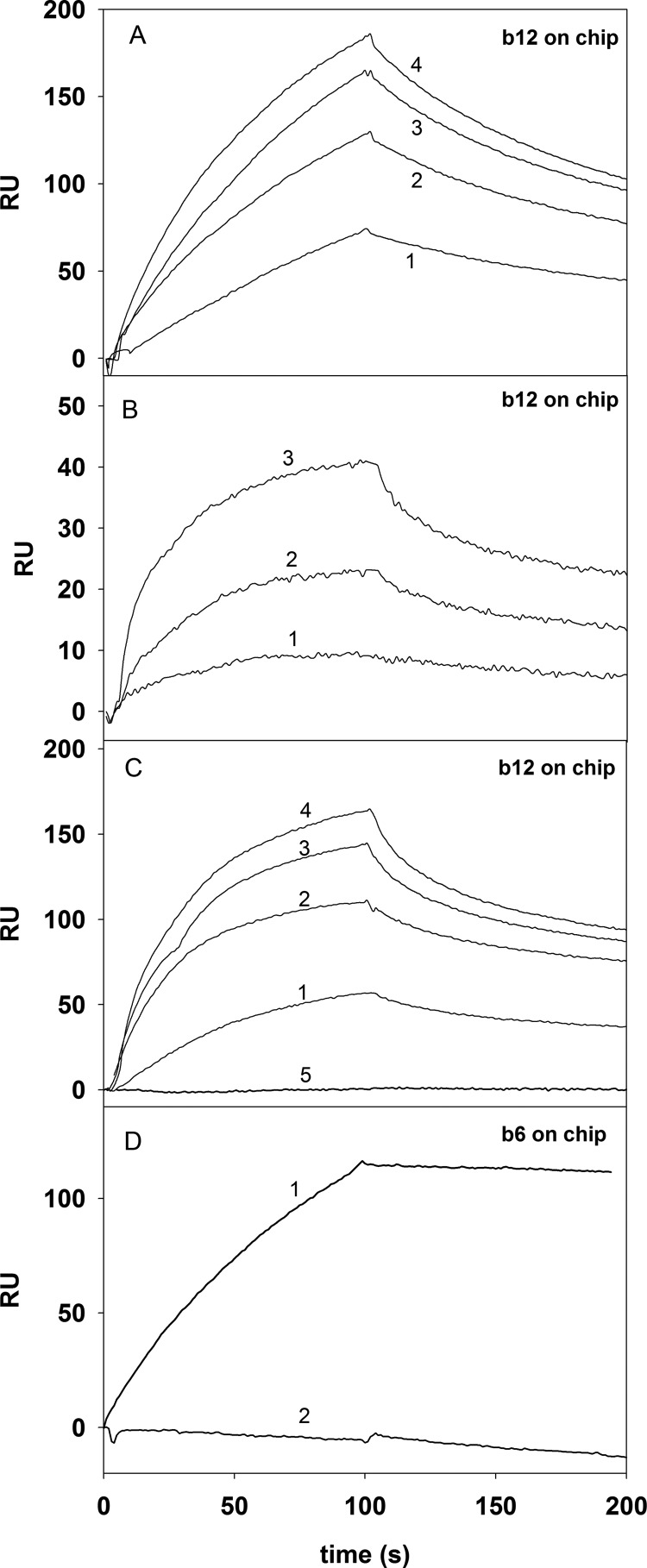
Binding of b121a, b122a, and RCAM-b122a to immobilized IgG1b12 was characterized by SPR on a Biacore 2000 instrument. b122a bound better than b121a, whereas RCAM-b122a did not bind (Fig. 3C), indicating that the disulfide bridges were very important for structure and binding of the fragments. None of the proteins bound to the non-neutralizing antibody b6 (Fig. 3D), confirming that the binding is specific for b12. Compared with core gp120 (KD = 76 nm), the binding of b122a to b12 is about 100 times weaker (Table 1). However, the fact that there is measurable binding to b12 with a small, non-glycosylated fragment of gp120 is encouraging. Designed disulfides have been used to further improve binding affinity by about 16-fold (see below).
FIGURE 3.
Binding studies on the Biacore 2000 instrument. Binding of HIV-1 gp120, b121a and b122a to surface-immobilized IgGb12 and b6 was monitored by SPR. A, curves 1, 2, 3, and 4, 50, 100, 150, and 200 nm concentrations of gp120 analyte, respectively. B, curves 1, 2, and 3, 78, 117, and 157 μm concentrations of b121a, respectively. C, curves 1, 2, 3, and 4, 8.5, 17, 34, and 51 μm concentrations of b122a, respectively. Curve 5, reduced and carboxymethylated b122a, which did not show any measurable binding to b12. D, curves 1 and 2, 100 nm full-length gp120 and 20 μm b122a. Surface density of both antibodies was 900 resonance units (RU); buffer was PBS, pH 7.4, 0.005% P20; and flow rate was 30 μl/min. The fact that b122a loses binding upon reduction and carboxymethylation of its cysteines shows that the binding is specific. b122a also does not show any binding to b6. Binding parameters are listed in Table 1.
TABLE 1.
Kinetic parameters for binding of full-length gp120, b121a, and b122a to b12 by surface plasmon resonance
| Protein | b12a |
||
|---|---|---|---|
| kon | koff | KD | |
| m−1 s−1 | s−1 | m | |
| gp120b | 6 × 104 | 4.6 × 10−3 | 7.6 × 10−8 ± 0.8 × 10−8 |
| b121a | 2 × 102 | 6.9 × 10−3 | 34.5 × 10−6 ± 15.6 × 10−6 |
| b122a | 6.6 × 102 | 5.6 × 10−3 | 8.4 × 10−6 ± 0.5 × 10−6 |
a The non-neutralizing antibody b6 binds gp120 with a kon of 1.1 × 105 m−1 s−1. There was very slow dissociation; hence, koff could not be measured. However, the published KD is 0.013 pm (7). There was no detectable binding of b121a or b122a to b6.
b Both full-length and core gp120 bind b12 with similar affinity.
Immunization and Neutralization Studies
It was hypothesized that because the designed fragments are originally part of a large protein, it is likely that a fraction of the fragment protein ensemble will not adopt exactly the same conformation as the corresponding regions in the whole molecule in the absence of b12. Therefore, a prime-boost rabbit immunization study was planned, which involved priming with the b121a/b122a protein fragments and boosting with full-length gp120. The hypothesis was that this regimen might elicit gp120 cross-reactive antibodies targeted to the b12 epitope that was present in the priming immunogen. The rationale of the protocol is summarized in Fig. 4. Rabbits were immunized with 20 μg of either core gp120, b121a, or b122a proteins in AdjuplexTM four times (at weeks 0, 4, 8, and 12). All three groups generated very low titer (∼102 or 103) of anti-gp120-specific antibodies when tested at week 14. Subsequently, animals were boosted with 20 μg of full-length JRFL gp120 at weeks 16 and 51, respectively. Four weeks following the last boost, the animals were terminated. Subsequently, sera were analyzed for anti-gp120 titers and titers against the priming immunogen by ELISA (Table 2), and HIV-1 neutralization assays were performed with Tier 1, 2, and 3 viruses. The long gap between the two boosts was to allow for extensive immune maturation because in HIV-1-infected patients, broad neutralization only develops after a couple of years of infection (29–31) or around 1 year from the time of infection, according to a recent study carried out in rhesus monkeys (32).
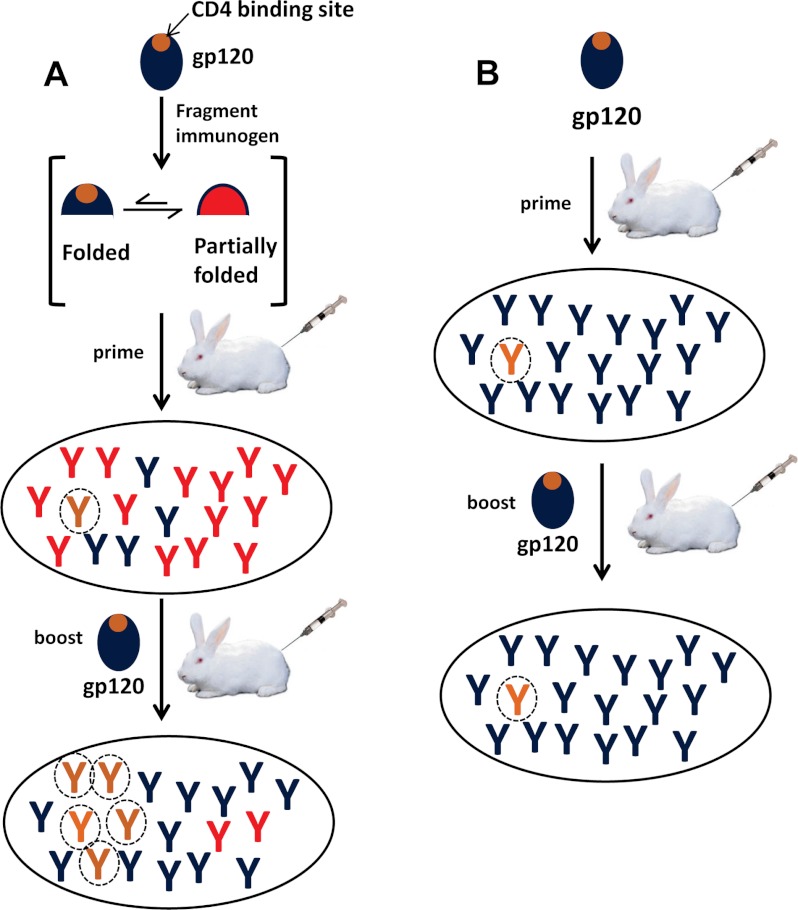
FIGURE 4.
Schematic representation of the prime-boost strategy. The fragment immunogen carrying the b12 binding site exists in equilibrium between the folded and partially folded forms. As shown in A, upon priming with the fragment immunogen, antibodies (red) elicited against the partially folded forms predominate. Upon boosting with full-length gp120, the cross-reactive antibodies (orange-encircled ones directed toward the CD4 binding site and blue ones directed toward regions other than the CD4bs) are selectively amplified, resulting in increasing amounts of CD4 binding site antibodies. B, a conventional gp120 prime/gp120 boost immunization, where lower levels of CD4 binding site antibodies (orange-encircled ones) are produced.
TABLE 2.
ELISA titers of sera from all three groups against immobilized JRFL gp120 and the priming immunogen
Following priming at weeks 0, 4, 8, and 12, all animals were boosted with JRFL gp120 at weeks 16 and 51.
| Group | Prime | Animal no. | Week 6 |
Week 10 |
Week 14 |
Week 18 |
Week 53, gp120 | Week 55 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gp120 | b121a/2a | gp120 | b121a/2a | gp120 | b121a/2a | gp120 | b121a/2a | gp120 | b121a/2a | ||||
| 1 | gp120 | 414 | <100 | ND | 3200 | ND | 25,600 | ND | 51,200 | ND | 409,600 | 409,600 | NDa |
| core | 415 | <100 | ND | 3200 | ND | 12,800 | ND | 51,200 | ND | 409,600 | 204,800 | ND | |
| 416 | 100 | ND | 3200 | ND | 6400 | ND | 12,800 | ND | 204,800 | 102,400 | ND | ||
| 2 | b121a | 418 | 100 | 6400 | 6400 | 102,400 | 3200 | 102,400 | 3200 | 102,400 | 409,600 | 102,400 | 25,600 |
| 419 | 100 | 25,600 | 400 | 25,600 | 100 | 25,600 | 1600 | 25,600 | 102,400 | 51,200 | 6400 | ||
| 421 | 400 | 102,400 | 400 | 102,400 | 400 | 102,400 | 6400 | 102,400 | 409,600 | 204,800 | 25,600 | ||
| 3 | b122a | 422 | 100 | 102,400 | <100 | 102,400 | <100 | 102,400 | 3200 | 25,600 | 409,600 | 409,600 | 25,600 |
| 423 | <100 | 102,400 | 100 | ND | 100 | 102,400 | 12,800 | 102,400 | 409,600 | 204,800 | 25,600 | ||
| 425 | 100 | 102,400 | 400 | 409,600 | 400 | 102,400 | 12,800 | 25,600 | 204,800 | 204,800 | 6400 | ||
a ND, not done; however, values for pooled, terminal bleed sera are shown in Table 3.
Although gp120 titers following priming were low, they rose to about 105 following the second boost. The terminal bleed sera were also analyzed for titers against CD4; core gp120; ODEC (20); b122a; reduced and carboxymethylated b122a (RCAM-b122a), which does not bind b12; and D368R gp120, which contains a mutation in the CD4bs that reduces b12 binding. Titers to both the entire outer domain and to the b122a fragment were significantly higher in b121a and b122a antisera relative to animals immunized with gp120 alone (Table 3). In addition, only the former two sera were sensitive to the D368R mutation. Coupled with the fact that these two sera also showed a reduction in titers with RCAM-b122a, it indicates that a higher fraction of CD4bs-directed Abs are present in groups 2 and 3 than in group 1.
TABLE 3.
ELISA titers of pooled terminal bleed sera from all three groups against various antigens
| Group | Prime | ELISA titers against |
||||||
|---|---|---|---|---|---|---|---|---|
| gp120 | gp120-D368R | CD4 | core gp120 | ODECa | b122a | RCAM-b122a | ||
| 1 | gp120 | 400,000 | 400,000 | 10,000 | 25,600 | 3500 | 1600 | 700 |
| 2 | b121a | 200,000 | 100,000 | <100 | 25,600 | 18,700 | 25,600 | 20,000 |
| 3 | b122a | 400,000 | 200,000 | <100 | 25,600 | 12,800 | 25,600 | 11,500 |
a E. coli expressed outer domain fragment of gp120 (20).
Neutralization assays in TZM-bl cells with week 53 sera from all three groups are summarized in Fig. 5. The 16 viruses initially tested comprise seven Tier 1, seven Tier 2, and two Tier 3 viruses (33). The b121a- and b122a-primed groups showed considerably broader neutralization compared with animals primed with core gp120 (Fig. 5). To compare this neutralization activity with broadly neutralizing sera obtained from HIV-1-infected patients, the sera were also tested against five additional viruses (94UG103, 92BR020, 93IN905, MGRM-C-026, and 92TH021, representing clades A, B, C, and CRF01_AE), comprising a five-virus panel that has been described previously and used to screen for broad neutralization (34). This panel was previously tested using over 1000 sera from HIV-1-infected individuals. Elite neutralizers were defined as those whose sera neutralized one or more pseudoviruses within a clade and across at least four different clades with an IC50 of at least 300. It was found that sera with elite neutralizing activity were found at a frequency of about 1% and, on average, were able to neutralize the five-virus panel with a geometric mean ID50 (GMT) of 500. In the present study, two of three sera in group 3 neutralized the five-virus panel with a GMT greater than 120, whereas all three sera from group 1 showed a GMT less than 40. The average GMTs of sera from groups 1, 2, and 3 were 28, 66, and 109, respectively, across the five-virus panel and 17, 56, and 104, respectively, across all Tier 2 and 3 viruses in the 16-virus panel. Overall, with a mean IC50 cut-off of 50, sera from gp120-immunized rabbits neutralized only one virus of the 14 non-Tier 1 viruses tested (7%), whereas sera from b121a- and b122a-immunized rabbits neutralized seven (50%) and twelve (86%) viruses, respectively. This reconfirms the broad neutralizing activity of sera from groups 2 and 3.
FIGURE 5.
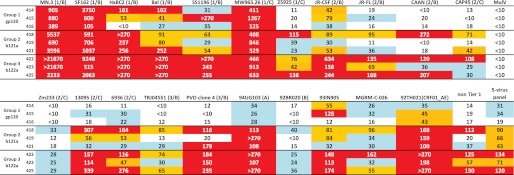
ID50 values for neutralization assays with week 53 sera in TZM-bl cells. Week 0 sera typically had ID50 values of <10, whereas MulV served as the negative control. For each virus, the tier category/clade is mentioned in parenthesis, where available. For 94UG103, 92BR020, 93IN905, MGRM-C-026, and 92TH021, the tier category information was not available. However, these comprise a five-virus panel described previously (34) and were chosen to evaluate the neutralization breadth of the sera. The difficulty of neutralization increases going from Tier 1 to Tier 3 viruses. The color code indicates the following: ID50 ≤ 20 (white); 20 < ID50 < 40 (blue); 40 < ID50 < 100 (yellow); ID50 >100 (red). The second from the right column lists the GMT values for each serum averaged across all Tier 2 and 3 viruses from the 16-virus panel, whereas the far right-hand column shows the average GMT values for each serum across the five-virus panel. For calculation of GMT, all ID50 values of <10 were taken as 10, and all ID50 values of >270 were taken as 270. Compared with group 1, sera from groups 2 and 3 (especially group 3) showed broad neutralization of the diverse panel of viruses tested.
To further explore the neutralization specificity of the sera, depletion studies were performed on pooled terminal bleed sera (week 55) for all of the groups due to limiting amounts of week 53 sera. Two rounds of depletion were carried out on gp120-coupled magnetic beads. The sera had to be diluted 5-fold in DMEM, 10% FBS prior to depletion in order to bind optimally to the gp120-coupled beads. The amount of sera that could be depleted was limited by the amounts of gp120 and D368R gp120 that could be expressed and purified. There was a significant decrease in the ELISA and neutralization titers upon depletion, which demonstrates that the observed neutralization is mediated by gp120-directed antibodies (Table 4). Upon depletion with a CD4 binding site-defective mutant of gp120 called D368R, it was seen that the depleted sera from groups 2 and 3 retained a substantially higher proportion of the neutralization titer as compared with group 1 (Table 4). These data indicate that groups 2 and 3 (especially group 3) contain a higher proportion of CD4bs-directed Abs than group 1. This is also consistent with the higher ELISA titers against b121a/2a and ODEC in the former sera. The undepleted week 55 terminal bleed sera showed a 2-fold lower ELISA titer and 3–5-fold lower neutralization ID50 than the corresponding week 53 sera. Coupled with the 5-fold dilution that occurred during depletion as well as the fact that a significant fraction of neutralizing antibodies bound to both gp120 and D368R gp120, this meant that it was not possible to reliably test the depleted sera against Tier 2 viruses. The fact that the undepleted sera showed higher neutralization ID50 values compared with the sera depleted on D368R-gp120 indicates that CD4BS antibodies sensitive to the D368R mutation are responsible for only a part of the observed neutralization. The remaining epitopes targeted by the sera remain to be elucidated.
TABLE 4.
Neutralization ID50 values obtained with pooled terminal bleed sera (week 55) before and after depletion
| ID50 in TZM-bl cells |
||
|---|---|---|
| MN.3 | SF162.LS | |
| Group 1 undepleted | 455 | 2305 |
| Group 2 undepleted | 2975 | 1015 |
| Group 3 undepleted | 13,545 | 4620 |
| Group 1 depleted on gp120 | 115 | 30 |
| Group 2 depleted on gp120 | 740 | 130 |
| Group 3 depleted on gp120 | 280 | 105 |
| Group 1 depleted on D368R | 145 | 75 |
| Group 2 depleted on D368R | 1135 | 450 |
| Group 3 depleted on D368R | 1105 | 270 |
| Group 1 depleted on BSA | 490 | 1945 |
| Group 2 depleted on BSA | 2470 | 750 |
| Group 3 depleted on BSA | 11,735 | 3980 |
a For groups 2 and 3, ID50 values of the sera depleted on gp120 are considerably reduced relative to the same sera depleted on gp120 D368R. This is not the case for sera in group 1.
To test for the presence of b12-like antibodies, competition binding experiments were carried out with b12 and purified antibodies from serum using surface plasmon resonance. Binding of gp120 was examined alone or in combination with purified IgG from all three groups to immobilized b12. As shown in Fig. 6A, gp120 prebound to group 3 IgG showed 4-fold reduced binding to b12 as compared with gp120 alone, whereas gp120 prebound to group 1 IgG showed increased binding. This latter observation is possibly due to the presence of higher amounts of antibodies directed toward epitopes other than the CD4 binding site in group 1 serum as compared with group 3 serum (Fig. 6D). The data show that group 3 serum has higher amounts of antibodies that compete for the CD4 binding site and therefore cause reduction in binding. However, because binding of the neutralizing mAb b12 to gp120 also prevents binding of the non-neutralizing CD4bs mAbs such as b6 (Fig. 6B), this experiment cannot distinguish between neutralizing and non-neutralizing CD4 binding site antibodies. As a negative control, competition experiments were carried out using PGT128, an antibody that binds glycan residues and the V3 loop on the outer domain of gp120. In order to enhance the binding signal to PGT128, we used a cyclically permuted trimeric gp120, which binds to PGT128 with 10-fold higher affinity than monomeric gp120. This molecule (ccmp-gp120 trimer) has been described previously (35). All three sera showed similar binding profiles (Fig. 6C), indicating that none of the sera have substantial amounts of PGT128-like antibodies. The similar increase in mass observed for all three sera also indicates that all three sera had equivalent amounts of anti-gp120 antibodies.
FIGURE 6.
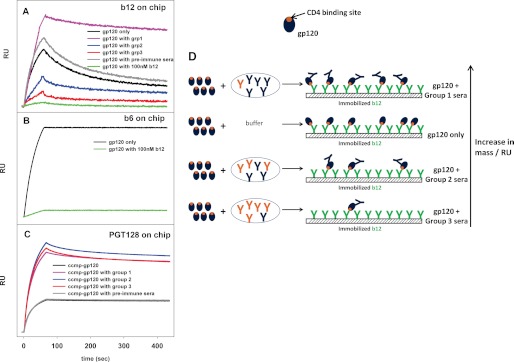
Competition binding assays of gp120 to immobilized monoclonal antibodies. A, b12; B, b6; C, PGT128. For b12 and PGT128, competition was carried out in the presence of antibodies purified from various sera. Binding was monitored by surface plasmon resonance. Prebinding of gp120 to sera from groups 2 and 3 resulted in reduced binding to b12, indicating the presence of substantial amounts of CD4 binding site antibodies. Prebinding to group 1 serum, however resulted in increased binding to b12, possibly due to large amounts of antibodies directed at epitopes other than the CD4bs in this serum. As a negative control, none of the sera showed any competition to PGT128. For all antibodies, incubation of gp120 with preimmune sera showed similar binding as gp120 alone, indicating the absence of gp120-specific antibodies in the preimmune serum. In B, binding of the neutralizing CD4bs antibody b12 prevents binding of the non-neutralizing CD4bs antibody b6. D, a schematic representation of the possible events during the b12 competition assay. CD4 binding site antibodies are shown in orange, whereas those that are directed toward epitopes other than CD4bs are shown in blue. Because the latter do not compete for the b12 binding site, gp120 precomplexed to these antibodies retains its ability to bind b12. The amount of CD4bs Abs increases going from group 1 to group 3, resulting in a gradual reduction in b12 binding in the competition assay. RU, resonance units.
DISCUSSION
Despite years of efforts to combat HIV/AIDS, an effective vaccine remains elusive. However, recent studies (36–38) indicate that several HIV-1 patients have sera able to neutralize diverse strains of HIV-1, although this broadly neutralizing activity takes several years to develop (29–31). Rational design of immunogens for HIV-1 has been done mainly by targeting several conserved regions of either the gp120 or gp41 subunits of Env (14–16, 39–41). In certain HIV-1-infected individuals, it has been found that neutralization is exclusively or substantially mediated by CD4 binding site antibodies (42, 43). Previous attempts to focus the immune response toward the b12 epitope were done in the context of whole gp120 by introducing mutations to selectively diminish non-neutralizing antibody binding and adding glycosylation sites to dampen responses to regions outside the CD4bs (14–16). However, the sera could neutralize the homologous JRFL virus weakly with an IC50 of 4–8, whereas good neutralization (IC50 >50) was obtained only for Tier 1 viruses like SF162 and HxBc2 (19). To reduce the conformational flexibility of gp120 and to stabilize it in the CD4-bound conformation, S375W/T257S mutations were made to fill the Phe-43 cavity (17, 18). The mutations improved sCD4 and b12 binding, both in the context of monomeric gp120 and trimeric GCN4-gp120. Rabbits immunized with the stabilized trimeric GCN4-gp120 showed better neutralization of YU2 (homologous virus) and some Tier-1 viruses than the corresponding WT gp120 group; however, no measurable neutralization was obtained against Tier 2 viruses like JRFL and TRJO.58 (18). In other recent studies, gp140-foldon containing the T257S/S375W mutations have been tested in rabbits in different homologous and heterologous regimens, but the results have been similar, with only homologous neutralization, good neutralization of Tier 1 viruses, and no neutralization of JRFL, JRCSF, and ADA (Tier 2) (44).
Because full-length or core gp120 does not elicit significant amounts of b12-like antibodies, an alternative target would be an outer domain (OD) only immunogen. However, previous immunizations with a glycosylated, wild-type version of the OD did not yield neutralizing sera (45). An engineered, non-glycosylated OD performed somewhat better with sera that showed improved but modest neutralization relative to animals immunized with monomeric gp120 (20). A recent study described grafting of portions of the b12 epitope onto heterologous scaffolds (40). Although the initial designs bound weakly, following mutagenesis and yeast display, good binders with KD values as low as 10 nm for b12 were obtained. However, no immunization or neutralization studies were described.
In the present study, two small fragments of gp120, encompassing the lower part of the outer domain, were designed. These immunogens (b121a and b122a) were partially folded; both showed binding to b12 and did not bind the non-neutralizing antibody b6. In prime-boost immunization studies, animals primed with b122a elicited sera that showed broad neutralization of a diverse panel of viruses in a TZM-bl assay. As described above, previous immunization studies have generally showed much reduced breadth and potency in terms of neutralization. In some other studies, rabbit immunizations have also been carried out in which DNA encoding a membrane-bound form of the cleavage-competent, disulfide-linked JRFL gp140 trimers (SOS) was used for the prime, followed by boost with soluble gp140 trimers containing an additional I559P mutation (SOSIP) (46). However, neutralization was mostly restricted to Tier 1 viruses and to the homologous JRFL virus. More recently, studies with KNH1144 SOSIP gp140 trimers have shown similar results (47). In a recent study (48), it was shown that gp140s derived from a clade A sequence (92UG037.8) and a clade C sequence (CZA97012) elicited measurably improved neutralization of Tier 1 viruses in a TZM-bl assay relative to gp120. No neutralization was seen for Tier 2 viruses in a TZM-bl assay, although it was present when a more sensitive A3R5 assay was used. In comparison with the studies described above, sera from the b122a group are unique in their ability to neutralize multiple Tier 2 and 3 viral isolates in a TZM-bl assay. Depletion studies revealed that a measurable fraction of neutralization in these sera was mediated by antibodies targeting the CD4bs and sensitive to the D368R mutation. Competition binding experiments with b12 clearly showed higher levels of CD4bs antibodies in group 2 and group 3 sera as compared with group 1 serum. From ELISA experiments, the former two sera were also found to have higher titers of CD4bs antibodies. In addition, the only known neutralization epitope in the b121a and b122a priming fragments is the b12 binding site. All of these data suggest that CD4bs antibodies make a significant contribution to the observed broad neutralization. However, the fact that the undepleted sera show better neutralization than the D368R-depleted sera shows that there are other types of antibodies also mediating neutralization. It is entirely possible that there may be CD4bs-directed neutralizing antibodies that are insensitive to the D368R mutation and would therefore bind to immobilized D368R gp120. Indeed one broadly neutralizing CD4bs mAb HJ16 (9) is insensitive to the D368R mutation. Mapping epitopes in polyclonal sera remains a nontrivial task. Nonetheless, it is apparent that sera from groups 2 and 3 (primed with b121a and b122a, respectively) show significantly better neutralization than those from group 1 (primed with core gp120). This study demonstrates that priming with a fragment immunogen and boosting with full-length gp120 is capable of inducing broadly neutralizing antibodies. Previous prime-boost studies have mainly used DNA for prime followed by a protein boost (46, 49), including the recently conducted RV144 trial, which showed partial protection of 31% (50–52), although some recent studies have used proteins for both (44). However, these were mainly restricted to full-length envelope proteins and were not carried out with fragment immunogens.
Besides the use of designed fragment immunogens, it is also likely that the long gap between the two boosts in the present studies contributed to immune maturation (53), leading to the observed neutralization. Although studies in HIV-1-infected individuals have shown that neutralizing antibodies develop only around 2.5 years after infection (31), in a recent study where rhesus monkeys were inoculated with CCR5-tropic SHIVAD8 molecular clone, neutralizing antibodies were found to develop in two distinct phases (32). The first phase (weeks 5–13) generally elicited antibodies that were able to neutralize sensitive viruses, whereas a second response dominated between weeks 41 and 51, which was able to neutralize more resistant viruses. Interestingly, in the present study, neutralizing antibodies were observed in the serum during week 53. ID50 value obtained using TZM-bl assays have been shown to correlate well with serum-neutralizing antibody titers required to provide passive protection from SHIV challenge in macaques (54–56); hence, the broad neutralization observed in this study is relevant to vaccine design. We are currently attempting to further improve the rigidity and binding affinity of b122a for b12. By introducing disulfides and other mutations, b122a derivatives have been made that bind b12 with KD values of 500 nm (supplemental Table S1), and it is very likely that, using library display methodologies (57–59), this can be improved substantially. Future studies will explore the use of such derivatives in monomeric or multimeric forms in different prime-boost immunization regimes. The present studies have used purified protein immunogens that are composed of gp120-derived sequence, and the observed neutralization is depleted by gp120. This is important because it shows that the broad neutralization is indeed gp120-directed and not a result of antibodies inadvertently elicited against human proteins.
Recently, several potent CD4bs-directed broadly neutralizing human antibodies, such as VRC01, have been isolated from HIV-1-infected people (10, 11). A common feature of these has been that they originate from a specific germ line VH allelic variant (60, 61). This might indicate that immunogens need to bind to this germ line product in order to eventually result in affinity-matured VRC01-like antibodies. Recently, it has also been shown that small animals, including rabbits and mice, do not possess any germ line VH fragments that contain the characteristic signature motifs present in potent VRC01-like antibodies (62). Given such a scenario, it is very likely that it would not be possible to elicit such antibodies in small animals. The present study suggests that it is possible to elicit broadly neutralizing sera in small animals. The presence of a significant fraction of CD4bs-directed Abs is correlated with the neutralizing ability of the sera. However, additional studies are required to definitively map the neutralizing epitopes targeted by these polyclonal sera.
Acknowledgments
We thank Dr. Dennis Burton for antibody b12 and the Neutralizing Antibody Consortium for gp120 and additional amounts of antibodies b12 and b6.

This article contains supplemental Methods, Table S1, and Figs. S1 and S2.
- CD4bs
- CD4 binding site
- IAEDANS
- 5-((2-((iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid
- RCAM
- reduced and carboxymethylated
- GMT
- geometric mean ID50
- OD
- outer domain.
REFERENCES
- 1. Kwong P. D., Wyatt R., Robinson J., Sweet R. W., Sodroski J., Hendrickson W. A. (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richman D. D., Wrin T., Little S. J., Petropoulos C. J. (2003) Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U.S.A. 100, 4144–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strain M. C., Günthard H. F., Havlir D. V., Ignacio C. C., Smith D. M., Leigh-Brown A. J., Macaranas T. R., Lam R. Y., Daly O. A., Fischer M., Opravil M., Levine H., Bacheler L., Spina C. A., Richman D. D., Wong J. K. (2003) Heterogeneous clearance rates of long-lived lymphocytes infected with HIV. Intrinsic stability predicts lifelong persistence. Proc. Natl. Acad. Sci. U.S.A. 100, 4819–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pantophlet R., Burton D. R. (2006) GP120. Target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24, 739–769 [DOI] [PubMed] [Google Scholar]
- 5. Wyatt R., Sodroski J. (1998) The HIV-1 envelope glycoproteins. Fusogens, antigens, and immunogens. Science 280, 1884–1888 [DOI] [PubMed] [Google Scholar]
- 6. LaBranche C. C., Galasso G., Moore J. P., Bolognesi D. P., Hirsch M. S., Hammer S. M. (2001) HIV fusion and its inhibition. Antivir. Res. 50, 95–115 [DOI] [PubMed] [Google Scholar]
- 7. Zhou T., Xu L., Dey B., Hessell A. J., Van Ryk D., Xiang S. H., Yang X., Zhang M. Y., Zwick M. B., Arthos J., Burton D. R., Dimitrov D. S., Sodroski J., Wyatt R., Nabel G. J., Kwong P. D. (2007) Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445, 732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roben P., Moore J. P., Thali M., Sodroski J., Barbas C. F., 3rd, Burton D. R. (1994) Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68, 4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corti D., Langedijk J. P., Hinz A., Seaman M. S., Vanzetta F., Fernandez-Rodriguez B. M., Silacci C., Pinna D., Jarrossay D., Balla-Jhagjhoorsingh S., Willems B., Zekveld M. J., Dreja H., O'Sullivan E., Pade C., Orkin C., Jeffs S. A., Montefiori D. C., Davis D., Weissenhorn W., McKnight A., Heeney J. L., Sallusto F., Sattentau Q. J., Weiss R. A., Lanzavecchia A. (2010) Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5, e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu X., Yang Z. Y., Li Y., Hogerkorp C. M., Schief W. R., Seaman M. S., Zhou T., Schmidt S. D., Wu L., Xu L., Longo N. S., McKee K., O'Dell S., Louder M. K., Wycuff D. L., Feng Y., Nason M., Doria-Rose N., Connors M., Kwong P. D., Roederer M., Wyatt R. T., Nabel G. J., Mascola J. R. (2010) Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou T., Georgiev I., Wu X., Yang Z. Y., Dai K., Finzi A., Kwon Y. D., Scheid J. F., Shi W., Xu L., Yang Y., Zhu J., Nussenzweig M. C., Sodroski J., Shapiro L., Nabel G. J., Mascola J. R., Kwong P. D. (2010) Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheid J. F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T. Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K., Hurley A., Myung S., Boulad F., Poignard P., Burton D. R., Pereyra F., Ho D. D., Walker B. D., Seaman M. S., Bjorkman P. J., Chait B. T., Nussenzweig M. C. (2011) Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333, 1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diskin R., Scheid J. F., Marcovecchio P. M., West A. P., Jr., Klein F., Gao H., Gnanapragasam P. N., Abadir A., Seaman M. S., Nussenzweig M. C., Bjorkman P. J. (2011) Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334, 1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantophlet R., Wilson I. A., Burton D. R. (2004) Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng. Des. Sel. 17, 749–758 [DOI] [PubMed] [Google Scholar]
- 15. Pantophlet R., Ollmann Saphire E., Poignard P., Parren P. W., Wilson I. A., Burton D. R. (2003) Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77, 642–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pantophlet R., Wilson I. A., Burton D. R. (2003) Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J. Virol. 77, 5889–5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiang S. H., Kwong P. D., Gupta R., Rizzuto C. D., Casper D. J., Wyatt R., Wang L., Hendrickson W. A., Doyle M. L., Sodroski J. (2002) Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76, 9888–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dey B., Pancera M., Svehla K., Shu Y., Xiang S. H., Vainshtein J., Li Y., Sodroski J., Kwong P. D., Mascola J. R., Wyatt R. (2007) Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state. Antigenicity, biophysics, and immunogenicity. J. Virol. 81, 5579–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selvarajah S., Puffer B., Pantophlet R., Law M., Doms R. W., Burton D. R. (2005) Comparing antigenicity and immunogenicity of engineered gp120. J. Virol. 79, 12148–12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhattacharyya S., Rajan R. E., Swarupa Y., Rathore U., Verma A., Udaykumar R., Varadarajan R. (2010) Design of a non-glycosylated outer domain-derived HIV-1 gp120 immunogen that binds to CD4 and induces neutralizing antibodies. J. Biol. Chem. 285, 27100–27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y., Svehla K., Louder M. K., Wycuff D., Phogat S., Tang M., Migueles S. A., Wu X., Phogat A., Shaw G. M., Connors M., Hoxie J., Mascola J. R., Wyatt R. (2009) Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83, 1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montefiori D. C. (2004) Evaluating Neutralizing Antibodies against HIV, SIV, and SHIV in Luciferase Reporter Gene Assays, John Wiley & Sons, Inc., New York: [DOI] [PubMed] [Google Scholar]
- 23. Bommakanti G., Citron M. P., Hepler R. W., Callahan C., Heidecker G. J., Najar T. A., Lu X., Joyce J. G., Shiver J. W., Casimiro D. R., ter Meulen J., Liang X., Varadarajan R. (2010) Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl. Acad. Sci. U.S.A. 107, 13701–13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma D., Balamurali M. M., Chakraborty K., Kumaran S., Jeganathan S., Rashid U., Ingallinella P., Varadarajan R. (2005) Protein minimization of the gp120 binding region of human CD4. Biochemistry 44, 16192–16202 [DOI] [PubMed] [Google Scholar]
- 25. Saha P., Barua B., Bhattacharyya S., Balamurali M. M., Schief W. R., Baker D., Varadarajan R. (2011) Design and characterization of stabilized derivatives of human CD4D12 and CD4D1. Biochemistry 50, 7891–7900 [DOI] [PubMed] [Google Scholar]
- 26. Lavinder J. J., Hari S. B., Sullivan B. J., Magliery T. J. (2009) High-throughput thermal scanning. A general, rapid dye-binding thermal shift screen for protein engineering. J. Am. Chem. Soc. 131, 3794–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niesen F. H., Berglund H., Vedadi M. (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2, 2212–2221 [DOI] [PubMed] [Google Scholar]
- 28. Murad W., Singh R., Yen T. Y. (2011) An efficient algorithmic approach for mass spectrometry-based disulfide connectivity determination using multi-ion analysis. BMC Bioinformatics 12, Suppl. 1, S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doria-Rose N. A., Klein R. M., Daniels M. G., O'Dell S., Nason M., Lapedes A., Bhattacharya T., Migueles S. A., Wyatt R. T., Korber B. T., Mascola J. R., Connors M. (2010) Breadth of human immunodeficiency virus-specific neutralizing activity in sera. Clustering analysis and association with clinical variables. J. Virol. 84, 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gray E. S., Madiga M. C., Hermanus T., Moore P. L., Wibmer C. K., Tumba N. L., Werner L., Mlisana K., Sibeko S., Williamson C., Abdool Karim S. S., Morris L. (2011) The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85, 4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mikell I., Sather D. N., Kalams S. A., Altfeld M., Alter G., Stamatatos L. (2011) Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7, e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shingai M., Donau O. K., Schmidt S. D., Gautam R., Plishka R. J., Buckler-White A., Sadjadpour R., Lee W. R., LaBranche C. C., Montefiori D. C., Mascola J. R., Nishimura Y., Martin M. A. (2012) Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc. Natl. Acad. Sci. U.S.A. 109, 19769–19774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seaman M. S., Janes H., Hawkins N., Grandpre L. E., Devoy C., Giri A., Coffey R. T., Harris L., Wood B., Daniels M. G., Bhattacharya T., Lapedes A., Polonis V. R., McCutchan F. E., Gilbert P. B., Self S. G., Korber B. T., Montefiori D. C., Mascola J. R. (2010) Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84, 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simek M. D., Rida W., Priddy F. H., Pung P., Carrow E., Laufer D. S., Lehrman J. K., Boaz M., Tarragona-Fiol T., Miiro G., Birungi J., Pozniak A., McPhee D. A., Manigart O., Karita E., Inwoley A., Jaoko W., Dehovitz J., Bekker L. G., Pitisuttithum P., Paris R., Walker L. M., Poignard P., Wrin T., Fast P. E., Burton D. R., Koff W. C. (2009) Human immunodeficiency virus type 1 elite neutralizers. Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83, 7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saha P., Bhattacharyya S., Kesavardhana S., Miranda E. R., Ali P. S., Sharma D., Varadarajan R. (2012) Designed cyclic permutants of HIV-1 gp120. Implications for envelope trimer structure and immunogen design. Biochemistry 51, 1836–1847 [DOI] [PubMed] [Google Scholar]
- 36. Walker L. M., Huber M., Doores K. J., Falkowska E., Pejchal R., Julien J. P., Wang S. K., Ramos A., Chan-Hui P. Y., Moyle M., Mitcham J. L., Hammond P. W., Olsen O. A., Phung P., Fling S., Wong C. H., Phogat S., Wrin T., Simek M. D., Protocol G Principal Investigators, Koff W. C., Wilson I. A., Burton D. R., Poignard P. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu X., Zhou T., Zhu J., Zhang B., Georgiev I., Wang C., Chen X., Longo N. S., Louder M., McKee K., O'Dell S., Perfetto S., Schmidt S. D., Shi W., Wu L., Yang Y., Yang Z. Y., Yang Z., Zhang Z., Bonsignori M., Crump J. A., Kapiga S. H., Sam N. E., Haynes B. F., Simek M., Burton D. R., Koff W. C., Doria-Rose N. A., Connors M., NISC Comparative Sequencing Program, Mullikin J. C., Nabel G. J., Roederer M., Shapiro L., Kwong P. D., Mascola J. R. (2011) Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333, 1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walker L. M., Phogat S. K., Chan-Hui P. Y., Wagner D., Phung P., Goss J. L., Wrin T., Simek M. D., Fling S., Mitcham J. L., Lehrman J. K., Priddy F. H., Olsen O. A., Frey S. M., Hammond P. W., Protocol G Principal Investigators, Kaminsky S., Zamb T., Moyle M., Koff W. C., Poignard P., Burton D. R. (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azoitei M. L., Ban Y. E., Julien J. P., Bryson S., Schroeter A., Kalyuzhniy O., Porter J. R., Adachi Y., Baker D., Pai E. F., Schief W. R. (2012) Computational design of high-affinity epitope scaffolds by backbone grafting of a linear epitope. J. Mol. Biol. 415, 175–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Azoitei M. L., Correia B. E., Ban Y. E., Carrico C., Kalyuzhniy O., Chen L., Schroeter A., Huang P. S., McLellan J. S., Kwong P. D., Baker D., Strong R. K., Schief W. R. (2011) Computation-guided backbone grafting of a discontinuous motif onto a protein scaffold. Science 334, 373–376 [DOI] [PubMed] [Google Scholar]
- 41. Correia B. E., Ban Y. E., Holmes M. A., Xu H., Ellingson K., Kraft Z., Carrico C., Boni E., Sather D. N., Zenobia C., Burke K. Y., Bradley-Hewitt T., Bruhn-Johannsen J. F., Kalyuzhniy O., Baker D., Strong R. K., Stamatatos L., Schief W. R. (2010) Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure 18, 1116–1126 [DOI] [PubMed] [Google Scholar]
- 42. Li Y., Migueles S. A., Welcher B., Svehla K., Phogat A., Louder M. K., Wu X., Shaw G. M., Connors M., Wyatt R. T., Mascola J. R. (2007) Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13, 1032–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheid J. F., Mouquet H., Feldhahn N., Seaman M. S., Velinzon K., Pietzsch J., Ott R. G., Anthony R. M., Zebroski H., Hurley A., Phogat A., Chakrabarti B., Li Y., Connors M., Pereyra F., Walker B. D., Wardemann H., Ho D., Wyatt R. T., Mascola J. R., Ravetch J. V., Nussenzweig M. C. (2009) Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640 [DOI] [PubMed] [Google Scholar]
- 44. Feng Y., McKee K., Tran K., O'Dell S., Schmidt S. D., Phogat A., Forsell M. N., Karlsson Hedestam G. B., Mascola J. R., Wyatt R. T. (2012) Biochemically defined HIV-1 envelope glycoprotein variant immunogens display differential binding and neutralizing specificities to the CD4-binding site. J. Biol. Chem. 287, 5673–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang X., Tomov V., Kurteva S., Wang L., Ren X., Gorny M. K., Zolla-Pazner S., Sodroski J. (2004) Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1. J. Virol. 78, 12975–12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beddows S., Schülke N., Kirschner M., Barnes K., Franti M., Michael E., Ketas T., Sanders R. W., Maddon P. J., Olson W. C., Moore J. P. (2005) Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 79, 8812–8827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang Y. K., Andjelic S., Binley J. M., Crooks E. T., Franti M., Iyer S. P., Donovan G. P., Dey A. K., Zhu P., Roux K. H., Durso R. J., Parsons T. F., Maddon P. J., Moore J. P., Olson W. C. (2009) Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine 27, 5120–5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kovacs J. M., Nkolola J. P., Peng H., Cheung A., Perry J., Miller C. A., Seaman M. S., Barouch D. H., Chen B. (2012) HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. U.S.A. 109, 12111–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang S., Arthos J., Lawrence J. M., Van Ryk D., Mboudjeka I., Shen S., Chou T. H., Montefiori D. C., Lu S. (2005) Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J. Virol. 79, 7933–7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roehr B. (2011) Researchers announce first correlates of protection for HIV vaccine. BMJ 343, d5880. [DOI] [PubMed] [Google Scholar]
- 51. Cohen J. (2011) AIDS research. Novel antibody response may explain HIV vaccine success. Science 333, 1560. [DOI] [PubMed] [Google Scholar]
- 52. Korber B. (2011) Building on the past to define an efficient path to an HIV vaccine. Expert Rev. Vaccines 10, 929–931 [DOI] [PubMed] [Google Scholar]
- 53. Sallusto F., Lanzavecchia A., Araki K., Ahmed R. (2010) From vaccines to memory and back. Immunity 33, 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willey R., Nason M. C., Nishimura Y., Follmann D. A., Martin M. A. (2010) Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res. Hum. Retroviruses 26, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hessell A. J., Poignard P., Hunter M., Hangartner L., Tehrani D. M., Bleeker W. K., Parren P. W., Marx P. A., Burton D. R. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15, 951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hessell A. J., Rakasz E. G., Poignard P., Hangartner L., Landucci G., Forthal D. N., Koff W. C., Watkins D. I., Burton D. R. (2009) Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5, e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chao G., Lau W. L., Hackel B. J., Sazinsky S. L., Lippow S. M., Wittrup K. D. (2006) Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 [DOI] [PubMed] [Google Scholar]
- 58. Bowley D. R., Labrijn A. F., Zwick M. B., Burton D. R. (2007) Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage. Protein Eng. Des. Sel. 20, 81–90 [DOI] [PubMed] [Google Scholar]
- 59. Walker L. M., Bowley D. R., Burton D. R. (2009) Efficient recovery of high-affinity antibodies from a single-chain Fab yeast display library. J. Mol. Biol. 389, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bonsignori M., Montefiori D. C., Wu X., Chen X., Hwang K. K., Tsao C. Y., Kozink D. M., Parks R. J., Tomaras G. D., Crump J. A., Kapiga S. H., Sam N. E., Kwong P. D., Kepler T. B., Liao H. X., Mascola J. R., Haynes B. F. (2012) Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor. Implications for vaccine design. J. Virol. 86, 4688–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haynes B. F., Kelsoe G., Harrison S. C., Kepler T. B. (2012) B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 30, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. West A. P., Jr., Diskin R., Nussenzweig M. C., Bjorkman P. J. (2012) Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. U.S.A. 109, E2083–E2090 [DOI] [PMC free article] [PubMed] [Google Scholar]