Abstract
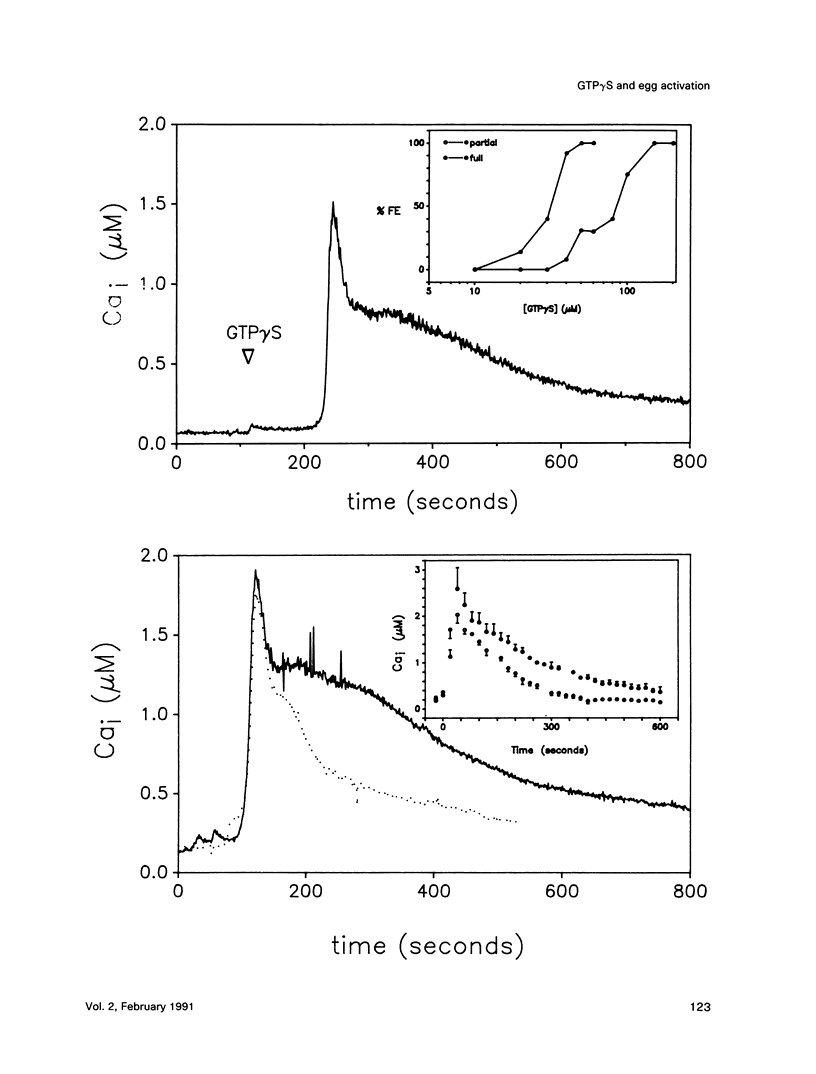
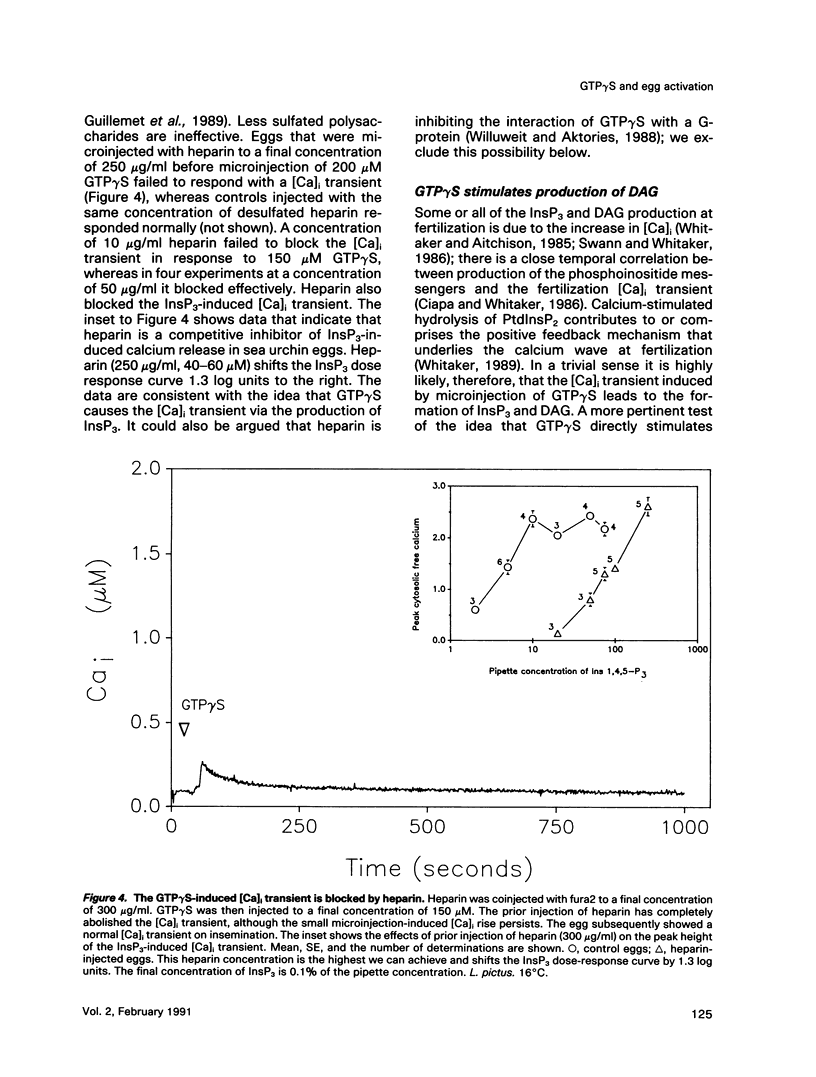
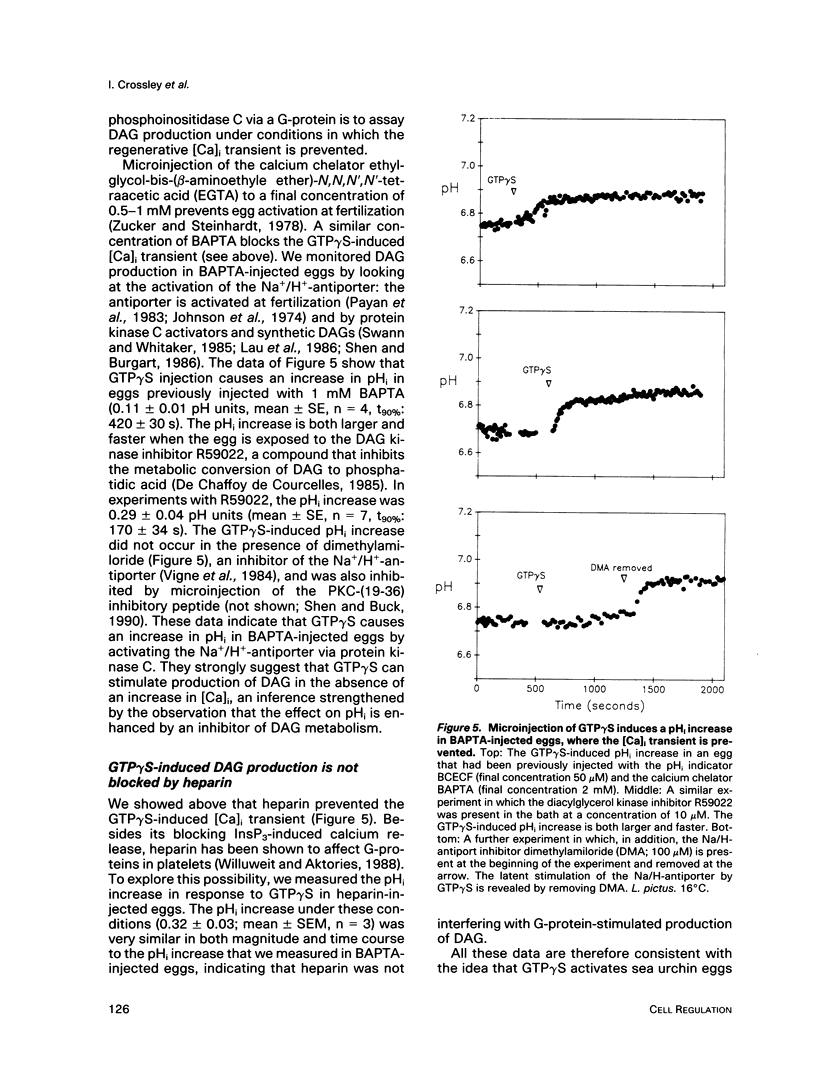
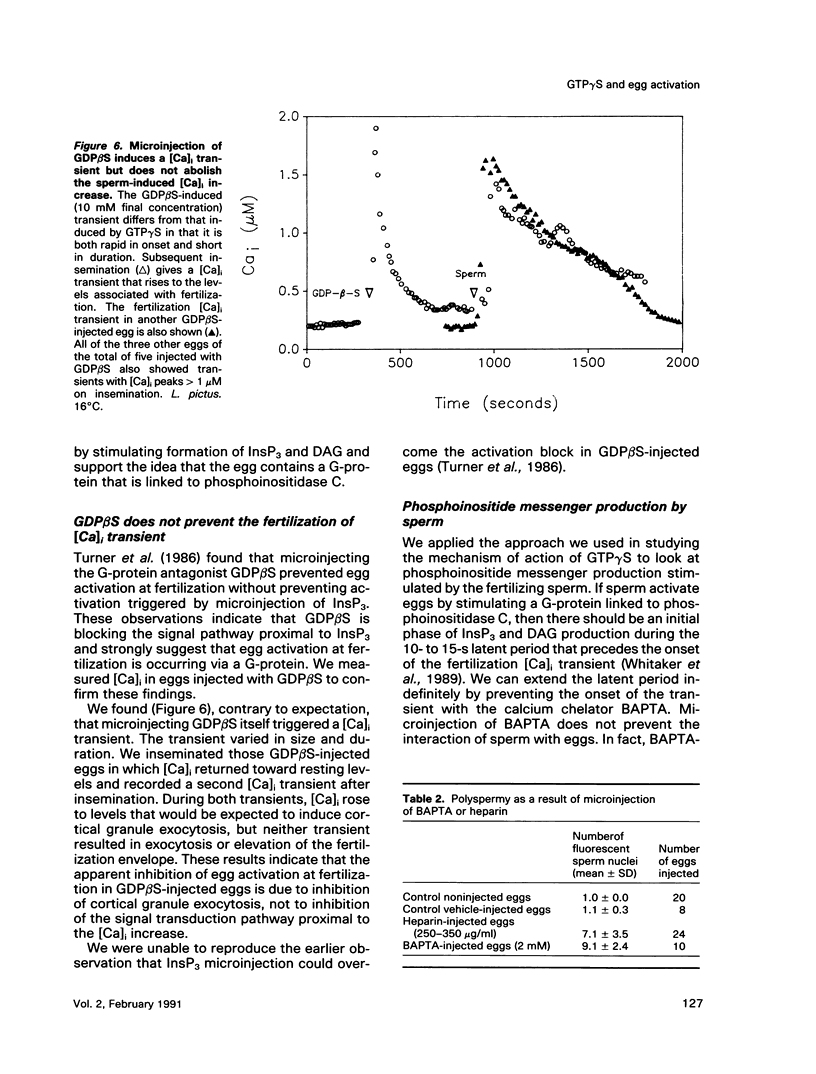
We show that microinjecting guanosine-5'-thiotriphosphate (GTP gamma S) into unfertilized sea urchin eggs generates an intracellular free calcium concentration [( Ca]i) transient apparently identical in magnitude and duration to the calcium transient that activates the egg at fertilization. The GTP gamma S-induced transient is blocked by prior microinjection of the inositol trisphosphate (InsP3) antagonist heparin. GTP gamma S injection also causes stimulation of the egg's Na+/H+ antiporter via protein kinase C, even in the absence of a [Ca]i increase. These data suggest that GTP gamma S acts by stimulating the calcium-independent production of the phosphoinositide messengers InsP3 and diacylglycerol (DAG). However, the fertilization [Ca]i transient is not affected by heparin, nor can the sperm cause calcium-independent stimulation of protein kinase C. It seems that the bulk of InsP3 and DAG production at fertilization is triggered by the [Ca]i transient, not by the sperm itself. GDP beta S, a G-protein antagonist, does not affect the fertilization [Ca]i transient. Our findings do not support the idea that signal transduction at fertilization operates via a G-protein linked directly to a plasma membrane sperm receptor.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res. 1979 Aug;122(1):203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Ciapa B., Whitaker M. Two phases of inositol polyphosphate and diacylglycerol production at fertilisation. FEBS Lett. 1986 Jan 20;195(1-2):347–351. doi: 10.1016/0014-5793(86)80191-0. [DOI] [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. G-proteins, the inositol lipid signalling pathway, and secretion. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):247–265. doi: 10.1098/rstb.1988.0075. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Comerford J. G., Dawson A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-induced Ca2+ release from rat liver microsomes. FEBS Lett. 1988 Feb 8;228(1):57–59. doi: 10.1016/0014-5793(88)80584-2. [DOI] [PubMed] [Google Scholar]
- David C., Halliwell J., Whitaker M. Some properties of the membrane currents underlying the fertilization potential in sea urchin eggs. J Physiol. 1988 Aug;402:139–154. doi: 10.1113/jphysiol.1988.sp017197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Larrodera P., Lopez-Barahona M., Cornet M. E., Barreno P. G., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is activated by muscarinic agonists. Biochem J. 1989 Oct 1;263(1):115–120. doi: 10.1042/bj2630115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Lamontagne S., Boulay G., Mouillac B. Differential effects of heparin on inositol 1,4,5-trisphosphate binding, metabolism, and calcium release activity in the bovine adrenal cortex. Mol Pharmacol. 1989 Mar;35(3):339–344. [PubMed] [Google Scholar]
- Hill T. D., Berggren P. O., Boynton A. L. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat liver cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Hinkley R. E., Wright B. D., Lynn J. W. Rapid visual detection of sperm-egg fusion using the DNA-specific fluorochrome Hoechst 33342. Dev Biol. 1986 Nov;118(1):148–154. doi: 10.1016/0012-1606(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Jaffe L. A., Turner P. R., Kline D., Kado R. T., Shilling F. G-proteins and egg activation. Cell Differ Dev. 1988 Nov;25 (Suppl):15–18. doi: 10.1016/0922-3371(88)90094-9. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Nicchitta C. V. Characteristics of GTP-mediated microsomal Ca2+ release. Biochim Biophys Acta. 1988 Nov 22;945(2):185–194. doi: 10.1016/0005-2736(88)90481-6. [DOI] [PubMed] [Google Scholar]
- Kline D., Simoncini L., Mandel G., Maue R. A., Kado R. T., Jaffe L. A. Fertilization events induced by neurotransmitters after injection of mRNA in Xenopus eggs. Science. 1988 Jul 22;241(4864):464–467. doi: 10.1126/science.3134693. [DOI] [PubMed] [Google Scholar]
- Lau A. F., Rayson T. C., Humphreys T. Tumor promoters and diacylglycerol activate the Na+/H+ antiporter of sea urchin eggs. Exp Cell Res. 1986 Sep;166(1):23–30. doi: 10.1016/0014-4827(86)90505-7. [DOI] [PubMed] [Google Scholar]
- Marty A., Zimmerberg J. Diffusion into the patch-clamp recording pipette of a factor necessary for muscarinic current response. Cell Signal. 1989;1(3):259–268. doi: 10.1016/0898-6568(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol. 1988 Feb;106(2):345–353. doi: 10.1083/jcb.106.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan P., Girard J. P., Ciapa B. Mechanisms regulating intracellular pH in sea urchin eggs. Dev Biol. 1983 Nov;100(1):29–38. doi: 10.1016/0012-1606(83)90197-5. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Buck W. R. A synthetic peptide of the pseudosubstrate domain of protein kinase C blocks cytoplasmic alkalinization during activation of the sea urchin egg. Dev Biol. 1990 Aug;140(2):272–280. doi: 10.1016/0012-1606(90)90077-v. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Burgart L. J. 1,2-Diacylglycerols mimic phorbol 12-myristate 13-acetate activation of the sea urchin egg. J Cell Physiol. 1986 May;127(2):330–340. doi: 10.1002/jcp.1041270222. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. Stimulation of the Na/H exchanger of sea urchin eggs by phorbol ester. Nature. 1985 Mar 21;314(6008):274–277. doi: 10.1038/314274a0. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986 Dec;103(6 Pt 1):2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swezey R. R., Epel D. Enzyme stimulation upon fertilization is revealed in electrically permeabilized sea urchin eggs. Proc Natl Acad Sci U S A. 1988 Feb;85(3):812–816. doi: 10.1073/pnas.85.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Jaffe L. A., Fein A. Regulation of cortical vesicle exocytosis in sea urchin eggs by inositol 1,4,5-trisphosphate and GTP-binding protein. J Cell Biol. 1986 Jan;102(1):70–76. doi: 10.1083/jcb.102.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Jaffe L. A., Primakoff P. A cholera toxin-sensitive G-protein stimulates exocytosis in sea urchin eggs. Dev Biol. 1987 Apr;120(2):577–583. doi: 10.1016/0012-1606(87)90260-0. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Sheetz M. P., Jaffe L. A. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature. 1984 Aug 2;310(5976):414–415. doi: 10.1038/310414a0. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Cragoe E. J., Jr, Lazdunski M. Structure-activity relationships of amiloride and certain of its analogues in relation to the blockade of the Na+/H+ exchange system. Mol Pharmacol. 1984 Jan;25(1):131–136. [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett. 1985 Mar 11;182(1):119–124. doi: 10.1016/0014-5793(85)81167-4. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Willuweit B., Aktories K. Heparin uncouples alpha 2-adrenoceptors from the Gi-protein in membranes of human platelets. Biochem J. 1988 Feb 1;249(3):857–863. doi: 10.1042/bj2490857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh G., Douglas A., Brighton V. Long-term culture of fetal rat hepatocytes in media supplemented with fetal calf-serum Ultroser SF or Ultroser G. Biol Cell. 1986;58(1):53–63. doi: 10.1111/j.1768-322x.1986.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Steinhardt R. A. Prevention of the cortical reaction in fertilized sea urchin eggs by injection of calcium-chelating ligands. Biochim Biophys Acta. 1978 Jul 17;541(4):459–466. doi: 10.1016/0304-4165(78)90155-1. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]


