Abstract
Several pathologic characteristics are associated with an adverse clinical outcome in papillary thyroid carcinoma (PTC), including the histological variant. This study aimed to investigate immunohistochemical expression and BRAF mutation status based on the histological variant and evaluated potential markers of aggressive behavior of PTC in Korean patients. In all, 407 PTC cases were classified to each histological variant, and the 94 representative cases were subjected to immunohistochemistry and BRAF mutation analysis. The classic type, follicular variant (FV) and tall cell variant (TCV) represented 76.9%, 14.2% and 6%, respectively. TCV showed a larger tumor size (P = 0.009), frequent extrathyroidal extension (P = 0.022) and cervical lymph node (LN) metastasis (P = 0.018). TCV and FV showed the reduced expression of galectin-3 (P = 0.003) and HBME1 (P = 0.114). Regardless of histology, PTEN loss and diffuse S100A4 expression were associated with LN metastasis (P = 0.007, P = 0.013). All TCVs harbored BRAF V600E mutation, and FV harbored less BRAF V600E mutation (P = 0.043). Immunohistochemical evaluation showed characteristic patterns in histological variants. PTEN and S100A4 expression are suggested as indicators of regional lymph node metastasis.
Keywords: Thyroid Carcinoma, Papillary; Histological Variant; Immunohistochemistry; PTEN; S100A4
INTRODUCTION
Histological grading, determined by marked nuclear atypia, tumor necrosis and vascular invasion, is a significant and independent parameter predicting papillary thyroid carcinoma (PTC) mortality, as demonstrated in multivariate analyses (1, 2). Although it is controversial whether the histological subtype or variant is an independent predictor of an adverse outcome (1), some histological variants appear to be of prognostic significance. The tall cell variant (TCV), columnar cell, diffuse sclerosing, and solid variants show regional LN metastasis, frequent local recurrence, and even distant metastasis (1, 3-5). LiVolsi argued that the portion showing "tall cell" histologic feature should be mentioned in pathologic reports because of its clinical significance, irrespective of the percentage of this histology (4).
Recently, novel immunohistochemical markers have been studied for the prediction of aggressive PTC behavior. Increased expression of vascular endothelial growth factor (VEGF), S100A4 and β-catenin are helpful markers of regional and distant metastasis (6-8), and increased expression of epidermal growth factor receptor (EGFR) and c-erbB2 plays a role in tumor recurrence and the progression of well-differentiated thyroid carciRecently, novel immunohistochemical markers have been studied for the prediction of aggressive PTC behavior. Increased expression of vascular endothelial growth factor (VEGF), S100A4 and β-catenin are helpful markers of regional and distant metastasis (6-8), and increased expression of epidermal growth factor receptor (EGFR) and c-erbB2 plays a role in tumor recurrence and the progression of well-differentiated thyroid carcinoma to undifferentiated carcinoma (9-11).
The phosphatase and tensin homolog (PTEN) tumor suppressor inhibits the phosphatidylinositol-3-kinase (PI3K)/AKT pathway, which is frequently activated in various types of cancer, including thyroid carcinoma (12). PTEN silencing through promoter methylation and PTEN mutations have also been found in sporadic PTCs (13, 14), indicating that PI3K/AKT pathway-mediated signaling may play a role in PTC tumorigenesis.
The immunoexpression of these markers based on histological variant has not been evaluated in a relatively larger number of cases. In this study, we selected histological variants of PTC in Korean patients and investigated the immunoexpression of markers known to be related to clinical outcome, with the diagnostic markers of galectin-3, HBME1 and CK19 and BRAF mutation analysis. The potential of these markers as indicators of aggressive clinical behavior is also evaluated.
MATERIALS AND METHODS
Patients
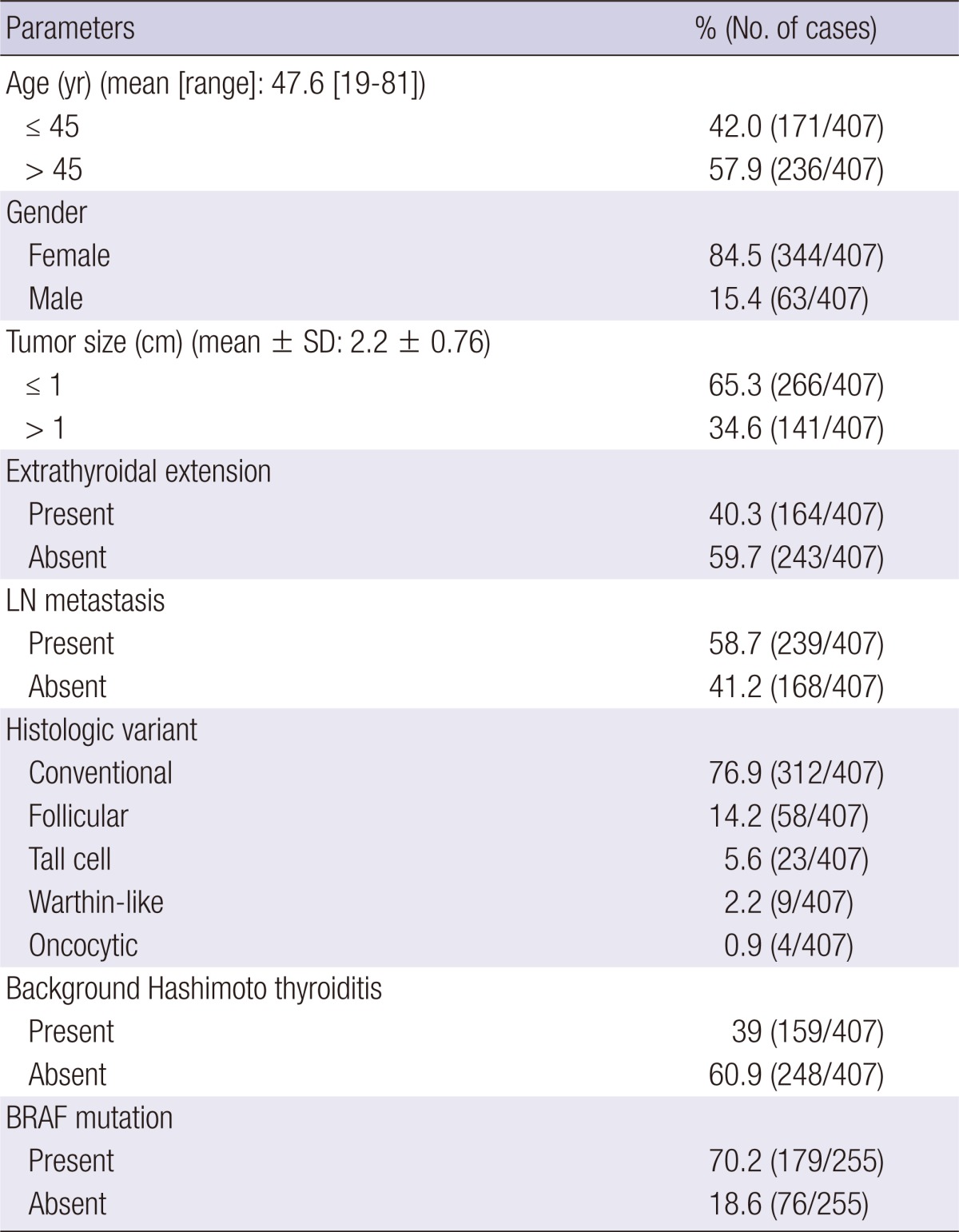
Eight hundred and seventy-five patients who underwent total thyroidectomy and had been diagnosed with PTC in 2009 at Seoul National University Hospital were reviewed. Among them, we selected 407 patients who have single PTC and underwent lymph node (LN) dissection that harbor more than 2 lymph nodes. Clinical and histological parameters are summarized in Table 1. The mean follow-up period was 33.1 months.
Table 1.
Summary of clinicopathologic traits
Evaluation of histological variants
Pathologic information was obtained from sections stained with hematoxylin and eosin (H&E). Histological subgroups of the follicular variant (FV), TCV, solid variant, clear cell variant, oncocytic variant, Warthin-like variant, cribriform-morular variant, and diffuse sclerosing variant were defined as described in the 2004 World Health Organization (WHO) classification. In particular, the diagnostic criteria for TCV were as follows: the height of the tall cell was at least three times its width and contained the nuclear features characteristic of PTC, a low nuclear cytoplasmic ratio and an eosinophilic granular cytoplasm, and the proportion of the tall cell was > 75%. All histological variants were diagnosed when the specific components represented > 75% of the tumor area, except for the follicular variant, which was defined when the follicular growth was exclusive. All cases were independently reviewed and confirmed by two endocrine pathologists.
Immunohistochemistry and interpretation
First, all slides were screened and tissue microarray blocks were then produced for immunohistochemistry (IHC) (Superbiochips, Seoul, Korea). Thirty-eight cases of classic PTC, 33 of FVPTC and 23 of TCVPTC were included (total = 94 cases). The specimens were processed with a DAKO-Envision detection kit (DakoCytomation, Carpinteria, CA, USA). The following antibodies were used: galectin-3 (9C4; 1:100; Novocastra, Newcastle, UK), HBME1 (1:50; DakoCytomation), CK19 (RCK108; 1:500; DakoCytomation), PTEN (6H2.1; 1:100; DakoCytomation), β-catenin (CAT-5H10; 1:150; Zymed, San Francisco, CA, USA), EGFR (31G7; 1:100; Invitrogen, Camarillo, CA, USA), c-erbB2 (CBII; 1:200; Novocastra), S100A4 (1:500, DakoCytomation), and VEGF(1: 500, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Galectin-3, HBME1, and CK19 expression were measured by H-score (intensity [0, 1, 2, or 3] multiplied by the distribution [%]). S100A4 expression was divided into 3 groups according to the positive distribution of the tumor area; 0, 0%; 1, 1-50%; and 2, > 50%. EGRF and c-erbB2 were scored as positive only on observation of membranous staining in ≥ 10% of the tumor cells, as previously described (9); 0, no membrane staining or < 10% of the tumor cells stained; 1+, incomplete membrane staining in ≥ 10% of the tumor cells; 2+ and 3+, complete membrane staining with moderate and strong intensity in ≥ 10% of the tumor cells, respectively. Lung and breast cancer tissues demonstrated as EGFR (3+) and c-erbB2 (3+), respectively, were routinely included as positive control. VEGF and PTEN immunoexpression was classified according to the staining intensity; 0, no staining; 1, weak; and 2, strong. β-catenin expression (score = 1) was classified into nuclear positivity and cytoplasmic and/or cytoplasmic membrane positivity.
Detection of the BRAF mutation
Among 407 cases, BRAF mutation analysis was done in 255 cases. Slides stained with H&E were reviewed and the representative tumor areas were marked as guides. Genomic DNA was extracted in the marked area and amplified for direct sequencing of the BRAF exon 15 (ABI 3730xl sequencer, Applied Biosystems, Foster City, CA, USA) using the following set of primers: forward, 5'-GCTTGCTCTGATAGGAAAATGAG-3'; reverse, 5'-GATACTCAGCAGCATCTCAGG-3', as described previously (8).
Statistical analysis
Statistical analysis was performed with the SPSS software (version 19.0, SPSS, Chicago, IL, USA). The chi-square or Fisher's exact test was used when comparing frequencies between groups. All numerical data are expressed as the means ± standard deviations (SD), and differences between the means were compared using an unpaired t-test and an analysis of variance (ANOVA). All significant factors by univariate analyses were subjected to multivariate analysis using the Cox proportional regression model. Probability values < 0.05 were considered to indicate statistical significance.
Ethics statement
Studies were performed with approval from the institutional review board, Seoul National University Hospital (H-1011-006-337). Informed consent was waived.
RESULTS
Clinicopathologic features
In the 407 cases (Table 1), the female: male ratio was 5.5: 1 with a mean age of 47.6 yr (range: 19-81 yr). Sixty-five percent (266/407) of cases had microcarcinoma, of which the tumor size was ≤ 1 cm, and 40.3% (164/407) of the tumors showed extra-thyroidal extension. Cervical LN metastasis was found in 58.7% (239/407) of cases, whereas background Hashimoto thyroiditis was present in 39% (159/407). Local recurrence was reported in 3/408 cases (0.7%); one of which was FV while the other two were of the classic type. Distant metastasis was found in 2/408 cases (0.4%) (one classic type and one TCV).
Histologically, the classic type represented 76.9% (312/407), FV represented 14.2% (58/407), and the proportion of TCV was 5.6% (23/407), among which the proportions of FV and TCV were slightly higher than those of Japanese patients (15). In addition, the Warthin-like variant and oncocytic variant represented 2.2% (9/407) and 0.9% (4/407) of patients, respectively. Other variants, including columnar cell, diffuse sclerosing, clear, solid, and cribriform-morular variant were not observed.
FV was frequently detected in patients > 45 yr of age, and in contrast, TCV was found in patients with age ≤ 45 yr (P = 0.008, Table 2). FV showed a striking female predominance compared to the other variants (P = 0.049). The tumor size of FV was smaller than the classic type, whereas the tumor size of TCV was significantly larger than the classic type (P = 0.009). TCV showed more frequent extrathyroidal extension (P = 0.022) and cervical LN metastasis (P = 0.018), particularly more than the FV.
Table 2.
Clinicopathologic characteristics of histological variant
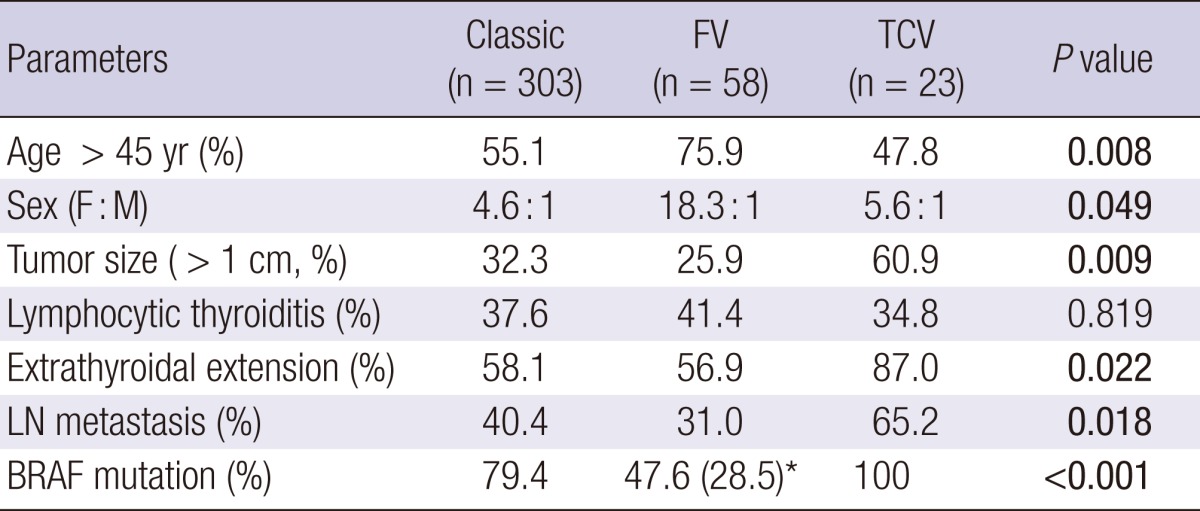
*Encapsulated follicular variant papillary carcinoma. FV, follicular variant papillary carcinoma; TCV, tall cell variant papillary carcinoma; LN, lymph node.
According to multivariate analysis, tumor size ( > 1 cm) was closely related with extrathyroidal extension and cervical LN metastasis (P < 0.001 and < 0.001, respectively), and cervical LN metastasis frequently occurred in patients > 45 yr old (P < 0.001). As in the univariate analysis, FV occurred more frequently in patients > 45 yr old (P = 0.009). However, TCV itself failed to show any independent association with clinicopathologic factors including extrathyroidal extension and cervical LN metastasis.
Expression of immunohistochemical markers
Among 407 cases, 38 of classic PTC, 33 of FVPTC and 23 of TCVPTC were selected and subjected to IHC study (total = 94 cases). Overall, galectin-3, HBME1 and CK19 were expressed in all cases, except for four cases of FVPTC. The proportions of cases with an H-score ≥ 100 were 55.9, 50.5 and 26.9% for galectin-3, HBME1, and CK19, respectively (Fig. 1). The VEGF expression rate was 68.5% (score ≥ 1, 61/89), whereas the expression rates of PTEN, S100A4, c-erbB2, and EGFR were 47.3% (score ≥ 1, 44/93), 82.7% (score ≥ 1, 77/93), 51.1% (score ≥ 1, 46/90), and 6.6% (score ≥ 1, 6/90), respectively (Fig. 2, 3). β-catenin was detected in the cytoplasm of most cases (96.8%, 90/93); however, nuclear localization was not observed. Among these, the membranous positive pattern was observed in 47.3% of cases and the cytoplasmic dot-like positive pattern in 49.5% of cases (Fig. 2).
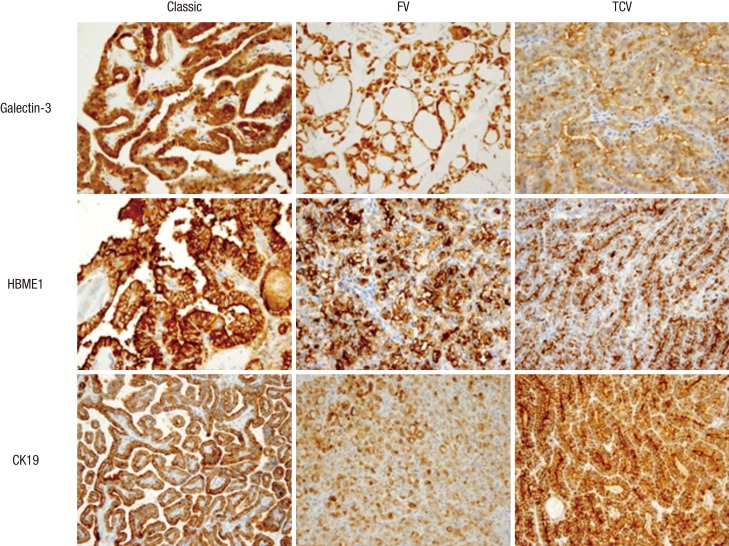
Fig. 1.
Immunohistochemical expression of galectin-3, HBME1, and CK19 in three PTC histological variants. Galectin-3 and HBME1 expression was relatively decreased in both FV and TCV (upper and middle row, ×400), while CK19 expression was decreased only in FV (lower row, ×400). FV, follicular variant; TCV, tall cell variant.
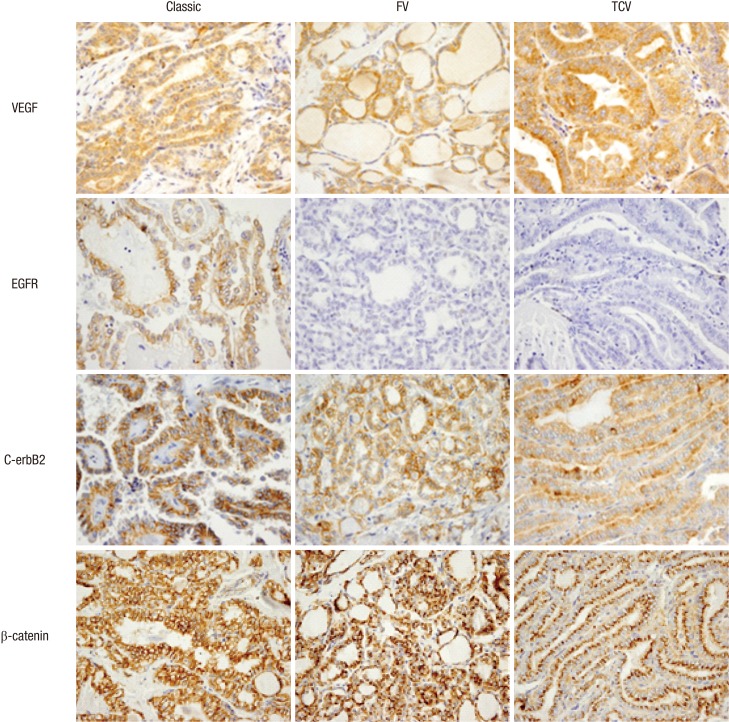
Fig. 2.
VEGF, EGFR, c-erbB2, and β-catenin expression in three PTC histological variants. VEGF expression was frequently increased in TCV (score ≥ 1, P = 0.012, first row, ×400) while no EGFR expression was detected in most FV and TCV cases (P = 0.002, second row, ×400). TCV showed reduced c-erbB2 positivity (score = 0, third row, ×400) and the characteristic paranuclear dot-like β-catenin positivity (fourth rows). FV, follicular variant; TCV, tall cell variant.
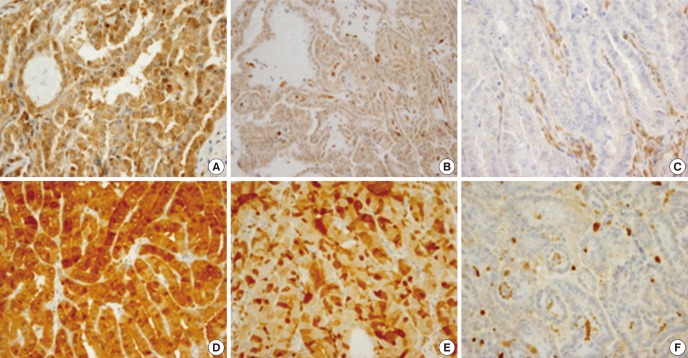
Fig. 3.
PTEN and S100A4 expression in PTC. PTEN expression was detected mainly in the cytoplasm of tumor cells (A, score = 2; B, score = 1, ×400) compared to the case with PTEN loss (C, score = 0). S1004 was expressed in both the nucleus and cytoplasm (D, score = 2; E, score = 1; F, score = 0, ×400).
Galectin-3 expression by H-score was decreased in both FV and TCV, which was more significant in TCV (P = 0.003, Fig. 1, Table 3). HBME1 showed a similar tendency, but not to statistically significant degree (P = 0.114). CK19 expression was markedly decreased in FV only (P < 0.001, Table 3). TCV showed frequent PTEN loss (score = 0), diffuse S100A4 expression (score = 2) and VEGF expression (score ≥ 1); however, PTEN loss and S100A4 expression were not statistically different (P = 0.168 and 0.059, respectively) when comparing the classic, FV and TCV. Nevertheless, when comparing TCV and non-TCV groups, S100A4 (score = 2) and VEGF (score ≥ 1) expression was dominant in the TCV group (P = 0.04 and 0.002, respectively).
Table 3.
Immunoexpression of histological variant
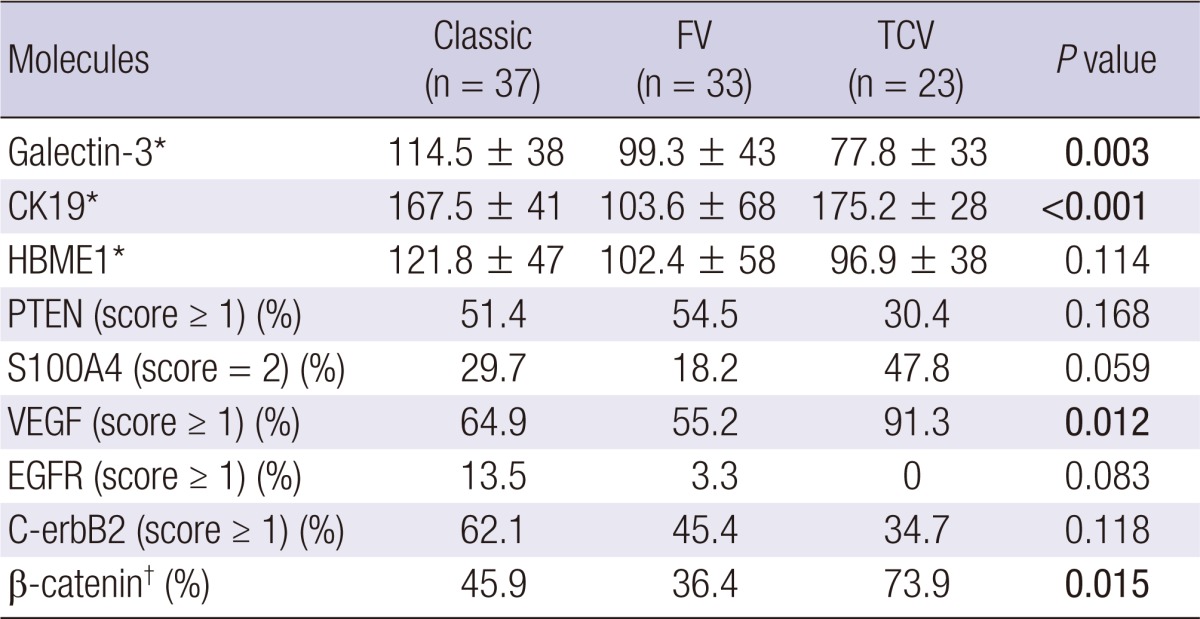
*Mean ± SD; †Cytoplasmic dot-like positive pattern. FV, follicular variant papillary carcinoma; TCV, tall cell variant papillary carcinoma.
EGFR was nearly negative in both the FV and TCV groups, but the overall positive rate (score ≥ 1), including the classic type, was low (6.6%). C-erbB2 expression was also generally weak (score = 1) in all PTC cases, exhibited a decreasing tendency in FV and TCV (P = 0.118). Characteristically, the cytoplasmic dot-like β-catenin positive pattern was observed more frequently in TCV than other histological subtypes (P = 0.015, Fig. 2).
Some markers showed an association with clinicopathologic factors regardless of histological variant. Cases with PTEN loss (score = 0) and diffuse S100A4 expression (score = 2) showed frequent regional LN metastasis (P = 0.007 and 0.013, respectively; Table 4). Moreover, cases with PTEN loss showed more extrathyroidal extension (P = 0.013) and a larger tumor size ( > 1 cm, P = 0.06). Based on multivariate analysis, PTEN loss was associated with LN metastasis (P = 0.02) and extrathyroidal extension (P = 0.029).
Table 4.
Clinicopathologic factors associated with PTEN and S1004 expression
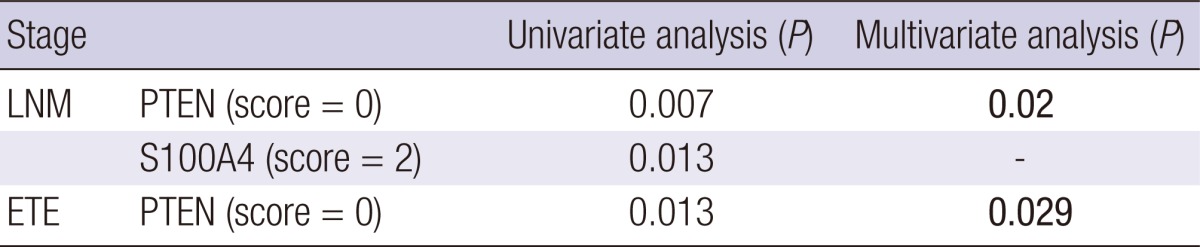
LMN, lymph node metastasis; ETE, extrathyroidal extension.
BRAF mutation study
The BRAF (V600E) mutation was detected in 70.2% (179/255) of patients, while other types of BRAF alterations were not found (Table 1). Cases with the BRAF mutation frequently showed extrathyroidal extension (P = 0.003), but not LN metastasis or a large tumor size (P > 0.05). Histological variants were statistically related with the BRAF mutation (P < 0.001, Table 2); specifically, FV showed a lower frequency of the BRAF mutation (47.6%) whereas TCV showed a higher frequency of the BRAF mutation. In particular, encapsulated FVPTC showed a lower BRAF mutation rate (28.5%, 2/7) whereas all TCV cases contained the BRAF mutation (100%, 23/23). According to multivariate analysis, the BRAF mutation status was associated with extrathyroidal extension (P = 0.010) and histological variants (P = 0.043). Additionally, tumors with Hashimoto thyroiditis in the background had a lower frequency of the BRAF mutation (57.1% vs 85.3%, P < 0.001). None of three cases of the Warthin-like variant that were subjected to direct sequencing harbored the BRAF mutation, which supports the inverse relationship between the BRAF V600E mutation and the presence of Hashomoto thyroiditis.
DISCUSSION
Of the histological PTC variants, TCV is a relatively common subtype that shows aggressive behavior (4). In our series of PTC, TCV showed a larger tumor size, frequent LN metastasis, extrathyroidal extension, and a higher BRAF mutation rate. TCV was detected frequently in younger patients ( ≤ 45 years old) compared to FV, likely due to its rapid growth. However, TCV failed to be an independent factor indicating an adverse pathologic parameter.
TCV showed characteristic immunoexpression of several markers. S100A4 (score = 2) and VEGF (score ≥ 1) were significantly overexpressed while PTEN was frequently lost in TCV (Table 3), which reflected the adverse prognosis of TCV. S100A4 was shown to be expressed in most of PTCs (16), however, high levels of S100A4 (positive area > 50%) has been reported as an indicator of LN metastasis (7, 8). VEGF expression has been associated with older age, tumor recurrence and LN metastasis in PTC (6, 17).
Although galectin-3, HBME1, and CK19 are regarded as the diagnostic markers of PTC (18), FV and TCV showed a unique galectin-3, HBME1, and CK19 expression pattern. The expression of these three markers in FV is known to be reduced (19, 20), which was evident in our data (Table 3). However, in TCV, the staining intensity of galectin-3 was generally weak and the distribution of HBME1 expression was occasional that the overall H-score was lower in TCV than in FV. One unique finding was the β-catenin expression pattern in TCV, which showed paranuclear dot-like positivity. PTC was reported to express β-catenin in the cytoplasm or membrane, except in the cribriform-morular variant in which β-catenin is localized in nucleus (21). Whereas the classic type and FV showed membranous or diffuse cytoplasmic positivity, TCV accumulated β-catenin predominantly in the paranuclear cytoplasm.
The prognostic value of immunohistochemical markers in PTC remains unclear. Loss of p27 and cyclin D1 overexpression has been suggested as independent predictors of regional LN metastasis (22); however, most immunohistochemical markers should be more verified as indicators of a poor clinical outcome. The expression of EGFR, c-erbB2 was nearly absent or weak (1+), and the VEGF expression was not strong in most cases (score = 1). The low expression of EGFR in PTCs was discrepant with the previous result (23), however, it is probably due to that the present data included only the membranous expression, excluding the cytoplasmic positivity. Likewise, the discrepancy with the previous data that showed the prognostic significance of c-erbB-2 (11) might be due to the small number of cases of the previous study (n = 32) and the subgroup analysis that divided to incomplete (1+) and complete (2+ or 3+) membranous expression of c-erbB2, which might limit the statistical significance in the present study.
Regarding PTEN immunoexpression, it was reported to be heterogenous and often puzzling to interpret (24). In the present study, PTEN was differentially expressed in the PTC cases, and the loss of expression was observed in both nucleus and cytoplasm compared to endothelial cells as internal positive controls (Fig. 3C). PTEN loss (score = 0) was independently associated with frequent LN metastasis (P = 0.02) and extrathyroidal extension (P = 0.029) in multivariate analysis. PTEN is a well-known negative regulator of the PI3K/AKT pathway, and alterations in its expression are commonly found in various tumors (25). Recently, evidences suggested that the PI3K/Akt pathway is also altered in PTC (13-14). In particular, the PTEN promoter was hypermethylated in 45.7% of PTC cases, which was significantly associated with PTEN loss in immunohistochemical studies (P = 0.001) (13). In the current study, PTEN loss was observed in 52.6% (49/93) of PTC cases, not only in FV but also in the classic type and TCV. Future studies should investigate the association between PTEN loss with promoter hypermethylation or mutation in these cases.
The overall BRAF V600E mutation rate was 70.4% and 100% in TCV, which is within the reported range for the Korean population (26). Considering the similar proportion of TCV (5.6%) with that of the Western population (5), this suggests that the higher BRAF mutation rate in the Korean population is not due to a higher frequency of TCV. However, BRAF V600E mutation failed to be associated LN metastasis, but it was associated with extrathyroidal extension and histological variants in multivariate analyses. On the other hand, the lower BRAF mutation rate in PTC cases with concurrent Hashimoto thyroiditis than in those without Hashimoto thyroiditis is consistent with previous reports (27). Although the association with tumorigenesis remains unclear, non-neoplastic follicular cells in Hashimoto thyroiditis harbor RET/PTC recombination (28). These results suggest RET/PTC recombination to be related to the development of PTC with concurrent Hashimoto thyroiditis rather than the BRAF mutation, since RET/PTC recombination and the BRAF mutation in PTC are mutually exclusive (29).
In summary, both TCV and FV showed the reduced immunoexpression of galectin-3 and HBME1. Regardless of histological subtype, PTEN loss and S100A4 may serve as indicators of an adverse outcome in PTC. An extended follow-up period may be required for further verification of these markers as prognostic predictors.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
References
- 1.Akslen LA, LiVolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000;88:1902–1908. [PubMed] [Google Scholar]
- 2.Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, Kihara M, Miyauchi A. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008;99:1908–1915. doi: 10.1111/j.1349-7006.2008.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid. 2010;20:153–158. doi: 10.1089/thy.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LiVolsi VA. Papillary carcinoma tall cell variant (TCV): a review. Endocr Pathol. 2010;21:12–15. doi: 10.1007/s12022-010-9106-y. [DOI] [PubMed] [Google Scholar]
- 5.Ghossein R, Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid. 2008;18:1179–1181. doi: 10.1089/thy.2008.0164. [DOI] [PubMed] [Google Scholar]
- 6.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–658. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 7.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min HS, Choe G, Kim SW, Park YJ, Park do J, Youn YK, Park SH, Cho BY, Park SY. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod Pathol. 2008;21:748–755. doi: 10.1038/modpathol.2008.51. [DOI] [PubMed] [Google Scholar]
- 9.Murakawa T, Tsuda H, Tanimoto T, Tanabe T, Kitahara S, Matsubara O. Expression of KIT, EGFR, HER-2 and tyrosine phosphorylation in undifferentiated thyroid carcinoma: implication for a new therapeutic approach. Pathol Int. 2005;55:757–765. doi: 10.1111/j.1440-1827.2005.01902.x. [DOI] [PubMed] [Google Scholar]
- 10.Landriscina M, Pannone G, Piscazzi A, Toti P, Fabiano A, Tortorella S, Occhini R, Ambrosi A, Bufo P, Cignarelli M. Epidermal growth factor receptor 1 expression is upregulated in undifferentiated thyroid carcinomas in humans. Thyroid. 2011;21:1227–1234. doi: 10.1089/thy.2011.0172. [DOI] [PubMed] [Google Scholar]
- 11.Freudenberg LS, Sheu S, Görges R, Mann K, Bokler S, Frilling A, Schmid KW, Bockisch A, Otterbach F. Prognostic value of c-erbB-2 expression in papillary thyroid carcinoma. Nuklearmedizin. 2005;44:179–182. 184. [PubMed] [Google Scholar]
- 12.Wang Y, Hou P, Yu H, Wang W, Ji M, Zhao S, Yan S, Sun X, Liu D, Shi B, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab. 2007;92:2387–2390. doi: 10.1210/jc.2006-2019. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Nuñez F, Bussaglia E, Mauricio D, Ybarra J, Vilar M, Lerma E, de Leiva A, Matias-Guiu X Thyroid Neoplasia Study Group. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006;16:17–23. doi: 10.1089/thy.2006.16.17. [DOI] [PubMed] [Google Scholar]
- 14.Sozopoulos E, Litsiou H, Voutsinas G, Mitsiades N, Anagnostakis N, Tseva T, Patsouris E, Tseleni-Balafouta S. Mutational and immunohistochemical study of the PI3K/Akt pathway in papillary thyroid carcinoma in Greece. Endocr Pathol. 2010;21:90–100. doi: 10.1007/s12022-010-9112-0. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: experience at a single institute. Pathology. 2008;40:617–622. doi: 10.1080/00313020802320630. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K, Miyauchi A. S100A4 expression is an early event of papillary carcinoma of the thyroid. Oncology. 2004;67:397–402. doi: 10.1159/000082924. [DOI] [PubMed] [Google Scholar]
- 17.De Araujo-Filho VJ, Alves VA, de Castro IV, Lourenço SV, Cernea CR, Brandão LG, Ferraz AR. Vascular endothelial growth factor expression in invasive papillary thyroid carcinoma. Thyroid. 2009;19:1233–1237. doi: 10.1089/thy.2008.0179. [DOI] [PubMed] [Google Scholar]
- 18.Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005;18:48–57. doi: 10.1038/modpathol.3800235. [DOI] [PubMed] [Google Scholar]
- 19.Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: galectin-3, HBME-1, and cytokeratin-19. Endocr Pathol. 2010;21:80–89. doi: 10.1007/s12022-010-9114-y. [DOI] [PubMed] [Google Scholar]
- 20.Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010;5:9. doi: 10.1186/1746-1596-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung CK, Choi YJ, Lee KY, Bae JS, Kim HJ, Yoon SK, Son YI, Chung JH, Oh YL. The cytological, clinical, and pathological features of the cribriform-morular variant of papillary thyroid carcinoma and mutation analysis of CTNNB1 and BRAF genes. Thyroid. 2009;19:905–913. doi: 10.1089/thy.2008.0332. [DOI] [PubMed] [Google Scholar]
- 22.Khoo ML, Beasley NJ, Ezzat S, Freeman JL, Asa SL. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002;87:1814–1818. doi: 10.1210/jcem.87.4.8353. [DOI] [PubMed] [Google Scholar]
- 23.Westermark K, Lundqvist M, Wallin G, Dahlman T, Hacker GW, Heldin NE, Grimelius L. EGF-receptors in human normal and pathological thyroid tissue. Histopathology. 1996;28:221–227. doi: 10.1046/j.1365-2559.1996.d01-427.x. [DOI] [PubMed] [Google Scholar]
- 24.Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung CK, Im SY, Kang YJ, Lee H, Jung ES, Kang CS, Bae JS, Choi YJ. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid. 2012;22:791–797. doi: 10.1089/thy.2011.0123. [DOI] [PubMed] [Google Scholar]
- 27.Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto’s thyroiditis. Pathol Int. 2005;55:540–545. doi: 10.1111/j.1440-1827.2005.01866.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, Qumsiyeh MB, Rothstein JL, Fusco A, Santoro M, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006;91:2414–2423. doi: 10.1210/jc.2006-0240. [DOI] [PubMed] [Google Scholar]
- 29.Bhaijee F, Nikiforov YE. Molecular analysis of thyroid tumors. Endocr Pathol. 2011;22:126–133. doi: 10.1007/s12022-011-9170-y. [DOI] [PubMed] [Google Scholar]