Abstract
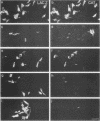
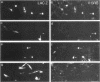
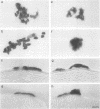
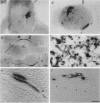
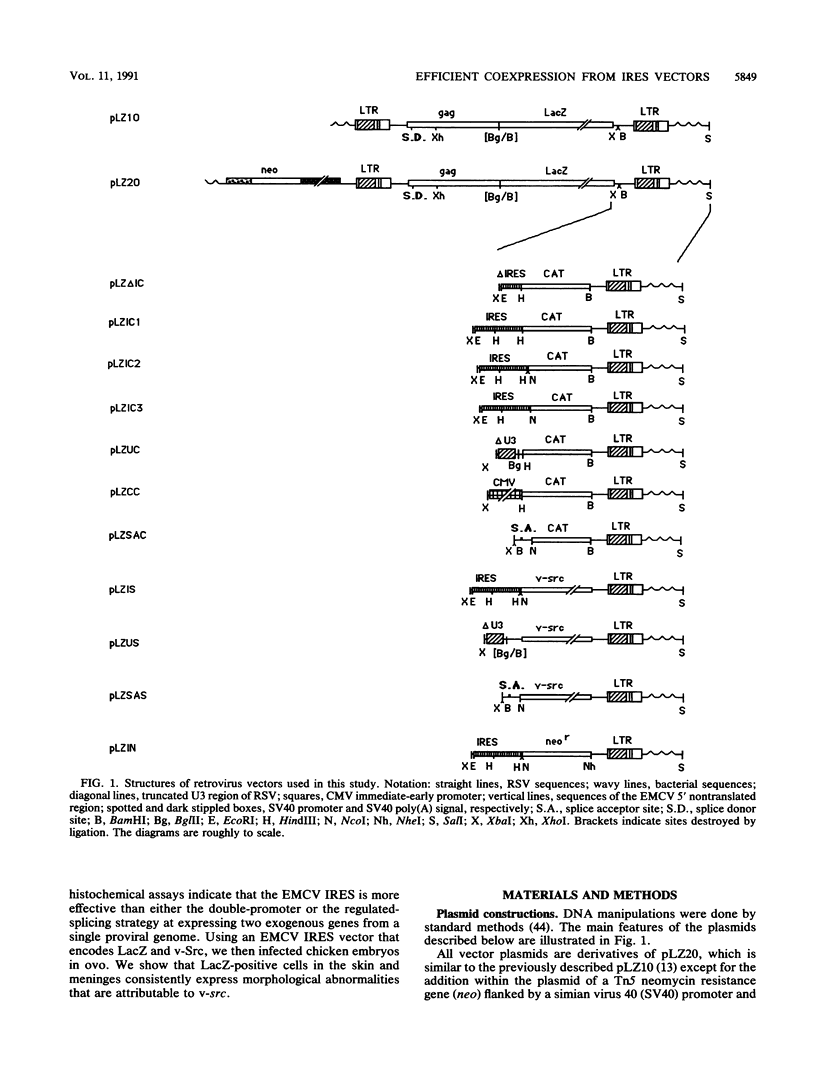
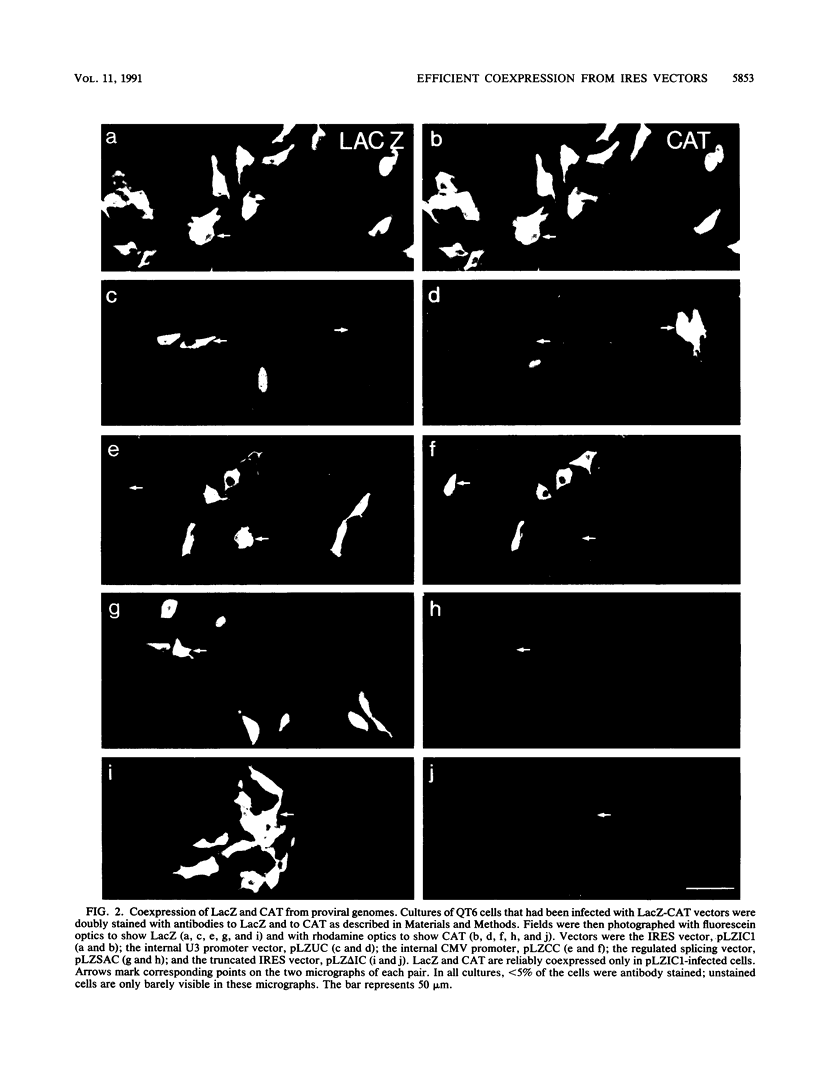
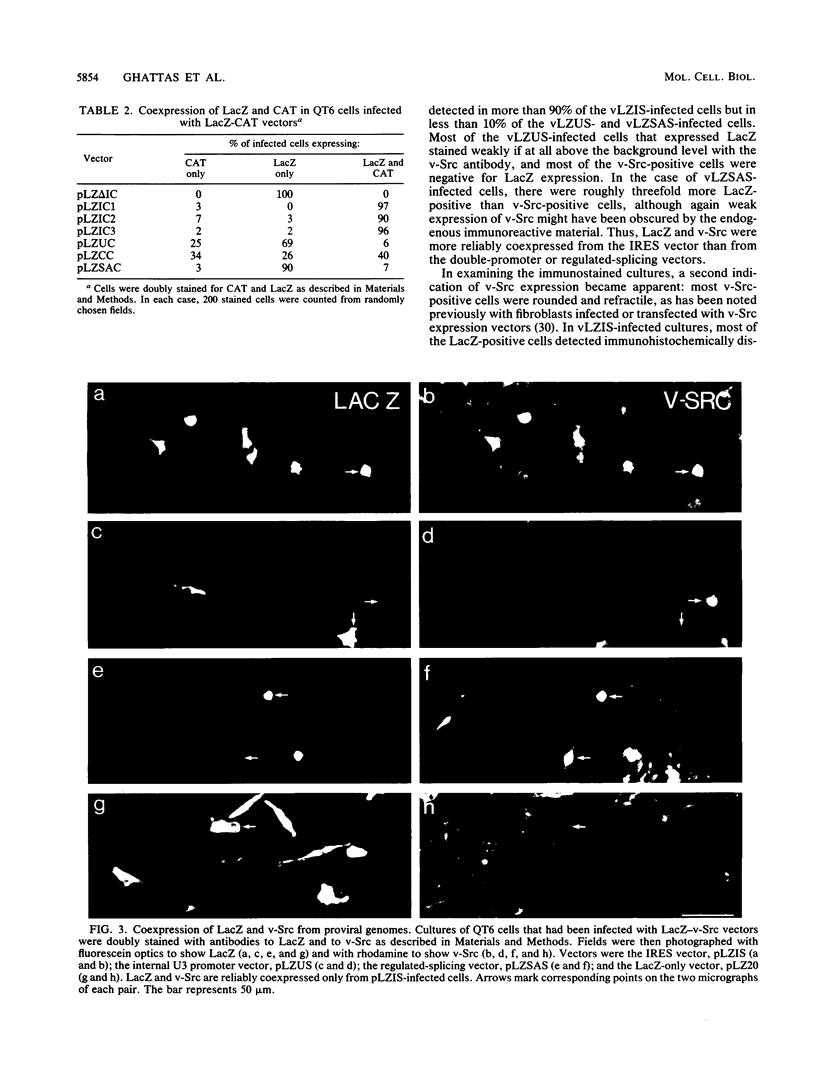
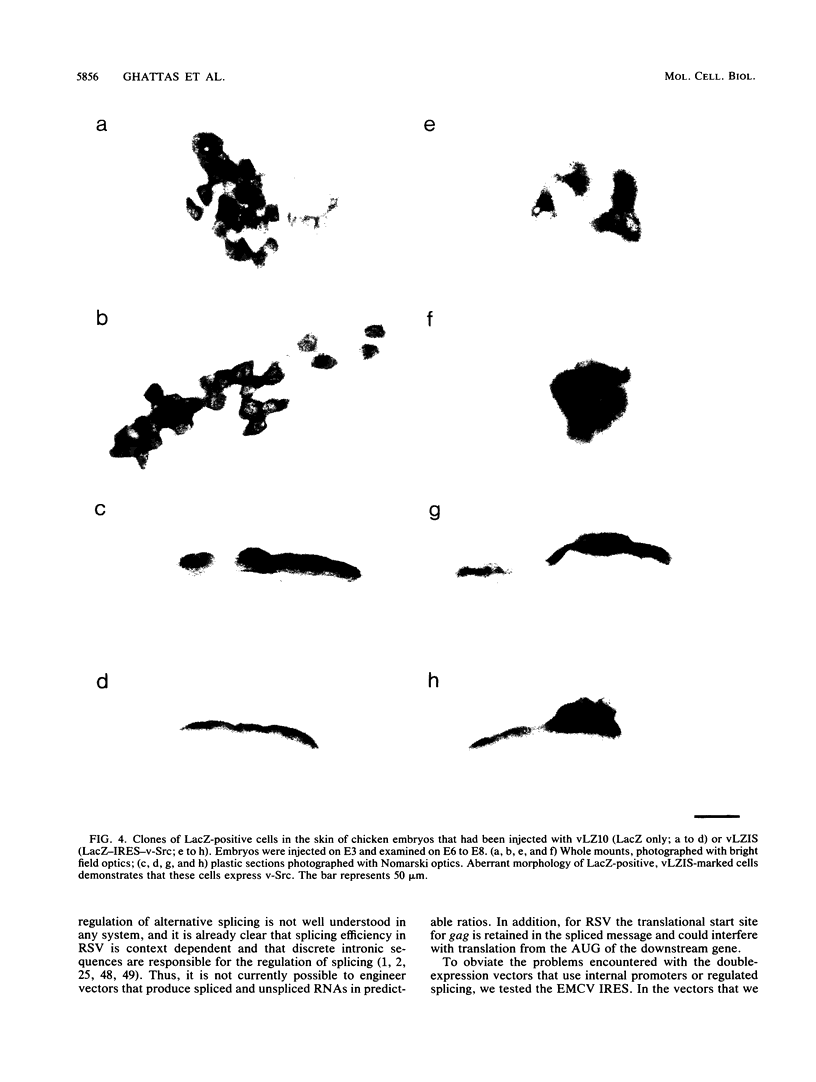
Rous sarcoma virus-based retroviral vectors were constructed to compare three different approaches for coexpressing two genes in individual infected cells. All vectors expressed the upstream gene (lacZ) from the Rous sarcoma virus long terminal repeat, while the downstream gene (the chloramphenicol acetyltransferase gene [cat] or v-src) was expressed in one of three ways: from a subgenomic mRNA generated by regulated splicing, from a strong internal promoter, or from the encephalomyocarditis virus internal ribosome entry site (IRES). Both biochemical and immunohistochemical assays of cultured cells showed that the encephalomyocarditis virus IRES provided the most efficient means for coexpressing two genes from a single provirus. Most importantly, most cells infected by a LacZ-IRES-CAT virus expressed both LacZ and CAT, whereas most cells infected by internal promoter or regulated splicing vectors expressed either LacZ or CAT but not both. In addition, viral titers were highest with IRES vectors. Presumably, use of the IRES avoids transcriptional controls and RNA processing steps that differentially affect expression of multiple genes from internal promoter and regulated splicing vectors. Finally, we injected a LacZ-IRES-v-Src virus into chicken embryos and then identified the progeny of infected cells with a histochemical stain for LacZ. LacZ-positive cells in both skin and mesenchyme displayed morphological abnormalities attributable to expression of v-src. Thus, IRES vectors can be used to coexpress a reporter gene and a bioactive gene in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988 Nov;8(11):4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S. L., Stoltzfus C. M. Mutations in the regions of the Rous sarcoma virus 3' splice sites: implications for regulation of alternative splicing. J Virol. 1991 May;65(5):2640–2646. doi: 10.1128/jvi.65.5.2640-2646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. Corrections to the nucleotide sequence of the src gene of Rous sarcoma virus. Nature. 1983 Feb 24;301(5902):736–738. doi: 10.1038/301736b0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking M. K., Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45(1):101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Frank E., Sanes J. R. Lineage of neurons and glia in chick dorsal root ganglia: analysis in vivo with a recombinant retrovirus. Development. 1991 Apr;111(4):895–908. doi: 10.1242/dev.111.4.895. [DOI] [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Galileo D. S., Gray G. E., Owens G. C., Majors J., Sanes J. R. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow M. M., Hayward W. S. 5' long terminal repeats of myc-associated proviruses appear structurally intact but are functionally impaired in tumors induced by avian leukosis viruses. J Virol. 1987 Aug;61(8):2489–2498. doi: 10.1128/jvi.61.8.2489-2498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. E., Glover J. C., Majors J., Sanes J. R. Radial arrangement of clonally related cells in the chicken optic tectum: lineage analysis with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7356–7360. doi: 10.1073/pnas.85.19.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. E., Leber S. M., Sanes J. R. Migratory patterns of clonally related cells in the developing central nervous system. Experientia. 1990 Sep 15;46(9):929–940. doi: 10.1007/BF01939386. [DOI] [PubMed] [Google Scholar]
- Gray G. E., Sanes J. R. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991 Feb;6(2):211–225. doi: 10.1016/0896-6273(91)90357-6. [DOI] [PubMed] [Google Scholar]
- Hoeben R. C., Migchielsen A. A., van der Jagt R. C., van Ormondt H., van der Eb A. J. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J Virol. 1991 Feb;65(2):904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Majors J. E., Varmus H. E. Hormonal regulation of the Rous sarcoma virus src gene via a heterologous promoter defines a threshold dose for cellular transformation. Cell. 1984 Oct;38(3):757–765. doi: 10.1016/0092-8674(84)90271-x. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Kotler M., Skalka A. M. cis-acting intron mutations that affect the efficiency of avian retroviral RNA splicing: implication for mechanisms of control. J Virol. 1988 Aug;62(8):2686–2695. doi: 10.1128/jvi.62.8.2686-2695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Wagner E. F. Expression of v-src induces a myeloproliferative disease in bone-marrow-reconstituted mice. Genes Dev. 1989 Jun;3(6):827–837. doi: 10.1101/gad.3.6.827. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber S. M., Breedlove S. M., Sanes J. R. Lineage, arrangement, and death of clonally related motoneurons in chick spinal cord. J Neurosci. 1990 Jul;10(7):2451–2462. doi: 10.1523/JNEUROSCI.10-07-02451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Brugge J. S. Biological and biochemical properties of the c-src+ gene product overexpressed in chicken embryo fibroblasts. Mol Cell Biol. 1989 Aug;9(8):3332–3341. doi: 10.1128/mcb.9.8.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F. pp60c-src encoded by the proto-oncogene c-src is a product of sensory neurons. J Neurosci Res. 1986;16(1):127–139. doi: 10.1002/jnr.490160113. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Overell R. W., Weisser K. E., Cosman D. Stably transmitted triple-promoter retroviral vectors and their use in transformation of primary mammalian cells. Mol Cell Biol. 1988 Apr;8(4):1803–1808. doi: 10.1128/mcb.8.4.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A. W., Hatier C., Bissell M. J. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J Cell Biol. 1990 Jul;111(1):217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A. W., Sieweke M. H. v-src induces clonal sarcomas and rapid metastasis following transduction with a replication-defective retrovirus. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10123–10127. doi: 10.1073/pnas.86.24.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Fogarty S. J. Multiple regions in the Rous sarcoma virus src gene intron act in cis to affect the accumulation of unspliced RNA. J Virol. 1989 Apr;63(4):1669–1676. doi: 10.1128/jvi.63.4.1669-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Lorenzen S. K., Berberich S. L. Noncoding region between the env and src genes of Rous sarcoma virus influences splicing efficiency at the src gene 3' splice site. J Virol. 1987 Jan;61(1):177–184. doi: 10.1128/jvi.61.1.177-184.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., DeLorbe W. J., Bishop J. M., Varmus H. E. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc Natl Acad Sci U S A. 1981 Jan;78(1):124–128. doi: 10.1073/pnas.78.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]