Abstract
Antibody glycosylation has been shown to change with various processes. This review presents mass spectrometric approaches for antibody glycosylation analysis at the level of released glycans, glycopeptides, and intact protein. With regard to IgG fragment crystallizable glycosylation, mass spectrometry has shown its potential for subclass-specific, high-throughput analysis. In contrast, because of the vast heterogeneity of peptide moieties, fragment antigen binding glycosylation analysis of polyclonal IgG relies entirely on glycan release. Next to IgG, IgA has gained some attention, and studies of its O- and N-glycosylation have revealed disease-associated glycosylation changes. Glycoproteomic analyses of IgM and IgE are lagging behind but should complete our picture of glycosylation's influence on antibody function.
BIOLOGICAL ROLE OF IMMUNOGLOBULIN GLYCOSYLATION
Immunoglobulins (Igs)1 are produced by the adaptive immune system in order to identify and neutralize foreign antigens and pathogens to which the host has been exposed. In humans, five known classes of Igs (IgG, IgM, IgA, IgE, and IgD) are secreted in variable amounts by B cells during an immune response. Although these Ig classes are built from Ig domains and are thus structurally related, they differ considerably in several aspects, such as their glycosylation (1). Over the past 30 years, numerous studies have explored the structural, biological, and clinical roles of Ig glycosylation, focusing mainly on IgG molecules, which are the most abundant serum Ig, occurring at 10 to 15 mg/ml (value for IgG1) in human circulation (1). Each IgG molecule consists of two heavy and two light chains that together form two fragment antigen binding (Fab) portions and one fragment crystallizable (Fc) portion (Fig. 1). Two N-glycans are linked to the heavy chains at Asn 297 in the CH2 domain of the protein backbone (Fc part). These Fc glycans are in part located in a cavity between the two heavy chains and influence the conformation of the protein (2, 3). Their removal by glycosidases or via mutation of the glycosylation sites reduces the binding of IgG to Fc-gamma receptors (FcγR) (4–6). The Fc-linked carbohydrates are complex-type biantennary N-glycans with a high level of core-fucosylation and a variable number of galactoses (Gal) resulting in the prevalent glycoforms G0F (no Gal), G1F (one Gal), and G2F (two Gal). A minor proportion of these glycans might contain a bisecting N-acetylglucosamine (GlcNAc) residue and/or terminal sialic acids substituting antenna Gal (7) (see Fig. 1).
Fig. 1.
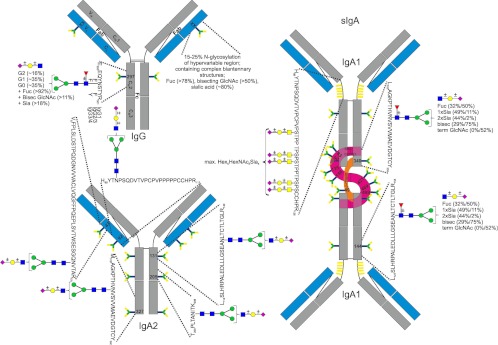
Glycoproteomic analysis of human IgG and IgA. Glycosylation of IgG1 (P01857), IgG2 (P01859), IgG3 (P01860), IgG4 (P01861), IgA1 (P01876) in secretory IgA (sIgA), and IgA2 (P01877). Heavy chains are depicted in gray, light chains in blue, secretory components (P01833) of sIgA in purple, and joining chain (P01591) in orange. Glycosylation sites are indicated by the respective amino acid number and schematic N- and O-glycans, respectively. Tryptic peptides for all constant region glycosylation sites are given except for the secretory component and the joining chain of sIgA. Glycans are depicted according to CFG notation; blue square, N-acetylglucosamine; green circle, mannose; yellow circle, galactose; red triangle, fucose; purple diamond, sialic acid. If known, further information on N-glycan structures is given (51, 74, 100). For IgA, values in parentheses indicate the abundance in plasma IgA/sIgA. The composition of O-glycans on the IgA HR peptide is reported according to Deshpande et al. (91).
Many reports have described variations of IgG Fc glycosylation, especially of the degree of galactosylation, related to age, sex, heritability, and pregnancy, as well as to autoimmune diseases, infectious diseases, and cancers (e.g. Refs. 8–15). For instance, an increase in IgG G0F is observed in the serum of patients with rheumatoid arthritis (7) and correlates with disease progression and severity (16, 17). These clinical observations have led researchers to examine in detail the relationship between Fc glycan structures, the biological properties of IgG, and the degree of inflammation. It was found that an absence of sialic acids and low levels of galactosylation might confer important pro-inflammatory properties to IgG by facilitating the formation of immune complexes and favoring the binding of IgG to activating FcγR (18–20). Similarly, the absence of core-fucose or the presence of bisecting GlcNAc improved the affinity of the Fc tail to FcγRIIIa, thereby enhancing antibody-dependent cellular cytotoxicity (21–23). On this basis, new glycoengineered anti-cancer antibodies carrying afucosylated Fc glycans are currently in clinical development, such as the anti-CD20 monoclonal antibody (mAb) obinutuzumab (GA101) for use against B-cell lymphoma (24, 25).
In addition, Fc-linked glycans appear to modulate the activation of the complement system. Whereas the classical complement pathway can be triggered by the preferential binding of C1q to fully galactosylated IgG, the lectin pathway is recruited through the recognition of agalactosylated IgG by mannose-binding lectin (26, 27). In contrast, the presence of terminal galactose and/or sialic acid residues on Fc glycans might confer anti-inflammatory properties to IgG via interaction with the human lectins Dectin-1 (28) and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (19, 29, 30). Thus, variations in the structure of IgG Fc glycans might skew the immune system toward a pro- or an anti-inflammatory response by modulating the interaction of IgG with several immune components, including FcγR, complement factors, and lectins. Interestingly, it was recently established that IgG Fc glycosylation may be modulated by factors such as hormones (e.g. estradiol and progesterone), cytokines (e.g. IFN-γ and IL-21), bacterial DNA (CpG oligodeoxynucleotide), and food metabolites (e.g. all-trans retinoic acid and drugs) (31–33).
The influence of glycosylation on the biological properties of other Ig classes has been poorly explored. Some reports have established that variations in the glycosylation of IgA and IgE modulate the affinity for their respective receptors, FcαR and FcεR (1). Results from clinical studies also support the idea that there is some structural and functional role of glycosylation in all classes of Ig. An example is IgA1, which exhibits O-glycosylation at various sites of its hinge region peptide (see Fig. 1). In nephropathy, lowered levels of IgA1 O-glycan sialylation and galactosylation have been observed (34). These abnormally glycosylated IgA1s were shown to have a longer half-life, to self-aggregate, and to form complexes with other molecules of the immune system, including IgG and mannose-binding lectin, thereby promoting IgA deposition in the kidney mesangium and exacerbating inflammation (1).
ANALYTICAL APPROACHES FOR Ig GLYCOSYLATION ANALYSIS
Glycosylation analysis of glycoproteins in general and of Igs in particular may be addressed via (a) intact glycoprotein analysis, (b) the characterization of glycopeptides, or (c) structural analysis of chemically or enzymatically released glycans (15, 35, 36). Mass spectrometric analysis of glycoproteins at the glycopeptide or released glycan level are currently the methods of choice for obtaining sensitive and comprehensive glycosylation information from complex biological samples (37).
Analysis at the glycopeptide level is the most favorable approach, as site-specific glycan heterogeneity can be characterized and glycan compositions can be correlated to their attachment sites on the protein (35). In particular, liquid chromatography–mass spectrometry (LC/MS) has been widely used for glycopeptide analysis. The advantage of LC-electrospray ionization (ESI)-MS analysis is the up-front chromatographic separation of the (glyco)peptides prior to MS analysis. Obviously the choice of an efficient chromatographic separation method for a glycopeptide mixture after proteolytic digestion is crucial. For this purpose, C18 reversed-phase (RP) HPLC is widely used, next to hydrophilic interaction liquid chromatography (HILIC) and graphitized carbon HPLC (15, 38, 39).
Released glycan samples are generally of lower complexity than glycopeptide samples, and various targeted and untargeted glycomics approaches are commonly applied at the released glycan level. A common high-throughput approach involves the permethylation of C18 RP and carbon solid phase extraction purified glycans followed by matrix-assisted lased desorption ionization (MALDI) time-of-flight (TOF) MS analysis (36, 40). This review focuses mainly on Ig glycosylation analysis via MS of glycopeptides, and we refer to other reviews for more in-depth coverage of released glycan analysis (41–44).
Total IgG Glycosylation Analysis
Polyclonal human IgG N-glycosylation has been studied extensively at the level of released N-glycans. A seminal 1985 work by Parekh et al. demonstrated increased levels of agalactosylated glycans associated with rheumatoid arthritis and osteoarthritis (7). That paper represents a major milestone in IgG research and gave rise to a continuing range of studies on human IgG glycosylation using a diverse range of methods for glycan analysis such as HILIC with fluorescence detection, capillary gel electrophoresis with laser-induced fluorescence detection, and MS, demonstrating IgG glycosylation changes with age, sex, pregnancy, and diseases (14, 15).
A high-throughput isolation and glycosylation analysis of IgG was published recently by Pucic et al. (10): IgGs of 2298 individuals were efficiently isolated from plasma using a 96-well protein G monolithic plate. The N-glycans were released using PNGase F, labeled with 2-aminobenzamide, and analyzed by means of HILIC HPLC with fluorescence detection. High variability in IgG glycosylation among individuals was observed and was found to be approximately three times higher than in the total plasma N-glycome. Heritability in this case was found to be between 30% and 50%, and gender appeared not to be an important predictor for any IgG glycans. Sialylation was found to be the most endogenously defined glycosylation feature, with up to 60% of variance explained by heritability.
Analysis of total IgG glycosylation at the level of released glycans registers mixtures of Fc and Fab glycans from the different subclasses of IgG. Approaches that provide more specific information on IgG glycosylation are presented in the following two sections.
IgG Fc Glycosylation Analysis
Mass spectrometric analysis of tryptic Fc glycopeptides allows the discrimination of different human IgG subclasses based on minor differences in amino acid sequences (Fig. 2). For IgG3, different allotypes appear to prevail in different ethnic groups (45, 46). Analysis of IgG3 from Caucasians mainly revealed allotype G3m(b*), which exhibits a phenylalanine (F) in position 296 (47). As a consequence, the resulting tryptic Fc glycopeptides of IgG2 and IgG3 show identical peptide moieties. In contrast, IgG3 from Asian donors was reported to exhibit a tyrosine (Y) in position 296, resulting in identical peptide moieties for IgG3 and IgG4 (45). Thus, allotypic variations have to be taken into account when comparing subclass-specific IgG Fc-glycosylation profiles of genetically different groups.
Fig. 2.
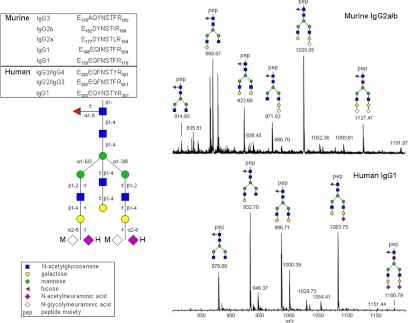
Murine and human plasma IgG Fc glycosylation differences. Tryptic glycopeptides (A) of murine (IgG1, BAE25911, BAC30871; IgG2a, P01863, P01864; IgG2b, P01867; IgG3, P03987) and human (Fig. 1) polyclonal IgG were analyzed via RP-nano-LC sheath-flow ESI-MS using a gradient of aqueous 0.1% trifluoroacetic acid and acetonitrile. Spectra represent the sum of a 45-s elution window depicting [M+3H]3+ species of murine IgG2a/b (B) and human IgG1 (C) Fc glycoforms.
A very convenient approach for IgG Fc glycosylation analysis is the measurement of (tryptic) Fc glycopeptides, which is generally performed via RP-LC-MS/MS (37, 48–50). Chromatographic separation is observed on the basis of small structural differences in a single amino acid side chain. Tryptic Fc glycopeptides of IgG1 carrying tyrosine residues in positions 296 and 300 elute in front of tryptic IgG3/4 glycopeptides (F296 and Y300), which again elute in front of tryptic IgG2/3 Fc glycopeptides (F296 and F300). In contrast, changes in the glycan structure with regard to galactosylation, fucosylation, and bisection hardly affect RP retention times. Consequently, IgG1, IgG2/3, and IgG3/4 glycopeptide clusters are observed in distinct retention time windows. Isomeric tryptic Fc glycopeptide species belonging to different IgG subclasses (i.e. fucosylated IgG1 and non-fucosylated IgG3/4, or fucosylated IgG3/4 and non-fucosylated IgG2/3) are consistently separated by RP-LC, allowing their unambiguous assignment to specific IgG subclasses upon mass spectrometric detection. Sialic acid, however, can have a strong influence on IgG Fc glycopeptide retention, depending on the solvent system. The use of an acetonitrile gradient in aqueous 0.1% formic acid results in greater retention of sialylated species than neutral glycopeptides (48, 50). We recently optimized an RP-nano-LC-ESI-MS setup for fast and robust subclass-specific Fc-glycosylation profiling in large sets of IgG samples (51). Tryptic (glyco)peptides of protein A or protein G affinity-purified polyclonal IgG were collected onto a porous particle C18 trap column and separated on a fused-core C18 column using a short gradient of aqueous 0.1% trifluoroacetic acid and acetonitrile (16 min total analysis time). When formic acid was replaced with trifluoracetic acid, sialylated glycopeptides eluted together with their non-sialylated counterparts. Long-term stable and robust mass spectrometric analysis was achieved by employing a sheath-flow ESI sprayer with isopropanol:water:propionic acid (50:30:20; v:v:v) as a sheath liquid. The relative standard deviations for the eight major observed glycopeptide species remained less than 4% over a time range of several months, thereby allowing the analysis of thousands of samples with high precision.
HILIC-LC-MS also has been reported as a versatile tool for the separation of glycans and glycopeptides (52–54). Tryptic IgG1 Fc glycopeptides experience more retention than IgG2 Fc glycopeptides as a result of the additional oxygen atoms presented by the tyrosine residues at positions 296 and 300 (54). Furthermore, greater retention is observed with increasing glycan size/complexity, and chromatographic distinction between the 3-arm and 6-arm isomers of monogalactosylated species is often possible because of the slightly greater retention of the 3-arm isomer (54, 55). The high organic modifier content applied in HILIC mobile phases makes this separation technique particularly well suited for MS interfacing.
Fast and straightforward analysis of IgG Fc glycosylation is achieved by enriching the tryptic Fc glycopeptides using HILIC solid phase extraction followed by direct-infusion ESI-MS(/MS) (56, 57). Alternatively, MALDI-MS of purified Fc-glycopeptides can be performed with either positive- or negative-mode ionization (37, 58–60). When combined with delayed-extraction TOF detection, MALDI analysis of sialylated Fc-glycopeptides might result in a vast degree of in-source decay, largely dependent on the matrix chosen for sample preparation. When α-cyano-4-hydroxycinnamic acid is used, sialylated species are almost completely degraded. In contrast, the analysis of sialylated Fc glycopeptides is possible with 2,5-dihydroxybenzoic acid and 4-chloro-α-cyanocinnamic acid, especially when combined with negative-mode ionization (61). Interestingly, MALDI Fourier transform ion cyclotron resonance (FTICR) MS, which features an intermediate-pressure ion source, allows the registration of sialylated glycopeptides with both 2,5-dihydroxybenzoic acid and α-cyano-4-hydroxycinnamic acid (59). This may be attributed to the efficient cooling of nascent ions, which limits in-source decay (62). Although direct infusion-ESI-MS and MALDI-MS have superior throughput relative to LC/MS approaches, the accurate relative quantification of polyclonal human IgG Fc glycoforms might be compromised by the presence of isomeric tryptic glycopeptides of different IgG subclasses. However, this is not an issue with mAbs. These high-throughput approaches show particularly high potential for biopharmaceutical quality control and fermentation monitoring (57). It has to be taken into account, however, that unlike human polyclonal IgG, which is over 99% glycosylated in the Fc moiety, biotechnologically produced IgG might contain Fc peptides holding the consensus N-glycosylation sequence but lacking glycosylation. As most non-glycosylated peptides will be lost upon HILIC solid phase extraction, RP and porous graphitized-carbon-based sample preparations might be advantageous for such samples in order to allow the simultaneous analysis of glycosylated and non-glycosylated versions of the Fc peptide covering the N-glycosylation site.
Another strategy for analyzing IgG Fc glycosylation on biopharmaceuticals involves ESI-high-resolution-MS(/MS) of intact mAbs or Fc portions prepared via reduction or enzymatic digestion (57, 63, 64). The high mass accuracy obtained with current high-resolution mass spectrometers allows one to determine the glycoform composition on intact monoclonal antibodies based on accurate mass with a typical 15-ppm (63) to 2-ppm (64) mass accuracy error. Moreover, up to 33% peptide sequence coverage has been reported for an intact commercial recombinant IgG using an LC-ESI-electron transfer dissociation high-resolution MS/MS approach in which time-domain transients recorded in different LC-MS/MS experiments were averaged prior to Fourier transform signal processing (64). Although intact glycoprotein analysis works well to profile Fc glycoforms on mAbs, it might not be applicable for highly complex samples such as human polyclonal IgG.
To elucidate the role of IgG Fc glycosylation in autoimmunity, inflammatory diseases, and cancer, many studies use murine disease models. Fc glycosylation of murine IgGs, however, considerably differs from that of human IgGs with regard to sialylation, fucosylation, and bisection (Fig. 2). The sialic acid on murine IgG appears to be exclusively N-glycolylneuraminic acid, whereas human IgG exclusively exhibits N-acetylneuraminic acid (27, 65). Moreover, serum-derived murine IgG1 and IgG2a/b both show high levels of disialylated Fc glycopeptides (signal at m/z 1127.41; Fig. 2A) (65–67). On human IgG, disialylated Fc N-glycopeptides have only recently been reported for recombinantly expressed mAb at a low relative abundance (57), but they can also be found on polyclonal IgG from human circulation, albeit at a low relative intensity (signal at m/z 1180.79; Fig. 2B). Fucosylation on murine IgG is even higher than on human IgG, with non-fucosylated glycoforms being almost completely missing. Also, bisected species are lacking on murine IgG Fc portions, making the overall glycoform repertoire of murine IgG much more restricted than that of human IgG. Thus, IgG Fc glycosylation variation observed in murine models might not directly translate to the human situation.
IgG samples that are biotechnologically produced or derived from human circulation are generally available in relatively large amounts (often microgram quantities), and the sensitivity of MS methods is therefore not an issue. It has been demonstrated, however, that IgG subpopulations might diverge considerably from total serum IgG in terms of Fc glycosylation profiles (68–71). Thus, the analysis of specific subpopulations of IgG has been found to be rewarding and has repeatedly revealed skewed glycosylation profiles that might have a profound influence on the biological activity of, for example, pathogenic autoantibodies and alloantibodies (68, 69, 71). Notably, these antibodies often may be obtained in only minute amounts by means of affinity purification, and conventional nano-LC/MS has in some cases been found to have insufficient sensitivity to analyze their Fc glycosylation. A recently reported transient-isotachophoresis separation in neutrally coated capillaries with a porous sheathless sprayer interfaced with an ultra-high-resolution TOF mass spectrometer addressed this issue, bringing the lower limit of detection down to ∼20 amol (72). This high sensitivity was reached as a result of reduced ion suppression, which is typical of ESI at very low flow rates such as those used with capillary electrophoresis sheathless ESI-MS (73).
Fab Glycosylation Analysis
Besides the conserved N-glycosylation sites on the Fc portion, additional carbohydrate chains can be linked to the hypervariable regions of Ig. For instance, between 15% and 25% of IgG molecules isolated from the serum of healthy human subjects have been reported to carry N-glycans on their variable domains (74–76). IgG populations with Fab glycans have been called asymmetric antibodies and were found to be bound by the lectin concanavalin A (77, 78). Interestingly, the amount of asymmetric IgG was found to increase during pregnancy, as well as after the treatment of antibody-producing cells with hormones (e.g. progesterone, estrogen) and cytokines (e.g. IL-6) (79–81). More recently, HPLC and MS analyses of Fab-linked glycans from human serum IgG have revealed primarily complex-type biantennary N-glycans with high contents of core-fucose (∼80%), bisecting GlcNAc (>50%), and sialic acid (∼80%) (74, 75, 82). Depending on their structures and locations, the Fab glycans may influence IgG effector functions by increasing or decreasing the affinity for the antigen (1). One report furthermore suggests that Fab glycosylation could modulate antibody half-life (83). Therefore, a better understanding of IgG functionality requires a detailed analysis of Fab specific glycosylation.
The choice of an appropriate strategy for the analysis of IgG Fab glycosylation is determined by the biological source (monoclonal IgG versus polyclonal IgG antibodies). Monoclonal IgGs, which exhibit well-defined Fab glycosylation sites, can be analyzed at the level of glycopeptides and IgG portions (Fc, Fab, heavy and light chains), as well as after the selective release of Fab-glycans using glycosidases. LC/MS allows the analysis of both Fc- and Fab-glycopeptides at the same time, thereby revealing site-specific N-glycan microheterogeneity on therapeutic antibodies (84). Alternatively, the glycosylation of heavy and light chains of IgG mAbs can be studied via LC/MS or direct-infusion ESI-MS after reduction (85, 86). The separation of Fab and Fc fragments of IgG is generally accomplished using the enzymes papain (75, 83, 86, 87) or pepsin (75). Papain cleaves IgG just above the disulfide bridges between the two heavy chains, resulting in two Fab portions and an Fc portion of similar molecular weight (∼50 kDa each). Pepsin cleaves below the disulfide bridges, generating a F(ab′)2 (±100 kDa) and two ½ Fc portions (±25 kDa each). In 2002, a streptococcal cysteine proteinase, IdeS, was reported to cleave IgG specifically at a unique site below the hinge region, leading to the formation of F(ab′)2 fragments with great yield and specificity (88). A recombinant version of this enzyme is now commercially available under the brand name FabRICATOR (Genovis, Lund, Sweden). A multitude of approaches have been used to purify F(ab) and F(ab′)2 fragments. After pepsin digestion, Fc glycopeptides and F(ab′)2 portions were separated using size exclusion (75). Papain digestion was followed by ion exchange chromatography (75) or affinity chromatography using Protein A (82, 87). In all cases, the Fab-linked N-glycans were released using PNGase F and analyzed via HPLC or MS.
Another way to separately analyze Fc and Fab glycans of a mAb is to release them from the entire IgG molecule using discriminating glycosidases and/or enzymatic conditions. For example, PNGase F and endoglycosidase F2 were reported to selectively release, in native condition, the Fc and Fab glycans, respectively (86).
Polyclonal IgGs exhibit a vast diversity of amino acid sequences of the variable regions created during somatic hypermutation, resulting in a multitude of Fab-glycosylation sites differing in number and location, as well as in the nature of their glycans. This enormous heterogeneity complicates, if not precludes, Fab glycosylation analysis at the glycopeptide level. Consequently, Fab glycosylation analysis of polyclonal IgG has hitherto relied on the analysis of released glycans from parts of IgG or from entire IgG molecules.
Recently, a method using sequential enzymatic release of Fc glycans and Fab glycans has been reported (74). Fab glycans, but not Fc sugars, were found to be resistant to PNGase F cleavage under native conditions (74). Therefore, IgG Fc glycans were first released under native conditions, and after IgG isolation, denaturing conditions allowed the liberation of Fab glycans.
For all techniques that use released glycans, a major drawback is that samples have to be extremely pure. Fc glycosylation is close to 100%, whereas Fab glycosylation is found on a only minor portion of polyclonal IgGs. Minor Fc contamination in Fab samples can bias the results. Fab and Fc glycosylation analysis at the released glycan level might be similarly compromised by the presence of other glycoprotein contaminants. This underlines the importance of highly specific purification methods.
Immunoglobulin A Glycosylation Analysis
Immunoglobulin A has several N- (IgA1 and 2) and O-glycosylation sites (IgA1 only; see Fig. 1), and both N- and O-glycosylation have been analyzed at the released glycan level (89, 90). In secretory fluids, such as mucosa and milk, two IgA molecules are dimerized by the N-glycosylated secretory component and the joining (J-)chain (91).
Site-specific N-glycosylation analysis of IgA has been done at the glycopeptide level after employing Asp-N endoproteinase (92). Two N-glycopeptides were identified, and the peptide sequences were obtained by means of Edman degradation. Based on the calculated masses of these sequences, different glycan compositions were deduced from MALDI-TOF-MS of desialylated glycopeptides. Differential treatment with galactosidase and fucosidase, as well as two-dimensional HPLC on released glycans using C18 and amide columns, revealed fully galactosylated complex-type biantennary structures with or without bisecting GlcNAc and fucose (92). More recently, tryptic glycopeptides of size-exclusion-chromatography-purified IgA1 have been analyzed using LC-FTICR-MS, with sequence confirmation using electron capture dissociation (ECD)-FTICR-MS/MS (93). Glycan compositions and linkages were established via gas-liquid chromatography. Interestingly, bi-, tri- and tetra-antennary complex type glycans were observed (93). N-glycosylation analysis of secretory IgA from human colostrum has recently been performed at the glycopeptide level using in-gel trypsin digestion and subsequent LC/MS and LC-MS/MS (91), revealing pronounced site-specific differences in glycosylation.
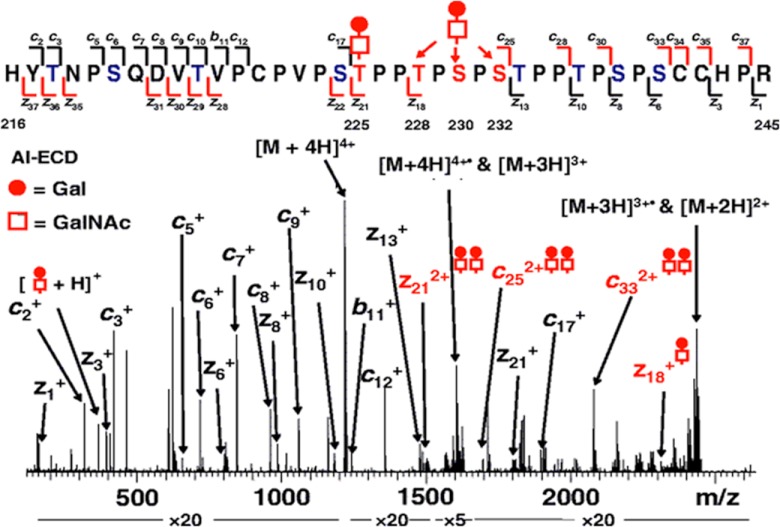
Also, the O-glycosylation of IgA has been extensively studied at the glycopeptide level (91, 94–98). Specific O-glycosylation changes were found in IgA nephropathy (34). More specifically, aberrantly glycosylated IgA1, with Gal-deficient hinge region (HR) O-glycans, plays a pivotal role in the pathogenesis of IgA nephropathy (95, 96, 99). Renfrow and coworkers showed IgA1 O-glycan heterogeneity via the use of FTICR-MS and LC-FTICR-MS to obtain accurate mass profiles of IgA1 HR glycopeptides from three different IgA1 myeloma proteins (95). Additionally, in that study, the first ECD fragmentation approach on an individual IgA1 O-glycopeptide from an IgA1 HR preparation that was reproducible for each IgA1 myeloma protein was obtained (Fig. 3). These results suggest that future analyses of IgA1 HRs from IgA nephropathy patients and healthy controls should be feasible.
Fig. 3.
ECD MS/MS spectrum of an HR IgA peptide. The O-glycosylated tryptic HR peptide ion [peptide + 2 GalNAc + 2 Gal + 4H]4+ from IgA1 was analyzed via ESI-FTICR ECD MS/MS. Sites of O-glycosylation were identified from series of differentially glycosylated product ions. The product ions localize one GalNAc-Gal disaccharide unambiguously to T225. A second GalNAc-Gal is narrowed to three possible sites, T228, S230, or S232. The remaining eight Ser/Thr residues are eliminated as sites of O-glycan attachment (in blue). N-terminal fragment ions (c and b) and C-terminal fragment ions (z) are indicated above and below the IgA1 HR sequence, respectively. Not all detected ECD fragments are labeled in the spectrum. Squares, GalNAc; circles, Gal. Taken from Renfrow et al. with permission (95).
Novel strategies for the analysis of clustered O-glycans involve the use of a combination of IgA-specific proteases and trypsin and ECD-FTICR-MS/MS. They provide a variety of IgA1 HR fragments that allow the unambiguous localization of all O-glycosylation sites for the six most abundant glycoforms, leading to the identification of Gal-deficient sites (96). Additionally, the published protocol was adapted for on-line LC-ECD-MS/MS and LC–electron transfer dissociation–MS/MS analysis. This work appears to be a relevant clinical approach for defining the molecular events leading to the pathogenesis of a chronic kidney disease, and at the same time it might be generally applicable for the analysis of clustered sites of O-glycosylation (96).
PERSPECTIVES
As demonstrated extensively for IgG, as well as for some IgA, a detailed structural analysis of N- and O-glycosylation is required in order for one to understand their three-dimensional structures and immune functions. To our knowledge, the glycosylation of other Igs (IgD, IgE, IgM) has hitherto not been addressed at the glycoproteomic level. The numerous O-glycosylation sites in the IgD HR and N-glycosylation sites (≥5 N-glycosylation sites) in IgM and IgE make their comprehensive glycosylation analysis at the glycopeptide level challenging. Additionally, the analysis of IgE and IgD from human circulation is particularly demanding, as these antibodies are generally present only in minute amounts (100). For IgE, glycoproteomic analysis would be needed in order to allocate its complex type and oligomannosidic glycans to their specific site(s) and analyze the NxS site on position 383, which has been predicted to be unoccupied (101). A particular analytical challenge will be the analysis of the variable region glycosylation of Ig subclasses other than IgG.
Ig glycosylation studies are routinely done in many different labs, and thus the amount of data produced is increasing tremendously. A recent approach combining genome-wide association and high-throughput glycomics analysis of plasma samples from 2705 individuals in three population cohorts showed that common variants in certain genes can influence N-glycan levels in human plasma (102). Based on a follow-up study, a high-throughput isolation and glycosylation analysis of IgG variability and heritability of the IgG glycome in three different populations was published (10). Although a variety of associations of clinical and physiological parameters with Ig glycosylation have been established, we believe that many more processes and diseases are marked by Ig glycosylation changes and that we have seen only the tip of the iceberg. Future Ig glycosylation profiling at the site-specific level by means of the mass spectrometric analysis of glycopeptides, when applied to human disease cohorts as well as in vitro and in vivo models of immunological processes, is expected to provide valuable new insights into the modulatory role of Ig glycosylation.
Footnotes
* This work has been supported by funding from the European Union's Seventh Framework Programme (FP7-Health-F5-2011) under Grant Agreement No. 278535 (HighGlycan) and the Dutch Athritis foundation projects nr. NR 10-1-411 (for Albert Bondt) and NR 10-1-204 (for Yoann Rombouts). Maurice H. J. Selman thanks Hoffmann la Roche for financial support.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- ECD
- electron capture dissociation
- ESI
- electrospray ionization
- Fab
- fragment antigen binding
- Fc
- fragment crystallizable
- FTICR
- Fourier transform ion cyclotron resonance
- Gal
- galactoses
- GlcNAc
- N-acetylglucosamine
- HILIC
- hydrophilic interaction liquid chromatography
- HR
- hinge region
- Ig
- immunglobulin
- mAb
- monoclonal antibody
- RP
- reversed-phase.
REFERENCES
- 1. Arnold J. N., Wormald M. R., Sim R. B., Rudd P. M., Dwek R. A. (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 [DOI] [PubMed] [Google Scholar]
- 2. Borrok M. J., Jung S. T., Kang T. H., Monzingo A. F., Georgiou G. (2012) Revisiting the role of glycosylation in the structure of human IgG fc. ACS Chem. Biol. 7, 1596–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krapp S., Mimura Y., Jefferis R., Huber R., Sondermann P. (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 325, 979–989 [DOI] [PubMed] [Google Scholar]
- 4. Jung S. T., Reddy S. T., Kang T. H., Borrok M. J., Sandlie I., Tucker P. W., Georgiou G. (2010) Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 107, 604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nesspor T. C., Raju T. S., Chin C. N., Vafa O., Brezski R. J. (2012) Avidity confers FcgammaR binding and immune effector function to aglycosylated immunoglobulin G1. J. Mol. Recognit. 25, 147–154 [DOI] [PubMed] [Google Scholar]
- 6. Tao M. H., Morrison S. L. (1989) Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 143, 2595–2601 [PubMed] [Google Scholar]
- 7. Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K., Takeuchi F., Nagano Y., Miyamoto T., Kobata A. (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 [DOI] [PubMed] [Google Scholar]
- 8. Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. (1989) A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 2, 101–114 [DOI] [PubMed] [Google Scholar]
- 9. Ercan A., Barnes M. G., Hazen M., Tory H., Henderson L., Dedeoglu F., Fuhlbrigge R. C., Grom A., Holm I. A., Kellogg M., Kim S., Adamczyk B., Rudd P. M., Son M. B., Sundel R. P., Foell D., Glass D. N., Thompson S. D., Nigrovic P. A. (2012) Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Rheum. 64, 3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pucic M., Knezevic A., Vidic J., Adamczyk B., Novokmet M., Polasek O., Gornik O., Supraha-Goreta S., Wormald M. R., Redzic I., Campbell H., Wright A., Hastie N. D., Wilson J. F., Rudan I., Wuhrer M., Rudd P. M., Josic D., Lauc G. (2011) High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteomics 10, M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruhaak L. R., Uh H. W., Beekman M., Koeleman C. A., Hokke C. H., Westendorp R. G., Wuhrer M., Houwing-Duistermaat J. J., Slagboom P. E., Deelder A. M. (2010) Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. PLoS. One 5, e12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saldova R., Royle L., Radcliffe C. M., Abd Hamid U. M., Evans R., Arnold J. N., Banks R. E., Hutson R., Harvey D. J., Antrobus R., Petrescu S. M., Dwek R. A., Rudd P. M. (2007) Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 17, 1344–1356 [DOI] [PubMed] [Google Scholar]
- 13. van de Geijn F. E., Wuhrer M., Selman M. H., Willemsen S. P., de Man Y. A., Deelder A. M., Hazes J. M., Dolhain R. J. (2009) Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11, R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bones J., Mittermayr S., O'Donoghue N., Guttman A., Rudd P. M. (2010) Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal. Chem. 82, 10208–10215 [DOI] [PubMed] [Google Scholar]
- 15. Huhn C., Selman M. H., Ruhaak L. R., Deelder A. M., Wuhrer M. (2009) IgG glycosylation analysis. Proteomics 9, 882–913 [DOI] [PubMed] [Google Scholar]
- 16. Gindzienska-Sieskiewicz E., Klimiuk P. A., Kisiel D. G., Gindzienski A., Sierakowski S. (2007) The changes in monosaccharide composition of immunoglobulin G in the course of rheumatoid arthritis. Clin. Rheumatol. 26, 685–690 [DOI] [PubMed] [Google Scholar]
- 17. van Zeben D., Rook G. A., Hazes J. M., Zwinderman A. H., Zhang Y., Ghelani S., Rademacher T. W., Breedveld F. C. (1994) Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br. J. Rheumatol. 33, 36–43 [DOI] [PubMed] [Google Scholar]
- 18. Jefferis R., Lund J., Pound J. D. (1998) IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol. Rev. 163, 59–76 [DOI] [PubMed] [Google Scholar]
- 19. Kaneko Y., Nimmerjahn F., Ravetch J. V. (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 [DOI] [PubMed] [Google Scholar]
- 20. Nimmerjahn F., Anthony R. M., Ravetch J. V. (2007) Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. U.S.A. 104, 8433–8437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 22. Ferrara C., Grau S., Jager C., Sondermann P., Brunker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., Umana P., Benz J. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U.S.A. 108, 12669–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou G., Ochiai H., Huang W., Yang Q., Li C., Wang L. X. (2011) Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. J. Am. Chem. Soc. 133, 18975–18991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salles G., Morschhauser F., Lamy T., Milpied N., Thieblemont C., Tilly H., Bieska G., Asikanius E., Carlile D., Birkett J., Pisa P., Cartron G. (2012) Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 119, 5126–5132 [DOI] [PubMed] [Google Scholar]
- 25. Sehn L. H., Assouline S. E., Stewart D. A., Mangel J., Gascoyne R. D., Fine G., Frances-Lasserre S., Carlile D. J., Crump M. (2012) A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 119, 5118–5125 [DOI] [PubMed] [Google Scholar]
- 26. Malhotra R., Wormald M. R., Rudd P. M., Fischer P. B., Dwek R. A., Sim R. B. (1995) Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1, 237–243 [DOI] [PubMed] [Google Scholar]
- 27. Raju T. S. (2008) Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20, 471–478 [DOI] [PubMed] [Google Scholar]
- 28. Karsten C. M., Pandey M. K., Figge J., Kilchenstein R., Taylor P. R., Rosas M., McDonald J. U., Orr S. J., Berger M., Petzold D., Blanchard V., Winkler A., Hess C., Reid D. M., Majoul I. V., Strait R. T., Harris N. L., Kohl G., Wex E., Ludwig R., Zillikens D., Nimmerjahn F., Finkelman F. D., Brown G. D., Ehlers M., Kohl J. (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat. Med. 18, 1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anthony R. M., Ravetch J. V. (2010) A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol. 30 Suppl 1, S9–S14 [DOI] [PubMed] [Google Scholar]
- 30. Anthony R. M., Kobayashi T., Wermeling F., Ravetch J. V. (2011) Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 475, 110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen G., Wang Y., Qiu L., Qin X., Liu H., Wang X., Wang Y., Song G., Li F., Guo Y., Li F., Guo S., Li Z. (2012) Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J. Proteomics 75, 2824–2834 [DOI] [PubMed] [Google Scholar]
- 32. Prados M. B., La B. J., Szekeres-Bartho J., Caramelo J., Miranda S. (2011) Progesterone induces a switch in oligosaccharyltransferase isoform expression: consequences on IgG N-glycosylation. Immunol. Lett. 137, 28–37 [DOI] [PubMed] [Google Scholar]
- 33. Wang J., Balog C. I., Stavenhagen K., Koeleman C. A., Scherer H. U., Selman M. H., Deelder A. M., Huizinga T. W., Toes R. E., Wuhrer M. (2011) Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteomics 10, M110.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Novak J., Julian B. A., Tomana M., Mestecky J. (2008) IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin. Nephrol. 28, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalpathado D. S., Desaire H. (2008) Glycopeptide analysis by mass spectrometry. Analyst 133, 731–738 [DOI] [PubMed] [Google Scholar]
- 36. Morelle W., Michalski J. C. (2007) Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2, 1585–1602 [DOI] [PubMed] [Google Scholar]
- 37. Kolarich D., Jensen P. H., Altmann F., Packer N. H. (2012) Determination of site-specific glycan heterogeneity on glycoproteins. Nat. Protoc. 7, 1285–1298 [DOI] [PubMed] [Google Scholar]
- 38. Zauner G., Deelder A. M., Wuhrer M. (2011) Recent advances in hydrophilic interaction liquid chromatography (HILIC) for structural glycomics. Electrophoresis 32, 3456–3466 [DOI] [PubMed] [Google Scholar]
- 39. Nwosu C. C., Seipert R. R., Strum J. S., Hua S. S., An H. J., Zivkovic A. M., German B. J., Lebrilla C. B. (2011) Simultaneous and extensive site-specific N- and O-glycosylation analysis in protein mixtures. J. Proteome Res. 10, 2612–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mechref Y., Hu Y., Garcia A., Zhou S., Desantos-Garcia J. L., Hussein A. (2012) Defining putative glycan cancer biomarkers by MS. Bioanalysis 4, 2457–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mechref Y., Muzikar J., Novotny M. V. (2005) Comprehensive assessment of N-glycans derived from a murine monoclonal antibody: a case for multimethodological approach. Electrophoresis 26, 2034–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruhaak L. R., Zauner G., Huhn C., Bruggink C., Deelder A. M., Wuhrer M. (2010) Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 397, 3457–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harvey D. J. (2012) Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2007–2008. Mass Spectrom. Rev. 31, 183–311 [DOI] [PubMed] [Google Scholar]
- 44. Leymarie N., Zaia J. (2012) Effective use of mass spectrometry for glycan and glycopeptide structural analysis. Anal. Chem. 84, 3040–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dard P., Lefranc M. P., Osipova L., Sanchez-Mazas A. (2001) DNA sequence variability of IGHG3 alleles associated to the main G3m haplotypes in human populations. Eur. J. Hum. Genet. 9, 765–772 [DOI] [PubMed] [Google Scholar]
- 46. Jefferis R., Lefranc M. P. (2009) Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs 1, 332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balbin M., Grubb A., de Lange G. G., Grubb R. (1994) DNA sequences specific for Caucasian G3m(b) and(g) allotypes: allotyping at the genomic level. Immunogenetics 39, 187–193 [DOI] [PubMed] [Google Scholar]
- 48. Perdivara I., Deterding L. J., Cozma C., Tomer K. B., Przybylski M. (2009) Glycosylation profiles of epitope-specific anti-beta-amyloid antibodies revealed by liquid chromatography-mass spectrometry. Glycobiology 19, 958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stadlmann J., Pabst M., Kolarich D., Kunert R., Altmann F. (2008) Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 8, 2858–2871 [DOI] [PubMed] [Google Scholar]
- 50. Wuhrer M., Stam J. C., van de Geijn F. E., Koeleman C. A., Verrips C. T., Dolhain R. J., Hokke C. H., Deelder A. M. (2007) Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 7, 4070–4081 [DOI] [PubMed] [Google Scholar]
- 51. Selman M. H., Derks R. J., Bondt A., Palmblad M., Schoenmaker B., Koeleman C. A., van de Geijn F. E., Dolhain R. J., Deelder A. M., Wuhrer M. (2012) Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. J. Proteomics 75, 1318–1329 [DOI] [PubMed] [Google Scholar]
- 52. Gilar M., Yu Y. Q., Ahn J., Xie H., Han H., Ying W., Qian X. (2011) Characterization of glycoprotein digests with hydrophilic interaction chromatography and mass spectrometry. Anal. Biochem. 417, 80–88 [DOI] [PubMed] [Google Scholar]
- 53. Singh C., Zampronio C. G., Creese A. J., Cooper H. J. (2012) Higher energy collision dissociation (HCD) product ion-triggered electron transfer dissociation (ETD) mass spectrometry for the analysis of N-linked glycoproteins. J. Proteome Res. 11, 4517–4525 [DOI] [PubMed] [Google Scholar]
- 54. Takegawa Y., Deguchi K., Keira T., Ito H., Nakagawa H., Nishimura S. (2006) Separation of isomeric 2-aminopyridine derivatized N-glycans and N-glycopeptides of human serum immunoglobulin G by using a zwitterionic type of hydrophilic-interaction chromatography. J. Chromatogr. A 1113, 177–181 [DOI] [PubMed] [Google Scholar]
- 55. Omtvedt L. A., Royle L., Husby G., Sletten K., Radcliffe C. M., Harvey D. J., Dwek R. A., Rudd P. M. (2006) Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum. 54, 3433–3440 [DOI] [PubMed] [Google Scholar]
- 56. Neue K., Mormann M., Peter-Katalinic J., Pohlentz G. (2011) Elucidation of glycoprotein structures by unspecific proteolysis and direct nanoESI mass spectrometric analysis of ZIC-HILIC-enriched glycopeptides. J. Proteome Res. 10, 2248–2260 [DOI] [PubMed] [Google Scholar]
- 57. Reusch D., Haberger M., Selman M. H., Bulau P., Deelder A. M., Wuhrer M., Engler N. (2012) High-throughput work flow for IgG Fc-glycosylation analysis of biotechnological samples. Anal. Biochem. 432, 82–89 [DOI] [PubMed] [Google Scholar]
- 58. Mysling S., Palmisano G., Hojrup P., Thaysen-Andersen M. (2010) Utilizing ion-pairing hydrophilic interaction chromatography solid phase extraction for efficient glycopeptide enrichment in glycoproteomics. Anal. Chem. 82, 5598–5609 [DOI] [PubMed] [Google Scholar]
- 59. Selman M. H., McDonnell L. A., Palmblad M., Ruhaak L. R., Deelder A. M., Wuhrer M. (2010) Immunoglobulin G glycopeptide profiling by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 82, 1073–1081 [DOI] [PubMed] [Google Scholar]
- 60. Wada Y., Tajiri M., Yoshida S. (2004) Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal. Chem. 76, 6560–6565 [DOI] [PubMed] [Google Scholar]
- 61. Selman M. H., Hoffmann M., Zauner G., McDonnell L. A., Balog C. I., Rapp E., Deelder A. M., Wuhrer M. (2012) MALDI-TOF-MS analysis of sialylated glycans and glycopeptides using 4-chloro-alpha-cyanocinnamic acid matrix. Proteomics 12, 1337–1348 [DOI] [PubMed] [Google Scholar]
- 62. O'Connor P. B., Budnik B. A., Ivleva V. B., Kaur P., Moyer S. C., Pittman J. L., Costello C. E. (2004) A high pressure matrix-assisted laser desorption ion source for Fourier transform mass spectrometry designed to accommodate large targets with diverse surfaces. J. Am. Soc. Mass Spectrom. 15, 128–132 [DOI] [PubMed] [Google Scholar]
- 63. Bondarenko P. V., Second T. P., Zabrouskov V., Makarov A. A., Zhang Z. (2009) Mass measurement and top-down HPLC/MS analysis of intact monoclonal antibodies on a hybrid linear quadrupole ion trap-Orbitrap mass spectrometer. J. Am. Soc. Mass Spectrom. 20, 1415–1424 [DOI] [PubMed] [Google Scholar]
- 64. Fornelli L., Damoc E., Thomas P. M., Kelleher N. L., Aizikov K., Denisov E., Makarov A., Tsybin Y. O. (2012) Analysis of intact monoclonal antibody IgG1 by electron transfer dissociation orbitrap FTMS. Mol. Cell. Proteomics 11, 1758–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blomme B., Van S. C., Grassi P., Haslam S. M., Dell A., Callewaert N., Van V. H. (2011) Alterations of serum protein N-glycosylation in two mouse models of chronic liver disease are hepatocyte and not B cell driven. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G833–G842 [DOI] [PubMed] [Google Scholar]
- 66. Mizuochi T., Hamako J., Titani K. (1987) Structures of the sugar chains of mouse immunoglobulin G. Arch. Biochem. Biophys. 257, 387–394 [DOI] [PubMed] [Google Scholar]
- 67. Mizuochi T., Hamako J., Nose M., Titani K. (1990) Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice. J. Immunol. 145, 1794–1798 [PubMed] [Google Scholar]
- 68. Scherer H. U., Wang J., Toes R. E., van der Woude D., Koeleman C. A., de Boer A. R., Huizinga T. W., Deelder A. M., Wuhrer M. (2009) Immunoglobulin 1 (IgG1) Fc-glycosylation profiling of anti-citrullinated peptide antibodies from human serum. Proteomics Clin. Appl. 3, 106–115 [DOI] [PubMed] [Google Scholar]
- 69. Scherer H. U., van der Woude D., Ioan-Facsinay A., el Bannoudi H., Trouw L. A., Wang J., Haupl T., Burmester G. R., Deelder A. M., Huizinga T. W., Wuhrer M., Toes R. E. (2010) Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 62, 1620–1629 [DOI] [PubMed] [Google Scholar]
- 70. Selman M. H., de Jong S. E., Soonawala D., Kroon F. P., Adegnika A. A., Deelder A. M., Hokke C. H., Yazdanbakhsh M., Wuhrer M. (2012) Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteomics 11, M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wuhrer M., Porcelijn L., Kapur R., Koeleman C. A., Deelder A., de Haas M., Vidarsson G. (2009) Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J. Proteome Res. 8, 450–456 [DOI] [PubMed] [Google Scholar]
- 72. Heemskerk A. A., Wuhrer M., Busnel J. M., Koeleman C. A., Selman M. H., Vidarsson G., Kapur R., Schoenmaker B., Derks R. J., Deelder A. M., Mayboroda O. A. (2013) Coupling porous sheathless interface mass spectrometry with transient-isotachophoresis in neutral capillaries for improved sensitivity in glycopeptide analysis. Electrophoresis 34, 383–387 [DOI] [PubMed] [Google Scholar]
- 73. Busnel J. M., Schoenmaker B., Ramautar R., Carrasco-Pancorbo A., Ratnayake C., Feitelson J. S., Chapman J. D., Deelder A. M., Mayboroda O. A. (2010) High capacity capillary electrophoresis-electrospray ionization mass spectrometry: coupling a porous sheathless interface with transient-isotachophoresis. Anal. Chem. 82, 9476–9483 [DOI] [PubMed] [Google Scholar]
- 74. Anumula K. R. (2012) Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 382, 167–176 [DOI] [PubMed] [Google Scholar]
- 75. Holland M., Yagi H., Takahashi N., Kato K., Savage C. O., Goodall D. M., Jefferis R. (2006) Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta 1760, 669–677 [DOI] [PubMed] [Google Scholar]
- 76. Stadlmann J., Pabst M., Altmann F. (2010) Analytical and functional aspects of antibody sialylation. J. Clin. Immunol. 30, 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Malan B. I., Gentile T., Angelucci J., Pividori J., Guala M. C., Binaghi R. A., Margni R. A. (1991) IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 20, 129–140 [DOI] [PubMed] [Google Scholar]
- 78. Margni R. A., Malan B. I. (1998) Paradoxical behavior of asymmetric IgG antibodies. Immunol. Rev. 163, 77–87 [DOI] [PubMed] [Google Scholar]
- 79. Canellada A., Blois S., Gentile T., Margni Idehu R. A. (2002) In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am. J. Reprod. Immunol. 48, 334–343 [DOI] [PubMed] [Google Scholar]
- 80. Gutierrez G., Malan B. I., Margni R. A. (2001) The placental regulatory factor involved in the asymmetric IgG antibody synthesis responds to IL-6 features. J. Reprod. Immunol. 49, 21–32 [DOI] [PubMed] [Google Scholar]
- 81. Zenclussen A. C., Gentile T., Kortebani G., Mazzolli A., Margni R. (2001) Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 45, 289–294 [DOI] [PubMed] [Google Scholar]
- 82. Stadlmann J., Weber A., Pabst M., Anderle H., Kunert R., Ehrlich H. J., Peter S. H., Altmann F. (2009) A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics 9, 4143–4153 [DOI] [PubMed] [Google Scholar]
- 83. Huang L., Biolsi S., Bales K. R., Kuchibhotla U. (2006) Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal. Biochem. 349, 197–207 [DOI] [PubMed] [Google Scholar]
- 84. Toyama A., Nakagawa H., Matsuda K., Sato T. A., Nakamura Y., Ueda K. (2012) Quantitative structural characterization of local N-glycan microheterogeneity in therapeutic antibodies by energy-resolved oxonium ion monitoring. Anal. Chem. 84, 9655–9662 [DOI] [PubMed] [Google Scholar]
- 85. Chevreux G., Tilly N., Bihoreau N. (2011) Fast analysis of recombinant monoclonal antibodies using IdeS proteolytic digestion and electrospray mass spectrometry. Anal. Biochem. 415, 212–214 [DOI] [PubMed] [Google Scholar]
- 86. Mimura Y., Ashton P. R., Takahashi N., Harvey D. J., Jefferis R. (2007) Contrasting glycosylation profiles between Fab and Fc of a human IgG protein studied by electrospray ionization mass spectrometry. J. Immunol. Methods 326, 116–126 [DOI] [PubMed] [Google Scholar]
- 87. Qian J., Liu T., Yang L., Daus A., Crowley R., Zhou Q. (2007) Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal. Biochem. 364, 8–18 [DOI] [PubMed] [Google Scholar]
- 88. von Pawel-Rammingen U., Johansson B. P., Bjorck L. (2002) IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21, 1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mattu T. S., Pleass R. J., Willis A. C., Kilian M., Wormald M. R., Lellouch A. C., Rudd P. M., Woof J. M., Dwek R. A. (1998) The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J. Biol. Chem. 273, 2260–2272 [DOI] [PubMed] [Google Scholar]
- 90. Royle L., Roos A., Harvey D. J., Wormald M. R., van Gijlswijk-Janssen D., Redwan e., Wilson I. A., Daha M. R., Dwek R. A., Rudd P. M. (2003) Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 278, 20140–20153 [DOI] [PubMed] [Google Scholar]
- 91. Deshpande N., Jensen P. H., Packer N. H., Kolarich D. (2010) GlycoSpectrumScan: fishing glycopeptides from MS spectra of protease digests of human colostrum sIgA. J. Proteome Res. 9, 1063–1075 [DOI] [PubMed] [Google Scholar]
- 92. Tanaka A., Iwase H., Hiki Y., Kokubo T., Ishii-Karakasa I., Toma K., Kobayashi Y., Hotta K. (1998) Evidence for a site-specific fucosylation of N-linked oligosaccharide of immunoglobulin A1 from normal human serum. Glycoconj. J. 15, 995–1000 [DOI] [PubMed] [Google Scholar]
- 93. Gomes M. M., Wall S. B., Takahashi K., Novak J., Renfrow M. B., Herr A. B. (2008) Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding. Biochemistry 47, 11285–11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Renfrow M. B., Cooper H. J., Tomana M., Kulhavy R., Hiki Y., Toma K., Emmett M. R., Mestecky J., Marshall A. G., Novak J. (2005) Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry. J. Biol. Chem. 280, 19136–19145 [DOI] [PubMed] [Google Scholar]
- 95. Renfrow M. B., Mackay C. L., Chalmers M. J., Julian B. A., Mestecky J., Kilian M., Poulsen K., Emmett M. R., Marshall A. G., Novak J. (2007) Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal. Bioanal. Chem. 389, 1397–1407 [DOI] [PubMed] [Google Scholar]
- 96. Takahashi K., Wall S. B., Suzuki H., Smith A. D., Hall S., Poulsen K., Kilian M., Mobley J. A., Julian B. A., Mestecky J., Novak J., Renfrow M. B. (2010) Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol. Cell. Proteomics 9, 2545–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wada Y., Dell A., Haslam S. M., Tissot B., Canis K., Azadi P., Backstrom M., Costello C. E., Hansson G. C., Hiki Y., Ishihara M., Ito H., Kakehi K., Karlsson N., Hayes C. E., Kato K., Kawasaki N., Khoo K. H., Kobayashi K., Kolarich D., Kondo A., Lebrilla C., Nakano M., Narimatsu H., Novak J., Novotny M. V., Ohno E., Packer N. H., Palaima E., Renfrow M. B., Tajiri M., Thomsson K. A., Yagi H., Yu S. Y., Taniguchi N. (2010) Comparison of methods for profiling O-glycosylation: Human Proteome Organisation Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1. Mol. Cell. Proteomics 9, 719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wada Y., Tajiri M., Ohshima S. (2010) Quantitation of saccharide compositions of O-glycans by mass spectrometry of glycopeptides and its application to rheumatoid arthritis. J. Proteome Res. 9, 1367–1373 [DOI] [PubMed] [Google Scholar]
- 99. Novak J., Julian B. A., Mestecky J., Renfrow M. B. (2012) Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 34, 365–382 [DOI] [PubMed] [Google Scholar]
- 100. Arnold J. N., Radcliffe C. M., Wormald M. R., Royle L., Harvey D. J., Crispin M., Dwek R. A., Sim R. B., Rudd P. M. (2004) The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J. Immunol. 173, 6831–6840 [DOI] [PubMed] [Google Scholar]
- 101. Dorrington K. J., Bennich H. H. (1978) Structure-function relationships in human immunoglobulin E. Immunol. Rev. 41, 3–25 [DOI] [PubMed] [Google Scholar]
- 102. Lauc G., Essafi A., Huffman J. E., Hayward C., Knezevic A., Kattla J. J., Polasek O., Gornik O., Vitart V., Abrahams J. L., Pucic M., Novokmet M., Redzic I., Campbell S., Wild S. H., Borovecki F., Wang W., Kolcic I., Zgaga L., Gyllensten U., Wilson J. F., Wright A. F., Hastie N. D., Campbell H., Rudd P. M., Rudan I. (2010) Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1alpha as a master regulator of plasma protein fucosylation. PLoS. Genet. 6, e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]