Abstract
Mice with null mutations in specific Golgi glycosyltransferases show evidence of glycan compensation where missing carbohydrate epitopes are found on biosynthetically related structures. Repetitive saccharide sequences within the larger glycan structures are functional epitopes recognized by animal lectins. These studies provide the first in vivo support for the existence of a feedback system that maintains and regulates glycan epitope density in cells. Receptor regulation by lectin–glycan interactions and the Golgi provides a mechanism for the adaptation of cell surface receptors and solute transporters in response to environmental cues and intracellular signaling. We suggest that other posttranslational modification systems might have similar conditional features regulated by density-dependent ligand–epitope interactions.
Cells must integrate multiple inputs (i.e. metabolites, trophic factors, pathogens) in order to maintain systemic control under a range of conditions. It is well established that many of these adaptive mechanisms involve posttranslational modifications (PTMs)1 of proteins such as phosphorylation, acylation, methylation, glycosylation, and others. The modifying enzymes recognize short consensus sequences in target proteins and a high energy donor substrate. The latter are metabolites, and their concentrations can also regulate PTMs (1). PTMs can exert conformation and allosteric effects on a target protein (2). However, PTMs also create binding sites (epitopes) for other proteins, thereby recruiting signaling complexes to biologically relevant regions in the cell. Most cytokine receptors and solute transporters are co-translationally N-glycosylated at NXS/T (X ≠ P) sites in the endoplasmic reticulum. Some of the Asn-(N-)glycans bind chaperones that promote protein folding, secretion, or degradation of misfolded proteins (3). On the cell surface, N-glycans can serve as ligands for animal lectins (galectin, siglec, and C-type lectins) (4) that regulate receptor clustering and dynamics, while phosphorylation at multiple PTM sites on the cytoplasmic tails of transmembrane receptors or transporters recruits adaptor complexes (5). Adaptor proteins are often structured as tandem domains that bind different and overlapping sets of PTMs, in which multivalency is a critical feature (6). Multivalent systems display partial redundancy that might buffer the mutational loss and gain of sites, thereby promoting the evolution of PTM networks.
Membrane microdomains such as ganglioside-rich lipid rafts, coated pits, cell junctions, and focal adhesions are dynamic and regulate the activity of receptors including glycoproteins. Here we describe lectin binding to transmembrane glycoproteins, which forms dynamic cross-linked “lattices” (7, 8). Lectin–glycan interactions occur widely and have been implicated in many systemic processes in mammals (9), but the molecular mechanisms remain poorly understood. We suggest that glycan epitope density is highly regulated and has a global impact on lectin-mediated regulation of receptors and transporters at the cell surface (10–12) (Fig. 1). In these important features, glycosylation can serve as a model for density-dependent ligand control of PTMs in general.
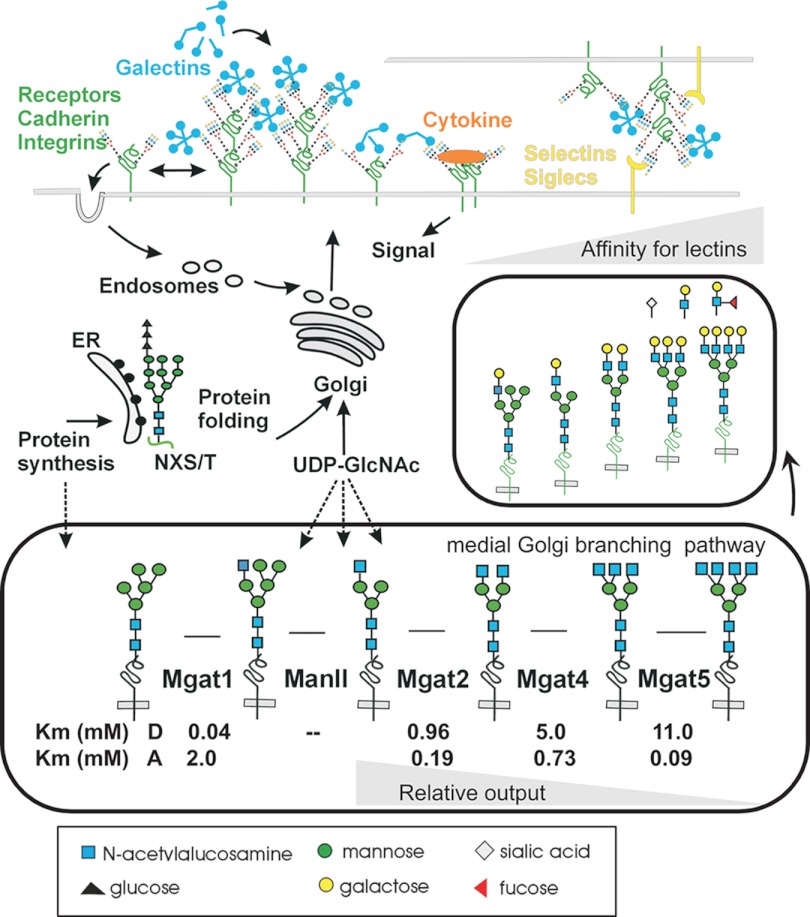
Fig. 1.
Multivalency and conditional regulation at the cell surface. At the cell surface, lectins bind glycan epitopes and cross-link glycoprotein receptors, altering receptor dynamics and interactions, such as reducing loss to endocytosis. Oligosaccharyltransferase recognizes NXS/T motifs in newly synthesized proteins in the endoplasmic reticulum (ER) and transfers the pre-assembled glycan from Glc3Man9GlcNAc2-pp-dolichol to the Asn. The chaperones calnexin and calreticulin bind N-glycans and promote protein folding. Glycoproteins traffic through the Golgi, where exposure to branching and extension enzymes in the medial- and trans-Golgi is dependent on multiple factors, including protein synthesis rates and the sugar-nucleotide supply. The reaction kinetics for the branching enzymes Mgat1, Mgat2, Mgat4, and Mgat5 differ as indicated by the Kms for UDP-GlcNAc “D” and glycoprotein acceptor “A.” The enzyme kinetics are tuned for pathway ultrasensitivity to UDP-GlcNAc and sensitivity to the concentration of glycoprotein substrates passing through the Golgi (protein synthesis rates). The epitopes are completed in the trans-Golgi, where efficient substitution with galactose generates the LacNAc epitope. Further extension with fucose, sialic acid, and LacNAc can modify affinities for galectins and generate epitopes for other lectins.
Glycan Density, Affinity Enhancement, and Cross-linking
Glycans on the surface of cells are present as multivalent epitopes at densities that are compatible with lectin binding and the formation of cross-linked lattices. The valency of epitopes on glycoprotein receptors depends on the structure of the N- and O-glycans, as well as on the number of glycan sites per molecule, and it controls the affinity for lectins and cross-linking dynamics (13, 14). The affinity (avidity) of lectins for multivalent glycan epitopes depends, in part, on the three-dimensional arrangement of the lectin binding sites. Lectins with multiple binding sites aligned to a matching array of glycan epitopes show large affinity enhancements as a result of simultaneous binding. For example, the binding of the asialoglycoprotein receptor to the N-acetyllactosamine (galactose-β1, 4GlcNAcβ, or LacNAc) branches of a triantennary N-glycan is ∼106-fold greater in affinity relative to monovalent LacNAc (15). Man-binding protein, a C-type lectin in the collectin family that functions in innate immunity, possesses multiple triple helical collagen-like arms and terminal trimeric carbohydrate-binding subunits with aligned binding sites. As a consequence, Man-binding lectin exhibits high affinity and specificity for multiple Man residues on the surfaces of pathogens (16).
Plant and animal lectins with binding sites on subunits oriented in different directions can also bind with high affinity to multivalent carbohydrates and glycoproteins. In such a case, lectin affinity increases with the number (valence) of glycan epitopes in a molecule or on a surface (glycan density) (17). An example is the binding of galectins, a family of β-galactose specific animal lectins, to asialofetuin, a globular glycoprotein possessing three N-linked triantennary chains and nine terminal LacNAc residues, which results in 50- to 80-fold enhanced affinity relative to monovalent LacNAc (18). A dramatic example is binding of the GalNAc-specific soybean agglutinin to a linear glycoprotein (mucin) possessing ∼2300 GalNAc residues, which results in a ∼106-fold enhanced affinity relative to monovalent GalNAc (19). The high affinity of the mucin is due to the large number of its glycans. The binding of lectins to glycan arrays also shows increasing affinity with increasing glycan epitope density (20). Affinity enhancement in these multivalent systems is believed to be primarily due to slower effective off-rates, although increases in on-rates may occur (20). In essence, a bound lectin is likely to rebind to proximate free glycan epitopes rather than diffuse away, even if the orientations of the binding epitopes are random (21, 22). Thus, lectin affinities are sensitive to the number of glycan epitopes on glycoconjugates and to the density of glycan epitopes on surfaces including glycan arrays and cells (14).
Lectins with multiple binding sites can also form two- and three-dimensional cross-linked complexes (lattices) with multivalent glycans (23). For example, the Man-specific lectin concanavalin A, in the presence of a mixture of two multivalent carbohydrates that differ in the number of Man epitopes, can bind and separate into distinct cross-linked lattices with each glycan (24). The structural basis for the formation of separate (homogeneous) cross-linked lattices has been shown to be crystal-packing interactions (25, 26). Lectin-mediated cross-linking interactions regulate the levels and activities of glycoprotein receptors and transporters on cells, as discussed below.
In vitro studies suggest that galectin-1, a symmetrical dimer, can form homogeneous lattices with asialofetuin (27). In vivo experiments by Baum and coworkers demonstrated that exogenous galectin-1 added to human T cells segregates CD43 and CD45 into different membrane microdomains and leads to apoptosis (28). The endogenous galectin-1 lattice on resting T cells partitions CD45 and T cell receptor (TCR) differentially and interacts with actin microfilaments on opposing sides of the plasma membrane to regulate basal growth signaling and the thresholds of T cell receptor activation (29). These findings are consistent with galectin-1 forming homogeneous lattices with glycoproteins in vivo. In contrast, in vitro studies indicate that galectin-3 is a mixture of monomers and pentamers in solution, and that the latter form disorganized heterogeneous lattices with multivalent carbohydrates (30). For example, galectin-3 binds but does not selectively aggregate CD45 on the surface of apoptosis-sensitive T cells, as does galectin-1 (31). In addition, galectin-3 cross-links different cytokine receptors on the surface of cells, slowing their mobility and loss to endosomes and enhancing cellular sensitivity to ligand-dependent signaling (32, 33). The galectin-3 lattice also regulates the dynamics of receptors in a context-dependent manner. For example, galectin-3 binding reduces GFP-tagged EGF receptor mobility in the lipid bilayer while preserving sensitivity to EGF, but it increases mobility in focal adhesions and promotes PI3K signaling (33, 34) (Table I). The bi-, tri-, and tetraantennary N-glycans display increasing LacNAc epitope densities and affinity for galectins, respectively (35). However, galectin-1 and -3 differ in their tolerance of additional modification to the LacNAc epitope, which might contribute to their distinct cross-linking activities and biological properties (36).
Table I. Interactions with lectins at the cell surface lattice.
| Receptor and transporter | Glycans (gene) | Lectins | Dynamics ina | Phenotype | Reference |
|---|---|---|---|---|---|
| T cell receptor | N-(Mgat5)b | Gal-3 | Immune synapse (+) | Autoimmunity | (8) |
| CTLA-4 | N-(Mgat5)b | Gal-3 | Membrane-endo | Autoimmunity | (10, 52) |
| CD45 phosphatase | N- and O- | Gal-1, -3 | Membrane (− and +) | T cell activation | (29, 64) |
| K+ channel Kv1.3 | N-branchingb | ND | Membrane-endo | Many cells | (65) |
| EGFR and TGF-β RII | N-(Mgat5)b | Gal-3 | Membrane-endo (−) | Cancer, stem cell | (32) |
| VEGF receptor | N-(Mgat5) | Gal-3 | Membrane-endo | Neovascularization | (66) |
| Integrins | N-(Mgat5) | Gal-3, -8, -9 | Focal adhesion (+) | Cancer, T cells | (34, 67, 68) |
| IL3Rβ | N-(Mgat5)b | ND | Membrane-endo | Growth control | (69) |
| N-cadherin | N-(Mgat5) | Gal-3 | Cell junctions (+) | Cancer invasion | (60) |
| TRPV5, Ca2+ channel | N- | Gal-1 | Membrane | Aging | (70) |
| GLUT2/SLC2A2 | N-(Mgat4a) | Gal-9 | Membrane-endo | Diabetes | (71) |
| GLUT4/SLC2A4 | N-b | ND | Membrane-endo | ND | (10) |
| B cell receptor | N-(ST6Gal1) | Siglec2 | Membrane, rafts, endo | B cell activation | (72) |
Gal, galactose; ND, no data.
a In the plasma membrane, the galectin lattice opposes receptor loss from the surface to endocytosis. Association with the galectin lattice decreases receptor dynamics in the membrane (−) but can increase dynamics in stable microdomains as indicated (+), as measured by fluorescence recovery after photobleaching (FRAP).
b Sensitivity to hexosamine (UDP-GlcNAc) regulation of N-glycan branching has been tested. The gene mutation used to show lattice dependency is presented in parentheses.
Epitope Density Maintenance in N-glycans
Lectins bind to epitopes within the larger glycan structure, suggesting that glycans might be grouped into equivalence classes by epitope number and used to compute the affinity of glycoproteins for lectins. Lau et al. (10) developed a model for the regulation of cytokine receptors at the surface of mammary tumor cells based on epitope density and galectin-3 binding (Fig 2A). Microheterogeneity at each NXS/T site results in a distribution of glycoforms for each glycoprotein receptor, and the various combinations can be grouped by affinity for galectin-3 and treated as biologically equivalent (10). Experimental data with cell lines fit this model well, as described below and in Table I. Support for the model as a homeostatic mechanism in vivo comes from recent structural analysis of glycans in mice with mutations in specific Golgi enzymes (37, 38).
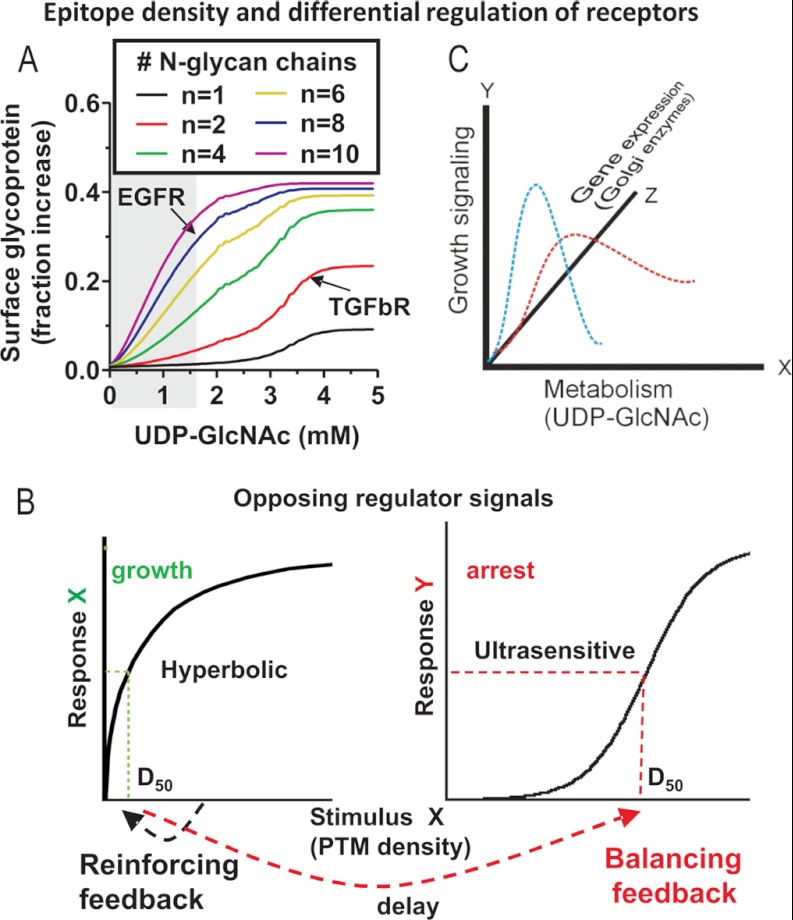
Fig. 2.
A, Ordinary Differential Equation (ODE) computational model of receptor regulation by the galectin lattice. N-glycosylation site (NXS/T) multiplicity interacts with the Golgi branching pathway to regulate glycoprotein affinities for the galectin lattice. Simulations of fractional change in surface receptors are shown as a function of the site number (n) and the UDP-GlcNAc supply to N-glycan branching (x-axis). Experimental validation of the model for EGFR (n = 8 occupied sites) and TβRI/II (n = 3) can be found in Ref. 10. B, epitope density and differential regulation. This is a more general model of opposing signaling pathways with high and low site PTM densities that allows differential regulation by the same PTM substrate and modifying enzymes. C, multidimensional regulatory space. Interacting pathways can be controlled by low affinity/specificity, multivalent, and density-dependent PTM systems. The curves indicate possible trajectories for growth signaling. Each is dependent on the site number in protein sequences (red or blue), where interaction with an opposing pathway (not shown) results in the specific trajectory. Specificity arises from low-affinity and ubiquitous PTM epitopes, based on conditional inputs such as metabolism, stress, and developmental cues.
Takamatsu et al. (37) generated N-acetylglucosaminyltransferase IV (GnT-IV) compound mutant mice, resulting in undetectable levels of GlcNAcβ1–4 branching activity and N-glycans in mouse tissues. N-acetylglucosaminyltransferases I, II, IVa/IVb, and V (encoded by Mgat1, Mgat2, Mgat4a/4b, and Mgat5) each substitute the trimannosyl core at a specific position in a sequential manner. GnT-IVa and GnT-IVb are catalytically redundant and initiate the synthesis of the GlcNAcβ1–4 branch on the core Manα1–3 arm. Loss of the branch decreases LacNAc epitopes per N-glycan and, consequently, glycoprotein affinities for galectins. Mgat4a expression is prominent in pancreatic and gastrointestinal tissues, whereas Mgat4b is widely expressed in most tissues. Mgat4a mutant mice develop type 2 diabetes with suppressed insulin secretion by β-cells due to the aberrant N-glycans on GLUT2, which reduce binding and surface retention by galectin-9. Mgat4b-deficient mice show compensation in the form of a marked up-regulation of Mgat4a expression in organs corresponding to a near-normal distribution of N-glycans. As such, the phenotype of Mgat4b-deficient mice is relatively normal, with modest decreases in coagulation factors and prolonged bleeding time. Although the Mgat4a/4b double deficiency eliminated expression of the GlcNAcβ1–4 branch, increased LacNAc epitope was seen as poly-LacNAc in compensating amounts on the remaining branches of the N-linked glycans (37).
Mgat4a/4b double-deficient mice displayed elevated resting glucose levels, similar to the Mgat4a mice, suggesting that compensation is insufficient. Although epitope compensation within the same classes of glycans and glycoproteins has been shown to maintain the residency of receptors at the cell surface in cell culture (10), homeostasis in vivo might fail at another level of regulation. In this regard, the mechanism of compensation in the double null mice was an up-regulation of multiple enzymes that act downstream of GnT-IV branching to generate poly-LacNAc and Lex epitopes, whereas compensation in Mgat4a was an up-regulation of Mgat4b encoding the same activity. Although these compensating enzymes are increased in the double null mice, they have different promoters and are not likely to mimic the normal epitope density and wild-type phenotype. In this regard, the Mgat4a gene is sensitive to metabolic regulation through the transcription factors FOXA2 and HIF1A (39). However, epitope density compensation in the double null mutant mice precludes a comparison with a truly epitope-deficient background, with which a more severe phenotype might be expected.
Mouse Mgat4a and Mgat4b segregate independently, and offspring from Mgat4a/Mgat4b heterozygote breeding shows reduced survival of Mgat4b−/− pups with one or two mutant Mgat4a alleles (37). Thus functional compensation, as measured in terms of pup survival, is less effective in double mutant embryos than Mgat4a alone, where GlcNAcβ1–4 branching is rescued via up-regulation of Mgat4b expression. This suggests a partially penetrant phenotype (stochastic) in which branch-extending activities and epitope densities are suboptimal in Mgat4a/Mgat4b mice. However, these studies reveal systemic feedback that appears to maintain epitope density by means of compensation on related structures, which, in turn, should support galectin lattices.
Epitope Compensation in O-linked Glycans
Following the report by Takamatsu et al. (37), Ismail et al. (38) reported on mice deficient in core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT), a key component in the O-glycan biosynthetic pathway encoded by three genes, C2GnT1, C2GnT2, and C2GnT3. Core 2 branched O-glycans are also preferentially modified by core 4- and I-GnT enzymes. The triple knockout mice lacked the immediate product and downstream core 4- and I-branched O-glycans. Similar to the Mgat4 example above, the missing O-linked branch is observed as small quantities of LacNAc epitope on the remaining linear arm of the O-linked glycans. This compensation was observed in the gastrointestinal tract but not in the kidneys, unlike in the GnT-IVa/IVb double-deficient mice, where N-glycan compensation was observed in all organs examined, including the kidneys. The apparent absence of O-glycan epitope compensation in the kidneys indicates that O-glycan compensation in the gastrointestinal tract and, by extension, N-glycan compensation are active processes that are not due to random biosynthetic processing. Surprisingly, O-linked mannosyl glycans were up-regulated in the triple knockout mice. This is an independent pathway that generates O-Man-GlcNAc-galactose-sialic acid at different sites on proteoglycans in the CNS and on α-dystroglycan in the muscle. Congenital muscular dystrophies are associated with mutations in this O-linked mannosyl pathway (40). The authors suggest that the metabolite UDP-GlcNAc might be the common factor in cases when compensation in the triple mutant results in up-regulation or increased flux through the hexosamine pathway as a compensatory mechanism. C2GnT triple knockout blocks the normal flux of UDP-GlcNAc into branched O-glycans, which might increase GnT activities in other pathways. The single C2GnT mutations have mild phenotypes, and synthetic phenotypes were not reported for the triple null mice. Nonetheless, evidence of epitope compensation in these mice indicates a wider generalization of the phenomena.
Glycan Epitope Maintenance in Brain Gangliosides
GM3 is the most widely distributed ganglioside in tissues and serves as a precursor for the biosynthesis of more complex gangliosides. In CNS axons, GM3 is converted in separate pathways to GD1a and GT1b, which are ligands for the receptor myelin associated glycoprotein (MAG). MAG/Siglec4 is a member of the Siglec family of sialic acid binding lectins (41), which bind GD1a and GT1b, stabilizing myelin–axon interactions (42). MAG/Siglec4-deficient mice show early neuronal cell death with aging, a phenotype associated with sialic acid binding activity (43). Surprisingly, mice deficient in GM3 synthase (CMP-NeuAc:lactosylceramide α2,3-sialytransferase) are grossly normal except for a heightened sensitivity to insulin in skeletal muscle (44). GM3 is absent, but a biosynthetic pathway normally absent in the CNS is activated in the mutant mice and produces GM1b and GD1α, which are also ligands of MAG/Siglec4 (45). Gangliosides are concentrated in lipid rafts where MAG/siglec4 might regulate signaling receptors, a possibility that is currently an active area of research (46). Nonetheless, elimination of the normal ganglioside ligands GD1a and GT1b in CNS axons of mutant mice induces feedback that stimulates an alternative pathway to MAG/Siglec4 ligands. As in the examples above, this suggests feedback from the cell surface to stimulate the compensation pathway to GM1b and GD1α. Beyond their being a backup system for a GM3-deficient CNS, we can speculate that the two pathways have overlapping functions and regulation in wild-type mice, again suggesting that glycan homeostasis is important. Axon regrowth following injury is enhanced by the disruption of MAG–sialic acid binding, consistent with a role in developmental signaling (42, 47).
Metabolic Regulation of Epitope Density on Receptors
Galectin lattice formation at the cell surface is highly dependent on N-glycan branching and the LacNAc epitope density on transmembrane glycoproteins (48). Therefore, the expression and biochemical properties of the Golgi enzyme have a considerable effect on the epitope density. Many of the Golgi enzymes function at subsaturating concentrations of a substrate, either the acceptor N-glycans on glycoproteins or donor sugar-nucleotide (Km values in Fig. 1). This results in a heterogeneous distribution of LacNAc epitopes at the various NXS/T sites, consistent with a model dependent on epitope density, rather than targeted occupancy at specific sites in glycoproteins. We developed a computational model of epitope-density-dependent regulation of receptor residency at the cell surface via binding to galectin-3 (10). The Golgi output of remodeled N-glycans was computed as a function of increasing hexosamine pathway activity (i.e. UDP-GlcNAc concentrations), and the probabilistic glycoform distributions were computed for EGF receptors (EGFR) and TGF-β receptors (TβR), and then surface receptor levels due to association with the lattice and the capacity for ligand-dependent signaling (10). TβRI/II has only three N-glycans and is therefore more dependent on the UDP-GlcNAc supply and branching to generate affinity for galectin-3 than EGFR, with eight N-glycans (Fig. 2A). In other words, more epitopes per glycan are required for TβRI/II, whereas with EGFR, a similar affinity for galectin-3 can be attained with more N-glycans (NXS/T sites) and less branching. Epitope equivalence or compensation is a critical feature of receptor regulation by the lattice (Table I).
Experimental data from cell lines and human autoimmune disease support the lattice model in which the N-glycan number and the Golgi pathways co-regulate receptor titration into the galectin lattice (10) (Fig. 2C). These intriguing dynamics are dependent on a conserved biochemical feature of the N-glycan branching pathway, namely, multi-step ultrasensitivity to UDP-GlcNAc. Multi-step ultrasensitivity arises from the decreasing affinities of Mgat1, -2, -4, and -5 enzymes for UDP-GlcNAc in sequential order of their action. Mass spectrometry analysis indicates that bi-, tri-, and tetraantennary N-glycans increase with intracellular UDP-GlcNAc concentrations (10). In Mgat5-deficient cells, the LacNAc density is reduced but can be restored via GlcNAc supplementation to UDP-GlcNAc, which generates more bi- and triantennary glycans in the absence of tetraantennary (Mgat5) structures. Titration of UDP-GlcNAc fully restores the ordered association of EGFR and TβR into the galectin-3 lattice, growth/proliferation, and then feedback inhibition via TGF-β/Smad signaling. A single stimulus, UDP-GlcNAc, promotes a Michaelis–Menten and a sigmoidal response for high and low multiplicity receptors, respectively, and the intervening “delay” allows growth signaling to prevail before the onset of negative regulation by the low multiplicity receptors (Fig. 2B). Consistent with this model, growth receptor kinases display roughly five times as many N-glycosylation sites (NXS/T), higher site densities, and longer extracellular domains than receptors that mediate differentiation and arrest (10). The evolution rate of glycoproteins is accelerated relative to that of proteins inside the cell (49). Moreover, the evolution of NXS/T multiplicity in receptors suggests that the delay in opposing signaling pathways has increased in humans since a common ancestor with mice.
The T cell co-receptors CD28 (n = 5 sites in humans, 4 in mice) and CTLA-4 (n = 2 in humans, 3 in mice) show a similar order of titration into the lattice in response to UDP-GlcNAc. UDP-GlcNAc levels, N-glycan branching, and poly-LacNAc increase with T cell activation (50, 51). CD28 stimulates growth, and as UDP-GlcNAc concentrations increase, surface CTLA-4 is recruited to the lattice, reaching a critical level that suppresses T cell proliferation (10, 52). A human polymorphism in CTLA-4 (49A/G, rs231775) (53) reduces N-glycan occupancy at one of the two NXS/T sites and increases the risk of autoimmune disease (54). A hyperactive variant of MGAT1 (IVAVT-T) suppresses branching, consistent with the ultrasensitive model of the branching pathway described above (52). Co-inheritance of MGAT1 (IVAVT-T) and CTLA-4 (49A/G, rs231775) additively weaken the affinity of CTLA-4 for the lattice and increase the risk of autoimmune disease (multiple sclerosis) (52). The effect of these alleles on T cell hypersensitivity is reversed by supplementation with GlcNAc, which is converted to UDP-GlcNAc and increases N-glycan branching. The MGAT5 (rs3814022, rs4953911) allele (55) has also been linked to multiple sclerosis severity. Moreover, IL2RA*T (rs2104286) and IL7RA*C (rs6897932) variants also drive T cell autoimmunity through N-glycan branching (52). Vitamin D3 deficiency, another well-documented risk factor, suppresses branching and T cell activation by up-regulating the expression of MGAT1. Moreover, oral GlcNAc treatment prevents spontaneous autoimmune diabetes (56) and inhibits experimental autoimmune encephalomyelitis in mice when treatment is initiated after disease onset (57). In the latter study, oral GlcNAc increased N-glycan branching and suppressed disease by inhibiting Th1 and Th17 T-helper cell responses. The “hexosamine branching lattice” provides a conceptual basis for the regulation of glycoprotein dynamics at the cell surface based on epitope density and allows for mechanisms of compensation and remarkable plasticity (11, 58) (see Figs. 2A–2C and Table I).
CONCLUSIONS
To summarize, glycan epitope compensation has been observed in mouse tissues for N- and O-linked glycans and brain gangliosides, which serves as strong evidence of systemic feedback. Density can be maintained through the gene expression of biosynthetic enzymes of the Golgi and the supply of metabolites to the hexosamine pathways. Changes in the lectin–glycan lattice-dependent regulation of receptors can act as an environmental sensor and result in altered signaling to metabolism and Golgi enzyme expression and thus epitope compensation. Importantly, different arrangements of epitopes in N-glycans produce distinct glycoform distributions but maintain comparable affinities for galectins. Therefore, epitope equivalence might allow the cell surface to adapt to various environmental inputs and stresses. Adaptation might involve the shifting of epitopes between different glycan classes. For example, gangliosides are concentrated in lipid rafts (59, 60) and might help recruit glycoproteins into lattices in rafts or, alternatively, compete for galectin that would otherwise bind N-glycans on receptors outside of rafts.
The ultrasensitive response of the branching pathway to UDP-GlcNAc, and by extension metabolism, is embedded in the kinetics and gene expression properties of MGAT enzymes (10, 11). More generally, ultrasensitive responses provide a means of decisive all-or-nothing transitions in the cell cycle and development, where multivalency often plays a role as well. A small shift in PTM epitope density can promote decisive transitions in molecular complex formation and signaling (61). These effects are present in other classes of PTMs, such as phosphorylation (61). For example, the S. cerevisiae cyclin dependent kinase (CDK) inhibitor Sic1 has nine sites in unstructured regions of the protein that are progressively phosphorylated as the G1 phase progresses (21, 22). Six phosphorylated sites are required for a threshold level of Ccd4 binding to Sic1, which triggers its ubiquitination and proteolysis, thereby relieving the inhibition of CDK and triggering the G1/S transition in a switch-like or ultrasensitive response. This ensures an all-or-nothing decisive start to DNA replication. Ultrasensitive responses are seen widely in regulatory systems, and many depend on affinity enhancement via conditional regulation through PTMs. Phosphorylated sites do not undergo secondary modification of the phospho-amino acids, but ubiquitinated sites can become polyubiquitin, generating multiple epitopes for ubiquitin-binding proteins, analogous to LacNAc units in branched N-glycans. For example, anaphase-promoting complex ubiquitinates substrates that are modified and degraded in a specific sequence that orders cell cycle events. Anaphase-promoting complex catalyzes the polyubiquitination of substrates with different relative processivity (62). Processive substrates obtain many ubiquitin chains within a single anaphase-promoting complex binding event, whereas distributive substrates frequently dissociate between substitutions (63). In a manner similar to that of N-glycan epitopes in the regulation of receptors, the differential modification of proteins by a common donor can time events in the cell cycle.
Lastly, PTMs such as phosphate, methyl, and acetyl groups on peptides and glycan epitopes generally present modest affinity to binding partners (17). Therefore, biological responses to the modification of PTM sites might depend on epitope densities and multivalent binding interactions (11). The encoded number of PTM sites in proteins, and their non- or partially ordered occupancy, often generates a characteristic ultrasensitive response to biologically important cues (Fig. 2B). Therefore, specificity arises from low-affinity and multiple PTM epitopes in target proteins and the threshold number of modifications required in order to reach the epitope density for binding partners. Many other factors affect the target proteins differentially, such as the modifying enzymes and substrate levels for modification and, ultimately, the homeostatic opposing pathways (Fig. 2C).
Footnotes
* Research was supported by grants from Genome Canada through the OGI and funding from CIHR (MOP-79405 and MOP-62975) and the Canadian Cancer Society to J.W.D.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- GalNAc
- N-acetylgalactosamine
- GlcNAc
- N-actylglucosamine
- GnT
- N-acetylglucosaminyltransferase
- LacNAc
- N-acetyllactosamine (Galβ1,4GlcNAc)
- MAG
- myelin associated glycoprotein
- Man
- mannose
- PTM
- posttranslational modification
- GM3
- N-acetylneuraminylgalactosylceramide.
REFERENCES
- 1. Metallo C. M., Vander Heiden M. G. (2010) Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev. 24, 2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuriyan J., Eisenberg D. (2007) The origin of protein interactions and allostery in colocalization. Nature 450, 983–990 [DOI] [PubMed] [Google Scholar]
- 3. Aebi M., Bernasconi R., Clerc S., Molinari M. (2010) N-glycan structures: recognition and processing in the ER. Trends Biochem. Sci. 35, 74–82 [DOI] [PubMed] [Google Scholar]
- 4. Drickamer K., Fadden A. J. (2002) Genomic analysis of C-type lectins. Biochem. Soc. Symp. 69, 59–72 [DOI] [PubMed] [Google Scholar]
- 5. Lim W. A., Pawson T. (2010) Phosphotyrosine signaling: evolving a new cellular communication system. Cell 142, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pawson T. (2004) Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116, 191–203 [DOI] [PubMed] [Google Scholar]
- 7. Brewer C. F., Miceli M. C., Baum L. G. (2002) Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12, 616–623 [DOI] [PubMed] [Google Scholar]
- 8. Demetriou M., Granovsky M., Quaggin S., Dennis J. W. (2001) Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 [DOI] [PubMed] [Google Scholar]
- 9. Di Lella S., Sundblad V., Cerliani J. P., Guardia C. M., Estrin D. A., Vasta G. R., Rabinovich G. A. (2011) When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50, 7842–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lau K., Partridge E. A., Silvescu C. I., Grigorian A., Pawling J., Reinhold V. N., Demetriou M., Dennis J. W. (2007) Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129, 123–124 [DOI] [PubMed] [Google Scholar]
- 11. Dennis J. W., Nabi I. R., Demetriou M. (2009) Metabolism, cell surface organization, and disease. Cell 139, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dam T. K., Brewer F. C. (2010) Maintenance of cell surface glycan density by lectin-glycan interactions: a homeostatic and innate immune regulatory mechanism. Glycobiology 20, 1061–1064 [DOI] [PubMed] [Google Scholar]
- 13. Brewer C. F. (1996) Multivalent lectin-carbohydrate cross-linking interactions. Chemtracts 6, 165–179 [Google Scholar]
- 14. Dam T. K., Brewer C. F. (2010) lectins as pattern recognition molecules. The effects of epitope density in innate immunity. Glycobiology 20, 270–279 [DOI] [PubMed] [Google Scholar]
- 15. Lee R. T., Ichikawa Y., Fay M., Drickamer K., Shao M.-C., Lee Y. C. (1991) Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A). J. Biol. Chem. 266, 4810–4815 [PubMed] [Google Scholar]
- 16. Drickamer K. (1993) Ca2+ dependent carbohydrate-recognition domains in animal proteins. Curr. Opin. Struct. Biol. 3, 393–400 [Google Scholar]
- 17. Dam T. K., Brewer C. F. (2008) Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry 47, 8470–8476 [DOI] [PubMed] [Google Scholar]
- 18. Dam T. K., Gabius H.-J., Andre S., Kaltner H., Lensch M., Brewer C. F. (2005) Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 44, 12564–12571 [DOI] [PubMed] [Google Scholar]
- 19. Dam T. K., Gerken T. A., Cavada B. S., Nascimento K. S., Moura T. R., Brewer C. F. (2007) Binding studies of α-GalNAc-specific lectins to the α-GalNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. J. Biol. Chem. 282, 28256–28263 [DOI] [PubMed] [Google Scholar]
- 20. Oyelaran O., Li Q., Farnsworth D., Gildersleeve J. C. (2009) Microarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J. Proteome Res. 8, 3529–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash P., Tang X., Orlicky S., Chen Q., Gertler F. B., Mendenhall M. D., Sicheri F., Pawson T., Tyers M. (2001) Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 [DOI] [PubMed] [Google Scholar]
- 22. Mittag T., Orlicky S., Choy W. Y., Tang X., Lin H., Sicheri F., Kay L. E., Tyers M., Forman-Kay J. D. (2008) Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proc. Natl. Acad. Sci. U.S.A. 105, 17772–17777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dam T. K., Brewer C. F. (2003) Carbohydrate-lectin cross-linking interactions: structural, thermodynamic, and biological studies. Methods Enzymol. 362, 455–486 [DOI] [PubMed] [Google Scholar]
- 24. Bhattacharyya L., Khan M. I., Brewer C. F. (1988) Interactions of concanavalin A with asparagine-linked glycopeptides: formation of homogeneous cross-linked lattices in mixed precipitation systems. Biochemistry 27, 8762–8767 [DOI] [PubMed] [Google Scholar]
- 25. Olsen L. R., Dessen A., Gupta D., Sabesan S., Sacchettini J. C., Brewer C. F. (1997) X-ray crystallographic studies of unique cross-linked lattices between four isomeric biantennary oligosaccharides and soybean agglutinin. Biochemistry 36, 15073–15080 [DOI] [PubMed] [Google Scholar]
- 26. Cheng W., Bullitt E., Bhattacharrya L., Brewer C. F., Makowski L. (1998) Electron microscopy and x-ray diffraction studies of Lotus tetragonolobus A isolectin cross-linked with a divalent lewisx oligosaccharide, an oncofetal antigen. J. Biol. Chem. 273, 35016–35022 [DOI] [PubMed] [Google Scholar]
- 27. Gupta D., Brewer C. F. (1994) Homogeneous aggregation of the 14-kDa β-galactoside specific vertebrate lectin complex with asialofetuin in mixed systems. Biochemistry 33, 5526–5530 [DOI] [PubMed] [Google Scholar]
- 28. Pace K. E., Lee C., Stewart P. L., Baum L. G. (1999) Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 163, 3801–3811 [PubMed] [Google Scholar]
- 29. Chen I. J., Chen H. L., Demetriou M. (2007) Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J. Biol. Chem. 282, 35361–35372 [DOI] [PubMed] [Google Scholar]
- 30. Ahmad N., Gabius H.-J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- 31. Stillman B. N., Hsu D. K., Pang M., Brewer C. F., Johnson P., Liu F. T., Baum L. G. (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176, 778–789 [DOI] [PubMed] [Google Scholar]
- 32. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 33. Lajoie P., Partridge E. A., Guay G., Goetz J. G., Pawling J., Lagana A., Joshi B., Dennis J. W., Nabi I. R. (2007) Plasma membrane domain organization regulates EGFR signaling in tumor cells. J. Cell Biol. 179, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lagana A., Goetz J. G., Cheung P., Raz A., Dennis J. W., Nabi I. R. (2006) Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell. Biol. 26, 3181–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E. G., Yagi F., Kasai K.-i. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 36. Zhuo Y., Bellis S. L. (2011) Emerging role of α2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 286, 5935–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takamatsu S., Antonopoulos A., Ohtsubo K., Ditto D., Chiba Y., Le D. T., Morris H. R., Haslam S. M., Dell A., Marth J. D., Taniguchi N. (2010) Physiological and glycomic characterization of N-acetylglucosaminyltransferase-IVa and -IVb double deficient mice. Glycobiology 20, 485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ismail M. N., Stone E. L., Panico M., Lee S. H., Luu Y., Ramirez K., Ho S. B., Fukuda M., Marth J. D., Haslam S. M., Dell A. (2011) High-sensitivity O-glycomic analysis of mice deficient in core 2 β1,6-N-acetylglucosaminyltransferases. Glycobiology 21, 82–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohtsubo K., Chen M. Z., Olefsky J. M., Marth J. D. (2011) Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat. Med. 17, 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Godfrey C., Foley A. R., Clement E., Muntoni F. (2011) Dystroglycanopathies: coming into focus. Curr. Opin. Genet. Dev. 21, 278–285 [DOI] [PubMed] [Google Scholar]
- 41. Varki A. (2010) Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U.S.A. 107 Suppl. 2, 8939–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schnaar R. L. (2010) Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett. 584, 1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mountney A., Zahner M. R., Lorenzini I., Oudega M., Schramm L. P., Schnaar R. L. (2010) Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl. Acad. Sci. U.S.A. 107, 11561–11566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J. L., Werth N., Sandhoff R., Sandhoff K., Proia R. L. (2003) Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. U.S.A. 100, 3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schnaar R. L., Lopez P. H. (2009) Myelin-associated glycoprotein and its axonal receptors. J. Neurosci. Res. 87, 3267–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujitani M., Kawai H., Proia R. L., Kashiwagi A., Yasuda H., Yamashita T. (2005) Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction. J. Neurochem. 94, 15–21 [DOI] [PubMed] [Google Scholar]
- 47. Murrey H. E., Hsieh-Wilson L. C. (2008) The chemical neurobiology of carbohydrates. Chem. Rev. 108, 1708–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patnaik S. K., Potvin B., Carlsson S., Sturm D., Leffler H., Stanley P. (2006) Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 16, 305–317 [DOI] [PubMed] [Google Scholar]
- 49. Julenius K., Pedersen A. G. (2006) Protein evolution is faster outside the cell. Mol. Biol. Evol. 23, 2039–2048 [DOI] [PubMed] [Google Scholar]
- 50. Togayachi A., Kozono Y., Ishida H., Abe S., Suzuki N., Tsunoda Y., Hagiwara K., Kuno A., Ohkura T., Sato N., Sato T., Hirabayashi J., Ikehara Y., Tachibana K., Narimatsu H. (2007) Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc. Natl. Acad. Sci. U.S.A. 104, 15829–15834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antonopoulos A., Demotte N., Stroobant V., Haslam S. M., van der Bruggen P., Dell A. (2012) Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation. J. Biol. Chem. 287, 11240–11251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mkhikian H., Grigorian A., Li C. F., Chen H. L., Newton B., Zhou R. W., Beeton C., Torossian S., Tatarian G. G., Lee S. U., Lau K., Walker E., Siminovitch K. A., Chandy K. G., Yu Z., Dennis J. W., Demetriou M. (2011) Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat. Commun. 2, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anjos S., Nguyen A., Ounissi-Benkalha H., Tessier M. C., Polychronakos C. (2002) A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J. Biol. Chem. 277, 46478–46486 [DOI] [PubMed] [Google Scholar]
- 54. Kavvoura F. K., Ioannidis J. P. (2005) CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE review and meta-analysis. Am. J. Epidemiol. 162, 3–16 [DOI] [PubMed] [Google Scholar]
- 55. Brynedal B., Wojcik J., Esposito F., Debailleul V., Yaouanq J., Martinelli-Boneschi F., Edan G., Comi G., Hillert J., Abderrahim H. (2010) MGAT5 alters the severity of multiple sclerosis. J. Neuroimmunol. 220, 120–124 [DOI] [PubMed] [Google Scholar]
- 56. Grigorian A., Lee S.-U., Tian W., Chen I.-J., Gao G., Mendelsohn R., Dennis J. W., Demetriou M. (2007) Control of T cell mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J. Biol. Chem. 282, 20027–20035 [DOI] [PubMed] [Google Scholar]
- 57. Grigorian A., Araujo L., Naidu N. N., Place D., Choudhury B., Demetriou M. (2011) N-acetylglucosamine inhibits T-helper 1 (Th1) / T-helper 17 (Th17) responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 286, 40133–40141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dennis J. W., Lau K. S., Demetriou M., Nabi I. R. (2009) Adaptive regulation at the cell surface by N-glycosylation. Traffic 11, 1569–1578 [DOI] [PubMed] [Google Scholar]
- 59. Lajoie P., Goetz J. G., Dennis J. W., Nabi I. R. (2009) Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J. Cell Biol. 185, 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boscher C., Zheng Y. Z., Lakshminarayan R., Johannes L., Dennis J. W., Foster L. J., Nabi I. R. (2012) Galectin-3 regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells. J. Biol. Chem. 287, 32940–32952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim S. Y., Ferrell J. E., Jr. (2007) Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128, 1133–1145 [DOI] [PubMed] [Google Scholar]
- 62. Rape M., Reddy S. K., Kirschner M. W. (2006) The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 [DOI] [PubMed] [Google Scholar]
- 63. Ferrell J. E., Jr. (1996) Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21, 460–466 [DOI] [PubMed] [Google Scholar]
- 64. Earl L. A., Bi S., Baum L. G. (2010) N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 285, 2232–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhu J., Yan J., Thornhill W. B. (2012) N-glycosylation promotes the cell surface expression of Kv1.3 potassium channels. FEBS J. 279, 2632–2644 [DOI] [PubMed] [Google Scholar]
- 66. Markowska A. I., Jefferies K. C., Panjwani N. (2011) Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 286, 29913–29921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bi S., Hong P. W., Lee B., Baum L. G. (2011) Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl. Acad. Sci. U.S.A. 108, 10650–10655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo H. B., Johnson H., Randolph M., Pierce M. (2009) Regulation of homotypic cell-cell adhesion by branched N-glycosylation of N-cadherin extracellular EC2 and EC3 domains. J. Biol. Chem. 284, 34986–34997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wellen K. E., Lu C., Mancuso A., Lemons J. M., Ryczko M., Dennis J. W., Rabinowitz J. D., Coller H. A., Thompson C. B. (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cha S. K., Ortega B., Kurosu H., Rosenblatt K. P., Kuro O. M., Huang C. L. (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. U.S.A. 105, 9805–9810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ohtsubo K., Takamatsu S., Minowa M. T., Yoshida A., Takeuchi M., Marth J. D. (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123, 1307–1321 [DOI] [PubMed] [Google Scholar]
- 72. Grewal P. K., Boton M., Ramirez K., Collins B. E., Saito A., Green R. S., Ohtsubo K., Chui D., Marth J. D. (2006) ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol. Cell. Biol. 26, 4970–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]