Abstract
Objectives
Physical exercise has been shown to benefit diverse medical and behavioral conditions. This study assesses the feasibility and efficacy of an 8-week endurance and resistance training program on fitness measures in individuals undergoing residential treatment for methamphetamine (MA) dependence.
Methods
A total of 39 MA-dependent individuals were randomized to 3 days/week of exercise training (ET, n=15) or health education without training (EA, n=14) over 8 weeks. Aerobic performance (VO2max) was measured by indirect calorimetry, body composition by skinfolds, muscle strength by 1-repetition maximum (1-RM) and endurance at 85% of 1-RM for both leg press (LP) and chest press (CP).
Results
A total of 29 individuals completed the study for a 74% adherence rate. Baseline characteristics (mean±SD) were balanced between groups: age 31±7 years; height=1.74±0.07 m; weight 82.0±15.0 kg. The ET group significantly improved VO2max by 0.63±0.22 L/min (+21%), LP strength by 24.4±5.6 kg (+40%) and CP strength by 20.6±5.7 kg (+49%). The ET group increased LP and CP endurance by 120% and 96%, respectively and showed significant reductions in body weight of 1.7±2.4 kg (−2%), % body fat of 2.8±1.3% (−15%) and fat weight 2.8±1.8 kg (−18%). All changes were significant (P<0.001) for ET, and no changes were seen for the EA group.
Conclusions
Individuals recovering from methamphetamine dependence showed substantial improvements in aerobic exercise performance, muscle strength and endurance, and body composition with exercise training. These findings demonstrate the feasibility of an exercise training intervention in these participants and also show excellent responsiveness to the exercise stimulus resulting in physiological changes that might enhance recovery from drug dependency.
Keywords: Methamphetamine, exercise, addiction
INTRODUCTION
Worldwide, it is estimated that between 13.7 and 52.9 million people used methamphetamine (MA) and other amphetamine-type stimulants for non-medical purposes in 2008 (United Nations Office on Drugs and Crime [UNODC], 2010). In 2007, one third of all individuals admitted to the publicly funded substance dependency treatment system in California had a primary diagnosis of MA dependence (Substance Abuse and Mental Health Services Administration [SAMHSA],2009) . This diagnosis exceeded all other substance dependency diagnoses, including alcohol (Gonzales et al., 2010). Behavioral approaches for MA dependence have proven moderately effective in treating MA dependence, but problems remain with substantial proportions of individuals dropping out early in treatment (Peirce et al., 2006; Rawson et al., 2004; Roll et al., 2006; Rawson et al., 2006). Furthermore, many individuals are unable to sustain gains from treatment and avoid post-treatment relapse (Rawson et al., 2006).
Participation in regular physical exercise may be an effective intervention to aid MA dependent individuals in avoiding relapse during attempted abstinence. Exercise has proven effective in ameliorating symptoms of depression and anxiety (Byrne & Byrne, 1993), which are commonly reported by MA users attempting abstinence (Glasner-Edwards et al., 2010; Zweben et al., 2004) and may be associated with relapse to drug use (Breslin et al., 2002; Marlatt, 1996). Prior studies have also demonstrated benefits of exercise in improving cognition (Angevaren et al., 2008; Etnier et al., 2006), and cognitive deficits found in chronic MA users (Simon et al., 2000). Furthermore, exercise may have a salutary effect on reducing cardiovascular risk factors, such as hypertension, that are associated with MA use (Mooney et al., 2009; Turnipseed et al., 2003). Increased aerobic and muscle performance, along with favorable changes in body composition resulting from exercise could provide additional health benefits in this population that might also help recovering MA users avoid relapse.
There are no published data in humans indicating that regular exercise training is feasible or beneficial in recovering MA-dependent individuals. The purpose of this study, therefore, was to assess the feasibility of a combined 8-week aerobic and resistance exercise training protocol in a sample of individuals undergoing residential treatment for MA dependence. In addition, the effectiveness of the 8-week exercise training intervention in improving selected measures of health-related physical fitness was also compared with a control group that received only an educational intervention.
METHODS
Study design
Individuals with MA dependence who were admitted for treatment to a residential facility in southern California were recruited within 10 days of admission to participate in the study. After obtaining informed consent, a physician-administered medical history and physical examination, clinical laboratory tests and a 12-lead resting electrocardiogram (ECG) were performed to determine study eligibility. Further baseline measures were completed during a 2-week data collection period. During this time, study candidates continued to participate in the standard schedule of treatment activities which included group and individual therapy and 12-step meetings, characteristic of residential treatment programs. Participants who met screening eligibility and successfully completed the 2-week baseline data collection period were randomized to either the exercise-training (ET) group or an equal-attention educational program (EA) and scheduled for subsequent study interventions. Participants in both groups received incentives to participate in the form of vouchers given out upon completion of the study. The overall study design is described in Table 1.
TABLE 1.
Study Design
|
Weeks
−2 - 0: Screening |
|
|
|
Weeks 1 - 8: Intervention (Exercise or Equal-Attention) |
Intervention:
Group 1: Exercise plus usual care Group 2: Equal Attention plus usual care |
|
|
Exercise (ET) (thrice weekly exercise training with incentive for participation) plus usual care |
Equal-Attention (EA) (thrice weekly health education sessions with incentive for participation) plus usual care |
|
| All participants allowed ad lib access to gym | ||
|
Weeks 9 – 10 Post-intervention |
Usual care plus end of study assessments | |
| Discharge from Residential Program (referral to continuing care) | ||
|
Post-discharge
follow- ups |
All participants re-contacted at 4, 8, 12, and 26 weeks after discharge per informed consent and scheduled for follow-up assessments |
|
Participants
Men and women aged 18-55 years were recruited for participation. Participants were required to be in-residence at the treatment center and meet DSM-IV-TR criteria for MA dependence as determined via the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Secondary current substance dependence by participants was reported as: Alcohol (ET Group, 40% vs. EA Group, 29%); Cocaine (ET Group, 13% vs. EA Group, 21%); Cannabis (ET Group, 40% vs. EA Group, 7%); Tranquilizers, i.e., Xanax, Valium and GHB (ET Group, 20% vs. EA Group, 14%). There were no group differences for any secondary substances. From the study time period of December 2010 through March 2012, 80 individuals were screened for study participation, of which 65 were consented and 39 were randomized into the study intervention. Those individuals who were not consented (15 individuals) failed to meet initial screening criteria for study participation (i.e., did not meet criteria for methamphetamine dependence, length of treatment less than 90 days, etc). Individuals who were consented but not randomized (26 individuals) failed to meet subsequent study criteria (i.e., untreated or unstable medical or mental illness, clinically significant hypertension, etc.), were discharged from the residential treatment facility, or left treatment against staff’s advice. Of the 39 participants randomized into the study intervention, 29 (74%) completed the full 8 week study intervention.
In addition to the comprehensive medical examination, all participants completed clinical laboratory tests including infectious disease testing for hepatitis and syphilis and a urine drug screen to assess for drugs of abuse; all females were given a pregnancy test. In addition, the medical health of all participants was assessed with the following requirements for inclusion: (i) resting heart rate between 50-99 beats/min, (ii) blood pressure between 150-85 mm Hg systolic and 90-45 mm Hg diastolic and, (iii) no clinically significant abnormalities of the resting ECG. Exclusionary criteria included any musculoskeletal conditions and unstable cardiovascular, pulmonary, metabolic, or other disorders that would preclude exercise training. A total of 31 participants (5 women) met all entry criteria. Thus the subjects were reasonably representative of the population at the rehabilitation center. This study was approved by the UCLA Institutional Review Board and participants gave written informed consent.
Compensation
Participants were compensated for study participation. They could earn a maximum of $515 for study visits and follow-up visit completion. All compensation during the 12 weeks of study was provided within the guidelines of the residential treatment program and money earned was held by research staff until the participants discharge from the residence. Vouchers, which could be exchanged for goods, food, and gas, were used to compensate participants for each intervention session. Participants received $10 per event, thrice weekly for eight weeks. They were also compensated $15 per cognitive assessment for a total of four and received $30 for each of the four post-discharge visits. Participants received a bonus of $40 for completion of all study intervention activities and another $40 bonus after completion of all four follow-up data visits.
Anthropometry
Body weight was measured on a calibrated digital scale and height was determined using a precision stadiometer. Body composition was determined using the skinfold method and standard techniques (Harrison et al, 1988). Body density was estimated from skinfold thickness using sex specific equations (Jackson and Pollock, 1978; Jackson et al., 1980). Relative body fat was calculated from these estimates of body density using equations specific for age, sex, and ethnicity (Heywood, 2006; Jackson and Pollock, 1978).
Aerobic performance
All potential participants completed a symptom-limited cardiopulmonary exercise test (XT) using an incremental treadmill protocol. Aerobic capacity (VO2max) was assessed by indirect calorimetry using a portable, metabolic measurement system (Oxycon Mobile, CareFusion, Yorba Linda, CA). Briefly, participants were asked to walk on a treadmill with gradually increasing speed and grade. They were asked to give their best effort and exercise as long as they could. A 12-lead ECG was obtained at rest and monitored throughout exercise and recovery; blood pressure was obtained at rest and during recovery. These safety measures were considered important because exercise could exacerbate cardiac risk factors associated with MA abuse.
Musculoskeletal fitness
Muscle strength was determined by the 1-repetition maximum (1-RM) method for the leg press and chest press exercises (Harman and Garhammer, 2008). Through progressive increments in resistance and use of rest intervals, this method assesses the maximum weight that can be lifted only once through a complete range of motion with good form. Muscle endurance was measured as the number of repetitions to failure using 85% of baseline leg press and chest press 1-RM values.
Exercise training intervention
General schedule
All participants randomized to exercise training met for approximately one hour, three days per week for eight weeks to complete both endurance and resistance exercise routines in the treatment facility gym. All exercise sessions were directly supervised by an experienced exercise trainer. Training schedules were developed in advance with exercise sessions typically conducted on Mondays, Wednesdays, and Fridays. The exercise trainer was present to supervise the subjects throughout each training session. Sometimes two individuals trained at the same time. Under these circumstances, a trained assistant monitored one of the subjects. Additional trainer time was devoted to data entry and analysis.
Endurance training
For the first three weeks, participants walked and/or jogged on a motor driven treadmill at an intensity based on the heart rate that coincided with the at the metabolic threshold during the baseline treadmill exercise test. Heart rate was monitored continuously for each participant throughout their training session using a heart rate monitor (Polar™RS400, Polar Inc, Lake Success, NY). Their training zone was defined as this heart rate ± 6 beats per minute. The objective was to accumulate 30 minutes of continuous aerobic exercise in the prescribed heart rate zone. During the first three week period, treadmill speed and or grade was adjusted at the trainer’s discretion to maintain the target intensity. For the remaining five weeks of training, intensity was increased to a level equivalent to a heart rate corresponding to the heart rate midway between the heart rate at metabolic threshold and maximum heart rate measured during the XT. Treadmill speed and grade were adjusted by the trainer to maintain the prescribed intensity. In the event a participant was unable to complete 30 continuous minutes of treadmill exercise, rest periods were given until the participant accumulated a total of 30-minutes at this intensity.
Resistance training
Following the endurance training session, participants completed a progressive, circuit-type resistance training program using selectorized machines and/or dumbbell resistance that included all the major muscle groups of the upper and lower body. The exercise trainer instructed all participants in proper exercise technique for all lifts and provided continuous monitoring during each training session. For the first three weeks, participants performed a warm-up set with very light weight then completed one set of 8-15 repetitions for each exercise using resistance that resulted in “substantial fatigue.” (Haskell et al., 2007). During the final five weeks of the training protocol, participants added a second set of each exercise and increased the resistance to a level equal to 8-12 RM. The target range of two sets of 8-12 RM was maintained through the end of the study.
Gym availability
Per the policy of the residential treatment center, all patients enrolled in treatment, including participants in both study groups, had ad libitum access to the facility gymnasium. Use of the gym and all other physical activity (i.e., walking/jogging/running and other sports) was recorded weekly using a 7-day recall for the entirety of the 8-week intervention.
Control group
Participants randomized to the control group, (equal attention, EA), participated in small-group health and wellness education sessions three times a week for approximately one hour. A trained counselor provided informational materials and facilitated discussion of educational content. Sessions consisted of an integrated multimedia program addressing a variety of health, wellness, and lifestyle topics such as healthy eating, dental care, acupressure, and cancer screening. As a result of the session activities and data collection, participants received the same amount of staff attention as participants in the exercise group.
Nutritional supplementation
In order to support the additional energy requirements of the thrice weekly endurance and resistance exercise, participants’ institutional diets were supplemented with a commercially available ready-to-drink product (Protein Rush, VPX, Weston, FL). This supplement consisted of 42 grams protein and 240 Kcal per serving to ensure caloric and protein adequacy although overall nutrition was not controlled. Participants were asked to consume a protein drink after each exercise or education session.
Statistical analysis
Participant characteristics are presented as mean (SD). Within group comparisons were evaluated by paired t-tests and between group comparisons for changes from baseline to eight weeks with independent t-tests. Progress in training volume was assessed by linear regression. Statistical significance was set at P<0.05.
RESULTS
Demographics
Of the 39 participants randomized, 29 (4 women) completed the study. Two participants dropped out of the study due to musculoskeletal problems unrelated to the exercise training (one strained knee and one twisted ankle). The mean baseline participant characteristics and performance variables for each condition are presented in Table 2. Participants had a mean (SD) age of 31±7 years, weight of 82.0±15.0 kg and height of 1.74±0.07 m. The majority of participants were White, Non-Hispanic (55%), followed by White, Hispanic (24%), Black (10%), Asian (7%), and Other (4%). This sample was representative of patients in the treatment facility. All baseline variables were balanced and not significantly different between groups.
TABLE 2.
Mean Baseline Subject Characteristics and Performance Variables between Conditions for Subjects Completing the 8-week Study
| Variable |
ET (n=15) mean ± SD |
EA (n=14) mean ± SD |
|---|---|---|
| Age (years) | 30 ± 7 | 32 ± 7 |
| Sex | M=13, F=2 | M=12, F=2 |
| Height (m) | 1.75 ± 0.06 | 1.74 ± 0.07 |
| Weight (kg) | 84.2 ± 16.9 | 80.5 ± 11.7 |
| BMI (kg/m2) | 27.6 ± 5.3 | 26.6 ± 3.5 |
| Body fat (%) | 22.1 ± 6.5 | 19.9 ± 6.3 |
| Fat weight (kg) | 18.5 ± 7.8 | 16.6 ± 7.4 |
| Fat-free weight (kg) | 65.6 ± 10.1 | 64.3 ± 7.1 |
| VO2max (L/min) | 2.32 ± 0.31 | 2.48 ± 0.72* |
| 1-RM chest press (kg) | 41.8 ± 14.0 | 44.5 ± 18.3 |
| 1-RM leg press (kg) | 60.9 ± 13.3 | 63.1 ± 15.0 |
| 85% of 1-RM chest press (reps) | 7.7 ± 1.7 | 7.0 ± 1.4 |
| 85% of 1-RM leg press (reps) | 8.1 ± 3.1 | 8.0 ± 3.3 |
n=13
Retention and adherence to program
Retention within each group and adherence to each treatment intervention was high. In the ET group, only two participants failed to complete the study intervention, citing medical problems as their reason to discontinue participation. Fifteen participants completed at least 22 of 24 training sessions (92%) and 13 (87%) participants completed every session. Additionally, during the last month of training (eight sessions) 100% adherence was obtained. Initially, a few participants were unable to maintain 30 minutes of continuous treadmill exercise. However, these interrupted sessions accounted for less than 10 out of 360 total training sessions. The need for rest intervals was overcome by appropriate adjustments of training intensity. Participants in the EA group attended an average of 23 out of 24 educational sessions, and all 14 participants in this group completed the intervention.
Performance variables
Table 3 highlights the mean changes from baseline in the performance variables for the ET and EA groups. Anthropometric changes observed in the ET group included significant reductions in percent relative body fat (−2.8±1.3%, −15%; P<0.001) and fat weight (−2.8±1.8 kg, −18%; P<0.001) with these differences also significant between groups (p<0.05). Although body weight decreased (−1.7±2.4 kg, −2%) and fat-free weight increased (+1.6±1.9 kg, +2%) in the ET group, the changes were not statistically significant. Body composition measures did not change within the EA group. For the ET group, maximum oxygen uptake increased by 0.63±0.22 L/min (+21%; P<0.001) while this measure did not change in the EA group. For the ET group, lower body strength increased by 24.4±5.6 kg (+40%; P<0.001) and upper body strength increased by 20.6±5.7 kg (+49%; P<0.001). Lower and upper body endurance improved by ten repetitions (+120%) and seven repetitions (+96%), respectively. These changes were all significantly greater than those seen in the EA group (P<0.001). These measures did not change in the EA group.
TABLE 3.
Changes from Baseline in Physical Performance Variables in the Exercise Training (ET) and Equal-Attention (EA) Groups. Data include Means, Standard Deviations and Percent Change from Baseline in Subjects Completing the 8-week Study.
| Variable |
ET (n=15) mean ± SE |
EA (n=14) mean ± SE |
|||
|---|---|---|---|---|---|
| Change | %Change | Change | %Change | p† | |
| VO2max (L/min) | 0.63 ± 0.06* | 21 | −0.02 ± 0.05* | −1 | <0.001 |
| Body fat (%) | −2.8 ± 0.3* | 15 | 0.7 ± 0.4 | 3 | <0.001 |
| Weight (kg) | −1.7 ± 0.6 | 2 | 1.7 ± 0.6 | 2 | 0.032 |
| Fat weight (kg) | −2.8 ± 0.5* | 18 | 1.0 ± 0.4 | 5 | <0.001 |
| Fat-free weight (kg) | 1.6 ± 0.5 | 2 | 0.6 ± 0.2 | 1 | 0.344 |
| 1-RM chest press (kg) | 20.6 ± 1.5* | 49 | 1.3 ± 0.7 | 3 | <0.001 |
| 1-RM leg press (kg) | 24.4 ± 1.4* | 40 | −0.2 ± 0.6 | 0 | <0.001 |
| 85% of 1-RM chest press (reps) | 7.4 ± 0.3* | 96 | −0.1 ± 0.2 | −1 | <0.001 |
| 85% of 1-RM leg press (reps) | 9.7 ± 0.9* | 120 | 0.6 ± 0.3 | 7 | <0.001 |
= P < 0.05 for within group changes from baseline
= P for changes between groups
Training progression
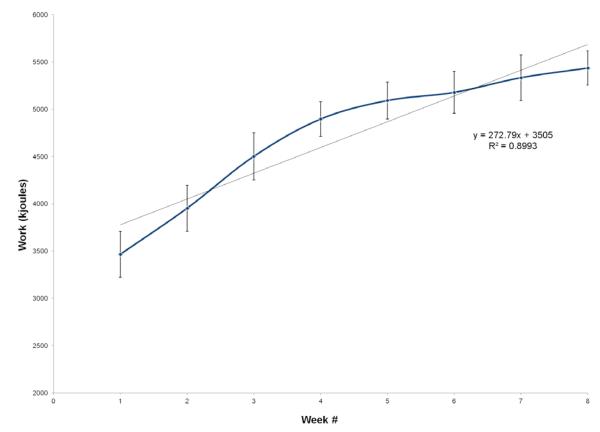
Figure 1 display the progression of total work (kJ) performed during endurance training for the ET group over the eight weeks. Total work increased weekly by 273 kJ on average, although there was a great deal of individual variability. The mean increase in total work performed from week one to week eight was 1900 kJ, representing a 56% increase (P<0.001). Figure 2 displays the progression of resistance training volume (kg/week) for the ET group over the eight weeks of the exercise program. Total volume of weight lifted (calculated as exercises x sets x repetitions x resistance) increased by a mean ± SD of 2126 ± 3777 kg per week over the eight weeks, exhibiting a greater than two-fold increase in total weight lifted over the course of the study. These changes were significant from week one to eight (P<0.001).
Figure 1.
Progression in total work performed over 8-weeks of endurance exercise training. Values represent means for total work by training participants each week. Error bars are SE. Grey diagonal represents regression line of work completed over the 8 weeks of treadmill exercise. Data are presented only for subjects completing the 8-week study.
Figure 2.
Progression in total volume performed over 8-weeks of resistance exercise training. Values represent means for total volume (total amount of weight lifted) by training participants each week. Error bars are SE. Grey diagonal represents regression line of volume completed over the 8 weeks of resistance exercise. Data are presented only for subjects completing the 8-week study.
Ad libitum exercise training
Using interviews conducted weekly by study personnel, participants in the EA group reported an average of 31±26 minutes of daily ad libitum physical activity over the eight-week course of the study. Participants in ET reported 18±19 minutes of daily activity that was in addition to their supervised training. Although the EA group reported over 70% more ad libitum exercise, this difference was not statistically different.
DISCUSSION
This study demonstrates the feasibility and efficacy of an exercise training program for individuals, mostly men, recovering from methamphetamine dependency in a residential treatment facility. We defined efficacy as statistically significant and clinically meaningful improvements in aerobic capacity ( max), muscle strength (1-RM) and endurance (85% of 1-RM) and percentage of body fat (FM%). Those randomized to a supervised, eight-week endurance and resistance training program significantly improved all of these measures and we believe this is the first study to describe such effects. Most of the literature on exercise as an adjunct to treatment for substance abuse concerns nicotine and alcohol addiction and studies of aerobic exercise training as a potential intervention to aid tobacco smoking cessation have shown mixed results. However, more consistent positive effects on cigarette cravings, withdrawal symptoms, and smoking-related behaviors after chronic exercise training have been demonstrated (Taylor et al., 2007).
In the present study, baseline measures of aerobic capacity, max, for men and women were 30.6 ml/kg/min and 23.2 ml/kg/min, respectfully. Based on wellestablished reference values for age and sex (Thompson, 2010) these participants are classified as having “poor” cardiorespiratory fitness with average rankings below the 10% percentile for age and sex . Participants randomized to ET showed that they were capable of improving max by 21% after just 8-weeks of training. The increases in max observed in both men and women in the ET group placed them within the 30th-40th percentiles of cardiorespiratory fitness for age and sex.
High aerobic capacity ( max) and greater skeletal muscle strength are both associated with lower prevalence of chronic diseases and lower mortality (Booth and Roberts, 2008). The impressive increases in max (21%) as well as muscle strength and endurance for upper body (49% and 120%, respectively) and lower body (40% and 90%, respectfully) demonstrated in this study illustrate the potential of the training program to reduce risks from chronic diseases such as heart disease, stroke and diabetes. Moreover, the improvements from exercise training may also have positive effects in ameliorating some of the conditions associated with chronic MA exposure including cardiomyopathy, coronary artery disease, dysrhythmias, myocardial ischemia, hypertension and cerebrovascular dysfunction (Henry et al., 2010).
Our resistance training protocol was based on recommendations from the American College of Sports Medicine, American Heart Association, and National Strength and Conditioning Association (Baechle et al., 2008; Haskell et al., 2007); i.e., one set of 8–12 repetitions of 8–10 resistance exercises involving major muscle groups (arms, shoulders, abdomen, back, hips, and legs) on 2–3 days per week with multiple sets potentially offering greater improvements (Haskell et al., 2007). As evidence of training adaptations in this study, total work performed during aerobic training and total volume of weight lifted in resistance training increased significantly over the 8-week training program (Figures 1 and 2). Furthermore, participants also demonstrated concomitant increases in FFM (+1.6 kg) and decreases in FM (−2.8 kg) over the 8 week period. This is potentially important for individuals newly abstinent from MA who tend to gain significant weight, especially fat mass, once the effects of MA on appetite suppression and metabolism are removed (Henry et al., 2010). This phenomenon of weight gain was exhibited by the control (EA) participants in this study who gained, on average 1.7 kg in body weight, with increases in percentage body fat up to 3% and fat mass up to 5%.
One common limitation to achieving a successful result from an exercise training intervention in substance abusers is adherence to the exercise prescription (Kinnunen et al., 2008; Marcus et al., 1999). Several studies have demonstrated that adherence to an exercise regimen results in better treatment outcomes (i.e., increased abstinence from nicotine). One study which assessed the effects of exercise with substance dependent individuals found that patients who attended at least 75% of the exercise sessions had better treatment outcomes than those who did not (Brown et al., 2009). Additionally, a recent review of exercise interventions for smoking cessation found that of the 13 studies examined, those that offered supervised exercise sessions had a higher attendance rate compared to those that relied on home-based exercise methods (Ussher et al., 2008). Finally, Williams and colleagues (2011) found that effects of exercise on affect and withdrawal symptoms appear to be short-lived and suggest that sustained adherence to exercise programs is imperative in order to impact smoking cessation outcome. We believe the success of this study is due in part to the individualized and progressive exercise training program and to the professional supervision of the exercise training sessions within the context of residential substance abuse treatment. For example, it has been demonstrated that supervised, one-to-one exercise training is superior to self-directed exercise (Mazzetti et al., 2000). In addition, we believe that the financial compensation offered to participants increased adherence with study procedures and encouraged participation in follow-up interviews.
Although the residential treatment center encourages ad libitum exercise, this activity in the control group, averaging 31 min per day, was not sufficient to cause any changes in physical performance or body composition. The lack of structure, instruction, and supervision were likely contributors to less rigorous training and lack of significant changes in this group. Our findings suggest that involvement of a qualified exercise specialist in residential treatment facilities can augment physical fitness and perhaps improve treatment outcomes of individuals recovering from methamphetamine addiction.
The results of this study should be interpreted in light of some limitations. First, the sample in this study was restricted to only those that were admitted to a residential treatment facility. Consequently, it is unclear to what extent the results of this study generalize to treatment in different settings such as outpatient facilities. Second, most of our participants were men and similar physiological changes may not be seen on average in women. Third, reliance on supervised exercise training and financial incentives might limit the generalizability of the study, depending on resources. Finally, continued adherence could not be guaranteed once the intervention ended and the participants left the facility.
CONCLUSIONS
This is the first study to report the feasibility and physiological effects of 8-weeks of exercise training on individuals in a residential facility recovering from methamphetamine-dependence. The positive adaptations resulting from endurance and resistance training in this group of individuals are consistent with those seen in the general population. The impact of successful exercise training on behavioral changes and ultimate recovery from MA-dependency remains to be elucidated in this ongoing study. However, based on our experience to date, structured and supervised exercise training should be considered as part of the overall treatment plan in recovering MA patients.
Acknowledgment
This study was funded by NIH/NIDA grant R01 DA027633 to Dr. Richard A. Rawson. We thank Patricia Ballesteros for her work on this project.
Source of Funding: This study was funded by NIH/NIDA grant R01 DA027633 to Dr. Richard A. Rawson. Dr. Christopher Cooper has acted as a consultant to Carefusion, whose OxyCon Mobile was used for the exercise testing.
Footnotes
Conflicts of Interest For the remaining authors, no conflicts of interest were declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity ands enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Baechle T, Earle R, Wathen D. Resistance Training. In: Baechle T, Earle R, editors. Essentials of Strength and Conditioning. Human Kinetics; Champaign, IL: 2008. pp. 381–411. [Google Scholar]
- Booth FW, Roberts CK. Linking performance and chronic disease risk: indices of physical performance are surrogates for health. Br J Sports Med. 2008;42:950–952. doi: 10.1136/bjsm.2008.052589. [DOI] [PubMed] [Google Scholar]
- Breslin FC, Zack M, McMain S. An information-processing analysis of mindfulness: implications for relapse prevention in the treatment of substance abuse. Clinical Psychology: Science and Practice. 2002;9:275–299. [Google Scholar]
- Brown RA, Abrantes AM, Read JP, et al. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33:220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J Psychosom Res. 1993;37:565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29:12–20. doi: 10.1111/j.1465-3362.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman E, Garhammer J. Administration, scoring, and interpretation of selected tests. In: Baechle TR, Earle RW, editors. Essentials of Strength and Conditioning. Human Kinetics; Champaign, IL: 2008. pp. 249–254. [Google Scholar]
- Harrison G, Buskirk E, Lindsay Carter J, Johnston F, et al. Skinfold thickness and measurement technique. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL: 1988. pp. 55–70. [Google Scholar]
- Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on heart rate variability. Addict Biol. 2010;17(3):648–658. doi: 10.1111/j.1369-1600.2010.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood V. Advanced Fitness Assessment and Exercise Prescription. Human Kinetics; Champaign, IL: 2006. 2006. [Google Scholar]
- Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- Kinnunen T, Leeman RF, Korhonen T, et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tob Res. 2008;10:689–703. doi: 10.1080/14622200801979043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Lest taxonomy become taxidermy: a comment on the relapse replication and extension project. Addiction. 1996;91(Suppl):S147–153. [PubMed] [Google Scholar]
- Mazzetti SA, Kraemer WJ, Volek JS, et al. The influence of direct supervision of resistance training on strength performance. Med Sci Sports Exerc. 2000;32:1175–1184. doi: 10.1097/00005768-200006000-00023. [DOI] [PubMed] [Google Scholar]
- Mooney LJ, Glasner-Edwards S, Marinelli-Casey P, et al. Health conditions in methamphetamine-dependent adults 3 years after treatment. J Addict Med. 2009;3:155–163. doi: 10.1097/ADM.0b013e3181a17c79. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006 Feb;101(2):267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, Galloway GP, Herrell J, Huber A, McCann MJ, Obert J, Pennell S, Reiber C, Vandersloot D, Zweben J, the Methamphetamine Treatment Project Corporate Authors A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(no.6):708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, et al. Effects of Lower-Cost Incentives on Stimulant Abstinence in Methadone Maintenance Treatment: A National Drug Abuse Treatment Clinical Trials Network Study. Arch Gen Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, Blaine J, MacDonald M, DiMaria J, Lucero L, Kellog S. Contingency management for the treatment of methamphetamine abuse. American Journal of Psychiatry. 2006;169:1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies . National Admissions to Substance Abuse Treatment Services. SAMHSA; Rockville, MD: 2009. Treatment Episode Data Set (TEDS). Highlights - 2007. DASIS Series: S-45, DHHS Publication No. (SMA) 09-4360. Available from http://wwwdasis.samhsa.gov/teds07/tedshigh2k7.pdf. [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Thompson WR, editor. ACSM’s Guidelines for Exercise Testing and Prescription. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med. 2003;24:369–373. doi: 10.1016/s0736-4679(03)00031-3. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report 2010. UNODC; Vienna: 2010. (United Nations Publication, Sales No. E.10.XI.13) Available from http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lores.pdf. [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2008:CD002295. doi: 10.1002/14651858.CD002295.pub3. [DOI] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36:894–897. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]