Abstract
Cells can sense, signal, and organize via mechanical forces. The ability of cells to mechanically sense and respond to the presence of other cells over relatively long distances (e.g., ∼100 μm, or ∼10 cell-diameters) across extracellular matrix (ECM) has been attributed to the strain-hardening behavior of the ECM. In this study, we explore an alternative hypothesis: the fibrous nature of the ECM makes long-range stress transmission possible and provides an important mechanism for long-range cell-cell mechanical signaling. To test this hypothesis, confocal reflectance microscopy was used to develop image-based finite-element models of stress transmission within fibroblast-seeded collagen gels. Models that account for the gel’s fibrous nature were compared with homogenous linear-elastic and strain-hardening models to investigate the mechanisms of stress propagation. Experimentally, cells were observed to compact the collagen gel and align collagen fibers between neighboring cells within 24 h. Finite-element analysis revealed that stresses generated by a centripetally contracting cell boundary are concentrated in the relatively stiff ECM fibers and are propagated farther in a fibrous matrix as compared to homogeneous linear elastic or strain-hardening materials. These results support the hypothesis that ECM fibers, especially aligned ones, play an important role in long-range stress transmission.
Introduction
A rapidly growing body of literature suggests that the passive mechanical environment, e.g., the local viscosity (1) and elastic modulus (2), impacts cellular function. For example, substrate stiffness affects the rate and direction of migration (3,4), focal adhesion (3), and stress-fiber formation (5,6), as well as responsiveness to exogenous growth factors (7) for cultured fibroblasts. The spreading of smooth muscle cells is dependent on both the density of surface ligands and the material’s compliance with spreading favored on stiffer surfaces (8). Additionally, neurons show increased neurite branching densities when cultured on malleable substrates while glial cells, which are normally cocultured with these neurons, do not survive on soft substrates (9). Biomaterial stiffness also influences the differentiation of mesenchymal stem cells (10), a cell’s force generation (11), and its own stiffness (10,12).
Polyacrylamide (PA) gels are frequently used for studies on the role of substrate stiffness because of the ability to control its mechanical properties (2–11). Computational modeling (13) and experimental studies (14,15) suggest that cells grown on top of PA gels are responsive to their local mechanical environment but cannot sense substrate stiffness beyond ∼20 μm away from the cell surface (16). For example, when human mesenchymal stem cells (hMSCs) are grown on a 70-μm-thick layer of PA adhered to a glass slide, they respond to the stiffness of the PA, not the glass (10). Similarly, endothelial cells cultured on PA gels appear to mechanically sense the presence of other cells within 25 μm and respond by migrating toward nearby cells, but they do not respond to those farther away (17). In contrast to cells on PA gels, cells grown in or on extracellular matrix (ECM) gels may be able to sense and propagate mechanical signals over longer distances. For example, hMSCs grown on type-I collagen gels appear to respond to the stiffness of a glass slide through more than 1000 μm of collagen gel (18). Fibroblasts grown on fibrin gels sense the presence of other cells up to 250 μm away and respond by aligning themselves relative to one another. Similarly, hMSCs cultured on fibrin gels have been shown to sense other cells up to 450 μm away (19), while the same cells on PA can only detect cells several tens of micrometers away (13). In these recent experimental studies, the ability of cells to mechanically sense rigid substrates (e.g., a glass slide) and other cells over longer distances in fibrin and collagen gels relative to short distances in PA gels has been attributed to the strain-hardening behavior of the ECM as opposed to the purely elastic behavior of PA (18,19).
In other experimental studies, such as those by Vernon and Sage (20) using in vitro models of capillary morphogenesis, a cell’s ability to sense a distant cell and migrate toward it has been attributed to the propagation of mechanical forces through the matrix. Others have suggested that directional migration of cells toward one another within fibrous gels is a result of contact guidance (i.e., preferential migration of cells along the matrix fibers) (21). In vitro models demonstrating the ability of cells to sense the presence of and move toward other cells that are many cell diameters away have been available since at least the 1930s (22,23).
While the overall phenomenon of cells effectively sensing and moving toward one another is clear, the underlying mechanism has been vigorously debated since at least the 1950s (24). Despite ongoing experimental work (10,13–19,21,25,26) and others studies reviewed by Janmey and Miller (27)), the debate over long-range sensing continues and “whether this directed movement and growth result from mechanical stresses (tension), the orientation of fibrin strands caused by forces generated by the tissues or some other mechanism is as highly controversial now as it was 60 years ago” (27). In experimental systems with fibrous matrices, it is extremely difficult to decouple the relative contribution of each potential mechanism. For example, matrix fibers between distant cells align, which would simultaneously allow for contact guidance, improved force transmission, and the matrix to strain-harden. Further, it is difficult to decouple the relative contributions of strain-hardening and the presence of fibers to an observed cellular response because biologically relevant fibrous materials (e.g., ECM or cytoskeletal elements) are also strain-hardening (19,28). To our knowledge, no efficient means to experimentally decouple these factors has yet been proposed.
In contrast to experimental systems, where it is difficult to isolate the potential contribution of different mechanisms, computational models allow for efficiently decoupling various physical variables. Therefore, in this study we have used confocal reflectance microscopy images of cells and their surrounding network of collagen fibers to generate finite-element models of stress transmission in fibrous networks. The advantage of these computational simulations is their ability to independently investigate the effects of strain-hardening and the presence of fibers on the long-range propagation of forces between cells. Simulation results indicate that the fibrous nature of the collagen gel as opposed to the strain-hardening material properties is important for long-range stress propagation.
Materials and Methods
NIH-3T3 fibroblasts (ATCC, Manassas, VA) were seeded on ∼40 μL bovine collagen gels (Advanced Biomatrix, San Diego, CA) at a density of 32 cells/mm2, and allowed to compact and reorganize the collagen fibers. Images of gels were collected at 4 h and 24 h postseeding using a model No. LSM 510 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany) configured to collect images in reflectance mode using a 63× objective and a 488-nm argon laser. Details on the processes used for cell culture, collagen gel formation, and confocal microscopy and image analysis can be found in the Supporting Material.
Two-dimensional geometry modeling and meshing
Thresholded microscopy images (software IMAGEJ; W. Rasband, National Institutes of Health, Bethesda, MD) were converted from TIFF to DXF formatted graphics files using the software PRINT2CAD (BackToCAD Technologies, Atlanta, GA). After smoothing the geometries using the software SOLIDWORKS (SolidWorks, Concord, MA), DXF files were imported into the COMSOL Multiphysics finite-element program (COMSOL Multiphysics, Burlington, MA). Nonfibrous material, collagen fibers, and cells were separately registered as two-dimensional solid geometry objects. Thus, two-dimensional solid plane stress models with three subdomains were built, based on their two-dimensional morphologies representing fibroblasts seeded on top of type-I collagen gels with dimensions of 202 μm × 202 μm; the thickness was set to be 10 μm. The combined two-dimensional solid objects were meshed using quadratic triangular elements in the program COMSOL Multiphysics (Fig. 1 c). The complex two-dimensional geometries required a large number of triangular elements to generate good quality meshes due to the curvature and roughness of the contour of collagen fibers and cells. The meshes generally consisted of 422,000–762,000 triangular elements and ∼1,670,000–3,050,000 degrees of freedom. The degree of required mesh refinement was objectively determined using a convergence test, and results demonstrated that the default mesh needed to capture the geometric features was sufficient for computing accurate displacements.
Figure 1.
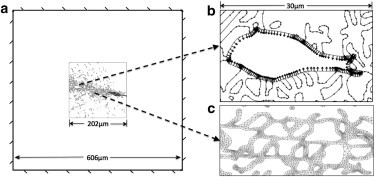
Two-dimensional model setup. (a) Fixed boundary conditions with the ROI surrounded by soft nonfibrous material. (b) A uniform contractile stress was imposed on the void simulating a contracting cell. (c) A dense mesh was used to compute stress distributions in the fibers.
Material properties
To examine the effect of stiff collagen fibers versus a strain-hardening matrix on long-range stress propagation, the ECM was modeled using three different assumptions:
Assumption 1. Fibrous material with two subdomains, i.e., collagen fibers and nonfibrous material.
Assumption 2. Linearly elastic homogeneous nonfibrous material.
Assumption 3. Strain-hardening homogeneous nonfibrous material.
For these assumptions, the two-dimensional models of the collagen fiber networks had several characteristics:
For Assumption 1, each intersection of collagen fiber segments was assumed to be a crosslink (29), and modeled as a welded joint.
For Assumption 2, the collagen fibers, nonfibrous material and cell subdomains were assumed to be linearly elastic (neglecting the viscoelasticity of collagen fibers and cells, as has been done in previous studies (30–32)).
For Assumption 3, the material constants were chosen from the literature for the collagen fibers (32,33), nonfibrous material (34–36) and cells (28,37) (Table 1).
Table 1.
Material properties chosen for collagen fibers, nonfibrous material, and fibroblasts
| Young’s modulus E (Pa) | Poisson’s ratio νυυ | |
|---|---|---|
| Collagen fiber | 300 × 106 | 0.45 |
| Nonfibrous material | 42.6 | 0.49 |
| Fibroblasts | 1000 | 0.49 |
For the enhanced set of assumptions, that of a homogeneous ECM, simulations were based on the same images but the moduli of the fiber- and non-fiber-extracellular regions were assumed to be equal (E = 42.6 Pa) and linearly elastic. For the third set of assumptions, the Young’s modulus was chosen to simulate the strain-hardening material seen in collagen gels as outlined in Fig. S1 in the Supporting Material.
Loading and boundary conditions
To investigate mechanical stress transmission, we focused on and created models for images in which two cells were located ∼100 μm apart (∼10 cell diameters). We chose to simulate cell contraction of one cell (we will call this the first cell) by imposing a centripetal stress on its membrane while monitoring the resultant displacement, strain, and stress fields on a second cell (we will call this the second cell) and in the surrounding materials. This scheme was intended to provide a simplified representation of the loading experienced by the cell and collagen gel under the experimental culture conditions (38). Cell contraction was simulated by applying an inward normal unit load on the boundary of the first cell, a constant stress σn = 1 Pa. Note that the first cell was modeled as a void to allow precise loading of the surrounding material (see Fig. 1 b), where n was the normal direction of the boundary. The 202 μm × 202 μm images of cells and ECM were defined as the region of interest (ROI) for the simulations. To minimize the potential contribution of edge effects, the ROI was surrounded by a linear elastic material with the same properties as the nonfibrous material and with a width equal to that of the ROI. The outer boundaries of this surrounding material were held fixed (Fig. 1 a) and prevented rigid body motion of the system. A small parameter study showed that increasing the width of the surrounding region did not influence the results in the ROI. Using the chosen width for the linearly elastic boundary, the computational results for idealized circular cells embedded in a homogeneous linear elastic matrix matched the analytical solution, as shown in Fig. S3.
Solution procedures and postprocessing
Three sets of images were modeled for two different experimental conditions: a cell pair (∼100-μm apart) at 4 h and a similar cell pair at 24 h. The models were solved as static problems after load application using the program COMSOL (Vers. 3.5a and 4.3a). Postanalysis results for stress distribution were obtained using COMSOL’s postprocessing features. All calculated values were compared across models using the same conditions to explore differences in the stress transmission to the second cell.
Characterization of stress on the boundary of the unloaded cell
To examine stress transmission, we characterized the normal and tangential stresses on the boundary of the unloaded second cell; stress components on the boundary of the second cell were obtained from COMSOL postprocessing features. Normal and tangential stresses on the boundary of the second cell were calculated using the equation (39)
| (1) |
where and were x, y components of the normal vector, and and were x, y components of the tangential vector.
Statistical analysis
One-way ANOVA was used for comparison of results between more than two groups with p < 0.05 considered as being statistically significant.
Results
Experimental results
Images taken 4 h after seeding fibroblasts on top of collagen gels revealed relatively circular cells (Fig. 2 a) and collagen fibers distributed evenly with little evidence of a preferred direction of fiber alignment (Fig. 2 b). In contrast, by ∼24 h after seeding, fibroblasts had elongated (Fig. 2 c) and reorganized the collagen fibers leading to areas of higher fiber density near the cells, with highly aligned collagen fibers in the area between the cells (Fig. 2 d). The reorganization observed one day after seeding was similar to observations reported by Stevenson et al. (38) and Vader et al. (40).
Figure 2.

Confocal reflectance (b and d) and differential interference contrast (a and c) microscopy images of cell-seeded type-I collagen gels showing pairs of nearby cells at 4 h (a and b) and 24 h (c and d).
Computational results
Stresses developed during centripetal contraction by a single cell (the ones on the tops of the images) were assessed by performing simulations based on images of two nearby cells on top of the collagen gel. For simulations based on images of the cells and collagen fibers taken 4 h after seeding, stresses were concentrated in fibers near the contracting cell (Fig. 3 a); after 24 h, stresses were transmitted through aligned fibers between the two cells that were ∼120 μm apart (Fig. 3 d). In contrast, for simulations based on the same images in which the moduli of the fiber and surrounding matrix regions were set to be equal (E = 42.6 Pa) and linearly elastic (i.e., homogenous conditions), peak stresses around the cell were much lower and decayed quickly with distance from the cell (Fig. 3, b and e). Changing the matrix properties to a strain-hardening material with a stress-strain relationship based on analysis of experimental measurements for collagen (see Fig. S1) did not visibly change the calculated stresses (Fig. 3, c and f) from that of the linearly elastic material.
Figure 3.
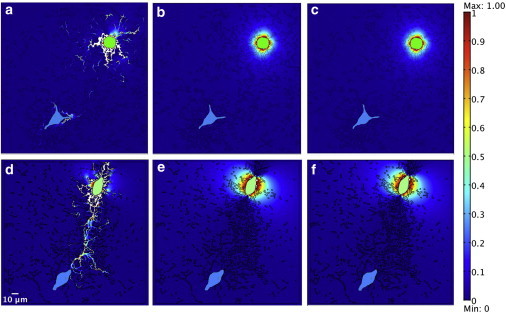
First principal stress plots show the mechanical inhomogeneity feature of gels, that contained a cell pair, which were remodeled to different extents with fibrous material (a and d), homogenous materials (b and e), and homogenous materials with nonlinear strain-hardening properties (c and f) at 4 h (a–c) and 24 h (d–f). The contracting cells are on the topside in the images. The unit of stress is Pa. The color bar is from 0 to 1 Pa. (White) Any stress value that exceeds the largest value in the color bar. (Green, manually colored) Contracting cells. (Cyan, manually colored) The second cells.
Fig. S5 shows a closeup view of Fig. 3 d and focuses on a contracting cell with fibrous material at 24 h. Both figures show that stresses are focused into concentrated stresses in the stiff fibers rather than in the surrounding matrix. The magnitudes of stresses in fibers attached to the contracting cell were thus even greater than the stress applied to the boundary of the contracting cell (1 Pa). Note that this level of applied stress gave a local cell boundary displacement up to 1.3 μm, consistent with experimental results of Yang et al. (41).
The close-up view of the second, noncontracting cell with fibrous material shows that stresses are concentrated in the fibers, whereas models without fibers—including material with strain-hardening properties—do not have stress concentrations around the second cell (Fig. 4). Fig. 5 shows the normal and tangential stress distribution on the boundary of the second cell. With fibrous material in the ECM, the normal and tangential stress distribution presented a complex pattern with several spikes or bursts of spikes corresponding to the connections between cell boundary and fibers. When the ECM was modeled as homogeneous, with either linear elastic or strain-hardening properties, the stress patterns were substantially smaller and smoother—they are indistinguishable when plotted on the scale needed to display the results with fibers. Analysis of data similar to that shown in Fig. 5 for all three models indicated that the normal and tangential stress gradients on the boundary of the second cell were up to four orders greater with fibrous ECM than nonfibrous ECM at both 4 and 24 h (Fig. 6). There were significant differences in maximum normal and tangential stress gradients between fibrous and strain-hardening ECM both at 4 h (p = 0.004 and 0.002, respectively) and 24 h (p = 0.011 and 0.017, respectively), and between fibrous and homogeneous ECM both at 4 h (p = 0.003 and 0.002, respectively) and 24 h (p = 0.008 and 0.012, respectively).
Figure 4.
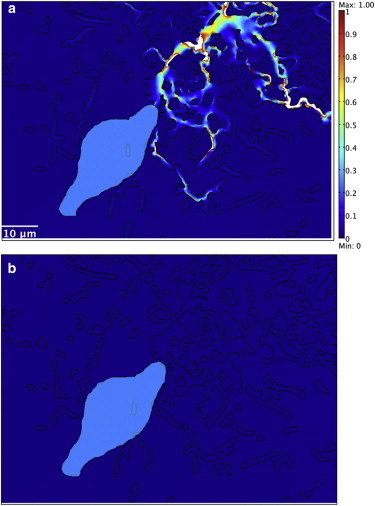
Higher first principal stresses were transmitted to the second cell through fibrous material (closeup view of the second cell with fibrous material (a)) than through strain-hardening (b) materials at 24 h. The color scale for both plots is 0–1 Pa. (White) Any stress value that exceeds the largest value in the color bar. (Cyan, manually colored) The second cells.
Figure 5.
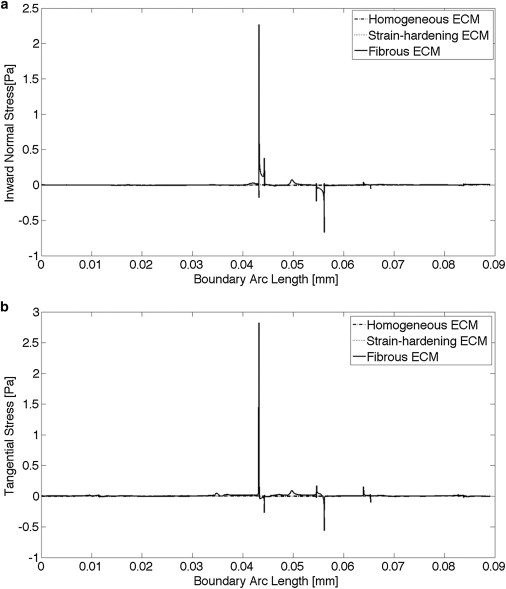
Plot of normal (a) and tangential (b) stresses on the boundary of the second cell. The scale required to show the peaks for the fibrous ECM (from −1 to +3 Pa) makes the results from the homogeneous and strain-hardening ECM indistinguishable; their absolute values were <0.006 Pa.
Figure 6.
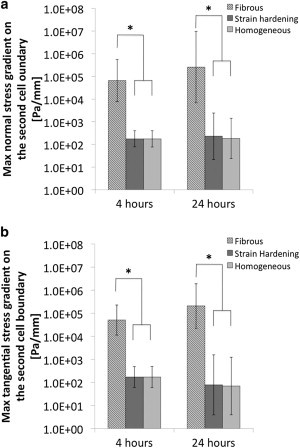
Plots of normal (a) and tangential (b) stress gradients on the boundary of the second cell for all three cases (mean ± SD, n = 3). p < 0.05 is considered statistically significant.
Peak normal and tangential stresses on the boundary of second cells in models with fibrous material were greater than in models with nonfibrous material using either linear elastic or strain-hardening properties. With fibrous ECM, the peak normal and tangential stresses on the boundary of the second cell were up to 10 times the applied stress at 24 h; while with nonfibrous material, the peak normal and tangential stresses on the boundary of the second cell were only 25% of applied stress. At 4 h, the peak normal stress on the boundary of the second cell was up to 140% of stress applied to the first cell, and the peak tangential stress on the boundary of the second cell was up to 50% of applied stress. With nonfibrous ECM, the peak normal and tangential stress on the boundary of the second cell was only 2% of applied stress (Fig. 7, a and b). At 24 h, mean normal and tangential stresses on the boundary of the second cell with fibrous ECM were up to three times greater than that with nonfibrous materials. At 4 h, mean normal and tangential stresses on the boundary of the second cell with fibrous ECM were comparable with nonfibrous materials (Fig. 7, c and d). There were significant differences in maximum tangential stress between fibrous and strain-hardening ECM both at 4 h (p = 0.006) and 24 h (p = 0.029) and between fibrous and homogeneous ECM both at 4 h (p = 0.004) and 24 h (p = 0.02). There were significant differences in maximum normal stress between fibrous and strain-hardening ECM at 4 h (p = 0.029), and between fibrous and homogeneous ECM at 4 h (p = 0.02).
Figure 7.

Plot of peak normal stress (a), peak tangential stress (b), mean normal stress (c), and mean tangential stress (d) on the boundary of the second cell for all three cases (mean ± SD, n = 3). p < 0.05 is considered statistically significant.
Our image-based models did not show a significant difference between the homogeneous material property and the strain-hardening material property. To test whether a different strain-hardening material could lead to an increase in stress propagation, we varied parameters a, b, and c from Eq. S2 in the Supporting Material and tested them using an idealized geometry described in Fig. S2. Details about the parameter ranges tested can be found in the Supporting Material. As a measure of the amount of strain-hardening present in a specific run, we calculated the ratio of Young’s modulus at the first cell boundary versus the Young’s modulus at the second cell boundary. Fig. 8 shows the mean normal stress on the second cell versus the modulus ratio for all 125 combinations of parameters. Stress transmission of all strain-hardening materials was less relative to the amount of strain-hardening that occurred in the simulation. Stress transmission for strain-hardening materials was never greater than the stress transmission for the homogeneous case. These studies indicate that the inability of the collagen strain–hardening material property to increase stress transmission in our image-based computational models is not due to the specific numerical values we chose for the strain-hardening material or boundary conditions.
Figure 8.

Mean normal stress on the second cell boundary versus the Young’s modulus ratio (Young’s modulus at the first cell boundary divided by the Young’s modulus at the second cell boundary) for all combinations of parameters a, b, and c in the four-paramenter Hill curve (Eq S2). (Dotted line) Value for a homogeneous material property.
Discussion
The results presented in this study suggest several possible mechanisms by which fibers could enhance the transmission of mechanical signals between cells. Our results show that centripetal contraction of a cell in a fibrous material with fibers that connect it to a second cell located ∼100 μm away can focus stresses into the stiff fibers and lead to a local increase in fiber stress near the first cell boundary that can be higher than the applied stress. Stress concentration in fibers could explain the higher peak stresses and stress gradients observed on the surface of the second cells. The increase in stress gradients is noteworthy because previous researchers have shown that stress gradients play an important role in cellular mechanotransduction (42–44). Specifically, at 24 h when the collagen fibers are aligned, the cell-contraction derived-stress is preferentially directed toward the second cell—this does not occur at 4 h before the fibers are aligned or if a mechanically homogenous matrix is used in the simulations. The stress concentration and higher peak stresses and stress gradients in fibrous materials were consistently found across simulation models.
Previous investigators have suggested that the strain-hardening properties of the fibrous materials such as collagen and fibrin gels might be responsible for long-range mechanical communication in these systems (18,19). A major advantage of the finite element calculation for cell-matrix mechanical interactions presented here is that it allows for decoupling contributions of the fibers and strain-hardening. As shown in Fig. 3, in this study we investigated this possibility by first specifying homogenous strain-hardening material properties mimicking collagen. Relative to a linear elastic homogeneous material, this strain-hardening material did not alter the amount of stress or stress gradients present at the second cell. It is possible that the strains in our experiments and/or simulations may not have been great enough to elicit increased stress transmission via strain-hardening, or perhaps, if another strain-hardening substrate were used, stress transmission would increase. To address these possibilities, we conducted an extensive parameter variation study in which the parameters of the homogeneous strain-hardening material model were altered to capture a wide range of material behaviors over a wide range of strain values (see Fig. S4). For all combinations of parameters and therefore material behaviors simulated, the stress at the boundary of the second cells was always less than or equal to the value for the case of a linear elastic material. This suggests that the conclusion that isotropic strain-hardening does not increase force propagation to the second cell is very robust. Fibers appear to be the essential element for the long-range transmission of stress in the simulated ECM models. Finally, we also performed a small study where just the fibers themselves were allowed to strain-harden, based on data from a study of isolated collagen fibers (33). Including the strain-hardening properties had a negligible (10−5 Pa) effect on the mean normal stresses on the boundary of the second cell compared to the linear elastic fiber properties used for the analyses presented here.
Our finite element study of cell-matrix interactions used geometries of the collagen fibers and cells based on their in vitro morphologies. Several previous finite element studies of mechanical interactions between cells and substrates used homogeneous substrates and idealized cell geometries (45,46). While there is at least one report where images of collagen fibers were used as the basis of computational simulations of the deformation of cell-free collagen gels to externally applied loads (47), we believe that we are the first to use image-based models to understand cell-matrix interactions. In our image-based models of cell-matrix interactions, we made several simplifying assumptions. We assumed uniformly distributed contractile stress on cell membrane and continuous adhesions between the cell and the ECM despite evidence of variation in stresses and adhesion on the subcellular length scale (48). While this assumption might significantly influence the predicted values close to the contracting cell, it should have less influence farther away from the contracting cell (e.g., at the second cell, which is ∼100 μm away). In addition, in our models, collagen fibers were assumed to be in their unstressed state when imaged 4 or 24 h after seeding the cells. This appears to be a reasonable first approximation given the viscoelastic nature of type I collagen gels and the modest (but clearly nonzero) recoil observed in such gels that have begun to compact if cellular contraction is inhibited (49). To more accurately account for the zero-stress state of the collagen fibers, time-lapse microscopy could be used to follow the displacement of fibers from the time that cells are initially seeded. Such time-lapse microscopy could also give insight into the best way to model fiber-fiber interactions. In our model, these are assumed to be welded (i.e., physically cross-linked); others have modeled them as torsional springs (47), but sliding of fibers that appear to be in contact might also occur. In our two-dimensional models, connected fibers acted more like a frame structure than as a net, as has been proposed by Wolinsky and Glagov (50). As a result, there were substantial compressive stresses in some regions with multiple-connected fiber segments. In real collagen networks, substantial compressive loads are unlikely to occur as the individual fibers are ∼100–1000 nm (51,52). In our image-based modeling, we considered contiguous regions with a fluorescent intensity greater than the threshold as a single collagen fiber, even if their diameter suggested that the image actually consisted of multiple thinner fibers. Higher resolution of microscopic images would make possible the modeling of individual collagen fibers. In this work, we considered a two-dimensional plane stress finite-element model based on a single confocal microscopy plane, which may be an appropriate first approximation for cells grown on the two-dimensional surface of a three-dimensional collagen gel. Future work could account for the three-dimensional nature of the fibrous gel; contemporary limitations motivate the need for detailed study of the fiber interaction issues in future models. Finally, in our simulations we considered a wide range of the isotropic strain-hardening cases but never observed a significant affect of stress propagation to the second cell. It is possible that relative to isotropic cases, anisotropic strain-hardening might yield increased stress propagation, although based on the several models we ran with strain-hardening in the fibers themselves—a form of anisotropic hardening—we saw very little effect on stresses at the second cell boundary.
Recognizing the role of ECM fibers on stress transmission gives insight into the selection of biomaterials for tissue-engineering applications. Especially relevant are cases with cells seeded on or in a biomaterial that are expected to organize and form multicellular structures, e.g., microvascular networks (53,54) and islet-like cell clusters (55,56). For example, initially isolated endothelial cells form microvascular networks in collagen (20,57–59) but they do not do so within Matrigel (BD Biosciences, Franklin Lakes, NJ) which lacks a stromal ECM fiber structure (60). Endothelial cells within PEG hydrogels functionalized with RGD, VEGF, and a MMP-degradable motif form interconnected, elongated structures, though the cells do not appear as elongated or interconnected as observed for endothelial cells in collagen gels or in vivo (61). Because PEG chains exhibit a random coil structure, as opposed to the fibrous structure of collagen, one might expect that PEG would not support the long-range fiber-mediated directional force propagation thought to be crucial for MVN formation in collagen gels (58,59). Consistent with this notion, endothelial cell density used in these studies with PEG gels was 30-fold greater than typically used for collagen gels, suggesting that higher cell densities and hence decreased intercellular distances might have been necessary to compensate for the impaired ability to transmit forces over long dimensions in nonfibrous materials.
Taken together, these results suggest that fibrous materials such as stromal ECM, concentrate stresses in their fibers but that this concentration of stresses, per se, is not adequate to account for the enhanced stress transmission to nearby cells, which appears to require the alignment of fibers. Treating the ECM as a homogenous isotropic strain-hardening material could not account for the increased stress transmission—suggesting an important role for the fibers themselves. The ability of fibers to facilitate the transmission of stresses over distances greater than several cell diameters likely impacts cell-cell (62) and cell-interface (63) interactions and should be considered in the design of biomaterials.
Acknowledgments
This work was supported by National Science Foundation grant No. NSF-CMMI-0928739 and the Edgar C. Hendrickson Fund at The Ohio State University.
Supporting Material
References
- 1.Edwards D., Gooch K.J., Langer R. The nucleation of receptor-mediated endocytosis. Proc. Nat. Acad. Sci. USA. 1996;93:1786–1791. doi: 10.1073/pnas.93.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyton S.R., Ghajar C.M., Putnam A.J. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem. Biophys. 2007;47:300–320. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 3.Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo C.M., Wang H.B., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday N.L., Tomasek J.J. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 1995;217:109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 6.Yeung T., Georges P.C., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 7.Arora P.D., McCulloch C.A. The deletion of transforming growth factor-β-induced myofibroblasts depends on growth conditions and actin organization. Am. J. Pathol. 1999;155:2087–2099. doi: 10.1016/s0002-9440(10)65527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engler A., Bacakova L., Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan L.A., Ju Y.E., Janmey P.A. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Paszek M.J., Zahir N., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Byfield F.J., Reen R.K., Gooch K.J. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech. 2009;42:1114–1119. doi: 10.1016/j.jbiomech.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen S., Engler A.J., Discher D.E. Matrix strains induced by cells: computing how far cells can feel. Cell. Mol. Bioeng. 2009;2:39–48. doi: 10.1007/s12195-009-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloney J.M., Walton E.B., van Vliet K.J. Influence of finite thickness and stiffness on cellular adhesion-induced deformation of compliant substrata. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008;78:041923. doi: 10.1103/PhysRevE.78.041923. [DOI] [PubMed] [Google Scholar]
- 15.Merkel R., Kirchgessner N., Hoffmann B. Cell force microscopy on elastic layers of finite thickness. Biophys. J. 2007;93:3314–3323. doi: 10.1529/biophysj.107.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buxboim A., Rajagopal K., Discher D.E. How deeply cells feel: methods for thin gels. J. Phys. Cond. Matter. 2010;22:194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhart-King C.A., Dembo M., Hammer D.A. Cell-cell mechanical communication through compliant substrates. Biophys. J. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leong W.S., Tay C.Y., Tan L.P. Thickness sensing of hMSCs on collagen gel directs stem cell fate. Biochem. Biophys. Res. Commun. 2010;401:287–292. doi: 10.1016/j.bbrc.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Winer J.P., Oake S., Janmey P.A. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS ONE. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernon R.B., Sage E.H. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am. J. Pathol. 1995;147:873–883. [PMC free article] [PubMed] [Google Scholar]
- 21.Davis G.E., Camarillo C.W. An α2 β1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 22.Weiss P. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J. Exp. Zool. 1934;68:393–448. [Google Scholar]
- 23.Katzberg A.A. Distance as a factor in the development of attraction fields between growing tissues in culture. Science. 1951;114:431–432. doi: 10.1126/science.114.2965.431. [DOI] [PubMed] [Google Scholar]
- 24.Weiss P. “Attraction fields” between growing tissue cultures. Science. 1952;115:293–295. doi: 10.1126/science.115.2985.293. [DOI] [PubMed] [Google Scholar]
- 25.Davis G.E., Senger D.R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 26.Karamichos D., Lakshman N., Petroll W.M. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest. Ophthalmol. Vis. Sci. 2007;48:5030–5037. doi: 10.1167/iovs.07-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janmey P.A., Miller R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storm C.C., Pastore J.J., Janmey P.A. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 29.Chandran P.L., Barocas V.H. Representative microstructure finite elements for collagen gels. In: Gladwell G., Huyghe J.M., Raats P., Cowin S., editors. Solid Mechanics and Applications, IUTAM Proceedings on Physicochemical and Electromechanical Interactions in Porous Media. Springer; Amsterdam: 2005. pp. 37–42. [Google Scholar]
- 30.Ohayon J., Chadwick R.S. Effects of collagen microstructure on the mechanics of the left ventricle. Biophys. J. 1988;54:1077–1088. doi: 10.1016/S0006-3495(88)83044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagenseil J.E., Okamoto R.J. Modeling cell and matrix anisotropy in fibroblast populated collagen vessels. Biomech. Model. Mechanobiol. 2007;6:151–162. doi: 10.1007/s10237-006-0019-0. [DOI] [PubMed] [Google Scholar]
- 32.Bischofs I.B., Schwarz U.S. Cell organization in soft media due to active mechanosensing. Proc. Natl. Acad. Sci. USA. 2003;100:9274–9279. doi: 10.1073/pnas.1233544100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Rijt J.A.J., van der Werf K.O., Feijen J. Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 2006;6:697–702. doi: 10.1002/mabi.200600063. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y.-L., Leone L.M., Kaufman L.J. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 2009;97:2051–2060. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arevalo R.C., Urbach J.S., Blair D.L. Size-dependent rheology of type-I collagen networks. Biophys. J. 2010;99:L65–L67. doi: 10.1016/j.bpj.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barocas V.H., Moon A.G., Tranquillo R.T. The fibroblast-populated collagen microsphere assay of cell traction force—part 2: measurement of the cell traction parameter. J. Biomech. Eng. 1995;117:161–170. doi: 10.1115/1.2795998. [DOI] [PubMed] [Google Scholar]
- 37.Solon J., Levental I., Janmey P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson M.D., Sieminski A.L., Gooch K.J. Pericellular conditions regulate extent of cell-mediated compaction of collagen gels. Biophys. J. 2010;99:19–28. doi: 10.1016/j.bpj.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beer F.P., Johnston E.R., Mazurek D. McGraw Hill; New York: 2011. Mechanics of Materials. [Google Scholar]
- 40.Vader D., Kabla A., Mahadevan L. Strain-induced alignment in collagen gels. PLoS ONE. 2009;4:e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z., Lin J.-S., Wang J.H. Determining substrate displacement and cell traction fields—a new approach. J. Theor. Biol. 2006;242:607–616. doi: 10.1016/j.jtbi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhou E.H., Xu F., Lim C.T. A power-law rheology-based finite element model for single cell deformation. Biomech. Model. Mechanobiol. 2012;11:1075–1084. doi: 10.1007/s10237-012-0374-y. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y., Haas C., Ghadiali S.N. Influence of transmural pressure and cytoskeletal structure on NF-κB activation in respiratory epithelial cells. Cell Mol. Bioeng. 2010;3:415–427. doi: 10.1007/s12195-010-0138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschumperlin D.J., Shively J.D., Drazen J.M. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L904–L911. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan R., Oommen B., van Vliet K.J. Modeling and simulation of chemomechanics at the cell-matrix interface. Cell Adhes. Migr. 2008;2:83–94. doi: 10.4161/cam.2.2.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appelman T.P., Mizrahi J., Seliktar D. A finite element model of cell-matrix interactions to study the differential effect of scaffold composition on chondrogenic response to mechanical stimulation. J. Biomech. Eng. 2011;133:041010. doi: 10.1115/1.4003314. [DOI] [PubMed] [Google Scholar]
- 47.Stein A.M., Vader D.A., Sander L.M. The micromechanics of three-dimensional collagen-I gels. Complexity. 2010;16:22–28. [Google Scholar]
- 48.McGarry J.P., Fu J., Deshpande V.S. Simulation of the contractile response of cells on an array of micro-posts. Philos. Transact. A Math. Phys. Eng. Sci. 2009;367:3477–3497. doi: 10.1098/rsta.2009.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signaling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- 50.Wolinsky H., Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ. Res. 1964;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- 51.Hasirci V., Vrana E., Aydin E. Nanobiomaterials: a review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006;17:1241–1268. doi: 10.1163/156856206778667442. [DOI] [PubMed] [Google Scholar]
- 52.Sander E., Stylianopoulos T., Barocas V.H. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc. Nat. Acad. Sci. USA. 2009;106:17675–17680. doi: 10.1073/pnas.0903716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieminski A.L., Padera R.F., Gooch K.J. Systemic delivery of human growth hormone using genetically modified tissue-engineered microvascular networks: prolonged delivery and endothelial survival with inclusion of nonendothelial cells. Tissue Eng. 2002;8(6):1057–1069. doi: 10.1089/107632702320934155. [DOI] [PubMed] [Google Scholar]
- 54.Liu W.F., Chen C.S. Cellular and multicellular form and function. Adv. Drug Deliv. Rev. 2007;59:1319–1328. doi: 10.1016/j.addr.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boretti M.I., Gooch K.J. Induced cell clustering enhances islet beta cell formation from human cultures enriched for pancreatic ductal epithelial cells. Tissue Eng. 2006;12:939–948. doi: 10.1089/ten.2006.12.939. [DOI] [PubMed] [Google Scholar]
- 56.Hardikar A., Marcus-Samuels B., Gershengorn M.C. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc. Nat. Acad. Sci. USA. 2003;100:7117–7122. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sieminski A.L., Hebbel R.P., Gooch K.J. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11:1332–1345. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- 58.Vernon R.B., Lara S.L., Sage E.H. Organized type I collagen influences endothelial patterns during “spontaneous angiogenesis in vitro”: planar cultures as models of vascular development. In Vitro Cell. Dev. Biol. Anim. 1995;31:120–131. doi: 10.1007/BF02633972. [DOI] [PubMed] [Google Scholar]
- 59.Vernon R.B., Sage E.H. Contraction of fibrillar type I collagen by endothelial cells: a study in vitro. J. Cell. Biochem. 1996;60:185–197. doi: 10.1002/(sici)1097-4644(19960201)60:2<185::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 60.Sieminski A.L., Padera R.F., Gooch K.J. Systemic delivery of human growth hormone using genetically modified tissue-engineered microvascular inclusion of nonendothelial cells. Tissue Eng. 2002;8:1057–1069. doi: 10.1089/107632702320934155. [DOI] [PubMed] [Google Scholar]
- 61.Leslie-Barbick J.E., Moon J.J., West J.L. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J. Biomater. Sci. Polym. Ed. 2009;20:1763–1779. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 62.Guo C.L., Ouyang M., Shen C.Y. Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc. Natl. Acad. Sci. USA. 2012;109:5576–5582. doi: 10.1073/pnas.1114781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebe R.J., Caldwell H., Milam S. Cells transmit spatial information by orienting collagen fibers. Matrix. 1989;9:451–458. doi: 10.1016/s0934-8832(11)80014-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


