Mechanosensitive (MS) ion channels are membrane proteins that display exquisite sensitivity to changes in the biophysical properties of the membrane. These channels are ubiquitous in all living cells where they are able to translate mechanical stimuli into an electrochemical signal, which ultimately leads to physiological or perceptual responses (1). Osmotic balance, cell division, morphogenesis, touch, pain, proprioception, blood pressure regulation, and hearing, are just some of the biological processes where MS ion channels play a crucial role. Unlike other ion channel families (e.g., voltage-dependent K+ or Na+ channels), MS channels do not necessarily share a common topology (2,3), making it rather difficult to identify homologs by sequence similarity. However, there are two families of so-called prokaryotic MS ion channels (note that the name “prokaryotic MS channels” no longer applies because they are also present in archaea, fungi, and plants) that share a reasonable sequence similarity, mainly within the pore-lining helix. Many of these MS channels conduct ions, water, and big solutes helping unicellular organisms to cope with sudden osmotic changes in the surrounding milieu. In this issue of Biophysical Journal, Petrov et al. (4) report an exhaustive electrophysiological characterization of one of these MS ion channels, revealing a surprising diversity within its subfamily.
MS channels of small and large conductance (MscS and MscL, respectively) were the first bacterial MS channels identified, whose electrophysiological activity was described in the inner membrane of Escherichia coli more than 20 years ago (5,6). The identification and cloning of the E. coli MscS (ecMscS) gene (7) laid the basis for relevant structure-function studies (8,9) toward understanding its gating mechanism, including three crystal structures trapped in different conformations (10–12). Therefore, ecMscS has been by far the most studied channel of the family, while only a few MscS-like channels have been electrophysiologically characterized (Fig. 1).
Figure 1.
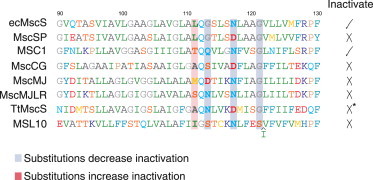
Sequence alignment of the pore-lining helix of MscS-like channels, which have been electrophysiologically characterized. ecMscS from E. coli accession number (AN) P0C0S2 (15,16); MscSP from S. pomeroyi AN Q5LMR6 (4); MSC1 from C. reinhardtii AN EDP05899 (18); MscCG from Corynebacterium glutamicum AN NP_600492 (23); MscMJ and MscMJLR from Methanocaldococcus jannaschii ANs Q57634 and Q58543, respectively (26); TtMscS from Thermoanaerobacter tengcongensis AN NP_624283 (21) (∗personal communication, Irene Iscla, 2013); and MSL10 from Arabidopsis thaliana AN Q9LYG9 (27). Residue numbering corresponds to ecMscS sequence.
The study by Petrov et al. (4) is an elegant electrophysiological characterization of MscSP, an MscS-like channel from the extremophilic marine bacteria Silicibacter pomeroyi. MscSP is quite similar to ecMscS, as they share 40% identity and MscSP is predicted to have the same ultrastructure with three transmembrane segments and a basket-like cytoplasmic domain, all key structural features of the ecMscS family (4). As expected from the sequence similarity (>70%), ecMscS and MscSP share many biophysical attributes. For instance, MscSP mechanosensitivity, conductance, slight rectification, and weak preference for anions are all similar to those previously reported for ecMscS (4,13,14). However, quite remarkably, MscSP does not display inactivation—a hallmark of ecMscS that has been extensively studied (15,16). MscSP activation also requires greater tension (≈1.5×) than ecMscS when reconstituted in liposomes or expressed in E. coli spheroplasts (4).
The work by Petrov et al. (4) leads to the following question: How could two related and similar channels differ so much in two of their core attributes?
The answer might be based on subtle sequence variations and will likely illuminate some of the underlying mechanisms. The ecMscS currents reveal an inactivation process (14,15) that can be completely abolished by increasing the helical propensity of the pore-lining helix by substituting residue Gly113 (17). However, MscSP does not inactivate even though it has a Gly at the equivalent position and shares 97% sequence identity in the pore-lining helix with the ecMscS. The Gly113 position is not entirely conserved across the members of the MscS family; in fact, those few channels that have been electrophysiologically characterized do not have Gly at this position (Fig. 1). In turn, these channels do not display inactivation, with the exception of MSC1 from Chlamydomonas reinhardtii that inactivates even with a Gln in an equivalent position (18). By looking at the sequence alignment between the pore-lining helix of ecMscS and MSC1, it is tempting to speculate that the equivalent position to Leu111, which corresponds to a Thr in MSC1, might promote inactivation. This notion is based on the fact that substituting Leu111 with polar residues in ecMscS promotes inactivation (16). The interpretation put forward by Petrov et al. (4) is that the absence of inactivation in MscSP is due to an Asp in the equivalent position to Asn117, which has been shown in ecMscS to allosterically influence inactivation by modulating the interaction with Gly168 from the cytoplasmic domain (19). Collectively, these results indicate that not a single position, but instead several residues along the pore, contribute to channel inactivation and that the interaction with the cytoplasmic domain might also be crucial for MscSP gating.
Another intriguing property of MscSP is the higher tension required for its activation, despite the high sequence similarity with ecMscS (4). In fact, the MscSP threshold for activation is more reminiscent of ecMscL, which requires membrane tension near the lytic limit of the bilayer to open. The MscSP homology model presented by Petrov et al. (4) highlights those residues that act as mechanosensors in ecMscS (16,20). These mechanosensors are strategically positioned at the membrane-protein interface, thereby sensing changes in the bilayer tension. The corresponding residues in MscSP are quite conserved, with the exception of Leu55, which is an Arg in MscSP. This hydrophilic substitution could account for the difference in sensitivity between both channels, because the Arg could interact electrostatically with the charged lipid headgroups, thus requiring more tension to break this interaction and open the channel.
The characterization of different members of the MscS-like channels in recent years underscores the fact that ecMscS is not necessarily an archetype of the family. Although MscS family members share distinctive elements, namely the pore-lining helix and the cytoplasmic domain, they also display differences in transmembrane residues that make them differentially sensitive to tension and determine their ability to inactivate (4). In addition, differences between the cytoplasmic domains of MscS family members determine their anionic preference or enable gating by nucleotide binding (21,22). Accordingly, MscS-like channel sequences are fine-tuned to serve physiological processes from osmoregulation (23) to organelle integrity and tissue morphology (24). In the particular case of MscSP, its functional role in S. pomeroyi remains to be determined. Given that the S. pomeroyi genome is quite adapted to its marine environment (25), it is likely that MscSP plays a critical role in survival and/or endows these organisms with the ability to sense and take advantage of brief changes in nutrient concentrations.
Acknowledgments
Dr. Vásquez's research is supported by the American Heart Association.
References
- 1.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 2.Haswell E.S., Phillips R., Rees D.C. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geffeney S.L., Goodman M.B. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron. 2012;74:609–619. doi: 10.1016/j.neuron.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrov E., Palanivelu D., Martinac B. Patch-clamp characterization of the MscS-like mechanosensitive channels from Silicibacter pomeroyi. Biophys. J. 2013;104:1426–1434. doi: 10.1016/j.bpj.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinac B., Buechner M., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukharev S.I., Martinac B., Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levina N., Tötemeyer S., Booth I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cell. Physiol. Biochem. 2011;28:1051–1060. doi: 10.1159/000335842. [DOI] [PubMed] [Google Scholar]
- 9.Booth I.R., Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J. Bacteriol. 2012;194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bass R.B., Strop P., Rees D.C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 11.Wang W., Black S.S., Booth I.R. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science. 2008;321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai J.Y., Poon Y.S., Rees D.C. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci. 2013 doi: 10.1002/pro.2222. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotomayor M., Vásquez V., Schulten K. Ion conduction through MscS as determined by electrophysiology and simulation. Biophys. J. 2007;92:886–902. doi: 10.1529/biophysj.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akitake B., Anishkin A., Sukharev S. The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 2005;125:143–154. doi: 10.1085/jgp.200409198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belyy V., Anishkin A., Sukharev S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat. Struct. Mol. Biol. 2010;17:451–458. doi: 10.1038/nsmb.1775. [DOI] [PubMed] [Google Scholar]
- 17.Akitake B., Anishkin A., Sukharev S. Straightening and sequential buckling of the pore-lining helices define the gating cycle of MscS. Nat. Struct. Mol. Biol. 2007;14:1141–1149. doi: 10.1038/nsmb1341. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama Y., Fujiu K., Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc. Natl. Acad. Sci. USA. 2007;104:5883–5888. doi: 10.1073/pnas.0609996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koprowski P., Grajkowski W., Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic β domain as a new gating element. J. Biol. Chem. 2011;286:877–888. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura T., Sokabe M., Yoshimura K. Lipid-protein interaction of the MscS mechanosensitive channel examined by scanning mutagenesis. Biophys. J. 2006;91:2874–2881. doi: 10.1529/biophysj.106.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Wang J., Yang M. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc. Natl. Acad. Sci. USA. 2012;109:18180–18185. doi: 10.1073/pnas.1207977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcolm H.R., Heo Y.-Y., Maurer J.A. Ss-bCNGa: a unique member of the bacterial cyclic nucleotide gated (bCNG) channel family that gates in response to mechanical tension. Eur. Biophys. J. 2012;41:1003–1013. doi: 10.1007/s00249-012-0855-z. [DOI] [PubMed] [Google Scholar]
- 23.Börngen K., Battle A.R., Krämer R. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim. Biophys. Acta. 2010;1798:2141–2149. doi: 10.1016/j.bbamem.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Jensen G.S., Haswell E.S. Functional analysis of conserved motifs in the mechanosensitive channel homolog MscS-Like2 from Arabidopsis thaliana. PLoS ONE. 2012;7:e40336. doi: 10.1371/journal.pone.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran M.A., Buchan A., Ward N. Genome sequence of Silicibacter pomeroyi reveals adaptations to the marine environment. Nature. 2004;432:910–913. doi: 10.1038/nature03170. [DOI] [PubMed] [Google Scholar]
- 26.Kloda A., Martinac B. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 2001;20:1888–1896. doi: 10.1093/emboj/20.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maksaev G., Haswell E.S. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc. Natl. Acad. Sci. USA. 2012;109:19015–19020. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]


