Abstract
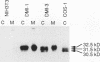
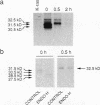
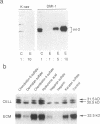
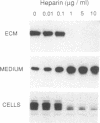
NIH3T3 cells transformed by mouse Int-2/Fgf-3 cDNA express a series of Int-2-related products representing discrete stages of processing and glycosylation. We confirm that in at least two highly transformed clonal lines, Int-2 products acquire further modifications and are efficiently secreted into the culture medium. Secreted proteins become associated with the cell surface and extracellular matrix and can be displaced by addition of soluble glycosaminoglycans, specifically heparin, heparan sulfate, and dermatan sulfate. Increasing concentrations of heparin not only compete for Int-2 binding in a dose-dependent manner but also inhibit the growth of these cells and revert the transformed phenotype. These findings reaffirm the notion that extracellular or surface-bound Int-2 protein is instrumental in the morphological transformation of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Blam S. B., Mitchell R., Tischer E., Rubin J. S., Silva M., Silver S., Fiddes J. C., Abraham J. A., Aaronson S. A. Addition of growth hormone secretion signal to basic fibroblast growth factor results in cell transformation and secretion of aberrant forms of the protein. Oncogene. 1988 Aug;3(2):129–136. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Dickson C., Acland P., Smith R., Dixon M., Deed R., MacAllan D., Walther W., Fuller-Pace F., Kiefer P., Peters G. Characterization of int-2: a member of the fibroblast growth factor family. J Cell Sci Suppl. 1990;13:87–96. doi: 10.1242/jcs.1990.supplement_13.9. [DOI] [PubMed] [Google Scholar]
- Dickson C., Deed R., Dixon M., Peters G. The structure and function of the int-2 oncogene. Prog Growth Factor Res. 1989;1(3):123–132. doi: 10.1016/0955-2235(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dixon M., Deed R., Acland P., Moore R., Whyte A., Peters G., Dickson C. Detection and characterization of the fibroblast growth factor-related oncoprotein INT-2. Mol Cell Biol. 1989 Nov;9(11):4896–4902. doi: 10.1128/mcb.9.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Kaufman R. J. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 1990;185:577–596. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- Goldfarb M., Deed R., MacAllan D., Walther W., Dickson C., Peters G. Cell transformation by Int-2--a member of the fibroblast growth factor family. Oncogene. 1991 Jan;6(1):65–71. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990 Oct;2(5):857–863. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Miyagawa K., Sakamoto H., Yoshida T., Yamashita Y., Mitsui Y., Furusawa M., Maeda S., Takaku F., Sugimura T., Terada M. hst-1 transforming protein: expression in silkworm cells and characterization as a novel heparin-binding growth factor. Oncogene. 1988 Oct;3(4):383–389. [PubMed] [Google Scholar]
- Muller W. J., Lee F. S., Dickson C., Peters G., Pattengale P., Leder P. The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J. 1990 Mar;9(3):907–913. doi: 10.1002/j.1460-2075.1990.tb08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A., Moon R., Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991 Jan 25;64(2):231–231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Papkoff J. Inducible overexpression and secretion of int-1 protein. Mol Cell Biol. 1989 Aug;9(8):3377–3384. doi: 10.1128/mcb.9.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico D., Basilico C. The K-fgf/hst oncogene induces transformation through an autocrine mechanism that requires extracellular stimulation of the mitogenic pathway. Mol Cell Biol. 1991 Feb;11(2):1138–1145. doi: 10.1128/mcb.11.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Thomas K. A. Transforming potential of fibroblast growth factor genes. Trends Biochem Sci. 1988 Sep;13(9):327–328. doi: 10.1016/0968-0004(88)90098-9. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Culpepper A., Reddy M., Loveless J., Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1(4):369–376. [PubMed] [Google Scholar]