Abstract
Pseudomonas aeruginosa is a major agent of hospital-acquired infections, and a pathogen of immunocompromised, cystic fibrosis and burn patients. It uses a type III secretion system for the injection of toxins directly into host cells, through a translocon assembled in the host cell membrane. The hydrophobic translocator subunits of this system, PopB and PopD, have membrane permeabilizing activity based on previous dye leakage experiments, but little is known about the mechanism of assembly and the pore properties of this translocon. Using electrophysiology, we have observed that an equimolar mixture of PopB and PopD induces current fluctuations in planar lipid bilayers, with a unitary conductance of 57 pS in 1 M KCl and numerous larger conductance levels. The activity depends on voltage magnitude and polarity, and increases with protein concentration and the duration of the voltage step. PopB alone is sufficient for producing current fluctuations. PopD rarely displays any transitions, but accelerates PopB onset of activity. The effects of pH, ionic strength, and lipid composition have also been explored. Our data provide new, to our knowledge, insights into the behavior of PopB and PopD by highlighting similarities with secreted pore-forming peptides, and by suggesting that PopB/PopD may form channels via the toroidal pore model. We believe that the events we report here represent the initial steps of insertion and assembly of these translocators in the membrane.
Introduction
Pseudomonas aeruginosa is a Gram-negative bacterium responsible for ∼10% of all nosocomial infections, most commonly in the form of pneumonias (1,2), and is the primary cause of chronic infection and pulmonary disease in patients suffering from cystic fibrosis (3). As an opportunistic pathogen, P. aeruginosa has developed a wide array of toxins to facilitate surviving the host immune system and manipulating host cells for its own advantage. One major pathway through which these toxins gain access to the host’s cells is the type III secretion system (T3SS) machinery (4–6). These supramolecular structures, named injectisomes, are located on the surface of the bacterium and act as molecular syringes to inject various exotoxins, hereafter referred to as effectors, directly into the host cell cytoplasm.
The injectisome is composed of three major structural parts (7,8): a basal body, a needle, and a translocon. From the basal body, effectors are thought to travel through the needle, a ∼80-nm long hollow tubule formed of helically arranged subunits (9,10), to finally pass through the translocon complex. This complex forms a pore in the host cell membrane and connects with the needle, creating a path for the effectors to move from pathogen to host cytoplasm (11).
In P. aeruginosa, four proteins are essential for forming the translocon: the hydrophobic translocators PopB and PopD, the hydrophilic translocator PcrV, and the hydrophobic translocator chaperone PcrH (6,11,12). The translocon is a prime target for drugs and vaccines due to the important role it plays in the virulence of the pathogen and its exposure to the extracellular environment. By studying the behavior of the translocon and the interactions between the translocon subunits, further methods of disrupting the T3SS can be discovered.
Previous studies have suggested that the PopB and PopD proteins of the translocon indeed participate in the formation of a pore in target membranes. Red blood cell lysis and macrophage membrane disruption (13) and the release of fluorescent dyes from reconstituted liposomes (14) have provided functional evidence that PopB/PopD permeabilize the membrane. In addition, electron microscopy showed that an equimolar mix of purified PopB and PopD, or either protein alone, forms ring-like structures on the surface of liposomes with a purported pore diameter of 40 Å (15). Functional assays also yielded an estimated PopB/PopD channel pore size between 28 and 61 Å (14,16). PopB is a 392 amino acid α-helical protein with two putative transmembrane regions. PopD contains 295 residues and has a putative transmembrane region and an amphipathic helix (15). Neither crystallographic structure has been solved to date, most likely because these proteins are intrinsically flexible (17,18). Purified PopB and PopD are membrane-active, but PopB shows significantly greater pore-forming activity in liposome leakage assays as compared to PopD (14). There are conflicting reports as to whether the translocators act cooperatively in pore formation (14,19), but they might form oligomeric assemblies (20). PcrV, the hydrophilic translocator, does not appear to be required for the translocon pore formation and does not interact with PopB, PopD, or the membrane in vitro (14,15). However, it has been observed that PcrV is required for pore assembly in red blood cells and lysis of these cells (13,21), and for translocation of effectors in target cells (13).
The ability of PopB/PopD to transfer from an aqueous environment to a membrane-bound one is akin to the insertion of toxin channels, antimicrobial peptides, and colicins into membranes. There is a large body of literature, including numerous electrophysiological studies, that provided insights into the molecular mechanism for protein insertion into the membrane and channel formation by these types of peptides, in particular with respect to the effect of physicochemical parameters and lipid composition on channel formation (22–24). The insertion of α-helical toxins into membranes is favored by acidic pH, which they may encounter in the close proximity of negatively charged lipids. Amphipathic helices participate in the direct interaction with the membrane. Aggregation and a trigger, such as membrane potential, play a role in driving membrane insertion (22,23,25,26). The conditions for PopB/PopD pore assembly in vitro are comparable to those of other well-studied α-helical toxins like the colicins and melittin (22,23). For example, negatively charged lipids favor the interaction of the Pop proteins with a membrane, whereas membrane permeabilization by the pore is enhanced by acidic pH in vitro (14,15,19).
So far, the pore-forming ability of PopB and PopD has been documented via biochemical assays, but these assays are only able to detect increased membrane permeability. Here, we have applied electrophysiological techniques to observe the kinetics of pore formation, document pore properties, and to analyze the effect of physicochemical parameters, such as membrane voltage. Electrophysiological techniques have been valuable in providing insights into the mechanism of translocation for pores like PopB/PopD, whose natural function is not ion flux, but rather the transport of other polypeptides (27–38). Although a previous study detected channel activity of the Yersinia homologs YopB and YopD using bacteria supernatants (39), here we report for the first time, to our knowledge, the real-time observation of current fluctuations in the presence of purified PopB and PopD components. Our work expands the current knowledge of PopB/PopD acquired from biochemical experiments and provides new, to our knowledge, insights into the mechanism of pore formation, while highlighting features that PopB/PopD share in common with toxins and antimicrobial peptides, in particular melittin.
Materials and Methods
Chemicals, media, and buffer composition
Bacteria were grown in Luria-Bertani medium (Becton Dickinson, Sparks, MD). For patch-clamp electrophysiology, the following buffers were used: buffer A (150 mM KCl, 10 μM CaCl2, 0.1 mM K-EDTA, 5 mM HEPES pH 7.2) and buffer B (= buffer A + 20 mM MgCl2). For planar lipid bilayer electrophysiology, we used 1 M or 150 mM KCl, 5 mM Hepes, pH 7.2, or pH 5.2. The pentane and hexadecane used in planar lipid bilayer experiments were from Burdick & Jackson (Honeywell, Muskegon, MI) and TCI America (Portland, OR), respectively. L-α-phosphatidylcholine Type II-S (asolectin) was purchased from Sigma (St. Louis, MO). The following lipids were obtained from Avanti Polar Lipids (Alabaster, Alabama): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC), 1,2-dieicosanoyl-sn-glycero-3-phosphocholine (DEPC), and 1,2-diphytanoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPhPG). All other chemicals were from Sigma.
Expression and purification of PopB, PopD, and PcrV proteins
The construction of the expression vector pET30b/popDpcrH has been described previously (15). The bicistronic vector allowing the expression of PopB and 6hisPcrH was constructed by replacing the popD sequence in pET30b/popDpcrH by the popB gene. The construction of the DNA fragment encoding full-length PcrV and the expression and purification of this protein have been described (15). Molecular masses of PopB, PopD, and PcrV are 40, 31, and 34 kDa, respectively.
Expression of PopB and PopD in complex with their chaperone PcrH was performed in Escherichia coli BL21(DE3)Star (Invitrogen, Carlsbad, CA) cells, in LB medium, and by inducing cultures with 0.5 mM isopropyl-beta-D-thiogalactopyranoside for 3 h at 37°C. Cells were harvested by centrifugation and lysed with a microfluidizer (Microfluidics, Newton, MA) in lysis buffer (25 mM HEPES; pH 7.8, 0.5 M NaCl, 20 mM imidazole). The supernatant was cleared by centrifugation at 180,000 × g for 30 min and applied to a HisTrap HP column (GE Healthcare Bio-Sciences, Uppsala, Sweden), preequilibrated in lysis buffer. The protein complexes were eluted with 200 mM imidazole. To separate PopB and PopD from their chaperone, 6HisPcrH, the complexes were dialyzed overnight in 25 mM HEPES pH 7.8, 6 M guanidine. Subsequently, the dissociated complexes were further loaded onto a HisTrap HP column. Untagged PopB and PopD were collected in the flow-through fraction, whereas 6HisPcrH was eluted with 200 mM imidazole in dialysis buffer.
Circular dichroism
Far-ultraviolet circular dichroism spectra of PopB and PopD were obtained in 25 mM Tris, pH 8.0 supplemented or not supplemented with 6 M guanidinium. Concentrated protein solutions were diluted >100 times to a final concentration of 1 μM. Spectra were recorded on a Jasco J-810 spectrophotometer (Jasco, Tokyo, Japan) at 20°C in a 0.1 cm cell with 15 accumulations.
Macrophage permeabilization assay
Recombinant proteins were diluted in 25 mM Hepes, pH 7.8, 6 M guanidine at 75, 37.5, and 18.75 μM. J774 murine macrophages (ATCC, Manassas, VA) were seeded in a 48-well plate at 0.4 million cells per well and cultured overnight in cell medium, DMEM Glutamax medium (Dulbecco’s Modified Eagle Medium, Life Technologies, Carlsbad, CA) containing 10% FBS (Fetal Bovine Sera, Basel, Switzerland). Before incubation with the proteins, the cells were washed two times with fresh cell medium. The medium was then replaced by proteins diluted extemporaneously in cell medium to reach a final concentration of 0.125, 0.25, and 0.5 μM, yielding a final guanidine concentration of 40 mM. As a control, guanidine buffer alone was used. After 4 h, LDH release in the cell supernatant was quantified with a Cytotoxicity Detection Kit from Roche Diagnostics (Mannheim, Germany), and normalized to a Triton extract of the same quantity of cells.
Planar lipid bilayer and patch-clamp electrophysiology
The purified recombinant PopB and PopD were kept separated and denatured until just before the individual electrophysiology experiments. Upon 100-fold dilution of the proteins from the guanidine containing buffer, PopB and PopD refold (Fig. 1). For electrophysiology, dilution and renaturation took place outside of the chamber for most of the experiments. A few control experiments were performed by diluting and renaturing the proteins in the chamber, i.e., in the presence of lipids, and no differences were observed (data not shown); this protocol was not used routinely to keep the guanidine concentration in the chamber to a minimum. Similarly, combining the translocators only after renaturation did not change the behaviors observed (data not shown). We will refer to the complex of PopB and PopD as PopBD, when experiments were performed using equimolar amounts of the subunits combined at the dilution stage.
Figure 1.
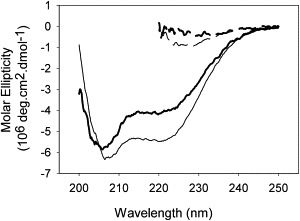
PopB and PopD refold after guanidine dilution. CD spectra of PopB (thin lines) and PopD (thick lines) in 25 mM Tris pH 8.0 supplemented (dashed lines) or not supplemented (continuous lines) with 6 M guanidine. The gain of specific signal with minima at 208 and 222 nm indicates that PopB and PopD adopt an α-helical structure upon dilution of the chaotropic salt. Trimmed spectra are presented for the guanidinium samples because high salt concentration precludes the recordings of good quality signal at shorter wavelengths.
For planar lipid bilayers, a 10-μm thick Teflon square (Goodfellow, Berwyn, PA) with an ∼100 μm hole was placed between two custom-made Teflon chambers. The hole was pretreated with a 1% hexadecane solution in pentane. Both chambers were filled with 1.5 mL of the desired buffer. A solution of 5 mg/mL of lipids in pentane was added to both sides. The technique of Montal and Mueller was used to form the bilayer (40). After bilayer formation, an aliquot of the protein samples diluted 100-fold in 150 mM KCl or 1 M KCl, 5 mM HEPES, pH 7.2., was added to the cis chamber, as the chamber was gently stirred. The amount of protein added to the 1.5 ml chamber ranged from ∼0.1 to 8 μg. A voltage of +90 mV (trans side grounded) was applied to the membrane to detect protein insertion in the bilayer. For recording the time of activity onset and membrane breakage, the +90 mV voltage and stirring were maintained from the start of the recording to a maximum of 10–20 min. For other recordings, voltage was switched to 0 mV shortly after detection of channel activity.
Most experiments were performed with planar lipid bilayers, to witness the real-time onset of activity of the proteins as they inserted into the bilayer. Patch-clamp experiments were also conducted to analyze a fixed amount of reconstituted proteins, without the complication of continuous insertion during the experiment. In this case, PopBD were first reconstituted into giant liposomes according to a published protocol (41,42), except that the step of detergent removal with Biobeads was omitted, and the incubation time of lipids with the diluted PopBD was increased to 2 h. Excised patches were obtained from blisters generated from the liposomes, as described. Protein/lipid ratios (w:w) of 1:1000 to 1:3000 were used. Even at the same ratio, each patch may contain a different number of channels. For both planar lipid bilayer and patch-clamp experiments, the preparation of L-α-phosphatidylcholine type II-S from Sigma was used most of the time; this is actually a mixture of lipids, also known as asolectin (27% phosphatidylcholine, 34% phosphatidylethanolamine, 14% phosphatidic acid, 21% phosphatidylinositol, 2% phosphatidylglycerol, 1% phosphatidylserine (43)). Some planar lipid bilayer experiments were done with 100% of pure DOPC, or DEPC, or DPhPC, or DPhPG.
For both planar lipid bilayer and patch-clamp experiments, the current was monitored with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA), under voltage-clamp condition, using a CV4 (patch clamp) or a CV4B (planar lipid bilayer) headstage. The current was digitized (ITC-18, Instrutech, Port Washington, NY), and stored on a PC computer using the Acquire software (Bruxton, Seattle, WA). Traces longer than 1 min in length were sampled at 1.25 ms intervals and filtered at 500 Hz. Traces 1 min in length or less were sampled at 100 μs intervals and filtered at 1 kHz. Traces used for analysis of unitary conductance were sampled at 50 μs intervals and filtered at 1 kHz. These experiments required that the chamber not be stirred to minimize insertions, which resulted in 30–60 min waiting times before insertions were observed.
Data display and analysis were done with pCLAMP (Axon Instruments). For analysis, we define activity onset as the time to first current transient after adding the protein sample into the cis chamber. This time includes the time required for the proteins to associate with the membrane and insert, and a possible activation time between insertion and first opening, which cannot be resolved in our experiments. Typically, insertions per se are not detectable unless the channels are already open. Experiments were performed to determine the cation- versus anion-selectivity of the pore, by using asymmetric conditions of 1 M KCl, 5 mM Hepes, pH 7.2 on the trans side, and 0.15 M KCl, 5 mM Hepes, pH 7.2 on the cis side. Ag/AgCl electrodes with pellets were used and the liquid junction potential electronically corrected. Because of the technical difficulty in measuring unitary conductance and because of the intrinsic voltage-polarity dependence of channel activity, the determination of reversal potentials was done by applying manual voltage ramps. Measurements were made of the voltages at which the first appearance of positive or negative currents was observed, and the reversal potential was then calculated as the average between the two observed voltages. The Nernst equilibrium potential for K+ is + 48.6 mV in these conditions.
Results
PopB and PopD refold upon guanidine dilution
The PopB and PopD proteins were purified in complex with their high-affinity chaperone PcrH. To remove PcrH from the translocators, the complexes were dialyzed into a buffer containing 6 M guanidine. The use of chaotropic agents to dissociate the translocators from their chaperone was shown to preserve the structural and functional properties of the translocators while preventing aggregation during long-term storage (17,19). This strategy ensures reproducible results by providing a maximum population of translocators active toward membranes. The electrophysiology experiments described below were conducted with purified protein samples initially denatured in 6 M guanidine and refolded by dilution immediately before the experiments. Far-ultraviolet circular dichroism demonstrated that PopB and PopD regain an α-helical structure after being refolded in this way (Fig. 1). The advantage of working with samples denatured before the experiments is that it mimics the in vivo scenario where PopB and PopD must be at least partially unfolded to travel through the T3SS needle complex before contacting the host cell membrane.
PopB and PopBD induce current fluctuations in artificial bilayers
A typical experiment consists of a series of sweeps recorded at a maintained voltage, separated by periods of time at 0 mV. At a membrane potential of +90 mV, PopB alone or an equimolar PopB/PopD mixture (PopBD) inserts into membranes readily and displays rapid transient current fluctuations (Fig. 2). However, PopD at similar concentrations rarely shows any conductance transients, and when it does so the events are smaller and very infrequent. Higher concentrations of PopD do occasionally show activity similar to that of PopB, but the high levels of protein also destabilize the lipid bilayer. The traces of PopB and PopBD show a characteristic delay in the appearance of activity, which is a reflection of both the time needed for channel insertion and an activation time (see below). In addition, the channel activity typically starts with a few events of low conductance, followed by a sustained activity involving events of large conductance. This increase in the size of the events may be due to the insertion of additional proteins, but it appears that there is also an intrinsic fluctuation in the size of the events because 1), consecutive sweeps obtained at the same voltage show low-conductance events at the beginning of a trace, even if the previous sweep ended with a sustained activity involving large events; and 2), as seen in the traces of Fig. 2, events of low conductance are seen interspersed amid events of larger conductance. Conductance levels and frequency of current transients are variable between recordings from the same bilayer in the same condition. However, the overall pattern of PopB and PopBD activity is extremely reproducible over the >400 bilayer recordings that we have performed. This high level of reproducibility and the fact that PopB and PopD alone have vastly different behavior makes it very unlikely that the observed fluctuations are due to contaminants or misfolded peptides. Such activity is not observed when guanidine alone is added to the chamber, and no detergent is used in these experiments. Dilution of the proteins to allow refolding and minimize the number of insertions is needed, precluding the possibility to perform a study of the activities over a broad range of concentration. However, we have found that the amount of activity obtained from the same PopBD diluted sample is concentration dependent (see Fig. S1 in the Supporting Material).
Figure 2.
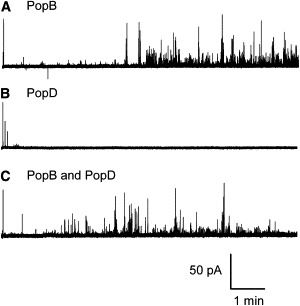
PopB and PopBD induce transient current fluctuations. Representative 10-min long recordings of the electrophysiological activity of PopB (3.5 μg), PopD (2 μg), and an equimolar mixture of PopB and PopD (1.4 μg for PopB and 1.2 μg for PopD), in planar lipid bilayers in 1 M KCl, 5 mM Hepes, pH 7.2. Upward deflections represent increased current transients, except for the first large deflection at the beginning of each trace, which is the capacitative transient. The membrane potential is +90 mV.
More details of the PopBD activity are illustrated in Fig. 3, where the bursting behavior is particularly evident in the expanded segment of the trace in panel A, and the fluctuations between bursts of events of low and high conductance are clearly seen in the full trace of panel A. The extreme brevity of the current fluctuations prevents the appearance of well-defined peaks on a current histogram (Fig. 3 B) (note that the histogram is turned sideways to match the conductance axis with the event sizes in panel A). However, by limiting the amount of insertions (no stirring of the bilayer chamber) and by recording the current at 50 μsec sampling intervals, we were able to catch well-defined unitary events with the canonical square-top signature, which allowed us to measure single-channel currents at several voltages (Fig. 3 C). The resulting current-voltage relationship (Fig. 3 D) yielded a conductance of 57 pS in 1 M KCl. As illustrated in the expanded traces of Fig. 3, C and E, and Fig. S2, transient events of larger conductance are abundant, but they rarely originate from the stacking of small unitary events. Rather, these events are direct transitions to larger conductance levels, resulting from either the simultaneous opening of many unitary pores or the openings of larger pores. PopB alone shows the same behavior as that of PopBD, with no discernible differences in conductances, dwell time, or frequency of current fluctuations.
Figure 3.
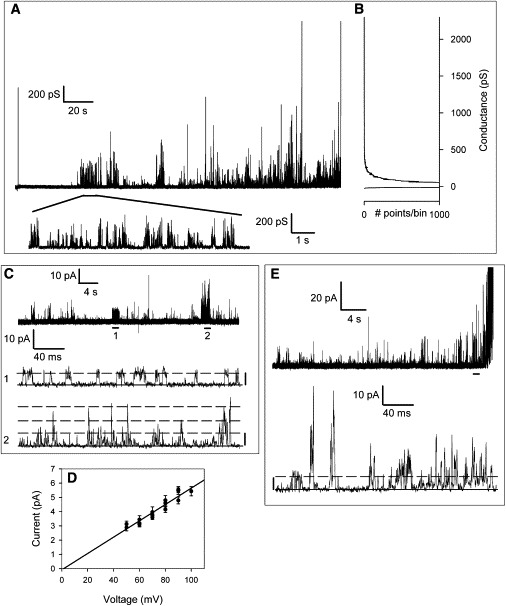
PopBD shows bursting kinetics and multiple conductance levels. (A) A 230-s long recording of PopBD activity at +90 mV in 1 M KCl, 5 mM Hepes, pH 7.2 illustrates the diversity of conductance of current transients (upward deflections). The segment marked by a thick horizontal bar is shown below on an expanded time scale. Note that the scale bars are given in pS, and not pA. (B) Amplitude histogram constructed from the same trace and rotated vertically to match the conductance values with the deflections of the trace. (C) A segment of a recording of PopBD activity at +90 mV in 1 M KCl, 5 mM Hepes, pH 7.2 sampled at 50 μsec sampling interval. The segments marked by thick horizontal bars 1 and 2 are shown below on an expanded timescale. Kinetic analysis of the whole 70-s trace gave 1363 events with a unitary current of 6 pA (Po = 0.025), 131 events of twice the unitary conductance (Po = 0.001), and 17 events with three times the unitary conductance (Po = 2 10−4). (D) Current-voltage relationship obtained by measuring individual unitary currents from traces with few inserted channels and recorded at 50 μsec sampling interval. The voltage threshold of +50 mV and the polarity dependence of channel activity prevented measurements at other voltages than those shown. (E) Another recording as in (C) to highlight transitions of larger conductance.
In planar lipid bilayers, PopBD activity is typically seen as ramping up during the sweep over a second-to-minute time period after the membrane potential has been switched on. This ramping up is characterized by a progressive increase in frequency and magnitude of the current transitions. This occurs even when consecutive sweeps are interspersed by a minute-long sojourn at 0 mV. Because this behavior does not occur in patch-clamp experiments (Fig. S2) where the proteins have been reconstituted into the bilayer before the recordings, we believe that the ramping-up behavior observed in planar lipid bilayers is due to the time-dependent insertion of new PopBD subunits into the bilayer. Indeed, progressively adding increasing amounts of PopBD in the chamber to the same bilayer yields recordings with more and more activity (Fig. S1). The fact that each sweep has a short delay before seeing current transitions suggests that the proteins might disengage from being inserted into the bilayer (either completely or while remaining associated with lipid headgroups) when the voltage is switched off at the end of the previous sweep (see Discussion for further elaboration on this point). The time-dependent increase of activity often leads to breakage of the membrane, which is rarely seen in patch-clamp experiments. PopB alone is less prone to cause membrane breakage, even during periods of high activity. On the other hand, PopD often breaks the membrane, even though this event is rarely associated with any prior activity. Exposing the lipid bilayer to similar concentrations of guanidine alone did not destabilize the membrane.
Voltage dependence of PopBD activity
The activity of PopBD is dependent on the membrane potential and requires a voltage threshold that, at steady state, varies between +30 and +70 mV (depending on the preparation and the time between purification and bilayer experiments), below which no current transitions are observable. In the experiment shown in Fig. 4, the threshold voltage was +50 mV. Frequency and conductance of current fluctuations both increase with membrane potential above this threshold (Fig. 4). Note that the traces are normalized to voltage (i.e., displayed in conductance units), making it clear that the voltage also influences the intrinsic size of the observed transients, as if promoting the opening of larger pores or more pores in unison. Transient events are only observable above the threshold at positive voltages at steady state. Upon switching the voltage polarity to negative, inactivation occurs, and residual current transients appear immediately after the polarity switch and then dwindle in size and frequency (Fig. 4 E). When switching back from a negative to a positive membrane potential, PopBD regains activity with a delay, and a ramping of activity is often seen. The effect of polarity is relative to the direction of PopBD insertion into the bilayer. If the grounding electrode is switched from the trans side to the cis side the polarity of inactivation also reverses. PopB alone shows the same voltage and polarity dependence. Note that the activity of cis-applied PopBD is only observed at voltages that are positive on the side of PopBD application; this polarity dependence mimics the in vivo situation where the translocators would approach the target eukaryotic membrane from the outside, i.e., the side of positive voltage relative to the cytoplasmic side.
Figure 4.
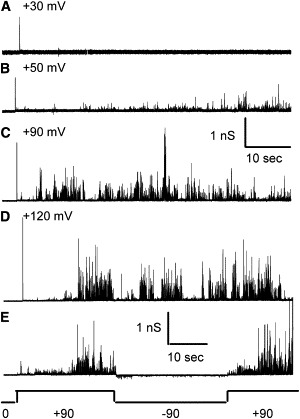
PopBD shows dependence on voltage magnitude and polarity. (A–D) Representative 1-min long recordings of PopBD activity in planar lipid bilayer in 1 M KCl, 5 mM Hepes, pH 7.2, at the indicated voltages. Increased current transients are upward deflections. For this experiment, a minimum threshold voltage of +50 mV was required, and no activity is seen on the trace at +30 mV. Note that the scale bar is given in pS to normalize the size of the current deflections to voltages. Increasing the membrane potential not only increases the frequency of events but also the conductance of the current transients. (E) For this recording, the membrane potential was held at the voltage shown below the trace (in mV) to illustrate the polarity-dependent inactivation of the current fluctuations, and reactivation upon reapplication of a positive voltage. The membrane broke at the end of the second +90 mV application.
Effect of protein composition on insertion and activity
Because PopB appears to be the pore-former of the PopBD complex, what is the contribution of PopD? Purified PopB and PopD incubated with liposomes have been shown to coimmunoprecipitate from the liposome fraction, indicating that they interact with each other within the proteoliposome membrane (13). Although the electrophysiological kinetic signature of the PopBD complex is not different from that of PopB alone, we found that the onset of activity after protein addition is affected by both the protein types and stoichiometries. Although PopD barely displays any activity, it helps PopB insert and/or activate. Fig. 5 A plots the activity onset (as defined in Materials and Methods) of various preparations. It clearly shows that PopB alone takes about three times longer to display the first current transient than an equimolar mixture of PopB and PopD (keeping the same total protein concentration). When comparing an equimolar mixture of PopB and PopD with a mixture with five times more PopD than PopB (keeping the same amount of PopB), the activity onset is increased, indicating that the appropriate stoichiometry is important for efficient channel formation. Neither of these alternative stoichiometries changed the overall electrophysiological behavior of the added protein mixture. Because an insertion per se is not detectable unless the channel is open while inserting, the effect of PopD may be on the efficiency of insertion and/or on the activation of pores that had inserted earlier. In agreement with the postulate that PopD promotes insertions, the presence of PopD speeds up the time of membrane rupture. When PopB was investigated alone, the bilayer remained intact for >600 s in 15 out of 21 experiments, whereas the average time after first current transient and before membrane breakage in experiments with PopBD is 114 s. Note, however, that at lower protein concentrations or in the absence of stirring to minimize insertions, bilayers with few inserted proteins can remain stable for up to 2 h.
Figure 5.
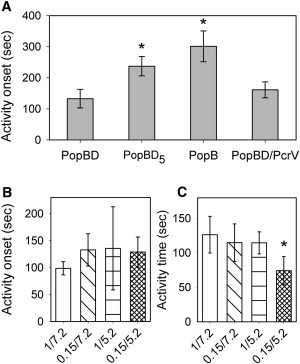
Dependence of activity onset and activity times on protein and buffer composition. (A) Activity onset was measured as the time of first event after adding the indicated protein samples into the cis chamber. The bars represent the averages ± SE of 3 to 8 experiments performed in 150 mM KCl, 5 mM Hepes, pH 7.2. PopBD corresponds to the equimolar mixture, PopBD5 to five times more PopD than PopB, and PopBD/PcrV to a 1:1:1 ternary mixture. Asterisks denote results that are statistically significant from the PopBD sample conditions (p < 0.005). (B and C) Activity onset (B) and activity times (C) of PopBD in the indicated buffer conditions, where the first number (1 or 0.15) represent the KCl concentration in M, and the second number (7.2.or 5.2) the pH. The bars represent the averages ± SE of three to six experiments. The activity onset was obtained as in (A). The activity times represent the times between first event and membrane breakage. The asterisk denotes results that are statistically significant from the 1M/pH 7.2 conditions (p < 0.005).
Effect of ionic strength and pH on channel insertion and activity
By measuring the release of a trapped fluorescent dye from liposome, previous studies have demonstrated that the PopBD-induced increase in membrane permeability is enhanced at lower pH and lower osmolarity (14,19). These experiments, however, cannot distinguish whether the enhanced membrane permeability is the result of facilitated insertion of the PopBD proteins or greater sustained channel activity. To answer this question, we have performed bilayer experiments with an equimolar mixture of PopB and PopD in the following conditions: 1M KCl at pH 7.2 and 5.2, and 0.15M KCl at pH 7.2 and 5.2. Experiments under conditions of varying pH and osmolarity showed no discernible differences in PopBD sustained activity (data not shown). Similarly, these conditions all promoted activity within the same time frame, and no significant differences were found in activity onset (Fig. 5 B). However, it appears that combining low ionic strength and acidic pH does promote breakage of the membrane, which results in the shortening of the time of sustained activity relative to neutral conditions in 1 M KCl (Fig. 5 C). No significant changes in activity times are observed upon lowering a single parameter and keeping the other constant, but the synergistic effect of both manipulations lead to a faster membrane breakage, which, as we indicated previously, is likely to be due to the insertion or activation of more subunits.
Effect of lipid composition
The type III system translocon proteins share with colicins and antimicrobial peptides the property of existing as soluble peptides capable of inserting into lipid bilayers. As proteins with proposed α-helical transmembrane domains (15) and an amphipathic helix (as predicted by the program AMPHIPASEEK for PopB and reference (13) for PopD), PopB and PopD have predicted secondary structure features that are also common to many α-helical channel-forming toxins (22,23). Some of these membrane-active peptides appear to form toroidal pores, in which the protein bends the surrounding lipids such that the resulting pore lumen is lined by both protein residues and the lipid headgroups, in contrast to the classical barrel-stave model, in which the barrel wall is composed entirely of the protein’s transmembrane α-helices. We wondered whether PopB and PopBD might form a toroidal pore, and for the sake of comparison, tested melittin, which is well known for toroidal pore formation (25,44). We found that indeed melittin shares an electrophysiological signature (Fig. S3) and many other characteristics with PopB and PopBD, such as bursting of transient events of variable conductance, voltage dependence, voltage polarity dependence, and potential for membrane rupture with sufficient activity. A prediction of the toroidal pore model is a pronounced dependence of channel properties—in particular those related to permeation—on lipid composition. To gain insight into the nature of the pore, we investigated the effect of lipid composition on PopBD properties.
We have analyzed the selectivity of PopBD in bilayers composed entirely of neutral DPhPC or negatively charged DPhPG. Voltage ramps were performed in asymmetric conditions, as described in Materials and Methods, and the average observed reversal potentials are given in Table 1. Although a voltage threshold is required at steady state to observe the current transients, we have found that applying fast voltage ramps allows the activity to remain within a voltage range that spans the equilibrium potentials for K+ and Cl− in our conditions. In asolectin bilayers (containing 38% of negatively charged lipids—see Materials and Methods) and in 100% DPhPG, mild cation selectivity is observed with PK/PCl ratios of 5–6. In neutral bilayers, however, the pore is even less selective. A lipid dependence of selectivity was also found for colicin E1 (45) and equinatoxin (46), and is highly suggestive of the involvement of the lipid headgroups in forming the channel walls, as in a toroidal pore. No effect on conductance or kinetic behavior of PopBD, though, was observed for proteins inserted into DPhPC versus DPhPG bilayers.
Table 1.
Selectivity of the PopB/PopD equimolar mixture in various lipid compositions
| Lipid | Observed Erev (mV) | PK/PCl | n |
|---|---|---|---|
| DPhPC | 14.4 ± 1.8 | 2.2 | 12 |
| DPhPG | 27.1 ± 0.5 | 4.8 | 8 |
| Asolectin | 30.5 ± 1.1 | 6.2 | 6 |
The observed reversal potential was measured as described in the Materials and Methods, in an asymmetric gradient of 150 mM KCl on the cis side and 1000 mM KCl on the trans side. The relative permeability ratio of K+ to Cl− was calculated from the Goldman-Hodgkin-Katz equation. n is the number of measurements.
One might also expect that thicker membranes would be less favorable for insertion of toroidal pores due to the increased hydrophobic interactions between the transmembrane helices and the lipids, with the resulting increased energy cost in bending the lipids. Because membrane insertion of PopBD is dependent on membrane potential, we have used the steady-state threshold potential required for activity as a reporter of the propensity for membrane insertion into lipid bilayers of varying composition. We have found that a threshold potential of +50 to +70 mV was required for PopBD activity in bilayers made of 100% DPhPC (fatty acid chains of 16 carbons) in 7 out of 8 experiments, whereas threshold potentials of +70 to +120 mV were necessary in 6 out of 8 experiments in bilayers made of 100% DEPC (fatty acid chains of 20 carbons). Bilayers made of 100% DOPC (fatty acid chains of 18 carbons) had an intermediate behavior. Therefore, higher membrane potentials are required to drive the insertion of the translocators into thicker bilayers.
Although a requirement for cholesterol has been reported for the delivery of effectors into cells (47), the inclusion of 20% or 40% cholesterol into asolectin membranes has not affected the activity onset, except for a slight delay with 40% cholesterol, perhaps because of the decreased fluidity of the membrane. Similarly, we have not found any consistent trend in the effect of negatively charged lipids on the activity onset, when comparing bilayers made of 100% DPhPC versus DPhPG. The onset of current fluctuations occurs within the same time frame in both types of bilayers (634 s in DPhPC vs. 746 s in DPhPG), but the success rate of insertion within 20 min is 83% in DPhPG and 50% in DPhPC (n = 6 for each condition).
Refolded PopB and PopD are cytotoxic
To verify that the refolded samples were membrane active, we measured the ability of recombinant refolded PopB, PopD, PcrV, and an equimolar mixture of PopBD to induce permeabilization of macrophage membranes. After incubation with these proteins, LDH leakage from macrophages was observed in a concentration-dependent manner, whereas PcrV or guanidine alone had only a minimal effect on membrane permeabilization (Fig. 6). These results confirm that the membrane insertion capacity of PopB and PopD recovers after refolding. The leakage of LDH is somewhat surprising, given the large size of this molecule, but it has been observed in previous studies and taken as evidence of pore formation by the translocators (16). We attempted to perform osmoprotection experiments as described for P. aeruginosa-infected macrophages (16), but the introduction of polyethylene glycols appears to precipitate the purified proteins and interfere with the assay (data not shown). The LDH release may be due to a deterioration of the permeability barrier of the macrophage or the formation of extremely large pores, which may result from the merging of toroidal pores. Note that 4 h of incubation were required to observe a significant LDH release, whereas the electrophysiological experiments take minutes. It is likely that LDH release involves the continued insertion of proteins, as we have seen for prolonged incubations in planar lipid bilayers. This timescale difference is also likely to explain why LDH release was observed with PopD alone. Indeed, current fluctuations and membrane breakage can occur in planar lipid bilayers with high concentrations of PopD alone. Simulations have suggested that toroidal pores, such as those formed by melittin, can lead to membrane micellization and rupture over time (44).
Figure 6.
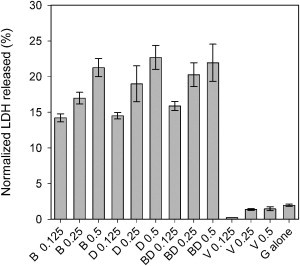
PopB and PopD, but not PcrV, have cytotoxic activity. Percentage of LDH released from J774 macrophages after 4 h of incubation with refolded recombinant PopB (B), PopD (D), PcrV, (V) or an equimolar mixture of PopB and PopD (BD) at the concentrations indicated in μM. The guanidine buffer alone (G alone) was used as a control. The data is normalized to a Triton extract of the same quantity of cells (set as 100%). The data are the averages of triplicate measurements and error bars are standard deviations.
Discussion
We have presented here the first, to our knowledge, electrophysiological study of the components of the T3SS translocon of P. aeruginosa. Our data confirm the previous findings that PopB alone and a PopB/PopD mixture are membrane active peptides, whereas PopD alone barely has any pore-forming activity (14). In addition, the renatured PopBD complex used in our study is cytotoxic toward macrophages and causes membrane permeabilization. Here, the use of planar bilayer electrophysiology has allowed us to monitor the onset of current fluctuations, presumably representing insertion events, separately from sustained activity, and to peer into the process of channel formation. We have found that PopB and PopBD display a voltage-dependent activity with bursting behavior, fast kinetics, and multiple levels of conductance. The smallest well-defined unitary conductance was found to be 57 pS in 1 M KCl, but events of larger conductance, some of them in the order of hundreds of pS, are commonly found. In fact, the activity appears to fluctuate between periods of bursts of low-conductance events interspersed by periods of transitions to larger conductance levels. The lipid composition dependence of the electrophysiological properties and the insertion ability is highly suggestive of the formation of toroidal pores by PopBD. Due to the large number of inserted proteins and the contribution of lipids to forming the channel, it is possible that the larger events result from the merging of neighboring pores diffusing in the plane of the membrane. The role of PopB and PopD in vivo is not to permeabilize and lyse cells, however, but to provide a translocation pathway for the four exotoxins that are directly delivered into the cytoplasm of the host cells. Perhaps, the variability of conductances might reflect the adaptability of the pore dimensions to accommodate the translocation of polypeptides of various sizes. Indeed, it is likely we have witnessed here only the initial steps of pore insertion and assembly, and other modulatory factors, such as the substrates themselves or other needle components, may come into play to stabilize the pores. In that respect, however, we have not found any effect of the accessory protein PcrV on channel insertion (Fig. 5 A) or activity (data not shown).
Proteins that form toroidal pores first bind to the lipid membrane parallel to the bilayer via an amphipathic helix domain. Aggregation of proteins at the surface of the bilayer is thought to play a major role as the triggering event that induces the embedding of the helices into the bilayer (25,44). Aggregation of PopB has been reported (19), and could act as the triggering mechanism for pore formation. In addition, PopD is predicted to contain an amphipathic helix, and was shown here to reduce the time required for the onset of activity by PopB. Membrane potential also plays an important role. Our own work (for melittin) and that of others (for colicin E1) (45) demonstrate that these channels, like PopBD display a robust polarity dependence, where they are opened by a cis-positive potential and closed by the opposite one. This effect of voltage on PopBD activity, which was not previously documented, is significant for several reasons. First, it is a physiologically relevant parameter, because all eukaryotic membranes exhibit an inside-negative membrane potential. In that respect, the presence of a voltage, combined with neutral pH and intermediate ionic strength represent more physiological conditions in which pore-forming activity was demonstrated than the low pH/low salt conditions (in the absence of voltage) used in prior studies of the translocators (14,19). Second, the role of a negative membrane potential to drive the insertion of the helices into the bilayer is widespread among antimicrobial peptides and toxin channels (22,23), and is likely to be a common trigger of the conformational changes necessary for such insertion of pore-forming peptides. We envision a concerted action of peptide aggregation at the bilayer surface and presence of a membrane potential to drive the formation of the toroidal pore. The fact that consecutive sweeps show a delay in activity suggests that the peptides might flip out of the bilayer every time the voltage is turned off. This might also happen upon application of negative voltages. Interestingly, the polarity dependence appears to be lost in bilayers made of 100% negatively charged lipids (data not shown), hinting that surface charges may provide a compensatory mechanism for protein insertion.
Electrostatic interactions are also important in the pore formation by α-helical peptides, and explain the dependency of pore formation on pH, membrane potential, and the presence of acidic phospholipids (22,23). Negatively charged lipids were necessary for permeabilization of liposomes by PopB and PopD in previous experiments (14), but we have not found this requirement in our experiments, although the reproducibility of insertion appears somewhat more favorable in bilayers made of 100% DPhPG than DPhPC. Acidic pH and low ionic strength were also reported to favor dye leakage in PopBD-loaded liposomes (14). We have found here similar effects, but only when these two parameters were combined. The need of a minimal KCl concentration for electrophysiological signal detection prevented us from investigating the same low concentration range (<100 mM) as in this previous report. The difference in dependencies on electrostatic parameters (negatively charged lipids, pH, or ionic strength) as compared with experiments in liposomes may be due to the use of a polarized membrane in electrophysiological experiments. The effect of an applied membrane potential on the proteins might supersede the effect of pH, ionic strength, or membrane surface charge.
Toroidal pores have been described for melittin (25), magainin 2 (48), equinatoxin (46), and colicin E1 (45). Melittin and magainin 2 are short peptides of 26 and 23 residues, respectively. Colicin E1 and equinatoxin, however, are longer peptides with 522 and 179 residues, respectively, which underscores that toroidal pore formation may not be confined to short peptides. Colicin E1 has a domain organization that includes a coiled-coil receptor domain, a translocation domain, and a globular 10 α-helix pore-forming domain. Recently, the coiled-coil region of pore-forming colicins has been found to be structurally superimposable with coiled-coil motifs in two PopB homologs (49). In addition, further homologies have been recognized in silico between a region within the pore-forming domains of colicin Ia and conserved motifs of the PopB homologs. It is an intriguing observation that the hydrophobic translocators of T3SS may share functional and structural properties with pore-forming colicins, a highly successful family of secreted pore-forming proteins of bacterial origin.
Acknowledgments
The authors are grateful to Michel Ragno (Bacterial Pathogenesis and Cellular Responses Team, Grenoble) for valuable technical help.
This work was funded in part from the DSV-CEA.
Footnotes
Beau Wager’s present address is Department of Microbial and Molecular Pathogenesis, Texas A&M Health Science Center, 8847 State Hwy 47, Campus Mail Stop 1359, Bryan, TX 77807.
Contributor Information
Ina Attree, Email: ina.attree-delic@cea.fr.
Anne H. Delcour, Email: adelcour@uh.edu.
Supporting Material
References
- 1.Diekema D.J., Pfaller M.A., Beach M. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 1999;29:595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 2.Crouch Brewer S., Wunderink R.G., Leeper K.V., Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 3.Murray T.S., Egan M., Kazmierczak B.I. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 2007;19:83–88. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 4.Coburn B., Sekirov I., Finlay B.B. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galán J.E., Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 6.Hauser A.R. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato H., Frank D.W. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front Microbiol. 2011;2:142. doi: 10.3389/fmicb.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornélis G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 9.Pastor A., Chabert J., Attree I. PscF is a major component of the Pseudomonas aeruginosa type III secretion needle. FEMS Microbiol. Lett. 2005;253:95–101. doi: 10.1016/j.femsle.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Deane J.E., Roversi P., Lea S.M. Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc. Natl. Acad. Sci. USA. 2006;103:12529–12533. doi: 10.1073/pnas.0602689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteï P.J., Faudry E., Dessen A. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J. 2011;278:414–426. doi: 10.1111/j.1742-4658.2010.07974.x. [DOI] [PubMed] [Google Scholar]
- 12.Mueller C.A., Broz P., Cornélis G.R. The type III secretion system tip complex and translocon. Mol. Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- 13.Goure J., Pastor A., Attree I. The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect. Immun. 2004;72:4741–4750. doi: 10.1128/IAI.72.8.4741-4750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faudry E., Vernier G., Attree I. Synergistic pore formation by type III toxin translocators of Pseudomonas aeruginosa. Biochemistry. 2006;45:8117–8123. doi: 10.1021/bi060452+. [DOI] [PubMed] [Google Scholar]
- 15.Schoehn G., Di Guilmi A.M., Dessen A. Oligomerization of type III secretion proteins PopB and PopD precedes pore formation in Pseudomonas. EMBO J. 2003;22:4957–4967. doi: 10.1093/emboj/cdg499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dacheux D., Goure J., Attree I. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 2001;40:76–85. doi: 10.1046/j.1365-2958.2001.02368.x. [DOI] [PubMed] [Google Scholar]
- 17.Faudry E., Job V., Forge V. Type III secretion system translocator has a molten globule conformation both in its free and chaperone-bound forms. FEBS J. 2007;274:3601–3610. doi: 10.1111/j.1742-4658.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 18.Dey S., Basu A., Datta S. Characterization of molten globule PopB in absence and presence of its chaperone PcrH. Protein J. 2012;31:401–416. doi: 10.1007/s10930-012-9416-7. [DOI] [PubMed] [Google Scholar]
- 19.Romano F.B., Rossi K.C., Heuck A.P. Efficient isolation of Pseudomonas aeruginosa type III secretion translocators and assembly of heteromeric transmembrane pores in model membranes. Biochemistry. 2011;50:7117–7131. doi: 10.1021/bi200905x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ide T., Laarmann S., Schmidt M.A. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 2001;3:669–679. doi: 10.1046/j.1462-5822.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- 21.Goure J., Broz P., Attree I. Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 2005;192:218–225. doi: 10.1086/430932. [DOI] [PubMed] [Google Scholar]
- 22.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 23.Parker M.W., Feil S.C. Pore-forming protein toxins: from structure to function. Prog. Biophys. Mol. Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Zakharov S.D., Kotova E.A., Cramer W.A. On the role of lipid in colicin pore formation. Biochim. Biophys. Acta. 2004;1666:239–249. doi: 10.1016/j.bbamem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Yang L., Harroun T.A., Huang H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H.W. Free energies of molecular bound states in lipid bilayers: lethal concentrations of antimicrobial peptides. Biophys. J. 2009;96:3263–3272. doi: 10.1016/j.bpj.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clantin B., Delattre A.S., Villeret V. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science. 2007;317:957–961. doi: 10.1126/science.1143860. [DOI] [PubMed] [Google Scholar]
- 28.Duret G., Szymanski M., Delcour A.H. The TpsB translocator HMW1B of haemophilus influenzae forms a large conductance channel. J. Biol. Chem. 2008;283:15771–15778. doi: 10.1074/jbc.M708970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mapingire O.S., Henderson N.S., Delcour A.H. Modulating effects of the plug, helix, and N- and C-terminal domains on channel properties of the PapC usher. J. Biol. Chem. 2009;284:36324–36333. doi: 10.1074/jbc.M109.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Méli A.C., Hodak H., Saint N. Channel properties of TpsB transporter FhaC point to two functional domains with a C-terminal protein-conducting pore. J. Biol. Chem. 2006;281:158–166. doi: 10.1074/jbc.M508524200. [DOI] [PubMed] [Google Scholar]
- 31.Grigoriev S.M., Muro C., Kinnally K.W. Electrophysiological approaches to the study of protein translocation in mitochondria. Int. Rev. Cytol. 2004;238:227–274. doi: 10.1016/S0074-7696(04)38005-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S., Udho E., Finkelstein A. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys. J. 2004;87:3842–3849. doi: 10.1529/biophysj.104.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basilio D., Jennings-Antipov L.D., Finkelstein A. Trapping a translocating protein within the anthrax toxin channel: implications for the secondary structure of permeating proteins. J. Gen. Physiol. 2011;137:343–356. doi: 10.1085/jgp.201010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basilio D., Kienker P.K., Finkelstein A. A kinetic analysis of protein transport through the anthrax toxin channel. J. Gen. Physiol. 2011;137:521–531. doi: 10.1085/jgp.201110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown M.J., Thoren K.L., Krantz B.A. Charge requirements for proton gradient-driven translocation of anthrax toxin. J. Biol. Chem. 2011;286:23189–23199. doi: 10.1074/jbc.M111.231167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert V., Volokhina E.B., Tommassen J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006;4:e377. doi: 10.1371/journal.pbio.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saparov S.M., Erlandson K., Pohl P. Determining the conductance of the SecY protein translocation channel for small molecules. Mol. Cell. 2007;26:501–509. doi: 10.1016/j.molcel.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Wirth A., Jung M., Wagner R. The Sec61p complex is a dynamic precursor activated channel. Mol. Cell. 2003;12:261–268. doi: 10.1016/s1097-2765(03)00283-1. [DOI] [PubMed] [Google Scholar]
- 39.Tardy F., Homblé F., Cabiaux V. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 1999;18:6793–6799. doi: 10.1093/emboj/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonet V.C., Baslé A., Delcour A.H. The Vibrio cholerae porins OmpU and OmpT have distinct channel properties. J. Biol. Chem. 2003;278:17539–17545. doi: 10.1074/jbc.M301202200. [DOI] [PubMed] [Google Scholar]
- 42.Delcour A.H., Martinac B., Kung C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 1989;56:631–636. doi: 10.1016/S0006-3495(89)82710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi M., Kasamo K. Modulation in the activity of purified tonoplast H+-ATPase by tonoplast glycolipids prepared from cultured rice (Oryza sativa L. var. Boro) cells. Plant Cell Physiol. 2001;42:516–523. doi: 10.1093/pcp/pce064. [DOI] [PubMed] [Google Scholar]
- 44.Sengupta D., Leontiadou H., Marrink S.J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta. 2008;1778:2308–2317. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Sobko A.A., Kotova E.A., Cramer W.A. Lipid dependence of the channel properties of a colicin E1-lipid toroidal pore. J. Biol. Chem. 2006;281:14408–14416. doi: 10.1074/jbc.M513634200. [DOI] [PubMed] [Google Scholar]
- 46.Anderluh G., Dalla Serra M., Menestrina G. Pore formation by equinatoxin II, a eukaryotic protein toxin, occurs by induction of nonlamellar lipid structures. J. Biol. Chem. 2003;278:45216–45223. doi: 10.1074/jbc.M305916200. [DOI] [PubMed] [Google Scholar]
- 47.Verove J., Bernarde C., Cretin F. Injection of Pseudomonas aeruginosa Exo toxins into host cells can be modulated by host factors at the level of translocon assembly and/or activity. PLoS ONE. 2012;7:e30488. doi: 10.1371/journal.pone.0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuzaki K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta. 1998;1376:391–400. doi: 10.1016/s0304-4157(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 49.Barta M.L., Dickenson N.E., Geisbrecht B.V. The structures of coiled-coil domains from type III secretion system translocators reveal homology to pore-forming toxins. J. Mol. Biol. 2012;417:395–405. doi: 10.1016/j.jmb.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


