Abstract
Xenopus laevis is an ideal organism in which to study the mechanisms linking genetics, the embryogenesis of the central nervous system, and the generation of cognitive behavior. Frog embryos facilitate the targeting of many pathways of importance to neuroscience via pharmacological, genetic, and surgical manipulations. A limiting factor for investigations of memory and learning has been the difficulty of eliciting learning in Xenopus. Here, we outline a simple strategy for aversive conditioning (associative learning) in Xenopus tadpoles, and present sample data using a quantitative automated analysis system. We also discuss the factors and variables that must be considered to ensure optimal learning and recall performance, for use as behavioral endpoints in any experiment.
INTRODUCTION
A common approach in cognitive science is to make specific modifications within the central nervous system and determine the resulting changes on animal behavior. As such, model organisms that are amenable to molecular developmental alterations are poised to provide great insight into cognitive function. Rodents are popular vertebrates for behavior studies, but are not ideal for large-scale rapid screens because of the low number of offspring per mother and the considerable development time needed before behavior studies can be completed. Zebrafish are receiving increased attention because of their amenability tomutagenesis and rapid screens in learning and memory tests, and have begun to be used in studies of addiction and circadian rhythm (Cahill et al. 1998; Darland and Dowling 2001; Gerlai et al. 2006; Bretaud et al. 2007; Pan et al. 2011). In comparison, few cognitive studies have been reported in frog species despite their acceptance as amodel system for both developmental biology and neurobiology. Although both frogs and zebrafish have rapid development, optically accessible internal organs, and a robust toolkit of molecular reagents, the frog model presents several advantages over fish for certain types of investigation. For example, the frog larva presents the opportunity to study the neurobiology and cognitive aspects of limb development and function, as well as the neurological rearrangements that take place during metamorphosis. Moreover, Xenopus is an ideal model for studies of lateralization because the cleavage pattern and fate map of early frog blastomeres allow injections of reagents (e.g., mRNAs and morpholinos) into precursors of just one side of the brain. Thus, robust learning protocols in Xenopus would be a highly useful addition to the molecular genetic, physiological, and other tools available for this versatile model system. Early reports noted difficulty in demonstrating associative learning in frog species, leading some authors to speculate that these animals may be incapable of learning in a laboratory environment (Thompson and Boice 1975). Aversive training was described as particularly problematic, with some frog species perishing during experiments after repeated failures to avoid electric shock (McGill 1960; Boice 1970).
Using an automated system, we have successfully created an associative learning protocol with Xenopus tadpoles. As part of this process, we investigated a number of variables independently in our experimental setup to determine how each affects the overall performance metrics in Xenopus. This article introduces the most important variables identified to date, in hopes of establishing standards for future studies in the field. Once consistent baselines of learning and memory are established for Xenopus species, comparisons can be made with animals subjected to molecular or pharmacological procedures, to determine the effects of specific central nervous system alterations. In addition, establishing standards for behavioral tests will enable researchers to make more accurate comparisons between studies without having to account for differences in training variables.
MEASURING BEHAVIOR
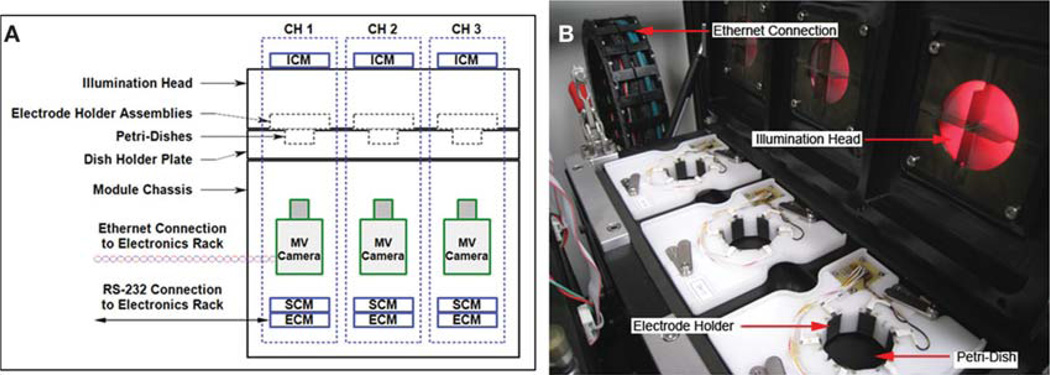
All behavior and learning results were obtained using a custom-made machine vision system (for full details, see Blackiston et al. 2010), although the basic principles we describe here are applicable to a wide variety of manual or automated implementations of such learning protocols. Briefly, the overall structure of the device contains a rectangular array of cells each containing a disposable Petri dish within the field of vision of a digital camera (Fig. 1). Each Petri dish houses a single animal that can be subjected to a series of individual conditions while at the same time being isolated from external stimuli (i.e., the conditions of the adjacent dishes). External illumination of each dish provided by a red light-emitting diode (LED) allows for automated tracking of animals by the cameras without generating unwanted confounding factors such as heat. Within each cell is also a set of six iridium-oxide-coated titanium electrodes, mounted flush with the edges of the Petri dish allowing the delivery of mild-to-strong electric shocks without disrupting the continuity of the chamber walls. Additionally, dishes can be divided into four quadrants allowing independent bright lighting conditions in each (distinct from the background weak red LED used for the monitoring). In effect, the device is a series of individual Skinner chambers, which can be monitored simultaneously and independently controlled by computer software. This allows shock punishments to be delivered based on tadpole position in an unbiased manner by a computer programmed to implement any desired relationship between light conditions, animal position, and shock punishment.
FIGURE 1.
Overview of the physical device used for all behavior trials. (A) The positions of individual animals are calculated from live video feeds recorded by machine vision (MV) cameras located below each Petri dish. (B) Illumination heads above each animal deliver red or blue light to each quadrant of the dish via LEDs. Shocks of varying strengths can be delivered across the dish through an array of six iridium-oxidecoated titanium electrodes in response to animal speed, proximity to edge of the dish, or position under specific colored quadrants. ICM, illumination control module; SCM, shock control module; ECM, excitation control module.
In addition to this device, other commercial systems are available for automated tracking of aquatic invertebrates. Noldus EthoVision and Viewpoint Zebralab are the most popular and complete hardware/software packages available at present (see Gerlai et al. 2000; Zhdanova et al. 2001; Gerlai et al. 2006; Prober et al. 2006; Berghmans et al. 2007; Lopez Patino et al. 2008; Irons et al. 2010). Neither system supports shock natively, but both have the option to set up custom “triggers” in the software to control outside stimuli based on the animal behavior. Delivering shocks similar to those reported in this report is possible with the use of an external function generator and the software triggers.
GENERAL HUSBANDRY
For all studies, Xenopus laevis embryos were cultured in standard Marc’s modified Ringer’s solution before behavior trials. Organisms were maintained under a 12 h/12 h light–dark cycle following fertilization at temperatures ranging from 16 to 22°C. No behavioral differences were noted between tadpoles at these temperatures in learning and memory trials. Embryos were cultured in standard 100 × 15-mm Petri dishes with up to 75 embryos per dish through stage 46, at which time they were transferred to 100 × 20-mm Petri dishes at a maximum concentration of 30 tadpoles per dish.
Age had a pronounced effect on learning results. Before feeding stage (~10–12 d of development at 16°C), we did not observe significant learning results in behavior trials. Following this “prelearning” period, we have tested tadpoles aged 12–22 d post fertilization at 16°C and have documented learning across the entire timeframe. For the studies described below, we used tadpoles at 14 d of age. Although we observed learning through the entire 12–22 d age range, we focused on 14-d tadpoles to begin experiments as soon as possible following fertilization, as well as to avoid tracking complications that arose rarely with younger tadpoles in our automated system.
FEEDING
Tadpole feeding regimes had a drastic effect on learning in conditioned place-preference tasks using electric shock. In many types of learning trials, it is customary to withhold food from experimental animals before training to encourage movement and exploration of the environment. Satiated animals may be reluctant to move because their basic food requirement ismet, thereby reducing the amount of data generated in a trial. However, this approach (withholding food) inhibited learning in all aversive training we have tried to date. Tadpoles starved for 6 h or more before testing showed no appreciable learning in trials, spending their time circling the experimental environment, regardless of whether or not they were being punished with electric shock. We believe that hunger and the desire to filter-feed overcome any negative reinforcement delivered with our current shock levels, and that the possibility of avoiding starvation is likely the highest order priority for Xenopus animals in our laboratory tests.
In contrast, we have observed that the most successful learning occurs when animals are satiated before testing. The feeding regime we have currently adopted for aversive training trials involves feeding animals twice per day on standard Sera micron powdered food. The first feeding is scheduled to correspond with the beginning of the 12-h light cycle, and the second is scheduled ~15 min before training animals. In the event that animals are not trained on a given day, they should be fed a second time 6 h after the first feeding.
Further, for learning trials lasting >3 h in our automated apparatus (e.g., continuous trials where the animals are kept in the machine for up to 12 h), it was necessary to add food directly to the media in the experimental environment, especially in the case of older tadpoles. Failure to do so resulted in the tadpoles reverting to the “circling” behavior of starved tadpoles after 3–6 h and diminished learning results. When adding powdered food to the experimental environment, a concentration of 0.13 mg/mL is sufficient to keep tadpoles satiated over the course of a 12-h trial. Adding additional food proved counterproductive to our automated tracking system, as nonconsumed food eventually settled and interfered with image processing. We have found this food concentration to be applicable for all ages of tadpoles tested to date, but it would likely have been increased for organisms older than 22 d post fertilization at 16°C.
INTENSITY OF ELECTRIC SHOCK
Throughout the literature, electric shock levels used in aversive training span a wide range of currents and are not described in consistent terminology (alternating versus direct current, waveform shape, voltage, amperage, etc.). We have examined multiple shock levels and methods of delivery to determine the minimum amount of current necessary to elicit aversive behaviors in Xenopus tadpoles. Pilot experiments determined AC current to be more effective than DC current because animals appear to be sensitive to the direction of current flow. In addition, preliminary data has focused on shocks alternating at a frequency of 25 Hz, although we have observed behavioral responses to frequencies as low as 8 Hz and as high as 100 Hz.
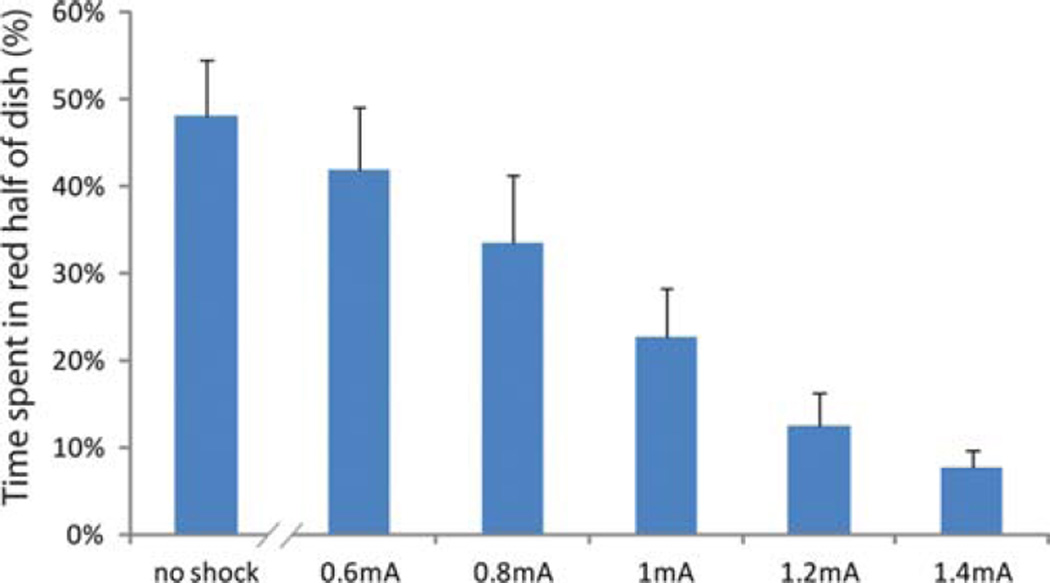
Concentrating on a 25-Hz AC current, we performed an intensity-response study of low shock levels to determine the minimum shock necessary to elicit an aversion behavior in 14-d-old Xenopus tadpoles. We placed tadpoles in an arena with half of the experimental space illuminated with red light and half with blue light. In the absence of punishment, tadpoles showed no preference for either color, spending roughly 50% of their time on each side. Experimental software was then programmed to deliver shock to the tadpole whenever it was observed on the red half of the dish, which should result in more time spent in the blue half. We tested tadpoles individually in the device over the course of 30 min and tested shock levels ranging from 0.2 to 1.8 mA in 0.2-mA steps (peak intensity 0.2, 0.4, 0.6 mA etc.).
Data are summarized in Figure 2. The lowest level of shock where we observed consistent behavioral responses was 0.6 mA, although responses were mild and animals still spent a large portion of time in the punishing red half of the dish. At increasing shock levels, we observed a linear decrease in the amount of time spent in punishing areas, until the response leveled out at 1.4 mA and stronger shocks. In further studies with well-fed tadpoles, the line plateaued at 1.2 mA, which is the current level we chose for future learning studies.
FIGURE 2.
Dose–response curve for electric shock punishment in Xenopus tadpoles. Tadpoles were placed individually into Petri dishes, with half of the dish illuminated with red light and half with blue. Whenever tadpoles occupied positions in the red half, a 25-Hz AC current was delivered to the dish, which was terminated when the tadpoles moved to the blue half. At current levels ranging from 0.2 to 0.6 mA, tadpoles showed no behavioral response to the shock, spending roughly half their time in the red and blue halves of the dish in the same manner as when no shocks were delivered. Above this level, the time spent in the red (punishing) half of the Petri dish decreased linearly with increasing shock levels, until the response plateaued at 1.4 mA. N = 12 individuals.
Furthermore, we have tested the response to constant current versus pulsed current. Constant current consisted of a continuous shock the entire time the tadpole was in the red half of the dish, whereas pulsed current consisted of 100-msec shocks spaced 400 msec apart (i.e., short breaks between each punishment). Intensity response curves between pulsed and continuous shock looked identical; however, pulsed shock regimes appeared to be less stressful for the animals than continuous shock regimes. Under the continuous method, there were rare occasions when the shock would interfere with normal swimming behavior, usually when the tadpole was directly adjacent and parallel to the electrode. In this case, the animal could still move but its swimming was erratic. This behavior was never observed with the pulsed shock regimes, all animals swam normally regardless of their position in the experimental environment. It is also important to note that we did not observe any mortality at the shock levels tested, all tadpoles survived the trials and showd normal swimming and feeding behavior upon being removed from the apparatus.
TRAINING SCHEDULE
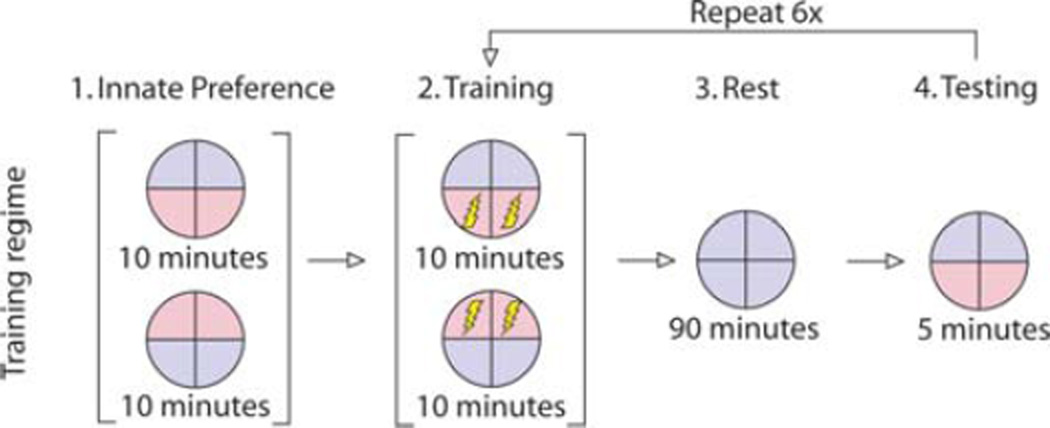
Having determined the minimum shock level necessary to induce aversive behavior in tadpoles, we attempted to find a training schedule that resulted in robust memory. Our work to date has focused on creating a red light aversion behavior by illuminating opposite halves of a Petri dish with red/blue light, and punishing animals with an electric shock when in the red half. The general setup consisted of: (1) an “innate preference” session where neither red nor blue halves of the dish were punished; (2) a training session where the red half of the dish was punished but the blue was not; (3) a rest session where the entire dish was illuminated with blue light; and (4) a memory session with unpunished red and blue halves of the dish, to determine if the animal would avoid red areas in the absence of shock. As part of this process, we have focused on the following variables in our training regime—the length of each training session, the total number of training sessions, length of rest sessions between training, and duration of the testing session.
Multiple options for training sessions (the portion of the trial where animals are punished for occupying the red half of a Petri dish) were examined. Continuous training periods of >90 min did not result in appreciable learning in our trials. This may be caused by fatigue in the absence of a rest period. Alternatively, many vertebrates require breaks between training sessions to consolidate short- to long-term memory, and it is not unlikely that tadpoles require a similar period to show learning. Ninety-minute training periods did result in red color avoidance in our behavior trials when separated by rest periods. Following this observation, we reduced the length of the training periods to 60, 30, 20, and 10 min in different groups of tadpoles. Learning rates were similar across all training time intervals except for the 10-min trials, in which animals showed reduced learning, leading us to use 20-min training sessions in future work. Animals explore their environment as a function of time, and it is possible that 10-min training sessions are too short for animals to cross into the punishing red half of the dish a sufficient number of times to learn.
Using 20-min training periods, we also varied the length of rest periods separating training and testing between 30, 60, 90, 120, and 180 min. Results for 90, 120, and 180-min rest periods were similar, and animals showd learning across all these time intervals. However, animals did not learn when training sessions were separated by either 30- or 60-min rests in our experiments, with results mirroring what we observed when no breaks were given in training.
Our training regime is summarized in Figure 3. Sessions began with a 20-min innate preference session. During this period, half of the dish was illuminated with red light, and half with blue light. We noticed on rare occasions that animals remained motionless during this period, which would result in a 100% preference for either blue or red light depending on their original position. To overcome this possibility, we inverted the lighting regime 10 min after initializing the trial, in which case motionless animals would result in “no-preference” for either color (50% of the innate preference period under red quadrants, 50% under blue). Following innate preference evaluation, the first training session was started. This session proceeded exactly as the innate period, except that animals now received a 1.2-mA shock when occupying the red quadrants of the dish. Following training, all animals were given a rest period where all quadrants were illuminated with blue light and no shocks were delivered. After the 90-min rest, animals were tested for color preference with a 5-min period without any shock, with half the dish illuminated with red light and half with blue. Importantly, the color positions in the testing period were opposite that at the end of the training period. In the event an animal remained motionless following training, they would have to move to the opposite half of the dish during testing to show conditioned color preference (this prevents simple inactivity upon finding a safe location from being scored as learning). After this sequence, the training, rest, and testing periods were then repeated sequentially for a total of six training sessions.
FIGURE 3.
Outline of the training regime used in learning trials. All trials began with an innate period, with half of the dish illuminated in red light, and half in blue light, with neither side being punished. After 10 min, the lighting regime was rotated 180° to ensure that stationary tadpoles were not scored as having a 100% preference recorded for one color or the other. After the 20-min innate trial period, the same lighting regimes were repeated, with the exception of a 1.2-mA shock being delivered to the dish when tadpoles occupied positions in the red half (training them to avoid red). Following training, all quadrants were illuminated with blue light for a 90-min rest period, allowing tadpoles to move within the dish without receiving any punishment. Immediately after the rest, a 5-min test occurs with half of the dish illuminated with red, and half with blue, to evaluate if tadpoles will move to the blue quadrant in the absence of shock. Training, rest, and testing periods were then repeated a total of six times to evaluate learning ability with repeated training sessions.
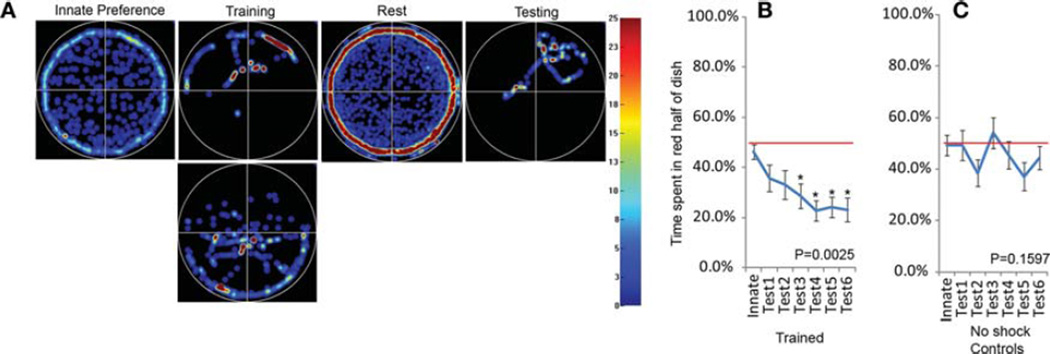
Learning results are summarized in Figure 4. During the innate phase animals showed no preference for red or blue light, spending roughly 50% of their time under each color. However, following each training period, the animals showed an increasing aversion to red light, spending more of their time under blue quadrants. These results are significant following three training periods. By comparison, in control experiments (identical to training trials except no shock is delivered during training sessions), animals did not show any significant changes from innate behavior. We also tested a small number of “yoked” controls, in which animals occupying even-numbered channels are punished according to their paired odd-numbered counterparts; none of these animals developed a blue preference over the course of the trial (n = 6; data not shown). In our training experiments, three of 48 animals remained motionless during the innate phase, but this behavior does not appear to be correlated with learning ability (two of the three tadpoles showed learning across the trial).
FIGURE 4.
After pairing red light with electric shock, tadpoles learn to avoid red areas of the experimental environment. (A) Heat map diagrams illustrate the behavior of individual tadpoles during the innate, first training, first rest, and first testing phase of a trial, with the amount of time an animal spends in each position represented by color (hotter colors indicate more time, as indicated in the heat map legend to the right). During the innate preference phase most animals explore the dish, spending the majority of their time along the edge, but showing no preference for blue or red halves. In comparison, during the training phase, animals spend the majority of their time in the nonpunishing blue half of the dish. After a rest period, animals that learn show a clear aversion to the red half of the dish compared to their innate preference. On a population level, tadpoles show no preference for the red or blue halves of the dish (B). However, following training, animals show an increasing aversion to the red half of the dish, which appears to plateau after four training sessions. In comparison, animals run through the exact same training regime but in which shock is disabled, show no statistical change in color preference across the trial (C). Red line indicates 50% preference for each color. N = 48 for both experiments. Error bars indicate ±1 SEM. Statistics in B and C were calculated with a repeated measure ANOVA followed by a Bonferroni comparison between innate values and all testing values.
SUMMARY
In an effort to create an aversive color training method for use with Xenopus tadpoles, we have examined the contribution of many variables to the learning output of this model species. We have formalized an aversive training regime that results in robust memory, thus overcoming a critical barrier to the use of this model system in neurobiological studies of learning and recall. Regardless of the particular implementation of the training apparatus, optimal learning requires that animals be: (1) Raised on a standard d/night cycle; (2) well fed both before and during the training; (3) punished with an appropriate intensity of current; and (4) given at least 90 min of rest between training sessions. Each of these variables were key to obtaining consistent learning results and should be considered for any future learning or memory studies using these organisms. With reproducible learning performance now attainable, it is possible to begin to leverage the molecular and developmental knowledge of Xenopus to determine the result of specific pharmacological/surgical/embryonic perturbations on learning and memory.
ACKNOWLEDGMENTS
D.B. was supported by National Institutes of Health T32-DE-007327. M.L. gratefully acknowledges the support of the G. Harold and Leila Y. Mathers Charitable Foundation and NIH grant MH081842.
REFERENCES
- Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Shomrat T, Nicolas CL, Granata C, Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 2010;5:e14370. doi: 10.1371/journal.pone.0014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice R. Avoidance learning in active and passive frogs and toads. J Comp Physiol Psychol. 1970;70:154–156. [Google Scholar]
- Bretaud S, Li Q, Lockwood BL, Kobayashi K, Lin E, Guo S. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Hurd MW, Batchelor MM. Circadian rhythmicity in the locomotor activity of larval zebrafish. Neuroreport. 1998;9:3445–3449. doi: 10.1097/00001756-199810260-00020. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebrafish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons TD, MacPhail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Lopez Patino MA, Yu L, Yamamoto BK, Zhdanova IV. Gender differences in zebrafish responses to cocaine withdrawal. Physiol Behav. 2008;95:36–47. doi: 10.1016/j.physbeh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE. Response of the leopard frog to electric shock in an escape-learning situation. J Comp Physiol Psychol. 1960;53:443–445. [Google Scholar]
- Pan Y, Kaiguo M, Razak Z, Westwood JT, Gerlai R. Chronic alcohol exposure induced gene expression changes in the zebrafish brain. Behav Brain Res. 2011;216:66–76. doi: 10.1016/j.bbr.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PA, Boice R. Attempts to train frogs: Review and experiments. J Biol Psychol. 1975;17:3–13. [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]