Abstract
Background:
Several studies have shown that the modulation of fibrotic scar in cardiac diseases has beneficial effects on cardiac arrhythmias. In addition, recent reports suggest a potential role of mineralocorticoid receptor upregulation in atrial fibrillation (AF). The role of spironolactone, a mineralocorticoid receptor blocker and a potent antifibrotic agent, in AF is as yet unexplored. The aim of this study was to determine if spironolactone, a mineralocorticoid receptor blocker with potent antifibrotic properties, has beneficial effects on AF.
Hypothesis:
Spironolactone therapy in patients with atrial fibrillation provides additional clinical benefits in addition to the current conventional pharmacological agents.
Methods:
A comprehensive retrospective analysis was performed on 83 patients with AF, including 23 who were treated with spironolactone for ≥3 months. The combined primary outcome of hospitalization for AF or direct current cardioversion (DCCV) was compared between patients treated with spironolactone in addition to the usual care for AF and those receiving conventional medical therapy alone.
Results:
Patients receiving spironolactone had significantly fewer primary outcome events (AF‐related hospitalizations or DCCV) (22% vs 53%, P = 0.027).
Conclusions:
Spironolactone therapy is associated with a reduction in the burden of AF, as reflected by a combination of hospitalizations for AF and DCCV. Larger randomized controlled studies should be performed to evaluate the efficacy and safety of spironolactone as an adjunctive therapy for patients with AF. © 2011 Wiley Periodicals, Inc.
This work was supported by American Heart Association grants 0705170Y, 0830313N (RHN), and 0640084N (JAH), and National Institutes of Health grants HL‐075173, HL‐090842, and HL‐080144 (JAH). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atrial fibrillation (AF) is associated with significant morbidity, including a high risk of stroke and mortality.1, 2, 3, 4, 5, 6, 7 Atrial remodeling processes underlie most, if not all, AF.8 Components of atrial remodeling include autonomic nervous system alteration, myocyte remodeling, electrical remodeling, and atrial fibrosis. Current therapies, including pharmacological therapies and catheter ablation, have major limitations. For decades, pharmacological therapies for AF have targeted electrical remodeling events. The utility of these therapies remains modest given their low effectiveness and high side‐effect profile.9
Cardiac fibrosis is a potential target for antiarrhythmic therapies. Cardiac fibrosis contributes to electrical remodeling by facilitating initiation and propagation of arrhythmias.10, 11, 12 We have recently shown that diminished fibrotic scar in the setting of dilated cardiomyopathy reduces the rate of induced ventricular tachycardia (VT) and leads to diminished VT burden.13 In addition, in an animal model we demonstrated that reduction in the scar content of the ventricle in the setting of dilated cardiomyopathy alters VT phenotype with a slowing of the VT rate.13 Recent reports also suggest that atrial fibrosis contributes to structural remodeling in the atria and propagates atrial arrhythmias, including AF.14 Therapies such as HMG‐CoA reductase inhibitors (statins), angiotensin‐converting enzyme inhibitors (ACEI), and spironolactone possess antifibrotic properties, in addition to their more well‐known mechanisms of action. In fact, both statins and ACEIs have been shown to have beneficial effects in AF.15, 16 In addition, the renin‐angiotensin system and mineralocorticoid receptors have recently been identified as potential contributors to AF.17 Spironolactone is an aldosterone receptor blocker with powerful antifibrotic properties, but its effects in patients with AF are currently unknown.
Methods
Study Design
A retrospective cohort study was performed at the Veterans Health Administration (VA) North Texas Healthcare System after receiving institutional review board approval. Using ICD‐9 search codes, we searched the Dallas electronic database for patients being discharged after implantable cardioverter defibrillator (ICD) placement from 2002 to 2009 with concomitant AF. One hundred eighty‐seven records with these discharge diagnoses were reviewed manually (by RW) and data were extracted to a standard case‐record form. Patients were excluded if they had incorrect diagnoses, had previously undergone atrioventricular junction (AVJ) ablation, or had incomplete medical records and/or device interrogation reports. The final cohort consisted of 83 patients (Figure 1). Patients were assigned to the spironolactone group if they had received spironolactone therapy for >3 months. All patients were followed up in the Dallas VA electrophysiology clinic and the VA records were screened for events. Average follow‐up was 1493 ± 628 days (1569 ± 621 d for those receiving usual care vs 1297 ± 616 d for those receiving spironolactone, P = 0.077).
Figure 1.
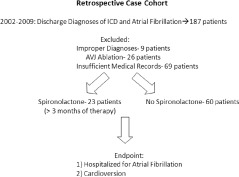
Schematic diagram representing trial design.
Study Endpoints
The primary endpoint of the study was prospectively specified as the composite of AF‐related hospitalizations, which was defined as AF with rapid ventricular response or symptomatic AF for which patients were admitted for loading of antiarrhythmic medication, and clinically indicated direct current cardioversion (DCCV). Secondary endpoints were each a component of the primary endpoint evaluated singularly.
Statistical Analysis
Analyses for this research project were carried out utilizing SAS version 9.1 software (SAS Institute, Inc., Cary, NC). Data were compiled for 83 patients. Categorical data items were summarized utilizing frequency counts and percentages, and means and SDs were calculated for the continuous measurements. We utilized χ 2 analyses for group comparisons of each of the categorical measurements and the Student t test for 2 independent groups for the continuous measurements.
A survival analysis was performed using the LifeTest method in SAS. The log rank test was calculated for significance.
Stepwise multiple logistic regression models were utilized to determine which demographic and risk factors were statistically related to the primary outcome event. Covariate factors tested for inclusion in the model were age, gender, heart rate, serum creatinine, serum potassium, coronary artery disease, diabetes, hypertension, hyperlipidemia, severe valvular disease, ACEIs, ARBs, statins, calcium channel blockers, amiodarone, digoxin, other antiarrhythmic medications, and spironolactone. The model entry criteria were relaxed to 10% to minimize a type 2 error. The Hosmer‐Lemeshow technique was utilized to assess the model fit. Similar subgroup analyses were performed for those 61 patients who had paroxysmal rather than permanent AF.
Results
Patient Characteristics
In total, 83 patients were included in this analysis; 60 patients were on usual medical therapy for AF and 23 patients were treated with spironolactone in addition to conventional therapy. All but one of the patients were male, and the mean age was 70 ± 7 years in the spironolactone group and 72 ± 9 in the usual therapy group (P = 0.36). Apart from left ventricular ejection fraction (LVEF), the 2 groups were evenly matched in all parameters including renal function, serum potassium, usual therapies for cardiac disease, and antiarrhythmic medications (Table 1). The LVEF was significantly lower in patients taking spironolactone (28% ± 8%) compared with those on usual therapies (35% ± 12%, P = 0.006) (Table 1).
Table 1.
Patient Characteristics
| Spironolactone (n = 23) | No Spironolactone (n = 60) | p | |
|---|---|---|---|
| Age | 70 ± 7 | 72 ± 9 | 0.36 |
| Sex | 0.53 | ||
| Male | 23 (100%) | 59 (98%) | |
| Female | 0 (0%) | 1 (2%) | |
| LVEF | 28 ± 8 | 35 ± 12 | 0.006 |
| Sinus Rhythm at Study Entry | 16 (70%) | 45 (75%) | 0.56 |
| Serum Creatinine | 1.3 ± 0.4 | 1.6 ± 1.2 | 0.31 |
| Average HR | 70 ± 9 | 72 ± 10 | 0.31 |
| Medications | |||
| Beta Blocker | 23 (100%) | 58 (97%) | 0.38 |
| ACE Inhibitor | 19 (83%) | 37 (62%) | 0.07 |
| ARB | 3 (13%) | 5 (8%) | 0.52 |
| Statin | 18 (78%) | 50 (83%) | 0.59 |
| Ca Channel Blocker | 0 (0%) | 2(3%) | 0.38 |
| Amiodarone | 4 (17%) | 14 (23%) | 0.56 |
| Other Antiarrhythmics | 4 (17%) | 9 (15%) | 0.79 |
| Digoxin | 9 (38%) | 18 (30%) | 0.43 |
| Severe Valvular Disease | 1 (4%) | 10 (17%) | 0.14 |
| Coronary Artery Disease | 19 (83%) | 43 (72%) | 0.30 |
| Diabetes | 8 (35%) | 19 (32%) | 0.79 |
| Hypertension | 18 (78%) | 49 (82%) | 0.72 |
| Hyperlipidemia | 21 (91%) | 56 (93%) | 0.75 |
| Atrial Fibrillation | 0.56 | ||
| Paroxysmal | 15 (65%) | 46 (77%) | |
| Persistent | 2 (9%) | 3 (5%) | |
| Permanent | 6 (26%) | 11 (18%) |
Abbreviations: ARB, angiotensin II receptor blocker; Ca, calcium; HR, heart rate; LVEF, left ventricular ejection fraction.
Study Endpoints
During the study period, 22% of patients receiving spironolactone (5/23) and 53% of patients in the usual‐care arm (32/60) had an AF‐related hospitalization or cardioversion for AF (P = 0.027) (Figure 2). Adjusted analysis in a stepwise logistic regression model shows a relative risk (RR) of 0.21 (95% confidence interval [CI]: 0.064–0.69, P = 0.009) (Table 2). With regard to our secondary endpoints, 13% of those on spironolactone (3/23) as compared with 42% of those on conventional therapy (25/60) were hospitalized for AF‐related reasons (P = 0.013) (Figure 3). Adjusted analysis reveals an RR of 0.13 (CI: 0.03–0.53, P = 0.014) (Table 2). There was no statistical difference between rates of DCCV, 9% (2/23) for those receiving spironolactone compared with 13% (8/60) for those on usual care (P = 0.44).
Figure 2.
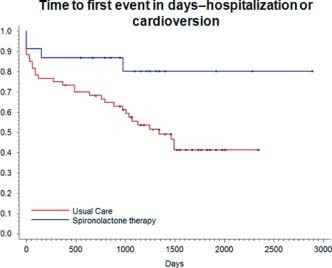
Kaplan‐Meier curve of combined endpoint of AF‐related hospitalizations and DCCV. Event rates for primary endpoint were significantly lower in patients receiving spironolactone therapy (P = 0.027). Abbreviations: AF, atrial fibrillation; AVJ, atrioventricular junction; DCCV, direct current cardioversion; ICD, implantable cardioverter defibrillator.
Table 2.
Multivariate Analysis in a Stepwise Logistic Regression Model
| Spironolactone | No Spironolactone | RR | 95% CI | P Value | |
|---|---|---|---|---|---|
| AF‐Hospitalization and cardioversion | 22% (5/23) | 53% (32/60) | 0.21 | 0.064, 0.689 | 0.009 |
| AF‐Hospitalization | 13% (3/23) | 42% (25/60) | 0.126 | 0.30,0.527 | 0.014 |
| Paroxysmal AF: primary endpoint | 19% (3/16) | 62% (28/45) | 0.184 | 0.051, 0.661 | 0.007 |
Abbreviations: ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CI, confidence interval; RR, relative risk.
Multivariate analysis in a stepwise logistic regression model. Covariate factors tested were age, gener, heart rate, serum creatinine, serum potassium, coronary artery deisease, diabetes, hypertension, hyperlipidemia, server valvular disease, ACE inhibitors, ARBs, statins, calcium blockers, amiodarone, digoxin, other antiarrhypthmic medications, and spironolactone. Diabetes, severe valvular heart deisease, and sprionolactone were in the final model.
Figure 3.
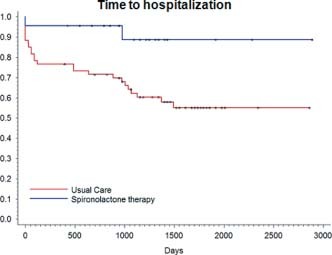
Kaplan‐Meier curve of secondary endpoint of AF‐related hospitalizations showed a significantly lower AF‐related hospitalization rate in patients on spironolactone compared with those receiving usual care (P = 0.013). Abbreviations: AF, atrial fibrillation; DCCV, direct current cardioversion.
Subgroup Analysis
Those with paroxysmal AF were evaluated using the same primary endpoint. Nineteen percent of patients on spironolactone (3/16) vs 62% of those not on the study drug (28/45) were either hospitalized and/or cardioverted for AF (P = 0.019). The RR was 0.18 in a stepwise logistic regression model (CI: 0.05–0.67, P = 0.007) (Table 2).
Discussion
Atrial fibrosis is the hallmark of atrial structural remodeling, which in turn promotes the occurrence and persistence of AF.8, 18 The major finding we describe here is that AF‐related events, including hospitalizations and clinically indicated cardioversions, were significantly decreased in patients receiving spironolactone. This association remained robust in multivariable analyses adjusting for baseline factors that were different between the treatment groups, including LVEF.
Although this study is limited by the constraints inherent to a retrospective analysis with relatively few subjects, our findings suggest that in patients with a cardiomyopathy, spironolactone has a positive effect on clinically meaningful AF endpoints. These findings are also supported by recent studies that suggest that amelioration of atrial fibrosis and downregulation of mineralocorticoid receptors in human subjects improve AF burden.17 Spironolactone modulates both these processes.17, 21 Interestingly, in our subgroup analysis of those with paroxysmal AF we also observed a benefit, even though the presumed amount of atrial structural remodeling is not as great in paroxysmal AF compared with persistent and permanent AF. Our study provides no evidence of the mechanism of action of the observed benefit; however, it is conceivable that the effect is mediated through modulation of the mineralocorticoid system and associated antifibrotic effects of spironolactone. Multiple stressors on the heart lead to structural remodeling and increase the risk of arrhythmias. This remodeling process includes altered gene expression, myocyte remodeling, and deposition of interstitial fibrosis. Whereas fibrosis is an important part of structural remodeling, current therapies do not target this aspect of remodeling. Spironolactone is a potent “antifibrotic” therapeutic agent that is currently used in clinical practice. The benefits of spironolactone seen in this study could be directly related to the fact that combination of this agent targets fibrosis, and this, in combination with therapies that target other aspects of structural remodeling, provides that observed additional benefit.
Our findings contribute to other data suggesting that targets for AF therapy should broaden from those solely focused on the ion channel. These data suggest that alteration of the structural, adverse remodeling seen in AF may prove incrementally beneficial. Better understanding of the mechanisms of cardiac fibrosis can potentially lead to novel therapeutic strategies for AF.
Spironolactone is attractive candidate drug for treating AF, as it has been extensively evaluated with regard to safety and efficacy in patients with advanced cardiac diseases, and is very inexpensive. If our findings are confirmed in prospective randomized trials, spironolactone could emerge as a useful adjunctive therapy for patients with AF. However, this should not stop the pursuit of additional potent antifibrotic agents, as further modification of fibrotic scar might provide additional benefit.
In summary, our findings identify a potential role of spironolactone in patients with AF and as such should be confirmed in a prospective fashion. Currently available therapies have limitations, requiring the need for better understanding of the underlying substrate abnormalities that are associated with AF. Understanding the mechanisms of cardiac fibrosis can potentially identify novel targets for pharmaceutical therapies.
Study Limitations
Our study provides evidence of important clinical benefits of spironolactone in patients with AF, but it does not address or provide the mechanism of action of this benefit. In addition, this is a retrospective study with a limited patient population, and results should be confirmed in a prospective fashion. Generalization of these findings outside the study population should not be made.
Conclusion
Spironolactone therapy is associated with a significant reduction in AF burden as measured by hospitalizations for AF and DCCV.
References
- 1. Preliminary report of the Stroke Prevention in Atrial Fibrillation Study. N Engl J Med. 1990;322:863–868. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. [DOI] [PubMed] [Google Scholar]
- 3. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 5. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 6. Cairns JA, Connolly SJ. Nonrheumatic atrial fibrillation: risk of stroke and role of antithrombotic therapy. Circulation. 1991;84:469–481. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 8. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 9. Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69:757–774. [DOI] [PubMed] [Google Scholar]
- 10. Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side‐to‐side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. [DOI] [PubMed] [Google Scholar]
- 11. Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. [DOI] [PubMed] [Google Scholar]
- 12. Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. [DOI] [PubMed] [Google Scholar]
- 13. Massare J, Berry JM, Luo X, et al. Diminished cardiac fibrosis in heart failure is associated with altered ventricular arrhythmia phenotype. J Cardiovasc Electrophysiol. 2010;21:1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- 15. Shiroshita‐Takeshita A, Brundel BJ, Burstein B, et al. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. [DOI] [PubMed] [Google Scholar]
- 16. Belluzzi F, Sernesi L, Preti P, et al. Prevention of recurrent lone atrial fibrillation by the angiotensin‐II converting enzyme inhibitor ramipril in normotensive patients. J Am Coll Cardiol. 2009;53:24–29. [DOI] [PubMed] [Google Scholar]
- 17. Tsai CT, Chiang FT, Tseng CD, et al. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J Am Coll Cardiol. 2010;55: 758–770. [DOI] [PubMed] [Google Scholar]
- 18. Kostin S, Klein G, Szalay Z, et al. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54: 361–379. [DOI] [PubMed] [Google Scholar]
- 19. Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Cui G, Esmailian F, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. [DOI] [PubMed] [Google Scholar]
- 21. Zhao J, Li J, Li Y, et al. Effects of spironolactone on atrial structural remodelling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Br J Pharmacol. 2010;159:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]


