Abstract
In this study, the reactions of presolvated electrons with glycine methyl ester and N-acetylalanylalanine methyl ester (N-aAAMe) are investigated by electron spin resonance (ESR) spectroscopy and DFT calculations. Electrons were produced by gamma irradiation in neutral 7.5 M LiCl-D2O aqueous glasses at low temperatures. For glycine methyl ester electron addition at 77K results in both N-terminal deamination to form a glycyl radical and C-O ester bond cleavage to form methyl radicals. For samples of N-acetylalanylalanine methyl ester electrons are found to add to the peptide bonds at 77K and cleave the carboxyl ester groups to produce methyl radicals. On annealing to 160K electron adducts at the peptide links undergo chain scission to produce alanyl radicals and further annealing to 170K α-carbon peptide backbone radicals are produced by hydrogen abstraction. DFT calculations for electron addition to the methyl ester portion of N-aAAMe show the cleavage reaction is highly favorable (free energy equals to −30.7 kcal/mol) with the kinetic barrier of only 9.9 kcal/mol. A substantial electron affinity of the ester link (38.0 kcal/mol) provides more than sufficient energy to overcome this small barrier. Protonated peptide bond electron adducts, also show favorable N-C chain cleavage reactions of −12.7 to −15.5 kcal/mol with a barrier from 7.4 to 10.0 kcal/mol. The substantial adiabatic electron affinity (AEA) of the peptide bond and ester groups provides sufficient energy for the bond dissociation.
Keywords: ESR spectroscopy, DFT calculations, electron affinity, electron-induced cleavage
Introduction
Low energy electrons (LEE) are produced in copious amounts by ionization radiation and most often lose energy to become less damaging solvated electrons.1,2 LEE are well known to induce facile bond cleavages in the gas phase and are used in mass spectrometry to cleave biomolecules at specific sites. 3, 4 In aqueous solution the action of LEE has been more controversial because of the many deactivation pathways for excess energy in condensed media as well as due to substantial stabilization (up to 3.4 eV) of solvated electrons in water.5 However, Sanche has shown that for DNA at low temperatures LEE can effectively cause both single and double strand cleavages in condensed media.1,2 Q-B. Lu was the first to suggest that presolvated electrons in aqueous solution may also produce DNA damage.6,7 For peptides and proteins it is well known that even solvated electrons can lead to bond cleavage reactions.8–13 Both the N-terminal amine group and the peptide bond are susceptible to such cleavage.8,9
In these previous investigations radicals formed by the reaction of electrons with amino acids, N-acetylamino acids and peptides or model systems were investigated in aqueous glasses.10–13 Each showed that electron attachment led to fragmentation reactions in these aqueous systems. Studies of direct gamma irradiation of N-acetyl amino acids in frozen aqueous solutions8,9 resulted in radicals from both electron and holes formed in these species. The electron reactions in the frozen ices followed the similar chemistry to those in aqueous glasses. Our recent efforts also have shown the cyclic peptide, N-acetylproline is susceptible to N-C bond cleavage; however in this case the cyclic nature of proline prevents the chain from full cleavage.10,14
The nature of the radiation produced electrons in LiCl aqueous glasses has been investigated by several workers. Several groups reported that in aqueous 6–8M LiCl glasses at low temperature there are two spectroscopically observable trapped electrons.15–17 The first is the dominant species and is a shallow trapped species (−0.5eV ground state below the continuum) that absorbs in the IR at >2400 nm (<0.5 eV). The second absorbs at 650 nm (−1.8 eV) and the ground state is ca. 2.6 eV below the continuum. The first was assigned by Kevan and coworkers to the presolvated electron17 while the second has the expected energy of more fully solvated electron (deeper trap). For the conditions used in this study (25mM peptides) the dominant reaction would be with the presolvated electrons and these electrons remain energetic by several eVs with respect to the fully solvated electron. Thus on addition this excess energy can contribute to reaction processes even at 77K. As suggested earlier the LEE range in solvated systems would be from ca. −1 to 10 eV.
In the current study we investigate the reactions of electrons with glycine methyl ester and N-acetylalanylalanine methyl ester at low temperatures and follow the radical species produced by use of electron spin resonance (ESR) spectroscopy. Previous work with methyl esters has shown the production of methyl radicals from the C-O bond cleavage even at 77K.18 Thus it is of interest to investigate the possibility of this cleavage in a peptide which can provide several other functional groups for electron attachment.14,18,19
Density functional theory is employed for the various radical species proposed to confirm our analyses and give additional insights to the mechanisms of action. Our results show that even at 77K attachment of a presolvated electron can result in cleavage of both N-terminal amine and methyl ester bonds in aqueous media whereas C-N peptide cleavages are observed only on annealing. The spontaneous 77K presolvated electron induced bond cleavage found in this work clearly shows the potential for presolvated electron induced C-O and C-N bond cleavages even in aqueous phases at low temperatures.
I. Materials and methods
Experiment
The glycine methyl ester and N-acetylalanylalanine methyl ether used in these experiments were purchased from Sigma-Aldrich as were the lithium chloride, ≥99% (LiCl) and deuterium oxide, 99.9% (D2O) used to prepare 7.5M LiCl/D2O. The gamma (Co-60) irradiator employed was a GR-9 producing 1.0 kGy/hr. All samples were irradiated at 77K. The ESR (electron spin resonance) spectrometer is a E-9 Century Series with dual microwave cavities. Suprasil quartz tubes (4 mm in diameter) used for samples were purchased from Wilmad Labglass.
Sample Preparation
Approximately 5 milligrams (25–80 mM) of solute was dissolved in 0.5ml of 7.5M LiCl/D2O. After the solution was fully dissolved, it was bubbled with nitrogen gas for 5 minutes to remove oxygen from the samples. The sample was then drawn into a 4 mm quartz tube and cooled to 77K in liquid nitrogen to form a clear glass.
Radical formation
The samples were gamma irradiated with Co-60 source for ca. 60 krads in a Styrofoam vessel filled with liquid nitrogen (77K). After sample irradiation was completed, the vessel with the sample was transported to the ESR instrument and placed in a quartz EPR dewar insert also at 77K. An ESR spectrum was then taken to view radicals produced from gamma irradiation. Since irradiation of the glass produces only electrons and Cl2−• and since only electrons are mobile at 77K, all radicals at 77K are from electron attachment to the solute. Annealing of the sample at temperatures of 152K–170K was done to view thermally induced radical reactions of the electron adducts. At these temperatures Cl2−• becomes mobile but is not able to oxidize the solutes chosen for this study.10 Thus only radicals originating from electron attachment are observed.
DFT calculations
In our theoretical work electron attachment to glycine methyl ester and N-acetylalanylalanine methyl ester was considered using DFT methods. All the calculations were performed using B3LYP20–22 and the 6-31++G(d,p) basis set. 23, 24 Additionally, to simulate an aqueous environment, the polarized continuum model (PCM25) with the UAHF solvation radii26 was employed.
All geometries presented here were fully optimized without any geometrical constraints, and the analysis of harmonic frequencies proved that all of them are either structures at energetic minima (all force constants positive) or first-order saddle points (all but one force constants positive). The starting conformation of the backbone of both the neutral glycine and N-acetylalanylalanine methyl esters were adopted after the literature. Namely, the conformation of neutral glycine methyl ester has been taken from the papers of Makowski et al. 27 and Simon et al. 28 On the other hand the conformation of the backbone of N-acetylalanylalanine methyl ester was adopted after the HF and B3LYP29 as well as MD conformational studies30 on the alanine dipeptide. This approach assured that optimization of the substrates geometries converged to one of the low energy conformations. Furthermore, one should realize that the starting conformation is not crucial as long as it is kept unchanged for particular stationary points along the reaction path. This is because one calculates the energy difference between a product and substrate (or a transition state and substrate) and if them both have a similar conformation their conformational energies cancel out.
All presented energetic values are given in the Gibbs free energy scale in aqueous solution. Moreover, the adiabatic electron affinity, calculated using Gibbs free energies at T=298 K of the fully optimized neutral and anion radical complex, is denoted by AEAG. If the considered reaction led to two molecular fragments, the reaction free energies were calculated as the difference between the free energy of the molecular complex between those fragments and that of substrate.
Calculations have been carried out with the GAUSSIAN0931 code and the pictures of molecules and orbitals were plotted with the GaussView 5 package.32
II. Results and Discussion
Glycine methyl ester
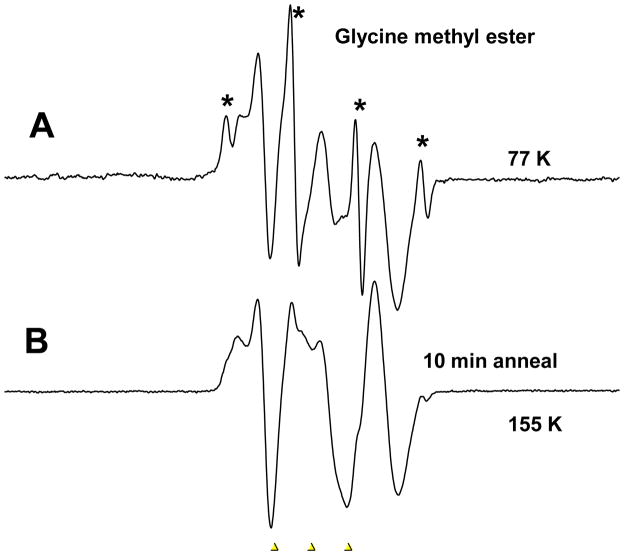
In Figure 1 we show the results of gamma irradiation at 77K of a 7.5M LiCl sample containing 80 mM N-glycine methyl ester. The radiation produces Cl2−• and electrons. For clarity the background signal of the Cl2−• has been subtracted. The electrons are mobile after formation and add to the solute whereas the Cl2−• remains fixed in the glass. The ESR spectrum shown in Figure 1A shows the results of electron addition to the solute at 77K. The sharp 4 line 1:3:3:1 quartet (marked with an asterisk) with 23 G separation is unambiguous evidence for methyl radicals formed on electron addition and immediate ester C-O bond cleavage to produce the methyl radical all at 77K. The second and more abundant radical in the spectrum is that formed by electron induced cleavage at 77K of the C-N bond to form •CH2CO2CH3 and ammonia. Annealing to 155K for 10 minutes results in the loss of almost all of the methyl radical signal and leaves the •CH2CO2CH3 without production of new radical species.
Figure 1.
Glycine methyl ester (5mg) in 7.5 M LiCl after 45 minutes of gamma irradiation at 77K. A. Sample at 77K after electron addition which results in both deamination by C-N bond cleavage to form •CH2CO2CH3 and C-O bond cleavage to form the methyl radical (CH3•) which gives rise to the sharp 1:3:3:1 spectrum with 23 G separations. The background signal of the Cl2−• has been subtracted. B. After annealing to 155K for 10 minutes which results in the loss of almost all the methyl radical signal and leaves the •CH2CO2CH3. This spectrum was recorded at 77K after annealing. The three markers are separated by 13.09 G each. The middle marker is at g=2.0056.
For the neutral glycine methyl ester two degradation paths were considered at the DFT level (see Figure 2). Path B, leading to the ester bond cleavage (ΔG=−27.1 and ΔG*=9.8 kcal/mol), is more likely than path A, leading to scission at the amine group (ΔG=4.3 and ΔG*=8.5 kcal/mol, see Figure 2). Nevertheless it should be underlined, that under the experimental conditions (pH ca. 5) the amine group is fully protonated and for the protonated species the situation is reversed (see Figure 3). Indeed, proton attachment to the amine group leads after electron addition to ammonia elimination with the negligibly low activation barrier (ΔG*=0.9 kcal/mol) and significantly negative thermodynamic stimulus (ΔG=−33.8 kcal/mol), while the molecule protonated at the ester site is less prone to degradation by cleavage of the ester C-O bond (ΔG=−13.0 and ΔG*=8.4 kcal/mol, see Figure 3). So, if the ester was protonated before electron attachment, path C in Figure 3 should be preferred. Thus, in order to explain the experimental picture one could assume that there is a mixture of protonated and the neutral forms of the ester in water glass and then the methyl radical would form on the path B in Figure 2 (from the neutral) while ammonia and the respective radical on the path C in Figure 3 (from the protonated form of the ester). However, this is not necessary as we note that the electron affinity of the glycine methyl ester is sufficient to provide the energy to overcome the barrier in protonated and nonprotonated cases (AEAG=34.7 kcal/mol, see Figure 2). The lower barrier for C-N cleavage does explain the predominance of the deamination pathway at 77K.
Figure 2.
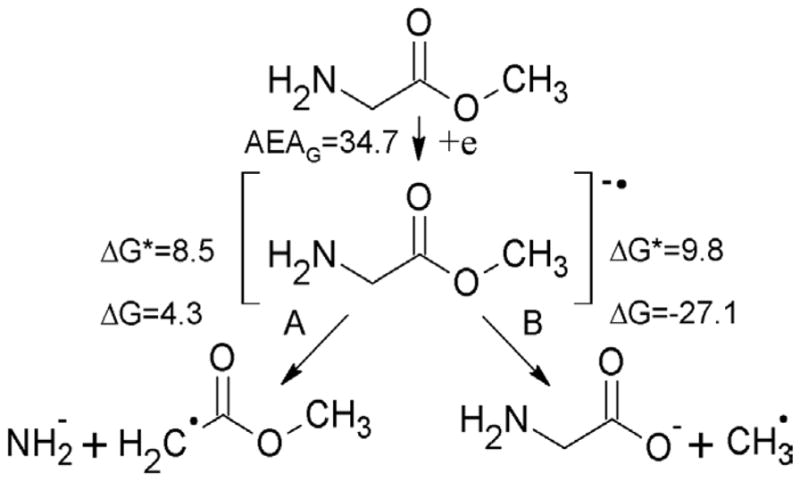
Electron attachment to neutral glycine methyl ester, leading to the cleavage at the amine (path A) or methyl (path B) group. AEAG, ΔG and ΔG* stand for adiabatic electron affinity, free energy and free activation energy of the reaction, all in kcal/mol.
Figure 3.
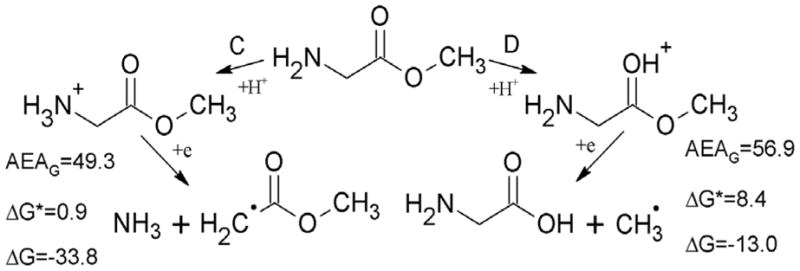
Electron attachment to protonated glycine methyl ester, leading to the cleavage at the amine (path C) or methyl (path D) group. AEAG, ΔG and ΔG* stand for adiabatic electron affinity, free energy and free activation energy of the reaction, all in kcal/mol.
N-acetyl-L-alanyl-L-alanine methyl ester
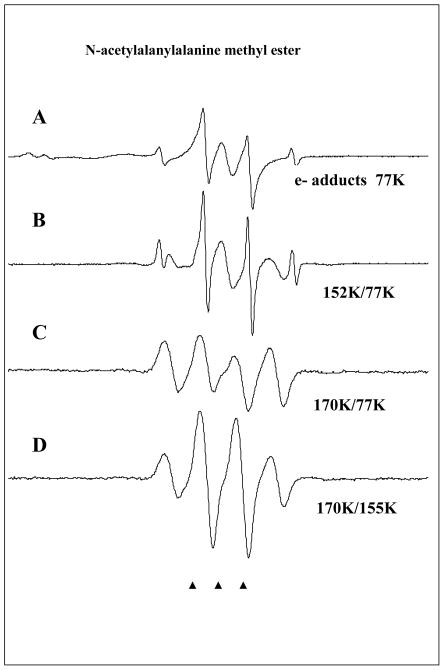
In Figure 4 we show the ESR results for a sample containing 25 mM N-acetyl-L-alanyl-L-alanine methyl ester. The ESR spectrum shown in Figure 4A shows the results of electron addition to the solute as well as line components of the Cl2− observed in the low field region of the spectrum. The sharp 4 line 1:3:3:1 quartet with 23 G separation is again unambiguous evidence for methyl radicals formed on electron addition at 77K. The central line component is assigned to the electron adducts at the peptide bonds which do not fragment at 77K. Line components of the Cl2−• are observed in the low field region of the spectrum.
Figure 4.
ESR spectra found after electron attachment to N-acetylalanylalanine methyl ester at 77K and annealing to temperatures shown in the figure. A. Spectrum at 77K immediately after gamma irradiation. The ESR spectrum consists of radicals formed by electron attachment to the three possible sites in the structure and Cl2−•. The strong signal from the methyl radical is a result of C-O bond cleavage at 77K induced by electron attachment. The doublet which broadens the central two CH3• radical lines is from electron attachment to the N-acetyl portion. The central singlet is assigned to electron attachment at the alanyl and ester sites. B. On warming the methyl radical increases and main chain cleavage occurs to form the alanyl radical, •CHCH3C(O)R, which shows a quintet from couplings to four nearly equivalent protons (24 G avg each). C. On annealing to 170 K all radicals abstract from the alpha hydrogens on the peptide backbone to produce the –NH-C(•)(CH3)-C(O)- radical with an 18 G coupling from 3 equivalent methyl group protons showing hindered rotation at 77K. All spectra in A–C are recorded at 77K after annealing to temperatures in the figure. D. This spectrum was recorded at 155K and shows the expected pattern from a methyl group in near free rotation. The change in spectra from C to D was reversible with temperature and is only a result of rotation of the methyl group.
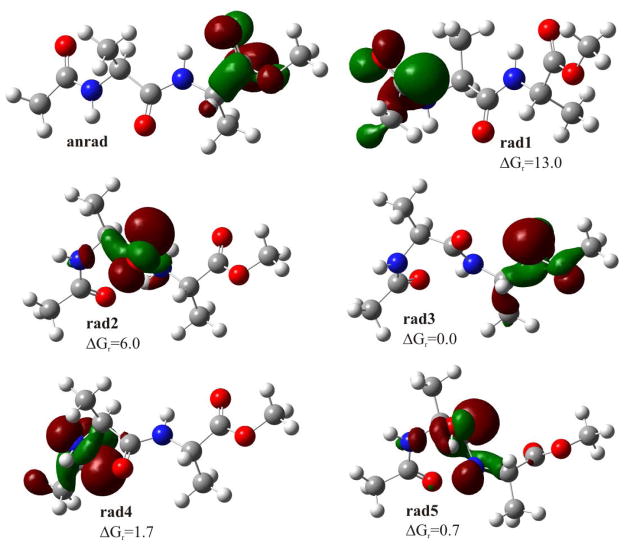
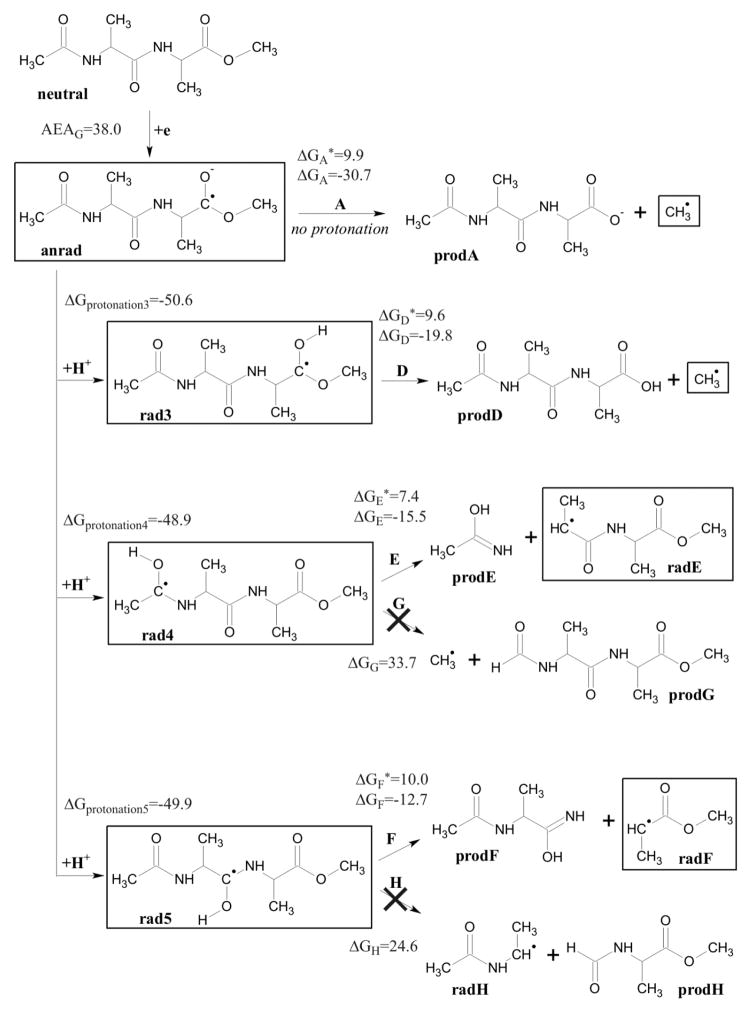
The ESR spectrum in Figure 4A consists of radicals formed by electron attachment to the three possible sites in the structure and Cl2−• in addition to methyl radicals. The doublet which broadens the central two CH3• radical lines is assigned to electron attachment to the N-acetyl group in the molecule. The central singlet is assigned to electron attachment at center peptide bond and ester carbonyl site. On annealing to 155K for 10 min (see Figure 4B) the methyl radical increases and main chain cleavage occurs to form the alanyl radicals, •CHCH3C(O)R1 and •CHCH3C(O)R2, which show a quintet from couplings to four nearly equivalent protons (24 G avg each) arising from the three β-protons from the -CH3 group and the single α-proton at the radical center. Figure 4C shows the results of further annealing to 170 K. At this temperature the CH3• and alanyl radicals abstract from the α-hydrogens on the peptide backbone to produce the peptide backbone α-carbon radical, i.e., –[NH-C(•)(CH3)-C(O)]-. This radical shows an 18 G coupling from 3 equivalent methyl group protons. The equal line intensities are evidence for hindered rotation of the methyl group at 77K. Note that all spectra in Figure 4A–4C are recorded at 77K after annealing to temperatures indicated in the figure. However, in Figure 4D the spectrum of the sample in 4C is recorded at 155K. At this temperature the spectrum shows line intensities more like the expected pattern from a methyl group in near free rotation. The change in spectra from 4C to 4D was completely reversible with temperature and is clearly not associated with any radical reactions. Such changes have been observed in previous work on glycyl alanine and alanyl alanine dipeptides and are simply the result of a small barrier of ca. 1 kcal/mol to free rotation for the methyl group. 33,34 As done for glycine methyl ester we carried out DFT calculations to model electron induced reactivity of N-acetylalanine methyl ester. The neutral form of N-aAAMe has an electron affinity high enough (AEAG=38.0 kcal/mol) to form a stable valence anion radical (anrad). The SOMO orbital is localized on the ester group (see Figure 5) and therefore this site is prone to direct cleavage (see path A in Figure 6). The slightly acidic environment of our experiment (pH=5) promotes protonation of anrad (negative ΔGprotonation value, see Figure 6). Five protonated radicals were considered (see Figure 5). Rad1 and rad2 are believed not to occur under experimental conditions due to their high relative free energies (ΔGr=13.0 and 6.0 kcal/mol, respectively; see Figure 6). For the remaining radicals (rad3–5) five degradation paths (D–H) were studied (see Figure 6). Two of them (G and H) are thermodynamically forbidden due to highly positive thermodynamic stimulus for the considered degradation reactions (ΔGG=33.7, ΔGH=24.6 kcal/mol), while paths D–F seem to be probable both thermodynamically and kinetically (see Figure 6). The radical degradation products, radE and radF (see Figure 6), are difficult to distinguish with an ESR technique (both should occur as 1:4:6:4:1 quintets at 77K). The same issue holds for distinguishing the initial anrad, rad3 and rad5 since all should produce singlets at 77K.
Figure 5.
Geometries and singly occupied molecular orbitals (SOMO) distribution in the anion radical (anrad) and radical substrates, along with the relative free energy for radicals, calculated with respect to the most stable one – rad3. All values given in kcal/mol, SOMO orbitals plotted with a contour value of 0.05 bohr−3/2.
Figure 6.
Degradation paths for N-acetylalanylalanine methyl ester after electron attachment, along with activation barriers (ΔG*), thermodynamic stimuli (ΔG) and adiabatic electron affinity (AEAG). All values given in the free energy scale, in kcal/mol, calculated at B3LYP/6-31++G** level, in aqueous solution (PCM model). Species that are observed in ESR spectra are framed.
The computational results are therefore in accord with the experimental interpretations. At first, anrad (ESR singlet; see Figure 6) can decompose directly via the ester bond cleavage (path A in Figure 6), giving the methyl radical (quartet in the ESR spectrum). On the other hand, the high pKa of a peptide bond electron adduct means that anrad will quickly protonate, giving two possible radicals: rad4 (ESR doublet at 77K) and rad5 (ESR singlet at 77K). The pKa of the ester link anion also suggests that rad3 will form by protonation of anrad which also produces an ESR singlet. Those radicals, after overcoming relatively low activation barriers, are predicted to react to produce the secondary radical products on path D (ester bond cleavage to form methyl radical), E and F (to form the alanyl radical) as found in the experiment.
III. Summary
In this study, the reactions of low energy electrons (LEE) with glycine methyl ester and N-acetylalanylalanine methyl ester are investigated. For glycine methyl ester electron addition at 77K results in N-terminal deamination to form the glycyl radical as well as methyl radical by electron induced ester C-O bond cleavage. For samples of N-acetylalanylalanine methyl ester electrons are found to add at 77K to the peptide bonds and to the carboxyl ester groups resulting in methyl radical formation. Our DFT calculations show the formation of methyl radicals has a significant barrier in both molecules. Thus the formation of the methyl radical at 77K is clearly a result of the LEE attachment which liberates the substantial electron affinity of the ester group as well as any residual kinetic/potential energy of the electron. On annealing to 160K electron adducts at the peptide links and ester carbonyl site undergo chain scission to produce alanyl radicals. On further annealing to 170K both the methyl radical and the chain scission radicals abstract from the α-carbon sites of the peptide forming the peptide backbone radical. Both of these processes are expected from previous work.7–13 Further the rotational hindrance of the methyl group found for the final peptide backbone radical in N-acetylalanylalanine methyl ester has been observed in earlier work.33,34 DFT calculations for electron addition to the methyl ester portion of N-acetylalanylalanine methyl ester show the cleavage reaction is highly favorable (−30.7 kcal/mol) with the barrier only 9.9 kcal/mol. The substantial electron affinity of the ester link (38.0 kcal/mol) provides more than sufficient energy to overcome this small barrier. The peptide bond protonation electron adducts, also show a favorable N-C chain cleavage reaction of (−12.7 to −15.5 kcal/mol) with a barrier from 7.4 to 10.0 kcal/mol) as well as a high AEA. The observation of low energy electron induced N-terminal deamination and methyl radical formation at 77K is attributed to both the exergonic nature of the reactions and the NH3 and CH3• mobility that allows for facile cleavage and some movement from the cleavage site.
Acknowledgments
The authors greatly appreciate the aid of the Department of Chemistry for granting the Thompson Undergraduate Research Award (JK) and the NIH (RO1 CA 045424) for partial support of this research. This work was also supported by the Polish Ministry of Science and Higher Education via the Young Scientists Grant No. 538-8221-1066-12 (L.C.) and DS/8221-4-0140-3 (J.R.). Calculations have been carried out in Wroclaw Centre for Networking and Supercomputing (http://www.wcss.wroc.pl), grant No. 209. Amitava Adhikary and Deepti Khanduri are acknowledged for their generous aid and helpful discussions.
References
- 1.Sanche L. Low Energy Electron-Driven Damage in Biomolecules. Eur Phys J D. 2005;35:367–390. [Google Scholar]
- 2.Boudaiffa B, Cloutier P, Hunting D, Huels MA, Sanche L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science. 2000;287:1658–1660. doi: 10.1126/science.287.5458.1658. [DOI] [PubMed] [Google Scholar]
- 3.Syrstad EA, Tureček F. Toward a General Mechanism of Electron-Capture Dissociation. J Am Soc Mass Spectrom. 2005;16:208–224. doi: 10.1016/j.jasms.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa S, Hashimoto M, Matsubara H, Tureček F. Dissecting the Proline Effect. Dissociations of Proline Radicals Formed by Electron Transfer to Protonated Pro-Gly and Gly-Pro Dipeptides in the Gas Phase. J Am Chem Soc. 2007;129:7936–7949. doi: 10.1021/ja0712571. [DOI] [PubMed] [Google Scholar]
- 5.Abel B, Buck U, Sobolewski AL, Domcke W. Phys Chem Chem Phys. 2012;14:22–34. doi: 10.1039/c1cp21803d. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Nguyen J, Lu Q-B. Bond Breaks of Nucleotides by Dissociative Electron Transfer of Nonequilibrium Prehydrated Electrons: A New Molecular Mechanism for Reductive DNA Damage. J Am Chem Soc. 2009;131:11320–11322. doi: 10.1021/ja902675g. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen J, Ma Y, Luo T, Bristow RG, Jaffray DA, Lu Q-B. Direct Observation of Ultrafast-Electron-Transfer Reactions Unravels High Effectiveness of Reductive DNA Damage. Proc Natl Acad Sci USA. 2011;108:11778–11783. doi: 10.1073/pnas.1104367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevilla MD, D’Arcy JB, Morehouse KM. An Electron Spin Resonance Study of Gamma-Irradiated Frozen Aqueous Solutions Containing N-Acetylamino Acids. J Phys Chem. 1979;83:2893–2897. [Google Scholar]
- 9.Sevilla MD, D’Arcy JB, Morehouse KM. An Electron Spin Resonance Study of Gamma-Irradiated Frozen Aqueous Solutions Containing Dipeptides. Mechanisms of Radical Reaction. J Phys Chem. 1979;83:2887–2892. [Google Scholar]
- 10.Kheir JF, Chomicz L, Rak J, Bowen KH, Sevilla MD. Radicals Formed in N-Acetylproline by Electron Attachment: Electron Spin Resonance Spectroscopy Computational Studies. J Phys Chem B. 2011;115:14846–14851. doi: 10.1021/jp207841m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Arcy JB, Sevilla MD. Radiat Phys Chem. 1979;13:119–126. [Google Scholar]
- 12.Sevilla MD, Brooks VL. Radicals Formed by the Reaction of Electrons with Amino Acids and Peptides in a Neutral Aqueous Glass. J Phys Chem. 1973;77:2954–2959. [Google Scholar]
- 13.Sevilla MD, D’Arcy JB, Suryanarayana D. Conformational Effects on the Electron Spin Resonance Spectra of Radicals Produced by Electron Attachment to Amino Acids and Peptides. J Phys Chem. 1978;82:2589–2594. [Google Scholar]
- 14.Chomicz L, Rak J, Paneth P, Sevilla MD, Ko YJ, Wang H, Bowen KH. Valence Anions of N-Acetylproline in the Gas Phase: Computational Anion Photoelectron Spectroscopic Studies. J Chem Phys. 2011;135:114301–7. doi: 10.1063/1.3625957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis HA, Teather GG, Buxton GV. Pulse Radiolysis of Deuterated Aqueous LiCl Glasses. Dependence of the Yields and Rates of Reaction of Visible and Infrared Absorbing Trapped Electrons on LiCl Concentration. Can J Chem. 1978;56:1889– 1897. [Google Scholar]
- 16.Klassen NV, Teather GG, Keifer F. A Study of Trapped Electrons in LiCl/D2O and Other Aqueous Glasses at Temperatures Down to 2 K by Radiolysis, Pulse Radiolysis, Photolysis, and Stimulated Luminescence. Can J Chem. 1979;57:1488– 1499. [Google Scholar]
- 17.Dolivo G, Kevan L. Effect of Irradiation Temperature from 77 to 1.6 K on the Optical Absorption and Scavenging Efficiency of Localized Electrons in Aqueous 10 M Lithium Chloride glasses. J Chem Phys. 1979;70:5489–5493. [Google Scholar]
- 18.Sevilla MD, Morehouse KM, Swarts S. An ESR Study of Electron Reactions with Esters and Triglycerides. J Phys Chem. 1981;85:923–927. [Google Scholar]
- 19.Sevilla MD, Swarts S, Bearden R, Morehouse KM, Vartanian T. An ESR Study of Electron Reactions With Carboxylic Acids, Ketones, and Aldehydes in Aqueous Glasses. J Phys Chem. 1981;85:918–923. [Google Scholar]
- 20.Becke AD. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys Rev A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 21.Becke AD. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 22.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti Conelation Energy Formula into a Functional of the Electron Density. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 23.Ditchfield R, Hehre WJ, Pople JA. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J Chem Phys. 1971;54:724–728. [Google Scholar]
- 24.Hehre WJ, Ditchfield R, Pople JA. Self Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J Chem Phys. 1972;56:2257–2261. [Google Scholar]
- 25.Tomasi J, Mennucci B, Cammi R. Quantum Mechanical Continuum Solvation Models. Chem Rev. 2005;105:2999–3093. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- 26.Barone V, Cossi M, Tomasi J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J Chem Phys. 1997;107:3210–3221. [Google Scholar]
- 27.Ejsmont K, Gajda R, Makowski M. Conformation of Tert-Butoxycarbonylglycyl-dehydroalanyl-glycine Methyl Ester in the Crystalline State and Calculated in the Gas Phase. Acta Cryst. 2007;C63:o80–o83. doi: 10.1107/S0108270106052590. [DOI] [PubMed] [Google Scholar]
- 28.Simon A, MacAleese L, Maitre P, Lemaire J, McMahon TB. Conformational Preferences Vibrational Frequency Distributions of Short Peptides in Relation to Multidimensional Infrared Spectroscopy. J Am Chem Soc. 2001;123:12886–12898. doi: 10.1021/ja011088z. [DOI] [PubMed] [Google Scholar]
- 29.Kang YK. Conformational Preferences of Non-Prolyl and Prolyl Residues. J Phys Chem B. 2006;110:21338–21348. doi: 10.1021/jp0647481. [DOI] [PubMed] [Google Scholar]
- 30.Gnanakaran S, Hochstrasser RM. Fingerprint Vibrational Spectra of Protonated Methyl Esters of Amino Acids in the Gas Phase Fingerprint Vibrational Spectra of Protonated Methyl Esters of Amino Acids in the Gas Phase. J Am Chem Soc. 2007;129:2829–2840. doi: 10.1021/ja0662321. [DOI] [PubMed] [Google Scholar]
- 31.Frisch MJ, et al. Gaussian 09: revision A.02. Gaussian Inc; Pittsburgh, PA: 2009. [Google Scholar]
- 32.Frisch Æ, Hratchian HP, Dennington RD, II, Todd AKeith TA, Millam J. GaussView. Vol. 5. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- 33.Suryanarayana D, Sevilla MD. Electron Spin Resonance Studies of Barriers to Hindered Rotation in Acetic Acid, Acetamide, and Peptide Radicals. J Phys Chem. 1979;83:1323–1327. [Google Scholar]
- 34.Suryanarayana D, Sevilla MD. INDO Study of the Anion Radicals of Acetic Acid and Acetamide. Nonplanarity and Barriers to Methyl Group Rotation. J Phys Chem. 1980;84:3045–3049. [Google Scholar]