Abstract
Background
Gastroesophageal reflux disease (GERD) in lung recipients is associated with decreased survival and attenuated allograft function. This study evaluates fundoplication in preventing GERD-related allograft dysfunction.
Methods
Prospectively collected data on patients who underwent transplantation between January 2001 and August 2009 were included. Lung transplant candidates underwent esophageal pH probe testing before transplantation and surveillance spirometry evaluation after transplantation. Bilateral lung transplant recipients who had pretransplant pH probe testing and posttransplant 1-year forced expiratory volume in the first second of expiration (FEV1) data were included for analysis.
Results
Of 297 patients who met study criteria, 222 (75%) had an abnormal pH probe study before or early after transplantation and 157 (53%) had a fundoplication performed within the first year after transplantation. Patients with total proximal acid contact times greater than 1.2% or total distal acid contact times greater than 7.0% demonstrated an absolute decrease of 9.4% (± 4.6) or 12.0% (± 5.4) in their respective mean 1-year FEV1 values. Patients with abnormal acid contact times who did not undergo fundoplication had considerably worse predicted peak and 1-year FEV1 results compared with recipients receiving fundoplication (peak percent predicted = 75% vs. 84%; p = 0.004 and 1-year percent predicted = 68% vs. 77%; p = 0.003, respectively).
Conclusions
Lung transplant recipients with abnormal esophageal pH studies attain a lower peak allograft function as well as a diminished 1-year FEV1 after transplantation. However a strategy of early fundoplication in these recipients appears to preserve lung allograft function.
Lung transplantation is an effective therapy that improves the quality of life and extends the survival of patients with end-stage pulmonary disease. However a lung allograft has one of the shortest graft survival times compared with other solid organ transplants, mainly because of the development of bronchiolitis obliterans (BO). The clinical correlate of BO is BO syndrome (BOS) reflected as a progressive decline in forced expiratory volume in the first second of expiration (FEV1) on spirometry. Important predictors of BOS development include the frequency and severity of acute rejection episodes, presence of gastroesophageal reflux disease (GERD), and cytomegalovirus (CMV) disease. [1–3] Unfortunately no therapeutic interventions have been shown to demonstrably delay the development of BOS and there are currently no reliable treatments for preventing the progression of BOS once it is diagnosed.
Gastroesophageal reflux disease is very common after lung transplantation, with an incidence of nearly 75% [4]. Supporting the role of GERD in causing lung allograft dysfunction, we reported the association between reflux disease and increased rates of BOS development and worse actuarial survival [3]. We also showed in a small subset of patients that surgical correction of the reflux might attenuate those effects [5]. Other transplantation centers have also linked GERD-related aspiration to the development of BOS and allograft dysfunction. For example, D'Ovidio and colleagues demonstrated an association between bile acids in bronchoalveolar lavage fluid (BAL) and a more rapid onset of BOS [6].
Building on this concept we hypothesized that the severity of the reflux and the timing of the fundoplication procedure to correct it would directly impact the severity of allograft dysfunction as reflected in the FEV1. The current study seeks to delineate acid contact times that will be predictive of worse allograft function and to determine if surgical prevention of GERD in the form of fundoplication will prevent the attenuation in pulmonary function associated with GERD.
Material and Methods
Patient Population and Surgery
We retrospectively evaluated all patients who underwent bilateral lung transplantation at Duke University Hospital between January 2001 and August 2009. Single-lung transplant recipients, redo transplantations, multiorgan transplantations, and recipients with less than 1 year of follow-up were excluded from the analysis. Only patients with pretransplantation esophageal pH studies and 1-year FEV1 values were included. All patients had a minimum of 1 year of follow up. Each patient was classified as 1 of 3 groups. In order to be included in the No GERD group, recipients must have had a normal pretransplantation and early (within 90 days) posttransplantation pH study. A posttransplantation pH study that did not demonstrate abnormal acid contact times was required to be included in the No GERD group secondary to the high rate of reflux development after lung transplantation [7].
Recipients with abnormal acid contact times before transplantation or early (within 90 days) after transplantation who did not undergo fundoplication in the first year were classified as the GERD group. Recipients with abnormal acid contact times before transplantation or early after transplantation who underwent fundoplication in the first year were classified as the fundoplication (FUNDO) group. Overall the study included 33 recipients who had normal acid contact times before transplantation but elevated acid contact times early after transplantation. Approval for the study was obtained from Duke University Hospital's Institutional Review Board (IRB) for Human Studies, and the requirement for informed consent was waived.
The transplantation procedures were performed in a standard fashion as previously described through a bilateral transverse sternothoracotomy or “clamshell” incision [8]. Per protocol, all recipients were provided proton pump inhibitors after transplantation. A small number of patients received H2 blockers because of financial considerations.
The laparoscopic Nissen fundoplication (360-degree wrap) was the procedure of choice unless esophageal manometry indicated significant esophageal dysmotility. Over the course of this study, 3 surgeons specializing in foregut surgery performed the fundoplications. Styles varied slightly among the 3, but in general 2 to 3 sutures were used to create the wrap with a length of 2 to 3 cm. This was typically done over a 54- to 58-cm bougie. Routine esophageal manometry testing was performed before fundoplication. If significant esophageal dysmotility was identified, a partial wrap (ie, Toupet procedure) was performed or fundoplication was deferred. Gastric emptying (ie, pyloroplasty) or esophageal lengthening (ie, Collis gastroplasty) procedures were done at the discretion of the attending foregut surgeon based on preoperative studies or intraoperative findings. When determining whether fundoplication was offered to lung recipients, the severity of reflux as determined by acid contact times provided the most important impetus. For the purpose of this study, patients were stratified into groups by pH results alone. No patients in this study received fundoplication for standard complications of GERD, such as Barrett's metaplasia, esophagitis, or esophageal strictures.
Immunosuppression
Immunosuppression regimens were similar among groups. All the patients received standard triple-drug therapy postoperatively that included corticosteroids, tacrolimus, and azathioprine or mycophenolate mofetil. The latter served as the antimetabolite for a very few patients as part of a previous randomized clinical trial. Otherwise all patients received azathioprine. Patients received the monoclonal interleukin-2 receptor immunoglobulin daclizumab as part of the induction treatment protocol.
Ambulatory 24-Hour pH Monitoring
Ambulatory 24-hour esophageal pH monitoring was performed using standard techniques [9]. Briefly, a dual esophageal probe was placed that records acid contact time over the following 24 hours while the individual maintains his or her normal routine of daily activities and diet. Acid-suppressing medications were discontinued 3 days (H2 blockers) or 14 days (proton pump inhibitors) before the study. Acid contact was recorded whenever the pH decreased to less than 4. Reports were provided as the percentage of acid contact time detected by the probe for the duration of the study (total), as well as with the patient in the upright and supine positions at both the distal and proximal esophageal locations. Normal laboratory values for distal acid contact times were less than 5% for total, 8% for upright, and 3% for supine positions. Normal proximal acid contact times were less than 0.9% for total, 1.3% for upright, and 0% for supine positions. Our laboratory does not determine a DeMeester score. Routine pH monitoring after fundoplication is not performed at our center.
Statistical Analyses
Standard descriptive statistics were used for patient demographic information. Values were calculated as mean ± SD for normally distributed data or median with interquartile range (IQR) for data not normally distributed. Comparisons between groups were made using 2 sample t tests (parametric) or the Wilcoxon rank sum test (nonparametric) for continuous data and the χ2 or Fisher's exact test for categorical variables.
We performed exploratory analyses to examine the relationship between acid contact times and pulmonary function at 1 year for all patients not undergoing fundoplication (ie, the analysis included patients in the GERD and No GERD groups) to determine an acid contact time associated with decreased FEV1. First, acid contact time was separated into 8 categories and then the average FEV1 for each category was calculated and plotted for inspection. This procedure was repeated for total distal acid contact time and total proximal acid contact time for each of the 2 outcomes: mean percent predicted peak FEV1 and mean percent predicted 1-year FEV1.
Univariate analyses were performed using 1-way analysis of variance to compare both 1-year FEV1 and peak FEV1 among the 3 groups (No GERD, GERD, and FUNDO). Newman-Keuls multiple comparison testing was performed to evaluate for statistical significance while accounting for multiple tests.
Linear regression using the least squares method was used to determine the effects of reflux and fundoplication on the peak and 1-year FEV1 measurements while controlling for potentially confounding effects of other variables. Separate models were created for peak percent predicted FEV1 measurements and 1-year percent predicted FEV1 measurements. Variables were selected for inclusion in the initial multivariate models based on previously published data and if the p value was less than 0.2 in initial testing between groups [3]. Ultimately the following variables were included in the multivariate models: underlying disease, recipient age, gender, GERD group, and CMV status. Patients with incomplete data for any of these variables were not included in the final models. The final multivariate models were created using the backward elimination method.
Results
Patients
From January 2001 to August 2009, 518 bilateral lung transplantations were performed at Duke University Medical Center. Of those, 297 bilateral lung transplant recipients met entrance criteria. One hundred forty-two patients who died during the first year after transplantation were excluded from the analysis. Of 297 included recipients, 222 (75%) demonstrated elevated acid contact times distally or proximally in at least 1 category: total, upright, or supine. Of these patients with abnormal pH studies, 157 received fundoplication within the first year after transplantation and were classified in the FUNDO group. The median time to fundoplication in this group was 68 days. The 65 patients with elevated acid contact times who did not undergo fundoplication populated the GERD group. Seventy-five (25%) patients had normal pretransplantation and early posttransplantation esophageal acid contact times and were classified as the No GERD group.
Demographics for each group are shown in Table 1. Overall there was a greater incidence of elevated acid contact times among recipients with cystic fibrosis and those with idiopathic pulmonary fibrosis. There were no other significant differences in patient characteristics among the groups. Median duration of follow-up was 39.5 months for the No GERD group, 49.2 months for the GERD group, and 37.6 months for the FUNDO group (p = 0.45). All patients received bilateral lung transplants and there was no appreciable difference in age or gender among the 3 groups. Median proximal and total acid contact times for the 3 groups are noted in Table 2. Acid contact times were significantly higher in the FUNDO group compared with either the GERD or No GERD group. Similarly the acid contact times were greater in the GERD group compared with the No GERD group. Additional confounding factors that are thought to influence BOS development, and therefore may play a role in posttransplantation allograft function include the lung allocation score, donor age, episodes of acute rejection, severe primary graft dysfunction, and CMV mismatch of donor and recipient. As can be found in Table 3, none of these factors was noted to be significantly different among the 3 groups.
Table 1.
Demographics for No GERD, GERD, and FUNDO Groups
| Recipient Characteristics | Normal Acid Contact (No GERD) | Elevated Acid Contact (GERD) | Fundoplication < 365 Days (FUNDO) | p Value |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (25) | 65 (22) | 157 (53) | |
| Age, median | 59 | 57 | 57 | 0.06 |
| Gender, n (%) | 0.96 | |||
| Women | 32 (42.7) | 29 (45.6) | 67 (42.7) | |
| Men | 43 (57.3) | 36 (54.4) | 90 (57.3) | |
| Ethnicity, n (%) | 0.25 | |||
| White | 65 (86.7) | 56 (86.2) | 145 (92.4) | |
| Nonwhite | 10 (13.3) | 9 (13.8) | 12 (7.6) | |
| Underlying disease, n (%) | 0.02 | |||
| COPD/A1A deficiency | 35 (47) | 20 (31) | 48 (31) | |
| CF/bronchiectasis | 7 (9) | 9 (14) | 38 (24) | |
| Interstitial pulmonary fibrosis | 27 (36) | 29 (45) | 64 (41) | |
| Primary pulmonary hypertension | 6 (8) | 7 (11) | 7 (4) | |
| Type of transplant, n (%) | 0.69 | |||
| Bilateral | 75 (100) | 65 (100) | 157 (100) | |
| Median follow-up, months | 39.5 | 49.2 | 37.6 | 0.45 |
A1A = alpha-1 antitrypsin deficiency; CF = cystic fibrosis; COPD = chronic obstructive pulmonary disorder; FUNDO = fundoplication; GERD = gastroesophageal reflux disease.
Table 2.
Esophageal Acid Contact Times for the No GERD, GERD, and FUNDO Groups
| Patient Position | No GERD | GERD | FUNDO | p Value |
|---|---|---|---|---|
| Acid contact times, % | ||||
| Proximal total, median | 0.2 (0.0–0.4) | 0.8 (0.45–1.2) | 0.85 (0.33–2.4) | 0.001 |
| Proximal upright, median | 0.3 (0.0–0.7) | 1.3 (0.75–2.0) | 1.5 (0.4–3.1) | 0.001 |
| Proximal supine, median | 0 (0.0–0.0) | 0.1 (0.0–0.4) | 0.0 (0.0–0.9) | 0.005 |
| Distal total, median | 0.9 (0.4–1.8) | 4.0 (2.4–6.3) | 9.1 (3.4–15) | 0.001 |
| Distal upright, median | 1.3 (0.6–2.8) | 4.5 (2.7–7.7) | 9.1 (4.3–16) | 0.001 |
| Distal supine, median | 0.0 (0.0–0.5) | 2.0 (0.15–4.8) | 5.3 (1.4–13) | 0.001 |
FUNDO = fundoplication; GERD = gastroesophageal reflux disease.
Table 3.
Potential Confounding Factors Affecting Allograft Function for the No GERD, GERD, and FUNDO Groups
| Confounding Factor | No GERD | GERD | FUNDO | p Value |
|---|---|---|---|---|
| Severe primary graft dysfunction requiring ECMO, n (%) | 4 (5.3) | 3 (4.6) | 2 (1.3) | 0.17 |
| CMV Mismatch (D + /R−), n (%) | 19 (25) | 14 (21) | 33 (21) | 0.75 |
| Acute rejection sum, median (IQR) | 4.0 (1–6) | 4.0 (1–6) | 4.0 (1–6) | 0.88 |
| Donor age, median (IQR) | 32 (22–46) | 30 (20–45) | 34 (22–47) | 0.52 |
| Lung allocation score, mean (SD) | 44 (15) | 41 (10) | 40 (10) | 0.18 |
CMV = cytomegalovirus; ECMO = extracorporeal membrane oxygenation; FUNDO = fundoplication; GERD = gastroesophageal reflux disease; IQR = interquartile range.
Acid Contact Time and Pulmonary Function
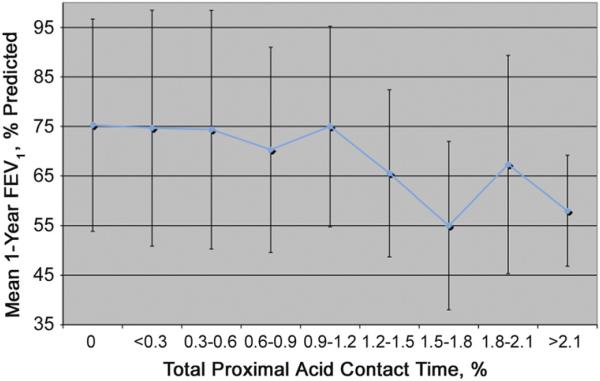
Among patients with GERD, mean 1-year FEV1 values decreased when total proximal acid contact times increased to greater than 1.19% (Fig 1). Similarly, mean peak FEV1 values decreased when proximal acid contact times increased to more than 1.19% (data not shown). Based on this threshold we then compared patients with proximal acid times less than 1.2% to patients with proximal acid times greater than or equal to 1.2% (Fig 2). The mean peak percent predicted FEV1 for recipients with a total proximal contact time less than 1.2% was 81% predicted compared with 72% predicted for recipients with proximal contact times greater than 1.2% (p = 0.04). Similarly the mean 1-year percent predicted FEV1 for recipients with a proximal contact time of less than 1.2% was 74% compared with 63% if greater than 1.2% (p = 0.04).
Fig 1.
Relationship of total proximal acid contact time to the mean percent predicted 1-year forced expiratory volume in first second of expiration (FEV1). Notably, the mean 1-year FEV1 decreases with total proximal acid contact times greater than 1.2%.
Fig 2.
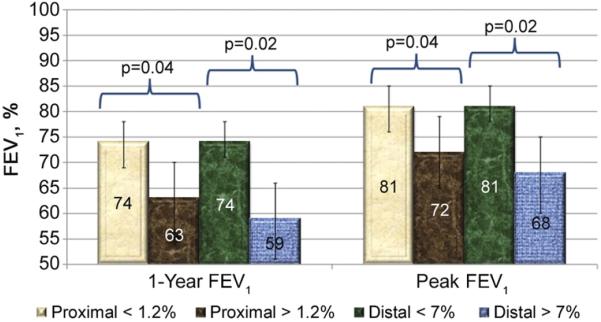
Acid contact time threshold for predicting allograft dysfunction was determined. Using 1.2% for total proximal acid contact times and 7% for total distal acid contact, clear distinctions were noted in both 1-year and peak forced expiratory volume in first second of expiration (FEV1) values.
A similar relationship was noted when total distal acid contact time and 1-year FEV1 were analyzed, with a threshold occurring at an acid contact time of 7.0% (data not shown). Based on this threshold we again divided recipients with GERD into 2 groups defined by a total distal acid contact time of greater or less than 7.0% (Fig 2). The mean 1-year FEV1 value for recipients with a distal acid contact time less than 7.0% was 74% but only 59% when acid contact times increased to greater than 7.0% (p = 0.02). Similarly the mean peak FEV1 for recipients with a distal contact time less than 7.0% was 81% compared with 68% for recipients with a distal contact time greater than or equal to 7.0% (p = 0.02).
Pulmonary Function and Fundoplication
Mean percent predicted 1-year FEV1 values were 76% for the No GERD group, 68% for the GERD group, and 77% for the FUNDO group (p = 0.003) (Fig 3). Of note, using multiple comparison testing, mean percent predicted 1-year FEV1 values for the No GERD versus GERD groups (p = 0.015) and FUNDO versus GERD groups (p = 0.0005) were significantly different; however there was no statistical difference between the No GERD and FUNDO groups (p = 0.80). Similarly the mean percent predicted peak FEV1 was 82% for the No GERD group, 75% for the GERD group, and 85% for the FUNDO group (p = 0.004). Again a difference between No GERD and GERD groups (p = 0.025), as well as the FUNDO and GERD groups (p = 0.001) was noted but not between the No GERD and FUNDO groups (p = 0.46).
Fig 3.
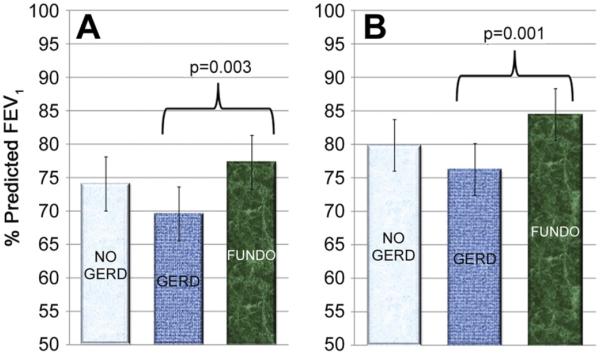
One-way analysis of variance demonstrating a significant difference between the GERD and No GERD and GERD and FUNDO groups for 1-year (A) and peak (B) FEV1. In neither instance was a difference noted between the No GERD and FUNDO groups.(FUNDO = fundoplicaion; GERD = gastroesophaeal reflux disease.)
We then created a multivariate model to determine the effects of reflux and fundoplication on the 1-year and peak percent predicted FEV1 measurements while controlling for potentially confounding effects of other variables, including recipient gender, recipient age, underlying diagnosis, and CMV status. In these models the GERD group was a significant independent predictor of 1-year (p = 0.002) and peak (p = 0.002) percent predicted FEV1 values. More specifically patients in the GERD group had an absolute decrease of 8.8% (95% confidence interval [CI], 2.4–15.2) in their mean percent predicted 1-year FEV1 compared with patients in the No GERD group (p = 0.01) and a 9.5% (95% CI, 3.9–15.0) lower percent predicted 1-year FEV1 than patients in the FUNDO group (p = 0.001). Similarly patients in the GERD group had an absolute decrease of 3.7% (95% CI, 1.9–9.3) in their mean percent predicted peak FEV1 compared with patients in the No GERD group (p = 0.20) and an 8.3% (95% CI, 3.4–13.1) lower peak FEV1 than patients in the FUNDO group (p = 0.001).
Comment
Chronic allograft rejection remains the most important contributor to lung transplant recipient morbidity and mortality. The clinical correlate, BOS, allows for a graded decline in pulmonary function that is useful both for monitoring patients clinically and as a tool for research purposes. Several groups have emphasized that certain nonimmune graft insults may have a role in BOS development. These include CMV pneumonitis, non-CMV viral infection, ischemia-reperfusion injury, and GERD [1, 2, 3, 5, 10 –13]. Earlier studies by our group demonstrated a relationship between GERD and premature graft dysfunction [3, 5]. A case report initially suggested that microaspiration events secondary to GERD might lead to graft failure [14]. After this correlation, we described a relationship between GERD and decreased FEV1 on formal pulmonary function testing in a small subset of patients, which improved after fundoplication [5]. Additional work from our group suggested that premature BOS secondary to GERD could be ameliorated with early fundoplication after transplantation [3].
Our work has been corroborated by findings from other transplantation centers that have also reported an association between GERD-related aspiration injury and BOS by examining bile acids in BAL samples. D'Ovidio and associates evaluated 120 BAL samples from lung transplant recipients at 3 months after transplantation. The 36 recipients with BOS had a significantly higher bile acid content compared with those without BOS. Moreover the recipients who experienced BOS within 12 months of their transplantation procedure had higher levels of bile acids compared with those who acquired BOS after 12 months [6].
Additionally animal models have been developed that demonstrate that repetitive microaspiration of gastric contents can induce lung injury. In 1 study involving orthotopic rat lung transplantation, chronic aspiration of gastric fluid led to more intense grades of acute rejection and fibrosis [15]. Similarly even with the use of cyclosporine for immunosuppression, chronic aspiration in the recipients led to monocytic infiltration of the bronchioles and increasing levels of fibrosis [16]. Both of these studies in rodent models of lung transplantation support the notion that repeated low-volume aspiration events can lead to obliteration of the bronchioles, the underlying pathologic condition that manifests clinically in humans as BOS.
In this study we tried to use a readily available clinical test, esophageal pH monitoring, as a surrogate marker for a recipient's risk of aspiration and subsequent allograft dysfunction. For our patients, distal and proximal total acid contact times predicted the pulmonary allograft function at 1 year, as well as the maximum allograft function as described by the peak percent predicted FEV1. Interestingly, even relatively mild abnormal increases in acid contact times (1.2% for proximal and 7% for distal) correlated with a diminished FEV1. Although based on these data we think that esophageal pH testing represents a ubiquitous clinical tool that can be used to help delineate recipients at high risk of GERD-associated allograft injury, this technique is only a surrogate marker for risk of repetitive aspiration of gastroduodenal contents.
The use of impedance testing for nonacid reflux may increase the sensitivity of identifying lung transplant recipients at risk. Although the acidity of the reflux was measured as a surrogate value for aspiration risk, acidity of the aspirated reflux material is not likely to be important. All of the patients in this study who had GERD and did not undergo fundoplication were essentially rendered achlorhydric through the use of medical therapy. The measurement of markers of aspiration such as bile, gastrin, and pepsin either in BAL fluid or in the exhaled gas is likely to be a more specific and possibly more sensitive predictor of aspiration-induced allograft injury. However those technologies are not yet widespread or reliable enough to be used in routine clinical practice, and there are insufficient data demonstrating their usefulness. Additionally their use to demonstrate the potential value of performing a fundoplication for a given patient has not been embraced by third-party payers or the surgical community performing the fundoplication procedures.
Despite mounting evidence from multiple transplantation centers demonstrating an association between GERD and diminished pulmonary function, no strategies have been demonstrated to assist in preventing GERD-associated allograft dysfunction. Medical management in the form of acid suppression and motility agents has clearly been ineffectual. The use of proton pump inhibitors after transplantation is routine at most centers, but does not abrogate the relationship between abnormal esophageal acid contact times and diminished FEV1. Animal models of aspiration injury show that the acidity of the aspirate is not the only contributor to lung injury [17]. In fact a neutral or alkaline gastric milieu may actually enhance the aspiration injury secondary to bacterial overgrowth normally suppressed by low gastric pH levels.
Conversely, fundoplication provides a physical barrier demonstrated to decrease the amount of esophageal reflux material. Based on preliminary data from earlier studies, we hypothesized that a timely and efficacious fundoplication in lung transplant recipients with GERD would help alleviate the negative effects of acid reflux on pulmonary function. Indeed in the current study, the patients who underwent fundoplication demonstrated a better 1-year FEV1 than those patients with medically managed GERD who did not undergo fundoplication. More importantly those undergoing fundoplication attained a higher percent predicted peak FEV1 than those with GERD who were managed medically. These findings are despite the FUNDO group having significantly higher acid contact times than the medically managed GERD group and held true when modeling to control for other predictors of FEV1, including underlying diagnosis.
The current study has limitations. First it was retrospective, which can lead to unanticipated biases. For example, we could not always identify why patients in the GERD group did not undergo fundoplication. Some may have been offered antireflux surgery and simply refused. Looking at our data, 1 obvious difference between the GERD and FUNDO groups is the severity of reflux. The FUNDO group had twice the acid contact times that the GERD group had, suggesting that severity of acid contact played a critical role in determining eligibility for fundoplication. Also there may have been some recipients who experienced perioperative complications that may have precluded fundoplication, regardless of their esophageal pH status. By using only those patients who survived to 1 year, we minimized the confounding effects of perioperative complications and their role in allograft function.
Our data are not robust enough to analyze for the optimal timing of fundoplication because most of our recipients underwent fundoplication less than 3 months after transplantation. However the effect of reflux and fundoplication may be more important the further out from transplantation the patients get. This correlates clinically if the fundoplication can reduce the amount of repetitive microaspiration thought to be occurring and suggests that the earlier the fundoplication can safely be performed, the more advantageous for the recipient it would be. Another limitation to this study is that we did not have confirmatory tests as to the efficacy of the fundoplication procedures. Most patients elect not to subject themselves to additional invasive testing, particularly if they are doing well clinically. Likewise third-party payers oftentimes will not support additional testing without a clinical indication.
In conclusion, the lung allograft differs from other transplanted solid organs because of the constant exposure to environmental elements. GERD represents a very common and potentially caustic exposure. Routine esophageal pH monitoring can help predict pulmonary allograft dysfunction. In fact even relatively mild abnormal elevations in acid contact times were associated with worse allograft function in our study. Fundoplication, probably through its mechanical barrier to reflux and subsequent reduction in the amount of gastroduodenal aspiration, improves 1-year and peak pulmonary allograft function.
Footnotes
Presented at the Fifty-seventh Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, Nov 3–6, 2010.
References
- 1.Girgis RE, Tu I, Berry GJ, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–8. [PubMed] [Google Scholar]
- 2.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., 3rd Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114:195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 3.Cantu E, Appel JZ, Hartwig MG, et al. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142–51. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–93. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 5.Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533–42. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 6.D'Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiov Surg. 2005;129:1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–93. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SM, Perfect JR, Howell DN, et al. Candidal anastomotic infection in lung transplant recipients: successful treatment with a combination of systemic and inhaled anti-fungal agents. J Heart Lung Transplant. 1998;17:1029–33. [PubMed] [Google Scholar]
- 9.Richter JE. Ambulatory esophageal pH monitoring. Am J Med. 1997;103:130S–4S. doi: 10.1016/s0002-9343(97)00338-0. [DOI] [PubMed] [Google Scholar]
- 10.Bando K, Paradis IL, Komatsu K, et al. Analysis of time-dependent risks for infection, rejection, and death after pulmonary transplantation. J Thorac Cardiovasc Surg. 1995;109:49–57. doi: 10.1016/s0022-5223(95)70419-1. discussion 57–9. [DOI] [PubMed] [Google Scholar]
- 11.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21:559–66. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 12.Hadjiliadis D, Howell DN, Davis RD, et al. Anastomotic infections in lung transplant recipients. Ann Transplant. 2000;5:13–9. [PubMed] [Google Scholar]
- 13.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119:1277–80. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 14.Palmer SM, Miralles AP, Howell DN, Brazer SR, Tapson VF, Davis RD. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest. 2000;118:1214–7. doi: 10.1378/chest.118.4.1214. [DOI] [PubMed] [Google Scholar]
- 15.Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiov Surg. 2006;131:209–17. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614–21. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popper H, Juettner F, Pinter J. The gastric juice aspiration syndrome (Mendelson syndrome). Aspects of pathogenesis and treatment in the pig. Virchows Arch A Pathol Anat Histopathol. 1986;409:105–17. doi: 10.1007/BF00705410. [DOI] [PubMed] [Google Scholar]