Abstract
Purpose
Establishing minimally clinically important difference (MCID) for patient-reported outcomes questionnaires is an important component of outcomes research to understand treatment effectiveness from the patient’s perspective. For patients with ulnar neuropathy at the elbow (UNE), these assessments are vital to examine how much change in the questionnaire scores equate to patient satisfaction.
Methods
We calculated the change in scores of Michigan Hand Outcomes Questionnaire (MHQ), Disabilities of the Arm, Shoulder and Hand questionnaire (DASH), and Carpal Tunnel Questionnaire (CTQ) from preoperative to 3,6 and 12 months postoperatively after ulnar nerve simple decompression procedure. We used the anchor based approach of receiver operating characteristic curves to determine the MCID.
Results
On average, MCID of 10, 12, and 7 points were identified for pain, function, and ADL domains of MHQ. Similarly, DASH, CTQ-symptom severity scale, and CTQ-function severity scale had an average MCID of 7, 0.7, and 0.3 points respectively. At the 3, 6, and 12 months’ time-points, an MCID of 9, 8, and 13 points for pain, 12, 12, and 12 points for function, and 6, 8, and 6 points for ADL domains of the MHQ were identified; similarly an MCID of 8, 7, and 7 points for DASH; 0.4, 0.7, and 0.7 points for CTQ- symptom severity scale; and 0.3, 0.3, and 0.4 points for CTQ-function severity scale were established.
Conclusion
The smaller MCIDs of MHQ, DASH, and even smaller MCIDs of CTQ found in our study indicate that a small change in the scores identified satisfied patients. Simple decompression surgery for UNE produced patient satisfaction with only a small change in their questionnaire scores. The implications of this finding are that simple decompression surgery for UNE is a highly effective procedure and the outcomes questionnaires used are highly responsive, which minimizes sample size requirements for future research studies relating to UNE.
Keywords: Minimal clinically important difference, Ulnar neuropathy at elbow, CTQ, DASH, MHQ
Patient-reported outcome (PRO) measures are commonly used to assess treatment effectiveness in clinical practice and research. PRO measures are easier and more efficient to use than physical assessments, particularly for studying outcomes of peripheral neuropathy such as carpal tunnel syndrome. (1) Reliability, validity and responsiveness to change are the 3 properties of an outcome instrument (questionnaire) that are commonly evaluated for various diseases. (2) Reliability and validity are constantly established by researchers in several disease settings but responsiveness to change is often difficult to measure and often not tested. Minimal clinically important difference (MCID) is an aspect of the PRO that helps in establishing the responsiveness to change in a questionnaire. It is the smallest change in scores perceived as beneficial by patients and would initiate a change in patient’s management in the absence of side effects and excessive cost. (3) This clinically relevant change as ascertained by patient is independent of statistical significance, as the later relies on sample size in the study. (4) In addition to presenting the minimum change important to a patient, MCID also informs about the minimum sample size required to achieve the clinically significant effect in a clinical trial.
Ulnar neuropathy at the elbow (UNE) is the second most common compressive peripheral neuropathy. (5) Patients often present with numbness and tingling of the ring and small finger accompanied by pain and weakness of these fingers. However, despite the 1% prevalence of this condition in the general population, (6) treatment effectiveness research for this disease lags behind the extensive amount of outcomes research for carpal tunnel syndrome, even though both have similar presentations marked by symptom and functional changes after treatment.
The aim of this study was to determine the MCID of 3 common questionnaires used in hand surgery, the Michigan Hand Outcomes Questionnaire (MHQ), the Disabilities of the Arm, Shoulder and Hand questionnaire (DASH), and the Carpal Tunnel Questionnaire (CTQ) to assess PRO after surgical treatment for UNE. The MCIDs derived can be used to guide future research on the effectiveness of treatment using PRO questionnaires.
METHODS
Study Cohort
Data for this project were obtained from a prospective cohort study conducted by a multicenter collaborative Surgery of the Ulnar Nerve (SUN) group. Five centers enrolled subjects with electrodiagnostically confirmed UNE and were subsequently treated with simple decompression surgery. All the centers obtained institutional review board approvals and informed consent documents from each of the enrolled subjects for this study. Inclusion criteria for this study were 18 years of age or older, ability to read, understand, and complete the questionnaires in English, electrodiagnostic confirmation of UNE, and treatment with simple decompression surgery. Exclusion criteria included history of trauma to the injured elbow, recurrent UNE or prior ulnar nerve surgery on the same elbow and concomitant conditions, such as carpal tunnel syndrome in the affected extremity, history of dementia, Alzheimer disease, traumatic brain injury or other serious psychiatric disorders, and history of substance abuse.
Questionnaire assessments
All the assessments were performed preoperatively and at 6 weeks, 3 months, 6 months, and 12 months postoperatively. We chose 3 common questionnaires that are used in outcome assessments of upper limb injuries, the MHQ, the DASH, and the CTQ. These were administered to all participants at the same time points as the physical assessments. The MHQ is a hand-specific outcomes instrument with 37 core questions in the 6 domains consisting of overall hand function, activities of daily living (ADL), pain, work performance, aesthetics, and patient satisfaction with hand function. (7, 8) Raw scores are converted to scores ranging from 0–100, with 0 being the worst score and 100 being the best. However, the pain domain scores are reversed and recoded. If 50% or more of the data items in a scale were missing, then that particular scale was not scored. For scales with less than 50% data missing, the average of the existing scale items was assigned for the missing items. (9) We calculated the MCID of MHQ domains; pain, ADL, and overall function to represent symptom and functional components.
The CTQ, a self-administered questionnaire consists of 2 components, symptom severity scale (SSS) with 11 questions and functional status scale (FSS) with 8 questions. Each question is scored on a 5 point scale (range 1–5) based on the severity of the disease. The individual scores are added and divided by the number of questions to give a final score for each scale, range 1–5, with a higher score indicating a worse condition. (10, 11)
The DASH is a self-reported questionnaire to measure physical function and symptoms in patients with musculoskeletal disorders of the upper limb. It consists of 30 questions, scored from 1–5. The scores of 2 optional modules of work and sports/music were not considered for the analysis of MCID. At least 27 questions have to be completed to obtain a DASH score for a patient. Final scores, range 0–100, were calculated from the raw score using the formula, DASH disability/symptom score = [(sum of n responses/n) −1] × 25, where n is the number of completed responses. (12)
Determination of MCID
We used statistical software to compute the final scores of MHQ domains and calculated the final scores of DASH and CTQ manually using the formulas mentioned above. We determined the change in questionnaire scores from pre-operative to 3, 6, and 12 months respectively after surgery. We used change in scores in the satisfaction domain of MHQ as an anchor to compare the change in questionnaire scores if they reflected the true change as perceived by the patient. Change in satisfaction scores of MHQ at 3, 6, and 12 months were calculated and divided by baseline standard deviation of the satisfaction scores to obtain effect size. This was used as a cut-off value to classify patients with questionnaire scores at an effect size of 0.8 or greater (Cohen’s large effect) to be considered satisfied and effect size below 0.8 to be considered as not satisfied with the treatment provided. (13) This method has been previously used to derive MCID of MHQ in patients with different hand conditions. (13,14) This essentially provided us with a dichotomous satisfaction variable to plot receiver operating characteristic (ROC) curves for MHQ domains, DASH, and CTQ-SSS and CTQ-FSS at 3, 6, and 12 months. The ROC curve is essentially a sequence of possible cut-off values in questionnaire change scores. The optimal cut-off point, with maximum sensitivity and specificity situated highest on the upper left side of the ROC curve represents MCID. (2) In addition, the area under the curve (AUC) denotes the probability of scores accurately differentiating improved and unimproved patients. AUC ranges from 0.5 to 1, with a score of 0.7 to 0.8 considered as an acceptable discriminating range and 0.8 to 0.9 considered as an excellent discriminating range. (15) We also performed paired sample t-tests to compare the means of change in satisfaction scores before and after surgery.
RESULTS
The demographic data of the subjects enrolled are presented in Table 1. The mean satisfaction score of MHQ had a significant improvement from 35 points before surgery to 68 points at 3 months after surgery, with a mean increase of 33 points and a standard deviation of 32 points (P<0.001). Similarly, at 6 and 12 months, there was a significant improvement from 35 points before surgery to 69 and 66 points (P<0.001, P<0.001) with a mean increase of 34 and 31 points and standard deviation of 26 and 27 points respectively. The cut-off point to categorize patients into satisfied or not was chosen to be 0.8 of the baseline standard deviation (Cohen’s large effect) of MHQ satisfaction domain. The number of patients satisfied and not satisfied according to each questionnaire at the 3 time points is presented in Table 2.
Table 1.
Participant Demographic Data
| MHQ | DASH | CTQ | |
|---|---|---|---|
| Number of patients | 41 | 38 | 40 |
| Mean Age, y (range) | 54.9 (23–72) | 49.3 (23–70) | 50.3 (23–72) |
| Affected hand | |||
| Right | 17 (40%) | 16(41%) | 17(41%) |
| Left | 24 (60%) | 22(59%) | 23(51%) |
| Severity of disease* | |||
| Mild | 16 | 16 | 16 |
| Moderate | 12 | 11 | 11 |
| Severe | 11 | 9 | 11 |
| Baseline PRO scores Mean (SD) | Pain: 49 (25.7) | 31.1(18.1) | CTQ-SSS: 2.6 (0.7) |
| Function: 50.6 (22.9) | CTQ-FSS: 2.0 (0.7) | ||
| ADL: 64.8 (22.7) | |||
| Change in PRO scores at 12 months Mean (SD) | Pain: 20 (25.5) | 12.6 (16.8) | CTQ-SSS: 0.7 (1) |
| Function: 21.3 (25) | CTQ-FSS: 0.4 (0.6) | ||
| ADL: 12 (19) | |||
MHQ- Michigan Hand Outcomes Questionnaire; DASH- Disabilities of the Arm, Shoulder and Hand; CTQ- Carpal Tunnel Questionnaire; PRO- Patient reported questionnaires;
Two patients with missing data on severity of disease; SD- Standard Deviation
Table 2.
Sample sizes of the subjects
| Time point | Satisfied | Not satisfied | |
|---|---|---|---|
| MHQ-Pain | 3 months | 29 | 12 |
| 6 months | 33 | 9 | |
| 12 months | 32 | 10 | |
| MHQ-Function | 3 months | 29 | 11 |
| 6 months | 32 | 9 | |
| 12 months | 31 | 10 | |
| MHQ-ADL | 3 months | 29 | 12 |
| 6 months | 33 | 9 | |
| 12 months | 32 | 10 | |
| DASH | 3 months | 28 | 10 |
| 6 months | 31 | 8 | |
| 12 months | 30 | 9 | |
| CTQ-SSS | 3 months | 28 | 12 |
| 6 months | 31 | 10 | |
| 12 months | 31 | 10 | |
| CTQ-FSS | 3 months | 28 | 12 |
| 6 months | 32 | 9 | |
| 12 months | 31 | 10 |
MHQ- Michigan Hand Questionnaire; DASH- Disabilities of the Arm, Shoulder and Hand; CTQ- Carpal Tunnel Questionnaire; SSS-Symptom Severity Scale; FSS- Functional Status Scale
MHQ
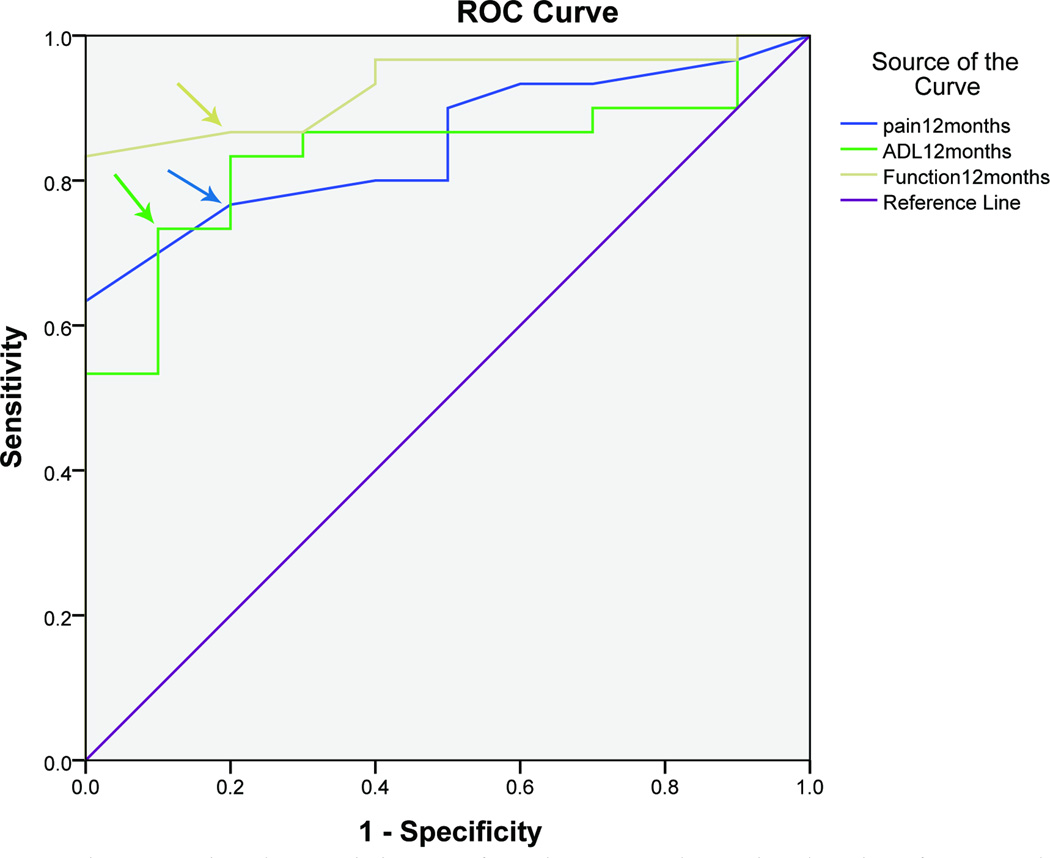
An MCID of 9, 8 and 13 points for pain domain, 12, 12, and 12 points for function domain and 6, 8, and 6 points for ADL domain were determined at 3, 6 and 12 months respectively. The MHQ domains were discriminating excellently between satisfied and dissatisfied patients with an AUC greater than 0.8 at all the time points. (Figures 1, Table 3)
Figure 1.
Receiver operating characteristic curve of Michigan Hand Outcomes Questionnaire domains, pain, activities of daily living, function, and patient satisfaction 12 months after surgery in UNE patients (n=41 patients)
Table 3.
Summary Results of MCIDs of 3 questionnaires
| Sensitivity | Specificity | AUC | MCID | ||
|---|---|---|---|---|---|
| MHQ-Pain | (100 point scale) | ||||
| 3 Months | 0.82 | 0.92 | 0.89 | 9 | |
| 6 months | 0.77 | 0.78 | 0.84 | 8 | |
| 12 months | 0.76 | 0.80 | 0.85 | 13 | |
| MHQ-Function | |||||
| 3 Months | 0.89 | 0.75 | 0.88 | 11 | |
| 6 months | 0.80 | 0.78 | 0.87 | 11 | |
| 12 months | 0.86 | 0.80 | 0.93 | 13 | |
| MHQ-ADL | |||||
| 3 Months | 0.89 | 1.00 | 0.95 | 6 | |
| 6 months | 0.80 | 0.78 | 0.88 | 8 | |
| 12 months | 0.73 | 0.90 | 0.83 | 6 | |
| DASH | |||||
| 3 Months | 0.85 | 0.90 | 0.89 | 8 | |
| 6 months | 0.61 | 0.50 | 0.63 | 7 | |
| 12 months | 0.73 | 0.89 | 0.75 | 7 | |
| CTQ-SSS | 5 point scale | 100 point scale | |||
| 3 Months | 0.78 | 0.75 | 0.83 | 0.4 | 8 |
| 6 months | 0.64 | 0.67 | 0.67 | 0.7 | 14 |
| 12 months | 0.63 | 0.70 | 0.63 | 0.7 | 14 |
| CTQ-FSS | |||||
| 3 Months | 0.67 | 0.83 | 0.81 | 0.3 | 6 |
| 6 months | 0.61 | 0.67 | 0.75 | 0.3 | 6 |
| 12 months | 0.56 | 0.70 | 0.73 | 0.4 | 8 |
MHQ- Michigan Hand Questionnaire; ADL- Activities of Daily Living; DASH- Disabilities of the Arm, Shoulder and Hand; CTQ-Carpal tunnel Questionnaire; SSS-Symptom Severity Scale; FSS- Functional Status Scale; AUC-Area Under the Curve; MCID- Minimal Clinically Important Difference
DASH
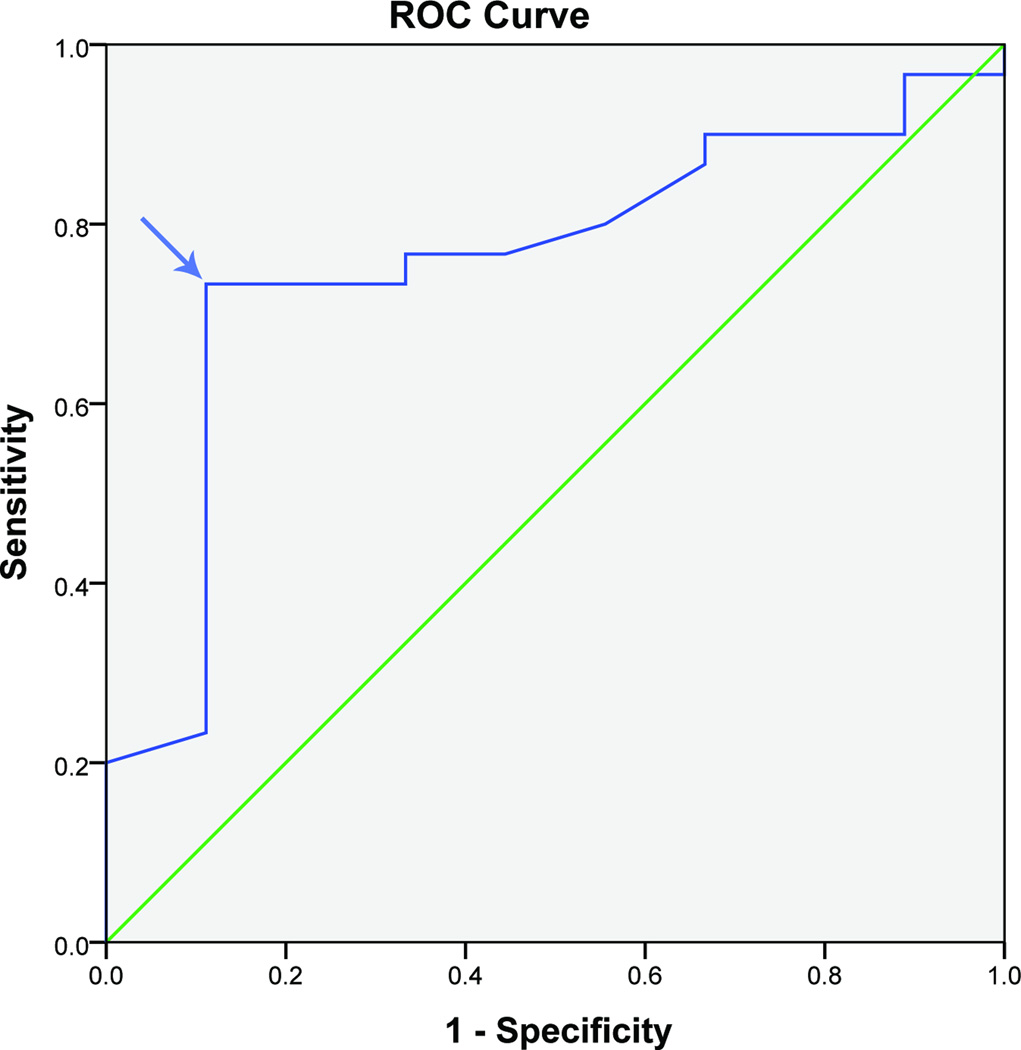
The number of satisfied patients differs from that of MHQ because some of the patients did not complete all the questionnaires. An MCID of 8, 7, and 7 points for DASH were determined at 3, 6, and 12 months. (Figure 2, Table 3) The DASH was discriminating acceptably between patients at 3 and 12 months with an AUC greater than 0.7 but not so good at 6 months with an AUC of 0.6.
Figure 2.
Receiver operating characteristic curve of DASH questionnaire and patient satisfaction at 12 months after surgery in UNE patients (n= 38 patients)
DASH: Disabilities of the Arm, Shoulder and Hand
CTQ-SSS
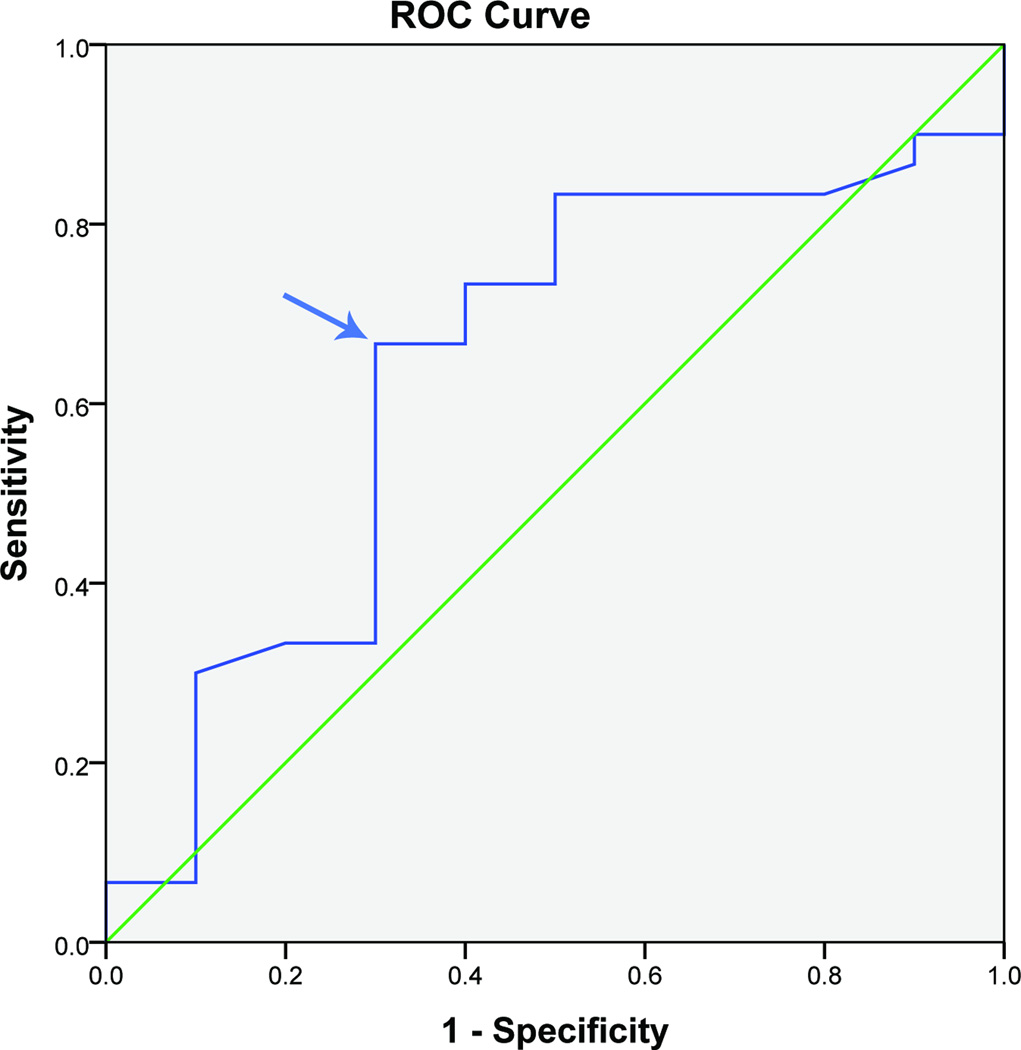
An MCID of 0.4, 0.7, and 0.7 points on a 5 point scale (8, 14, and 14 on a 100 point scale) for CTQ-SSS were determined at 3, 6, and 12 months respectively. (Figure 3, Table 3) The CTQ-SSS was able to discriminate between patients excellently at 3 months (AUC 0.8) but not so good at 6 and 12 months (AUC 0.6).
Figure 3.
Receiver operating characteristic curve of CTQ-SSS and patient satisfaction at 12 months after surgery in UNE patients (n= 40 patients)
CTQ-SSS: Carpal Tunnel Questionnaire-Symptom severity scale
CTQ-FSS
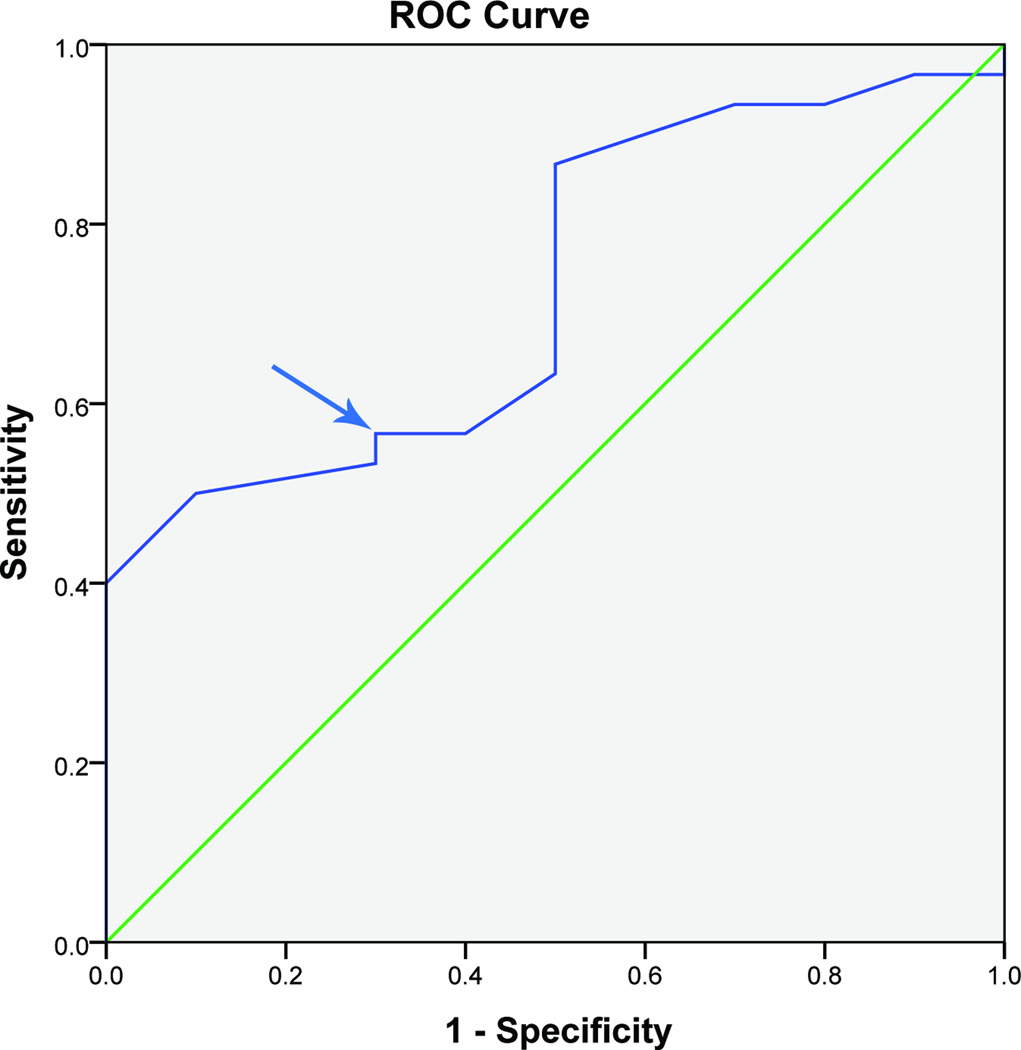
An MCID of 0.3, 0.3, and 0.4 points on a 5 point scale (6, 6, and 8 points on a 100 point scale) for CTQ-FSS were determined at 3, 6, and 12 months with an AUC of 0.8, 0.7, and 0.7 at the 3 time points respectively. (Figure 4, Table 3) The CTQ-FSS was able to discriminate between patients well at 3 months and acceptably at 6 and 12 months.
Figure 4.
Receiver operating characteristic curve of CTQ-FSS and patient satisfaction at 12 months after surgery in UNE patients (n= 40 patients)
CTQ-FSS: Carpal Tunnel Questionnaire-Functional status scale
In summary, an MCID of a 10, 12, and 7 points for MHQ pain, function, and ADL domains respectively, on average meant an improvement of those points after surgery was important to the patient. Similarly for DASH, an MCID of 7 points on average signified that a decrease of 7 points after surgery was considered important by the patient. A decrease of 0.6 points on average in CTQ-SSS scores and a decrease of 0.3 points on average in CTQ-FSS after surgery were considered important by the patients.
DISCUSSION
Our study determined the MCID of 3 outcomes questionnaires used in hand conditions for a sample of patients who underwent simple decompression surgery for UNE. The questionnaires used showed meaningful clinical improvement after the surgical procedure. Patients perceived an important clinical change after the surgical intervention through a small change in scores as evident by the MCIDs of 8, 7, and 12 points on MHQ domains and 7 points on DASH. Patients with UNE are treated conservatively and proceed to surgery if symptoms persist. Our study cohort achieved adequate symptom relief after surgery such that satisfied patients are identified with even smaller changes in questionnaire scores.
The MCIDs of CTQ in our cohort were found to be even smaller, 0.6 and 0.3 points (SSS and FSS scales) based on the 5 point scale. Conversion to a 100 point scale similar to the MHQ and DASH resulted in an MCID of 12 and 6 points for CTQ respectively, which are quite consistent with the findings for the MHQ and DASH. In CTS, MCID of 1.0 points for CTQ-SSS and MCIDs of 23, 13, and 8 points for pain, function, and work domains of MHQ were established in previous studies. (2, 13)
In 2012, a systematic review and meta-analysis of randomized controlled clinical trials conducted in UNE were performed to determine the effectiveness and safety of available conservative and surgical treatments for UNE. The review concluded that there was no optimal treatment for this condition based on clinical, neurophysiological, and imaging characteristics alone with the existing evidence. (16) The first step in deriving evidence for the treatment of UNE is to establish the MCIDs of potential outcomes instruments that can be applied for this disease to derive sample size estimates when structuring a multicenter clinical trial to determine the optimal treatment of this disease.
MCID achieves several functions beyond serving as a measure for meaningful clinical improvement. First, MCID helps to estimate the required sample size so that a study has sufficient power to detect the true differences and therefore assess treatments better. (17,18) It is encouraging that there is an increase in the number of surgical trials including sample size estimates in the manuscripts over the last 3 decades; however, a study conducted by Kashani et al. revealed that only 21% of the surgical trials considered MCID in their sample size calculations. (17) For to be adequately powered and not report negative results, appropriate MCID values should be considered in the sample size estimation at the beginning of trials.
Second, the ability to interpret study results accurately increases by understanding implication of the change of scores. It would be quick and simple to determine the patient satisfaction achieved and success of a treatment by comparing the scores to that of the condition of interest. (18) It also accounts for the patient’s perception of function, quality of life, and an overall feeling of wellbeing as a result of the treatment. Third, MCID can assess the responsiveness of measuring tools in this context of outcomes questionnaires in detecting a real change that has occurred.
Despite several advantages, MCID has some limitations. MCID is not a fixed value, it varies with several assessment methods used to determine it. Some suggest using both anchor and distribution methods to derive MCID that incorporates an external criterion (anchor) and a variability measure (distribution). (19, 20) There exists a need to identify an ideal method to quantify MCID through further research. The methodological limitation of our study was that we used only the anchor based approach to determine MCIDs of the questionnaires. Another limitation in our study was that we used the scores of satisfaction domain of MHQ as a standard to plot ROC curves and derive MCID for all the questionnaires. Our approach is valid in using satisfaction scores of MHQ as standard to represent patient satisfaction for other questionnaires because the DASH and CTQ do not have any satisfaction domain and all the 3 questionnaires were administered at the same time to participants at each visit.
Owing to the fluidity of the MCID concept, one should be cautious when assessing interventions based on MCID to not be overly critical if the study participants have not attained the required MCID in an intervention group. In our study, the questionnaires used had good discriminating ability between satisfied and not satisfied patients and the majority of patients were satisfied with the surgical procedure on all the questionnaires at the 3 time points they were estimated. We therefore conclude that estimation of MCID was relevant and useful in our study cohort in the absence of a standardized assessment tool specific for this condition.
Acknowledgement
This project was supported by the Ruth L. Kirschstein National Research Service Awards for Individual Postdoctoral Fellows (1F32AR058105-01A1) (to Dr. Jae W. Song) and by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) and a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute of Aging (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06) (to Dr. Kevin C. Chung). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank the following study coordinators for their assistance: Patricia Burns, MPH, Connie McGovern, Miriana Popadich, RN, BSN, Ann E. Coppage, PA-C, Mollie K. Hanlon, NP-C, MSN, MBA, Benjamin Connell, BA, Sara Defendorf, BS, and Allison W. McIntyre, MPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pynsent PB. Choosing an outcome measure. J Bone Joint Surg Br. 2001;83(6):792–794. doi: 10.1302/0301-620x.83b6.11973. [DOI] [PubMed] [Google Scholar]
- 2.Ozyurekoglu T, McCabe SJ, Goldsmith LJ, LaJoie AS. The minimal clinically important difference of the Carpal Tunnel Syndrome Symptom Severity Scale. J Hand Surg Am. 2006;31(5):733–738. doi: 10.1016/j.jhsa.2006.01.012. discussion 739–740. [DOI] [PubMed] [Google Scholar]
- 3.Cook CE. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J Man Manip Ther. 2008;16(4):E82–E83. doi: 10.1179/jmt.2008.16.4.82E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. Comparative responsiveness and minimal change for the Oxford Elbow Score following surgery. Qual Life Res. 2008;17(10):1257–1267. doi: 10.1007/s11136-008-9409-3. [DOI] [PubMed] [Google Scholar]
- 5.Mondelli M, Padua L, Giannini F, Bibbo G, Aprile I, Rossi S. A self-administered questionnaire of ulnar neuropathy at the elbow. Neurol Sci. 2006;27(6):402–411. doi: 10.1007/s10072-006-0719-3. [DOI] [PubMed] [Google Scholar]
- 6.Shin R, Ring D. The ulnar nerve in elbow trauma. J Bone Joint Surg Am. 2007;89(5):1108–1116. doi: 10.2106/JBJS.F.00594. [DOI] [PubMed] [Google Scholar]
- 7.Chung KC, Hamill JB, Walters MR, Hayward RA. The Michigan Hand Outcomes Questionnaire (MHQ): assessment of responsiveness to clinical change. Ann Plast Surg. 1999;42(6):619–622. doi: 10.1097/00000637-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23(4):575–587. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 9.MHQ. Michigan Hand Outcomes Questionnaire. [Accessed June 7, 2012]; http://sitemaker.umich.edu/mhq/ [Google Scholar]
- 10.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585–1615. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Gelberman RH, Wright EA, Lew RA, Liang MH. Responsiveness of self-reported and objective measures of disease severity in carpal tunnel syndrome. Med Care. 1994;32(11):1127–1133. doi: 10.1097/00005650-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 12.The DASH Outcome Measure. [Accessed July 10, 2012]; http://www.dash.iwh.on.ca/home. [Google Scholar]
- 13.Shauver MJ, Chung KC. The minimal clinically important difference of the Michigan hand outcomes questionnaire. J Hand Surg Am. 2009;34(3):509–514. doi: 10.1016/j.jhsa.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waljee JF, Chung KC. Objective functional outcomes and patient satisfaction after silicone metacarpophalangeal arthroplasty for rheumatoid arthritis. J Hand Surg Am. 2012;37(1):47–54. doi: 10.1016/j.jhsa.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Caliandro P, La Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD006839.pub3. CD006839. [DOI] [PubMed] [Google Scholar]
- 17.Kashani I, Hall JL, Hall JC. Reporting of minimum clinically important differences in surgical trials. ANZ J Surg. 2009;79(4):301–304. doi: 10.1111/j.1445-2197.2009.04865.x. [DOI] [PubMed] [Google Scholar]
- 18.Chuang-Stein C, Kirby S, Hirsch I, Atkinson G. The role of the minimum clinically important difference and its impact on designing a trial. Pharm Stat. 2011;10(3):250–256. doi: 10.1002/pst.459. [DOI] [PubMed] [Google Scholar]
- 19.de Vet HC, Ostelo RW, Terwee CB, van der roer N, Knol DL, Beckerman H, et al. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131–142. doi: 10.1007/s11136-006-9109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatchel RJ, Mayer TG, Chou R. What does/should the minimum clinically important difference measure? A reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain. 2012;28(5):387–397. doi: 10.1097/AJP.0b013e3182327f20. [DOI] [PubMed] [Google Scholar]