Abstract
Purpose
The effect of sleep quality on asthma control independent from common comorbidities like gastroesophageal reflux disease (GERD) and obstructive sleep apnea (OSA) is unknown. This study examined the association between sleep quality and asthma control and quality of life after accounting for OSA and GERD in non-severe (NSA) and severe (SA) asthma.
Methods
Cross-sectional data from 60 normal controls, 143 with NSA, and 79 with SA participating in the Severe Asthma Research Program was examined. Those who reported using positive airway pressure therapy or were at high risk for OSA were excluded.
Results
Both SA and NSA had poorer sleep quality than controls, with SA reporting the worst sleep quality. All asthmatics with GERD and 92% of those without GERD had poor sleep quality (p =.02). The majority (88%–100%) of NSA and SA participants who did not report nighttime asthma disturbances still reported having poor sleep quality. In both NSA and SA, poor sleep quality was associated with worse asthma control and quality of life after controlling for GERD and other covariates.
Conclusions
These results suggest that poor sleep quality is associated with poor asthma control and quality of life among asthmatics and cannot be explained by comorbid GERD and nighttime asthma disturbances.
Keywords: Asthma control, Gastroesphogeal reflux disease, Sleep
Asthma is a chronic inflammatory disease of the airways characterized by reversible airflow obstruction and bronchial hyperresponsiveness. The majority of the asthma population has mild to moderate asthma that can be controlled with conventional therapy. However, about 5–10% of patients in the United States have severe asthma that remains symptomatic as standard therapies are unable to completely control symptoms. Although severe asthma represents a small proportion of all asthma, it accounts for a disproportionate share of the health care costs associated with this disease[1]. Control of asthma is the main goal of therapy, but many patients with severe asthma remain symptomatic or uncontrolled despite appropriate treatments[2]. Severe asthma that is uncontrolled is associated with substantially more healthcare costs and an increased risk of severe asthma-related healthcare events including unscheduled office visits, emergency room visits, and hospitalizations as compared to controlled severe asthma[3].
Asthma control is a complex clinical problem with multiple contributing factors. Apart from disease severity or resistance to therapy, poor asthma control may be due to poor adherence, poor inhaler technique, smoking, environmental triggers, and patients’ knowledge and attitudes[4]. Additionally, common comorbidites in asthma such as gastroesophageal reflux disease (GERD), obstructive sleep apnea (OSA), and chronic rhinitis affect the airways and can complicate asthma control[5–7]. Sleep quality, independent of OSA, may be another factor affecting asthma control. Sleep disturbances, such as difficulty initiating and maintaining sleep and early morning awakenings, are commonly reported by patients with asthma[8–11]. Although nocturnal exacerbations can indicate inadequate asthma control and disturb sleep, poor sleep has been reported in patients with well-controlled asthma suggesting that poor sleep may be independent from nocturnal asthma symptoms[8, 12]. In the few studies that investigated the association between sleep quality and asthma control, the potential effects of common comorbidities were not taken into account when examining this relationship[8, 10, 11]. Given that GERD and OSA can disturb sleep[13, 14], it is unknown if poor sleep in asthma is distinct from the effects of comorbidities and thus can independently affect asthma control.
The present study examined if sleep quality is a predictor of asthma control and quality of life after controlling for GERD and other known risk factors in individuals with non-severe (NSA) and severe (SA) asthma. We excluded participants using positive airway pressure therapy and those at high risk for OSA, to eliminate the effect of OSA on both sleep quality and asthma control. Given that GERD is highly prevalent in asthma and can disturb sleep[13, 14], we explored whether the presence of GERD can account for all complaints of poor sleep in NSA and SA. Additionally, we sought to determine if poor sleep quality was present in those who do not report nighttime asthma disturbances. We hypothesized that poorer sleep quality will be independently associated with worse asthma control and quality of life, and that poor sleep quality will not be entirely explained by GERD, OSA, or nighttime asthma disturbances.
Materials and Methods
Study population
The data in this study was obtained from participants enrolled in the Severe Asthma Research Program (SARP), a multi-center collaborative study sponsored by the National Heart, Lung, and Blood Institute[15]. Current smokers or persons with 5 or more pack-years of tobacco use were excluded from SARP[15]. Participants were categorized as normal controls (NC), NSA, and SA. Normal controls were healthy individuals with normal lung function and a negative methacholine challenge and no history of asthma. Participants with asthma were evaluated and classified according to the American Thoracic Society’s definition of refractory asthma[16]. Participants with SA were required to meet one of the 2 major criteria (continuous oral corticosteroid use or high-dose inhaled corticosteroid use) and at least 2 of the 7 minor criteria. Those not meeting criteria for SA were categorized as NSA.
The goal of SARP is to determine factors that differentiate individuals with severe from those with milder asthma and to understand pathophysiologic mechanisms in severe asthma. Participants were enrolled from 4 study sites located in Pittsburgh, PA, Wake Forest University, University of Wisconsin, and the Cleveland Clinic. All studies were approved by the local institutional review boards, and all participants gave informed consent. Out of 1562 participants, 438 participants had complete data on the variables of interest. Those younger than 18 years of age were excluded (n=69). In order to eliminate the known effects of OSA on sleep quality and continuity[13], participants who reported using continuous positive airway pressure or bilevel positive airway pressure (n=15) or were at high risk for OSA (according to the Sleep apnea Scale of the Sleep Disorders Questionnaire[17]: ≥ 36 for men and ≥ 32 for women) (n=72) were excluded from the analyses, thus a total of 282 participants were included in the current study.
Data Collection and Measures
The current study used cross-sectional data from a retrospective cohort of participants in SARP. All participants completed comprehensive questionnaires, allergy skin testing, blood collection for complete blood counts and differentials and total IgE level, exhaled nitric oxide, and pulmonary function testing including baseline and post-bronchodilator spirometry and a methacholine challenge (see Moore et al. (2007)[15] for details of questionnaires and collections).
Asthma Control
Our asthma control variable was a composite of questions that assessed different components of asthma control defined in the National Heart Lung and Blood (NHBLI)/National Asthma Education Prevention Program (NAEPP) 2007 asthma guidelines, Expert Panel report[2] (Table 1). Asthma symptoms including cough, wheezing, shortness of breath, and nighttime asthma symptoms during the past 3 months, rescue β-agonist use, provocation of asthma symptoms with routine physical activities and exercise, and urgent care visits in the past year were assessed with SARP-specific questionnaires. FEV1 % predicted recorded from pre-bronchodilator spirometry was categorized as 0 = >80% and 1 = ≤80% to reflect (near) normal vs. reduced pulmonary function. Responses to each item were summed, with higher scores indicating worse asthma control (range of scores = 0–13).
Table 1.
Assessment of Asthma Control based on National Heart Lung and Blood Institute 2007 Asthma Guidelines
| NHBLI 2007 Asthma Guidelines | Current Study Assessments | ||
|---|---|---|---|
| Components of Asthma Control | Well-Controlled Asthma Criteria |
Responses | |
| Symptoms | ≤2 days/week | 1. Chronic cough 2. Wheeze 3. Shortness of breath |
0 = never or 1x/month or weekly but <2x/week 1 = >2x/week but <1x/day 2 = at least 2x/day or daily |
| Nighttime awakening | <2x/month | 1. Nighttime asthma symptoms: waking from sleep, nighttime use of albuterol, early morning chest tightness |
0 = never or 1x/month 1 = weekly but <2x/week or >2x/week but <1x/day 2 = at least 2x/day or daily |
| Interference with activities | None | Asthma symptoms with: 1. routine physical activities 2. physical exercise |
0 = No 1 = Yes |
| Rescue short-acting β2-agonist use | ≤2 days/week | β-agonist inhaler use daily or near daily (at least 5 of 7 days) |
0 = No 1 = Yes |
| FEV1 | >80% predicted | FEV1% predicted |
0 = >80% 1 = ≤80% >80% |
| Exacerbations | 0–1/year | Urgent care visits or emergency room visits in last year |
0 = No 1 = Yes |
Asthma Quality of Life Questionnaire
The Asthma Quality of Life Questionnaire (AQLQ) is a validated 32-item condition-specific health-related quality of life measure[18]. The AQLQ contains two items referring to sleep (“asthma interfered with getting a good night’s sleep” and “woken at night by your asthma”) and participants may also choose sleeping as one activity being limited by asthma. Omitting these items from the AQLQ total score did not change the results, thus these items were retained in the AQLQ total score.
Pittsburgh Sleep Quality Index
Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI)[19]. The PSQI assesses quality of sleep during the past month and contains seven component scales: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is scored from 0 to 3, yielding a global PSQI score ranging from 0 to 21, with higher scores indicating worse sleep quality. A global PSQI score > 5 has been found to have a sensitivity of 89.6% and specificity of 86.5% in differentiating good from poor sleepers[19].
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) is a validated and widely used questionnaire designed to measure daytime sleepiness[20]. The ESS rates the likelihood of dozing in 8 specific situations, with scores from 0 (no chance of dozing in the situation) to 3 (high chance of dozing in the situation). Total scores range from 0 to 24. A score of > 10 on the ESS is indicative of excessive daytime sleepiness. The scale has excellent test-retest reliability, with a Pearson correlation coefficient of 0.82[21].
Sleep apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ)
The SA-SDQ contains 12 items with eight OSA symptom items and 4 items related to age, current smoking status, weight, and body mass index (BMI)[17]. Total scores range from 0 to 60. To define high OSA risk, we used the SA-SDQ cutoff scores of ≥ 36 for men and ≥ 32 for women, which have been validated with polysomnography in a large sample of sleep clinic patients[17].
Pulmonary Function
All participants underwent pulmonary function testing according to American Thoracic Society guidelines[22]. For these analyses, data from pre-bronchodilator spirometry was used.
Data Analysis
Frequencies were determined for categorical variables and means ± standard deviations (SD) or medians ± SD were calculated for normally and nonnormally distributed continuous variables, respectively. Normality plots were constructed to check that assumptions of normality were met. Comparisons between the 3 groups used the chi-square or Fisher's exact test, as appropriate, for categorical variables and ANOVA or Kruskal-Wallis test, as appropriate, for continuous variables. In variables showing significant differences among the three subgroups, we performed posthoc pairwise comparisons (Tukey post hoc test for ANOVA and Mann-Whitney U test for Kruskal-Wallis) to determine which pair of subgroups had significant differences. Differences in continuous variables between the NSA and SA groups were calculated using Student's t test or Mann-Whitney U test. Spearman correlation coefficients were used to examine the relationships between the study variables within the NSA and SA groups. Within each group of asthma patients (NSA and SA), multiple regression analyses were conducted to examine the association of sleep quality with asthma control and quality of life. Covariates included age, gender, BMI, presence of GERD, daytime sleepiness, allergic rhinitis, and oral corticosteroid use (only in SA due to small number in NSA group). All tests were two-tailed, with significance set at p <0.05. In case of multiple comparisons, an alpha adjustment (Bonferroni) was used, and p values <0.02 were considered significant.
Results
Participant Characteristics
The participant characteristics for NC (n = 60), NSA (n = 143), and SA (n = 79) are shown in Table 2. The groups did not differ in gender or race. Those with SA were on average older than NSA and NC (p’s <.001). The NSA and SA groups had similar median BMI values (p = .67) and both groups had higher BMI than the NC group (p’s <.01). A greater proportion of participants with SA reported having co-morbidities than NSA and NC. Over 40% (n = 34) of those with SA reported having GERD, whereas only 17.5% (n =25) of those with NSA reported having GERD. As expected, those with SA had worse asthma control and asthma quality of life as compared to those with NSA (p <.001 for each).
Table 2.
Patient Characteristics (Median ± SD and %(n))
| Variable | Controls (n=60) | Non-Severe Asthma (n=143) |
Severe Asthma (n=79) | Statistic | Post Hoc |
|---|---|---|---|---|---|
| Age | 23.8 (9.9) | 28.4 (12.2) | 42.8 (14.1) | H = 58.83, p <.001 | SA>NSA>NC |
| Gender (% female) | 45% (n = 27) | 54% (n = 77) | 56% (n = 44) | Χ2 = 1.78, p =.41 | |
| Race: | Χ2 = 0.73, p =.69 | ||||
| Caucasians | 73% (n = 44) | 63% (n = 90) | 70% (n = 55) | ||
| African-American | 10% (n = 6) | 24% (n = 34) | 27% (n = 21) | ||
| Other | 17% (n = 10) | 13% (n = 19) | 3% (n = 3) | ||
| Education: | Χ2 = 38.87, p <.001 | ||||
| Didn’t complete high school/other | 0% (n = 0) | 0% (n = 0) | 8% (n = 6) | ||
| High School/GED | 5% (n = 3) | 15% (n = 22) | 28% (n = 21) | ||
| College | 55% (n = 33) | 66% (n = 94) | 49% (n = 39) | ||
| Post-college/graduate | 40% (n = 24) | 19% (n = 27) | 15% (n = 12) | ||
| BMI | 24.3 (0.9) | 26.5 (0.5) | 27.7 (0.7) | H = 8.94, p =.09 | SA=NSA>NC |
| CAD | 0% (n = 0) | 1% (n = 1 ) | 0% (n = 0) | Χ2 = 0.98, p =.61 | |
| GERD | 3% (n = 2) | 17.5% (n = 25) | 43% (n = 34) | Χ2 = 34.47, p <.001 | |
| Diabetes | 0% (n = 0) | 1.5% (n = 2) | 9% (n = 7) | Χ2 = 11.41, p =.003 | |
| Osteoporosis | 2% (n = 1) | 1% (n = 1) | 14% (n = 11) | Χ2 = 21.58, p <.001 | |
| CHF | 0% (n = 0) | 0% (n = 0) | 1% (n = 1) | Χ2 = 2.59, p =.27 | |
| Hypertension | 3% (n = 2) | 8% (n = 12) | 20% (n = 16) | Χ2 = 12.01, p =.002 | |
| Allergic rhinitis | 23% (n = 14) | 76% (n = 109) | 79% (n = 62) | Χ2 = 63.44, p <.001 | |
| Nasal corticosteroids | 7% (n = 4) | 22% (n = 32) | 56% (n = 44) | Χ2 = 43.55, p <.001 | |
| Inhaled corticosteroids | 0% (n = 0) | 15% (n = 22) | 36% (n = 29) | Χ2 =13.76, p <.001 | |
| Oral corticosteroids | 0% (n = 0) | 2% (n = 3) | 42% (n = 33) | Χ2 = 57.34, p <.001 | |
| Long acting β2 agonist | 0% (n = 0) | 2% (n = 3) | 8% (n = 6) | Χ2 = 4.22, p =.07 | |
| Inhaled β2 agonist | 0% (n = 0) | 82% (n = 117) | 81% (n = 64) | Χ2 = 0.03, p =.86 | |
| Leukotriene modifiers | 0% (n = 0) | 15% (n = 22) | 48% (n = 38) | Χ2 = 27.83, p <.001 | |
| FEV1% | 95.0 (10.5) | 89.0 (17.4) | 62.0 (20.7) | H = 90.63, p <.001 | SA<NSA<NC |
| FVC % | 99.0 (11.1) | 95.0 (15.0) | 81.0 (17.6) | H = 54.53, p <.001 | SA<NSA<NC |
| # positive skin test responses | 0 (2.5) | 5.0 (3.2) | 3.5 (4.0) | H = 39.10, p <.001 | SA=NSA>NC |
| Total serum IgE (IU/ml) | 16.0 (125.3) | 118.0 (770) | 90.0 (455.2) | H = 44.12, p <.001 | SA=NSA>NC |
| Blood eospinophils (%) | 2.0 (1.8) | 3.0 (2.5) | 3.6 (4.5) | H = 14.65, p =.001 | SA=NSA>NC |
| Exhaled nitric oxide (ppb) | 17.5 (17.5) | 24.6 (29.5) | 43.6 (63.2) | H = 20.00, p <.001 | SA>NSA>NC |
| Asthma controla | 3.0 (0.2) | 7.0 (0.4) | U = 28.46, p <.001 | ||
| Asthma quality of lifeb | 5.0 (1.1)d | 4.2 (1.3)c | t(220) = 5.12, p <.001 |
Note: CAD = coronary artery disease, GERD = gastroesophageal reflux disease, CHF = congestive heart failure, FEV1 = forced expiratory volume in first second, FVC = forced vital capacity, PSQI = Pittsburgh Sleep Quality Index,
Higher scores = poorer control (range = 0–13),
Lower scores = worse quality of life,
Mean ± SD. Missing cases exist for some variables.
Table 3 presents the sleep characteristics of the 3 groups. Participants with SA had worse sleep quality than NSA and NC (p’s <.01). Both SA and NSA groups had longer sleep latency and greater daytime sleepiness than NC (p’s <.01). The SA group had a significantly shorter total sleep time than NC (p =.001). Over 90% of participants in each of the SA (96%) and NSA groups (93%) were “poor sleepers” as defined by a PSQI global score of > 5[19].
Table 3.
Sleep Characteristics
| Controls (n=60) |
Non-Severe Asthma (n=143) |
Severe Asthma (n=79) |
Statistic | Post Hoc | |
|---|---|---|---|---|---|
| PSQI global score | 6.8 ± 2.5 | 9.1 ± 2.9 | 10.4 ± 2.7 | F(2,279) = 28.49, p <.001 | SA>NSA>NC |
| PSQI > 5 | 63% | 93% | 96% | ||
| Sleep latency (minutes) | 15.8 ± 10.9 | 26.8 ± 23.8 | 31.2 ± 27.9 | H = 17.35, p <.001 | SA=NSA>NC |
| Estimated sleep time (hours) | 7.2 ± 1.1 | 6.9 ± 1.7 | 6.4 ± 1.5 | H = 11.11, p =.004 | SA<NSA=NC |
| Epworth Sleepiness Scale | 4.8 ± 3.0 | 6.8 ± 3.8 | 7.2 ± 4.5 | H = 8.92, p = .01 | SA=NSA>NC |
| Epworth Sleepiness Scale, ≥ 10 | 7% | 23% | 26.5% |
Note: PSQI = Pittsburgh Sleep Quality Index
Nighttime Asthma Disturbances and Sleep Quality
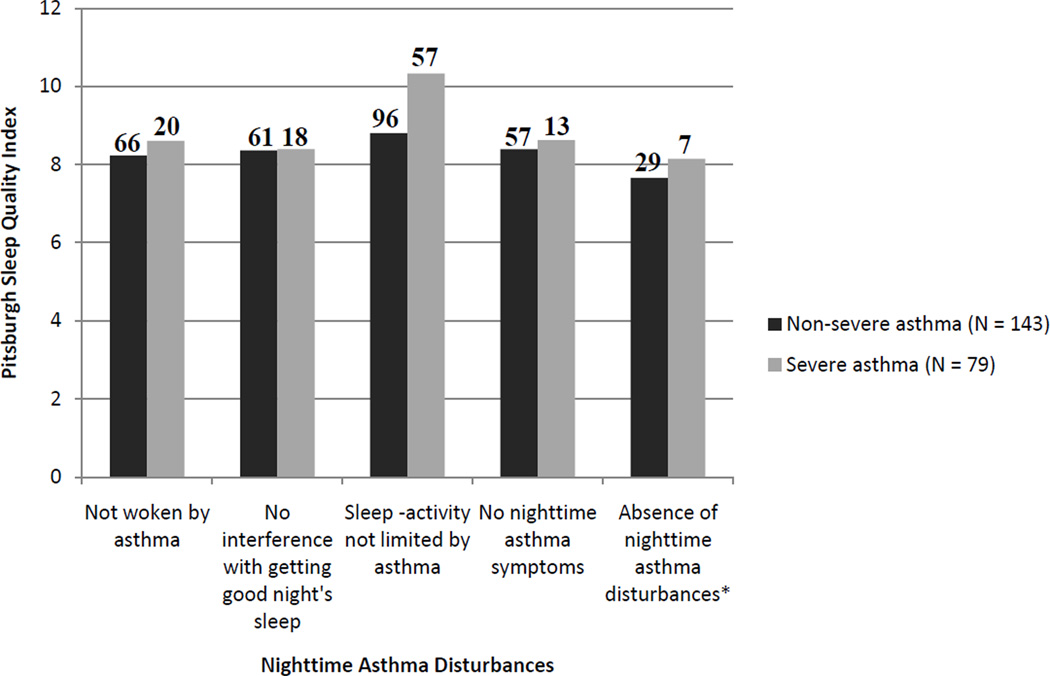
Figure 1 shows the average PSQI total score for those with NSA and SA who reported no nighttime asthma disturbances on the three sleep-related items on the AQLQ and the SARP-specific question asking about nighttime asthma symptoms. For both the NSA and SA groups, the mean PSQI score for each of these items was higher than the PSQI cutoff of >5, indicating those who did not complain of nighttime asthma disturbances reported, on average, poor sleep quality. Despite reporting no nighttime asthma disturbances for these individual items and even for those who reported no nighttime asthma disturbances for all 4 items, between 88%–92% of those with NSA and 90%–100% of those with SA had a PSQI score >5.
Fig. 1.
Pittsburgh Sleep Quality Index scores for those without nighttime asthma disturbances. Nighttime asthma disturbances represent 3 items from the Asthma Quality of Life Questionnaire (i.e., “woken at night by your asthma”, “asthma interfered with getting a good night’s sleep”, and sleep chosen as one of the top 5 most important activities limited by asthma) and 1 item from a SARP-specific questionnaire (i.e., “nighttime asthma symptoms: waking from sleep, nighttime use of albuterol, early morning chest tightness). *No nighttime asthma disturbances reported for all 4 items.
GERD and Sleep Quality
Among all participants with asthma, those with GERD symptoms had significantly worse sleep quality than those without GERD symptoms (t(216) = −2.69, p = .008). Although those with GERD symptoms had a higher mean score on the PSQI (10.41 ± 2.94), those without GERD symptoms still had a mean score on the PSQI (9.23 ± 2.94), indicating poor sleep quality. All of the participants with asthma and GERD had poor sleep quality (PSQI global score > 5) and almost all of those without GERD also had poor sleep quality (92%) (χ2 = 5.13, p =.02). The results were similar when examining PSQI and GERD in the NSA and SA groups separately.
Correlational Analyses of Sleep Quality Among NSA and SA Patients
Among those with NSA, poorer sleep quality was associated with worse asthma control (rs = .31, p <.001) and asthma quality of life (rs = −.31, p <.001), and greater daytime sleepiness (rs = .27, p =.001). Longer sleep latency was correlated with worse asthma control (rs = .20, p =.02) and quality of life (rs = −.26, p =.002). Sleep quality was not associated with age or BMI and weakly correlated with FEV1 (rs = −.25, p =.003). In contrast to NC in which no difference in sleep quality was found between males and females, females with NSA reported poorer sleep quality than males with NSA (t(141) = −2.76, p =.007). Sleep quality did not differ between those with and without allergic rhinitis (U = −.72, p =.47). Sleep quality was not associated with the number of positive skin test responses, total serum IgE levels, blood eosinophil count, or exhaled nitric oxide. The AQLQ sleep item"asthma interfered with getting good night’ sleep”, was negatively correlated with sleep quality as measured by the PSQI (rs = −.33, p <.001).
Among those with SA, worse sleep quality was correlated with worse asthma control (rs = .36, p <.001) and worse asthma quality of life (rs = −.32, p =.004). There was a trend towards shorter sleep duration being correlated with worse asthma control (rs = .20, p =.06). Sleep quality was not associated with age, BMI, or daytime sleepiness and weakly correlated with FEV1 (rs = −.23, p =.04). The difference in sleep quality between females and males approached significance (p =.06). Sleep quality did not differ between those with and without allergic rhinitis (U = 1.21, p =.23). Sleep quality was not associated with the number of positive skin test responses, total serum IgE levels, blood eosinophil count, or exhaled nitric oxide. The AQLQ sleep item was negatively correlated with sleep quality as measured by the PSQI (rs = −.41, p <.001). Asthma quality of life was negatively associated with daytime sleepiness (rs = −.25, p =.03).
Association of Sleep Quality with Asthma Control and Asthma Quality of life
Multivariate analyses controlling for covariates were conducted to examine the associations between sleep quality and asthma control and asthma quality of life among NSA and SA patients (Table 4 and 5). In those with NSA, sleep quality was a significant predictor of asthma control after adjusting for age, BMI, and presence of GERD (β = 0.31, p <.001). Age, BMI, and presence of GERD were not associated with asthma control. Similarly, sleep quality was a significant predictor of asthma quality of life after controlling for the above covariates and presence of allergic rhinitis (β = −0.28, p < 0.001).
Table 4.
Multiple Regression Analyses for Sleep Quality Predicting Asthma Control
| Non-Severe Asthma | |||||||
|---|---|---|---|---|---|---|---|
| Step | Variables | Β | p-value | ΔR2 | Total R2 | F | p-value |
| 1. | Age (years) | 0.10 | 0.26 | 0.07 | 0.07 | 3.62 | 0.02 |
| BMI | 0.15 | 0.09 | |||||
| GERD | 0.14 | 0.11 | |||||
| 2. | Sleep quality (PSQI) | 0.31 | <.001 | 0.10 | 0.17 | 6.92 | <.001 |
|
Severe Asthma | |||||||
| Step | Variables | Β | p-value | ΔR2 | Total R2 | F | p-value |
| 1. | Age (years) | 0.16 | 0.16 | 0.24 | 0.24 | 5.78 | <.001 |
| Daytime sleepiness | 0.18 | 0.10 | |||||
| GERD | −0.07 | 0.54 | |||||
| Oral corticosteroid use | 0.46 | <.001 | |||||
| 2. | Sleep quality (PSQI) | 0.26 | 0.01 | 0.06 | 0.19 | 3.37 | <.001 |
Table 5.
Multiple Regression Analyses for Sleep Quality Predicting Asthma Quality of Life
| Non-Severe Asthma | |||||||
|---|---|---|---|---|---|---|---|
| Step | Variables | β | p-value | ΔR2 | Total R2 | F | p-value |
| 1. | Age (years) | −0.07 | 0.43 | 0.09 | 0.09 | 3.10 | 0.02 |
| BMI | −0.14 | 0.11 | |||||
| GERD | −0.12 | 0.20 | |||||
| Allergic Rhinitis | −0.17 | 0.04 | |||||
| 2. | Sleep quality (PSQI) | −0.28 | <.001 | 0.08 | 0.74 | 5.19 | <.001 |
|
Severe Asthma | |||||||
| Step | Variables | β | p-value | ΔR2 | Total R2 | F | p-value |
| 1. | BMI | −0.24 | 0.03 | 0.17 | 0.16 | 3.65 | 0.01 |
| Daytime sleepiness | −0.26 | 0.02 | |||||
| GERD | −0.10 | 0.37 | |||||
| Oral corticosteroid use | −0.18 | 0.10 | |||||
| 2. | Sleep quality (PSQI) | −0.24 | 0.03 | 0.05 | 0.22 | 4.03 | 0.03 |
Note: BMI = body mass index, ESS = Epworth Sleepiness Scale, GERD = gastroesophageal reflux disease, PSQI = Pittsburgh Sleep Quality Index
Among those with SA, sleep quality was a significant predictor of asthma control (β = 0.26, p =.01), after controlling for BMI, daytime sleepiness, presence of GERD, and oral corticosteroid use. Sleep quality was a significant predictor of asthma quality of life after controlling for the above covariates (β = −0.24, p = 0.03).
Discussion
The present study examined the association between sleep quality and asthma control and quality of life independent of GERD and other relevant factors. Those with NSA and SA reported worse sleep quality than NC. As expected, patients with SA also had worse asthma control and asthma quality of life as compared to those with NSA. The majority of patients with asthma in our sample had poor sleep quality irrespective of the presence of GERD symptoms and nighttime asthma disturbances. In both NSA and SA, poorer sleep quality was an independent predictor of worse asthma control and quality of life after accounting for GERD and other covariates.
Consistent with evidence suggesting that GERD is more common in those with difficult to control asthma[15], we found a greater prevalence of GERD in SA as compared to NSA, with 43% vs. 17.5% reporting having GERD. Given that the presence of GERD was self-reported, it is likely that the prevalence of GERD in our sample may be higher as up to 62% of patients with asthma have asymptomatic acid reflux[23]. Epidemiologic and observational studies have documented sleep difficulties and poor sleep quality in individuals with GERD[24]. Consistent with these findings, all of our patients with asthma and GERD reported poor sleep quality. Interestingly, over 90% of those without GERD also reported poor sleep quality. These findings suggest that disturbed sleep in our sample may be indicative of sleep problems that are independent from the effects of GERD.
Nighttime asthma disturbances, such as being woken at night by asthma and rescue inhaler use during the night, are indications for poorly controlled asthma and can disturb sleep[2, 10]. Circadian activation of inflammatory cells and reduction in lung volume as mechanisms for poor sleep quality among asthmatics have been proposed[25]. Aside from the potential effect of nocturnal asthma on sleep, disrupted sleep in asthma may represent a comorbid sleep condition (i.e., insomnia) that is at least partly independent from nocturnal asthma symptoms[8, 12]. Insomnia has been reported in 45% of adults with asthma[11]. In our study, we found that the majority (88%–100%) of those with NSA and SA who did not report nighttime asthma disturbances still reported having poor sleep quality. These findings suggest that nocturnal asthma disturbances may not fully explain the high prevalence of poor sleep quality found in our sample of NSA and SA.
Previous findings that have linked sleep quality and asthma control did not take into account the potential effect of comorbidities such as GERD and OSA [8, 10, 11]. By excluding patients who reported using PAP therapy or who were at high risk for OSA, we hoped to eliminate OSA a possible contributor to poor sleep in our sample. Poor sleep quality was associated with worse asthma control and quality of life independent from the presence of GERD, BMI, and other relevant covariates in both NSA and SA. Although the causal direction of these relationships cannot be determined, these findings suggest that considering sleep as a factor affecting asthma control is noteworthy as previous examinations[8, 10] have mainly focused on asthma control as a factor that affects sleep. Improvements in sleep quality were associated with improvements in asthma control and quality of life over a six month period in mild-moderate asthmatics with poorly controlled asthma[11]. Asthma that is not well controlled is associated with impairments in asthma quality of life, work loss productivity, activity limitations, and increased healthcare utilization and costs[3], thus identification of a modifiable risk factor such as sleep has important clinical implications.
Insomnia is often perceived as a symptom of poorly controlled asthma and it is sometimes assumed that improving control will resolve sleep problems. However, previous investigations suggest that insomnia symptoms persist even in those with well-controlled asthma[8, 12]. The development of insomnia can involve several etiologic factors, including predisposing factors, precipitating factors, and perpetuating factors[26]. These etiologic factors may play a role in the development of insomnia in patients with asthma. Predisposing factors increase the individual’s vulnerability to develop insomnia and can include personality traits such as anxiety or hyperarousal or genetic factors such as female sex, aging, or family history of insomnia[27]. Precipitating events (e.g., stressful life events and health and psychological problems) can trigger the onset of insomnia. Specifically, asthma itself can serve as a precipitating factor as nocturnal asthma attacks, nighttime inhaler use, and medications disrupt sleep. Perpetuating factors contribute to self-sustained sleep problems. Sleep disruption associated with precipitating events usually subside over time when the event is resolved or as the individual adapts to the presence of the event. However, maladaptive sleep habits and dysfunctional beliefs and attitudes about sleep that are adopted to cope with initial sleep problems can lead to chronic insomnia. In the case of asthma, anxiety about a possible asthma attack during the night could contribute to chronic insomnia. These perpetuating factors are the most opportune targets for behavioral treatment[28]. The benefit of behavioral treatment of insomnia on disease-related outcomes has been demonstrated in medical and psychiatric disorders[29]. The only study to specifically address sleep problems in asthma administered 3mg of melatonin over 28 days to patients with mild-moderate asthma in a well-controlled stable condition[30]. This is notable due to the fact that melatonin is a weak hypnotic. As compared to placebo, the melatonin treatment group reported significant improvements in sleep quality. No differences in daily peak expiratory flow rate, asthma symptoms, or rescue inhaler use were found between the two groups.
A limitation of this study is that we are unable to make causal inferences about the directionality of the relationship between sleep quality and asthma control due to the cross-sectional nature of the assessments. Our study is limited by patients’ self-report of physician-diagnosed GERD, which may not be accurate. Additionally, although we attempted to exclude patients with OSA to eliminate its known effects on sleep quality, polysomnography was not performed to determine diagnosis, thus we may not have captured all patients with OSA. To better understand the distinct effect of poor sleep on asthma control and quality of life, future prospective studies that obtain objective sleep parameters, data from validated insomnia specific questionnaires, and physician-diagnosed comorbidities are needed.
In summary, this study demonstrates that patients with NSA and SA have significant impairments in sleep quality that may be independent from GERD, OSA, and nighttime asthma disturbances. Poor sleep quality was associated with worse asthma control and asthma quality of life in both NSA and SA, even after controlling for GERD and other relevant covariates. Future research is warranted to determine if behavioral and/or medical treatment of sleep problems can improve asthma control and quality of life.
Acknowledgements
Supported by National Institute of Health grants: HL69116, HL69130, HL69155, HL69167, HL69170, HL69174, HL69349, HL091762, KL2RR025009, M01 RR02635, M01RR03186, M01 RR007122-14, 1UL1RR024153, 1UL1RR024989, 1UL1RR024992, 1UL1RR025008, 1UL1RR025011
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, Eichler HG, Zhang Q, Yin DD. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–729. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 2.National Heart Lung and Blood Institute. [Accessed: 1 February 2011];National Education and Prevention Program (2007) Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 3.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007;62:126–133. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 4.Haughney J, Price D, Kaplan A, Chrystyn H, Horne R, May N, Moffat M, Versnel J, Shanahan ER, Hillyer EV, Tunsater A, Bjermer L. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102:1681–1693. doi: 10.1016/j.rmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest. 1997;111:1389–1402. doi: 10.1378/chest.111.5.1389. [DOI] [PubMed] [Google Scholar]
- 6.Ponte EV, Franco R, Nascimento HF, Souza-Machado A, Cunha S, Barreto ML, Naspitz C, Cruz AA. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy. 2008;63:564–569. doi: 10.1111/j.1398-9995.2007.01624.x. [DOI] [PubMed] [Google Scholar]
- 7.Teodorescu M, Polomis DA, Hall SV, Teodorescu MC, Gangnon RE, Peterson AG, Xie A, Sorkness CA, Jarjour NN. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138:543–550. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braido F, Baiardini I, Ghiglione V, Fassio O, Bordo A, Cauglia S, Canonica GW. Sleep disturbances and asthma control: a real life study. Asian Pac J Allergy Immunol. 2008;27:27–33. [PubMed] [Google Scholar]
- 9.Janson C, De Backer W, Gislason T, Plaschke P, Bjornsson E, Hetta J, Kristbjarnarson H, Vermeire P, Boman G. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9:2132–2138. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 10.Krouse HJ, Yarandi H, McIntosh J, Cowen C, Selim V. Assessing sleep quality and daytime wakefulness in asthma using wrist actigraphy. J Asthma. 2008;45:389–395. doi: 10.1080/02770900801971800. [DOI] [PubMed] [Google Scholar]
- 11.Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–189. doi: 10.1080/02770900801890224. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick MF, Engleman H, Whyte KF, Deary IJ, Shapiro CM, Douglas NJ. Morbidity in nocturnal asthma: sleep quality and daytime cognitive performance. Thorax. 1991;46:569–573. doi: 10.1136/thx.46.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 14.Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am. 2005;25:131–148. doi: 10.1016/j.iac.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 17.Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VP, Jr, Guilleminault C, Dement WC. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med. 162:34–39. doi: 10.1164/ajrccm.162.1.9907072. (200) [DOI] [PubMed] [Google Scholar]
- 24.Jansson C, Nordenstedt H, Wallander MA, Johansson S, Johnsen R, Hveem K, Lagergren J. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol. 2009;7:960–965. doi: 10.1016/j.cgh.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Martin RJ, Banks-Schlegel S. Chronobiology of asthma. Am J Respir Crit Care Med. 1998;158:1002–1007. doi: 10.1164/ajrccm.158.3.9712132. [DOI] [PubMed] [Google Scholar]
- 26.Spielman AJ, Glovinsky P. Case studies in insomnia. In: Hauri PJ, editor. The Varied Nature of Insomnia. New York, NY: Plenum Press; 1991. pp. 1–15. [Google Scholar]
- 27.Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med. 1992;152:1634–1637. [PubMed] [Google Scholar]
- 28.Glovinsky PB, Yang C, Dubrovsky B, Spielman AJ. Nonpharmacologic strategies in the management of insomnia: Rationale and implementation. Sleep Med Clin. 2008;3:189–204. [Google Scholar]
- 29.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Campos FL, da Silva-Junior FP, de Bruin VM, de Bruin PF. Melatonin improves sleep in asthma: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2004;170:947–951. doi: 10.1164/rccm.200404-488OC. [DOI] [PubMed] [Google Scholar]