Abstract
Positron emission tomography (PET) is a nuclear medicine imaging technology which allows for four-dimensional, quantitative determination of the distribution of labeled biological compounds within the human body. PET is becoming an increasingly important tool for the measurement of physiological, biochemical and pharmacological functions at the molecular level in healthy and pathological conditions. This review will focus on Flouride-18, one of the common isotopes used for PET imaging, which has a half life of 109.8 minutes. This isotope can be produced with an efficient yield in a cyclotron as a nucleophile or as an electrophile. Flouride-18 can be thereafter introduced into small molecules or biomolecules using various chemical synthetic routes, to give the desired imaging agent.
1. INTRODUCTION
Positron emission tomography (PET) is becoming increasingly important for the measurement of physiological, biochemical and pharmacological functions at the molecular level under healthy and pathological conditions. This modality allows accurate measurement of radioactivity concentration in small volume elements in vivo as well as the ability to follow tracer kinetics. The use of PET requires administration of a molecule labeled with positron-emitting nuclides such a 15O, 13N, 11C, or 18F, which have half-lives of 2.037, 9.965, 20.39, and 109.8 min, respectively [1]. In order to be suitable for PET, the radiotracers required to have high affinity and selectivity towards the receptor, low nonspecific binding and optimal biological half-life. The most common radiotracers that are being used in the clinic for measurements of different processes in the human body are [18F]-FDG [2] and [11C]-Choline [3] for the measurements of energy and cell membrane metabolism, [13N]-NH3 for perfusion imaging [4], [15O]-H2O for cerebral blood flow [5], [11C]-raclopride [6] and [18F]-FDOPA [7] for the measurements of dopamine system.
Ideally, the “physiological” radionuclides 11C, 13N and 15O are preferred for labeling an organic radiopharmaceutical. However, the very short half-lives of these isotopes are somewhat limiting, and 18F is considered to be a practical alternative. To date, the use of PET for diagnosing local recurrence and metastastic sites of various cancers is mainly based on the fluoride-18 radiolabeled glucose analogue tracer, fluorodeoxyglucose ([18F]-FDG), which measures glucose metabolism. Following malignant transformation, and probably due to high proliferation rate, a range of tumors can be characterized by elevated glucose consumption and subsequent increased uptake of the radiolabeled [18F]-FDG. The transport of FDG through the cell membrane via glucose transport proteins and subsequent intracellular phosphorylation by the enzyme hexokinase have been identified as key steps for the accumulation of FDG in the tissue [8]. In contrast to glucose-6-phosphate, FDG-6-phosphate cannot be further metabolized, thus, it accumulates in cells and is visualized by PET imaging.
However, the use of FDG is suboptimal since it is not a cancer specific agent and also accumulates in normal tissues such as brain and bladder and in inflammatory lesions. Hence, there is an increasing need for specific and selective biological radiotracers which will yield specific biochemical information and allow for noninvasive molecular imaging. The possibility of targeting receptors for imaging is an attractive concept since it may provide the opportunity to evaluate agents for the diagnosis of several disorders, and allow conducting experiments with agonists or antagonists for guiding and monitoring treatment.
18F is a commonly used isotope in nuclear medicine due to its ideal nuclear properties for PET imaging; ninety seven percent of the isotope decay is by positron emission [9-12], fairly low energy positron emission (maximum 0.635 MeV) and short linear range in the live tissue (maximum 2.4 mm) which consequential high resolution in PET imaging in accordance to other PET isotopes, such as 11C, 13N and 15O. 18F has an optimal half-life of 109.8 minutes, which is considered acceptable for chemical syntheses and favorable when investigating biological processes with a time frame longer than 100 min [13]. It has reasonable radiation dosimetry to patients, therefore, multiple studies can be done.
18F is readily produced in a reactor or on a cyclotron using a variety of nuclear reactions and can be produced with the theoretical specific activity value of 1710 Ci/μmol [13-15]. The specific activity of the tracer, which reflects the radiolabeled fraction out of the total administered mass, should be high enough to avoid saturation of binding-sites or toxicity to the biological system. 18F has been used for labeling of various biochemical systems. The high resolution of 18F in PET imaging and the low concentration of this tracer which gives high specific activity, allow the visualization of low capacity systems such as ligand binding to the receptor [16-22].
18F can be manufactured in two chemically forms: positively charged (electrophile) or electron-rich (nucleophile).
2. 18F -ELECTROPHILE
There are two major processes for 18F-electrophile production: 1) Two-bombardment method 18O2(p, n)18F [23], In this approach, the first bombardment is done on passivated nickel target charged with 95.2% enriched O2 and irradiated with 10 MeV protons to give 18F. 18F sticks to the target walls, while 18O2 is recovered. Then, the target is refilled with a noble-gas and F2 mixture and again irradiated, inducing isotopic exchange reactions between the adsorbed 18F and the molecular F2 [23]. 2) 20Ne(d, α)18F [24-27], Ne gas, containing 0.1-2% F2 as carrier, is used as a target and 18F can be obtained as [18F]-F2. This method is very useful for anhydrous precursor preparation, however the recovery yield of [18F]-F2 from the target is rather low and correlates with the carrier concentration. [18F]-F2 fluoride gas produced in the cyclotron can be directly used for electrophilic fluorination, or alternatively be used as a progenitor of other fluorination reagents. Fluoride is a violently reactive gas that erratically reacts with organic molecules to give poor regioselectivity and mixtures of products that result from the addition across the double bond [15, 28, 29] The use of fluoride diluted with an inert gas gives a more controllable reagent that can react selectively with organic compounds. If the double bond is substituted with electron donating groups, the intermediate cation is more stable, resulting in less product mixtures. In addition, substitution of arenes with organometallic precursor, can afford higher and further specific regioselectivity during the electrophilic fluorination [15, 28-30]. Therefore, it is important to take into account the biological system that is being investigated when using [18F]-F2, since increasing the yield of [18F]-F2 by adding carrier will result in very low specific activity.
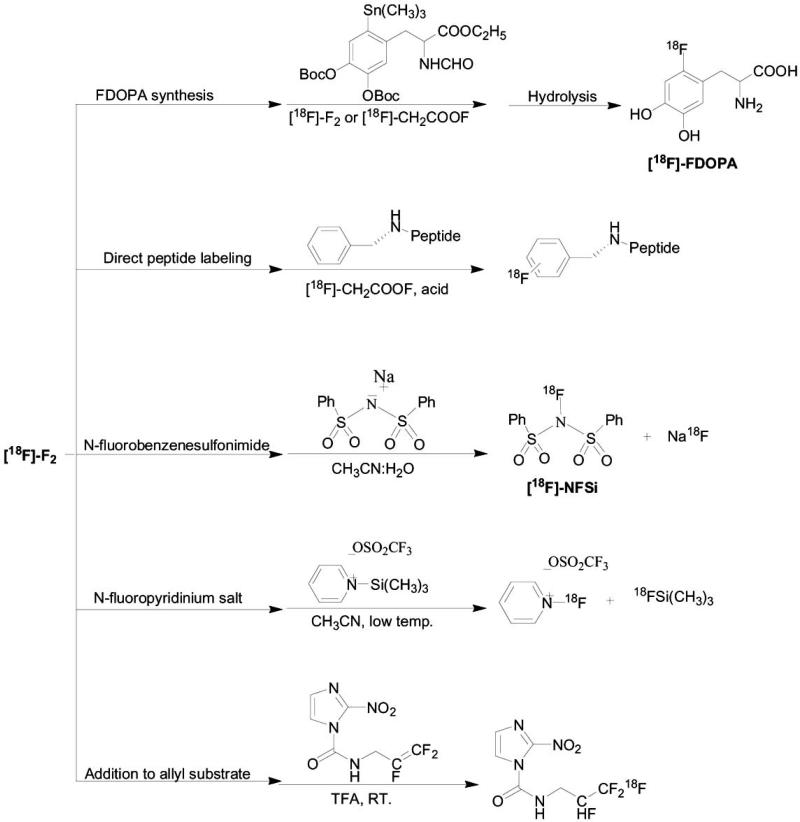
Another alternative for the use of the reactive F2 electrophile is to convert it to the less vigorous electrophile, acetyl hypofluorite (AcOF) [15, 31]. [18F]-F2 is produced by either of the two methods described above, and subsequently converted to [18F]-AcOF by passing through a CH3CO2Na·3H2O-column. [18F]-AcOF is then bubbled into a vial containing the precursor for radiolabeling in the presence of acid to give the desired product. This method can be applicative for a direct labeling of small molecules [32, 33] or peptides (Scheme 1) [31].
Scheme 1.
Primary and secondary precursors used in electrophilic fluorination reactions.
[18F]-F2 can also be converted to N-[18F]-fluorobenzene-sulfonimide ([18F]-NFSi) fluorination reagent by bubbling [18F]-F2 through 0.01 M NaN(SO2Ph)2 solution in CH3CN-water. [18F]-NFSi can be further reacted with various substrates such as silyl-enol-ether derivatives and allylsilanes [34] (Scheme 1). In addition, [18F]-F2 can be bubbled through a cold solution of N-trimethylsilylpyridinium triflate in CH3CN, to give the corresponding N-[18F]-fluoropyridinium electrophilic reagent which can further react selectively with various nucleophilic substrates which contain high electron density, such as enolate ions, carbanions and Grignard compounds [28, 35]. The widest use of 18F-electrophile is in the synthesis of [18F]-FDOPA electrophilic fluorodestannylation reaction (Scheme 1) [36-42].
There are other application for electrophilic substitution displacement with 18F-electrophile. One of them was reported by Dolbier et al. [43] as electrophilic addition was done by bubbling [18F]-F2 on allyl precursor with partially fluorinated terminal double bond in trifluoroacetic acid at room temperature, for the synthesis of [18F]-EF-5 analogue for imaging tumor hypoxia [43] (Scheme 1).
3. 18F-NUCLEOPHILE
For 18O(p, n)18F reaction [44], enriched water (H218O) is used as a target. The nucleophilic 18F is achieved as fluoride ion in aqueous solution and removed from the target by pressure. This reaction can easily lead to curie amounts of 18F with no carrier added and high specific activity.
Fluoride ion in aqueous solution forms hydrogen bonds with the surrounding water and consequentially is not labile to nucleophilic reactions [28, 45]. It is crucial to form reactive fluoride in an anhydrous environment. The reactivity of the fluoride is enhanced by the addition of a cationic counter ion prior to the evaporation of the water. The water is removed by either distillation or the use in resin column, from added base (potassium carbonate complexes with kryptofix, cesuim or tetraalkylammonium salts) and the fluoride salt is dried by azeotropic distillation with CH3CN or microwave heating.
Using resin column for the separation of fluoride can also exclude metal ions impurities and increase the reactivity of the fluoride [46, 47]. The solubility and nucleophilicity of the fluoride ion in the solvent is also a major concern. In order to solve these problems, bulky phase transfer catalysts, such as crown ether (kryptofix 2.2.2) and tetrabutylammonuim hydroxide are being used in the preparation of the reactive fluoride. The solvent is an important parameter and dipolar aprotic solvents such as dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF) and CH3CN are generally chosen.
With reactive fluoride ion available one can choose a variety of methods for its introduction into an organic compound. The methods can be conveniently divided into two major classes: aliphatic and aromatic fluorinations. In contrast to the wide variety of electrophilic reagents that have been developed and used with varying success, there is only one nucleophilic fluorinating reagent: fluoride ion. The diversity of the applications of this reaction is achieved by the use of a wide range of leaving groups, substrates, and reaction conditions.
3.1. Aliphatic Nucleophilic Fluorination
Nucleophilic displacement that is usually used for aliphatic fluorination reactions, involves the SN2 substitution of 18F ion and alkyl substrate which contain leaving groups such as halogens or sulfonic ester. The reactivity of halogens as leaving groups increases down the periodic table, whereas iodine is the best leaving group, and flouride is the poorest. Substitution of fluoride-19 with fluoride-18 can occur only under conditions that eliminate solvation effects, however this substitution will give low specific activity. These substitutions are usually done in a moderate temperature of 80-115°C for 5-15 minutes. The efficiency of nucleophilic displacement can be low in non-coordinating solvents in which the fluoride is too basic and there is higher possibility of side reactions such as β-elimination or hydroxylation [28].
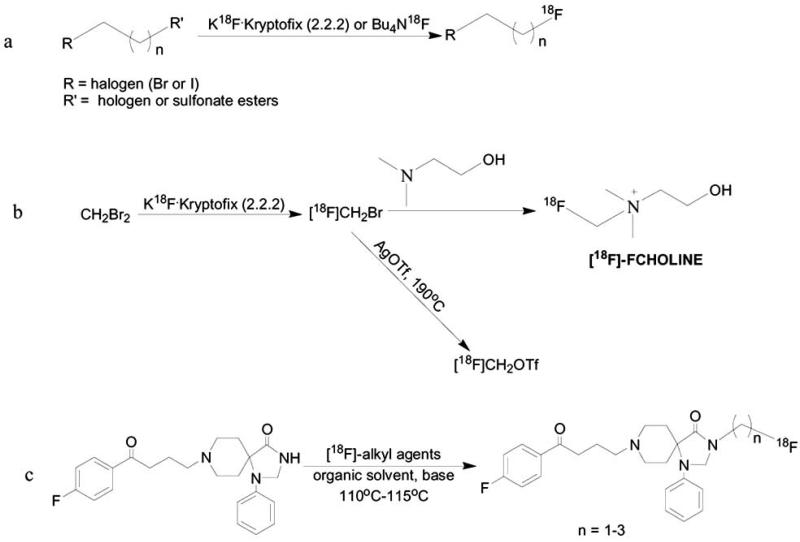
Depending on the stability of the precursor for labeling, the reactivity and simplicity of the incorporation, aliphatic fluorination can be formed by either direct labeling [20, 22, 48] or formation of [18F]-fluoroalkyl agents (Scheme 2). [18F]-fluoroalkyl agents are synthesized by substitution of halogens or sulfonate esters with non-carrier added fluoride ion in the presence of K2CO3/Kryptofix complex or tetrabutylammonuim hydroxide in organic solvents such as acetonitrile, o-dichlorobenzene or tetrahydrofurane [13, 49] (Scheme 2a). After the formation of [18F]-fluoroalkyl agent, it can be used for the second alkylation reaction, however it might contain impurities which may affect the radiochemical yield. [18F]-fluoroalkyl agents have relatively low boiling point and can be purified using gas chromatography separation, or distillation from the reaction mixture into disposable Sep-Pak cartridges [50]. Purification of [18F]-fluoroalkyl agent before the next alkylation reaction gives more chemically and radiochemically pure product. Moreover, it eliminates non-volatile impurities and therefore increases the radiochemical yield of the second alkylation reaction [13, 49, 51-54]. A well-known aliphatic substitution reaction using [18F]-fluoroalkyl agents is the radiosynthesis of [18F]-fluorocholine, in which dibromomethane is fluorinated to generate 18F-fluorobromomethane, which reacts with the precursor, dimethylethanolamine to produce [18F]-fluorocholine (Scheme 2b). In some cases, [18F]-fluorobromomethane can be converted to the more reactive synthon, [18F]-fluoromethyltriflate, which would react more efficiently with 2-dimethylethanolaminel to give [18F]-fluorocholine. Another example is the radiosynthesis of dopamine antagonist, spiperone and its derivatives (Scheme 2c) [49].
Scheme 2.
[18F]-Aliphatic alkylation. a; synthesis of [18F]-fluoroalkyl agents. b; radiosynthesis of [18F]-FCHOLINE. c; synthesis of spiperone derivative via alkylation with [18F]-fluoroalkyl agent.
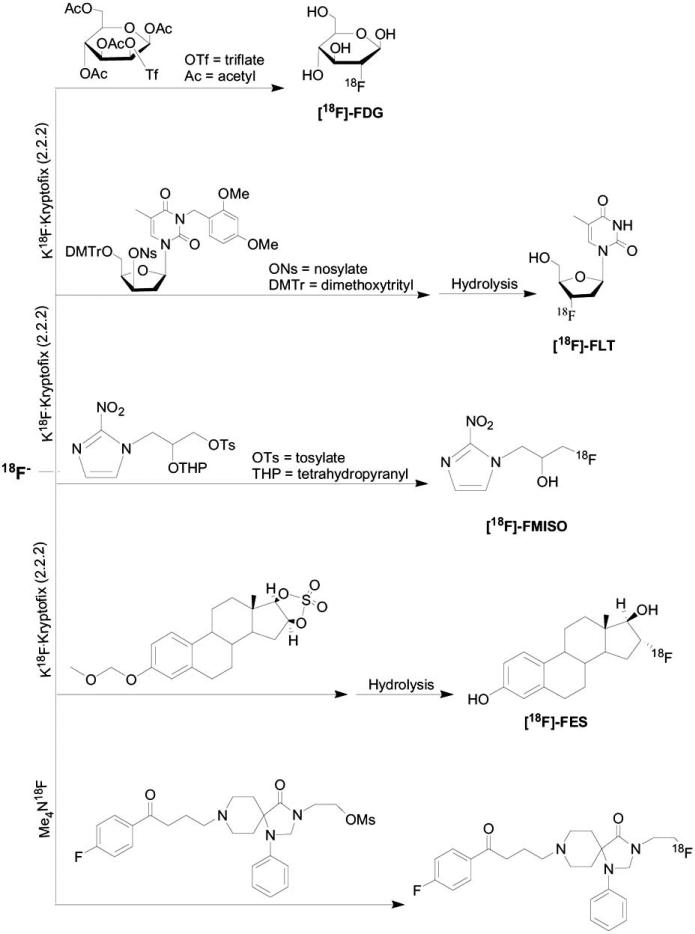
Sulfonates are more reactive than halogens as a leaving group. There is a variety of sulfonate leaving groups available for aliphatic nucleophilic fluorination, such as p-toluenesulfonate (tosylate), methanesulfonate (mesylate), trifluoromethanesulfonate (triflate) and p-nitrosulfonate (nosylate). Among the sulfonates, the tosylate group has the lowest reactivity, and therefore is associated with lowest labeling yields. The mesylate and nosylate groups are intermediate in reactivity and the triflate is the most reactive group for 18F-labeling. In some cases, in order to get more rapid reaction, such as in the case of [18F]-fluoroalkyl agents, it is better to use triflate as a leaving group than halogen [50, 55]. Nevertheless, due to its high reactivity, triflate compounds may be unstable during the fluorination reaction temperatures, they cannot tolerant any water and are subject to side reaction such as elimination. There are a variety of fluorination reactions involving aliphatic substitution on sulfonate esters, such as syntheses of 2-fluoro-2-deoxyglucose ([18F]-FDG) [43], [18F]fluoro-3-deoxy-L-thymidine ([18F]-FLT) [56,57], [18F]fluoroestradiol ([18F]-FES) [48,58], [18F]Fluoromisonidazole ([18F]-FMISO) [59-66], and a direct labeling to obtain spiperone derivative [22] (Scheme 3).
Scheme 3.
Direct [18F]-aliphatic fluorination on sulfonate esters precursors.
Kim et al. reported the use of ionic solvents, such as tertiary alcohols as a reaction media for the nucleophilic fluorination with alkali metal fluoride [45, 67, 68]. The use of nonpolar, protic tert-alcohols increases the nucleophilicity and reactivity of the alkali metal fluoride, in comparison to conventional fluorination with dipolar aprotic solvents and reducing formation of typical byproducts (e.g., alkenes, alcohols, or ethers) [68].
3.2. Aromatic Nucleophilic Substitution
Introduction of non-carrier added 18F into aromatic compounds is mostly limited to substitution on electron-deficient arenes. Fluoride is of similar size to hydrogen and both form strong bonds with carbon, however its high electronegativity may affect the electron density of the arene. The most common leaving groups for aromatic nucleophilic substitution are nitro and quaternary trimethylammonuim. Typically, fluoride substitution of trimethylammonuim group requires lower temperature (105-110°C), therefore, acetonitrile is mostly used as a solvent. Substitution of nitro group with fluoride ion requires much higher temperatures (120-180°C) and DMF or DMSO are used as solvents.
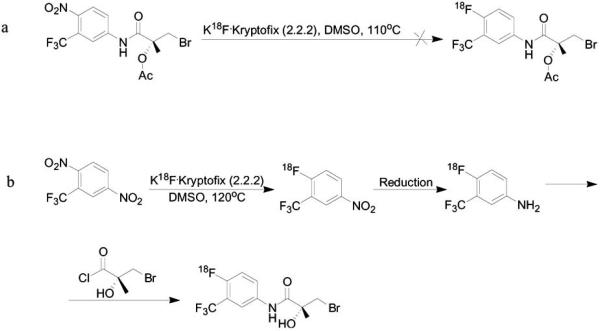
The presence of electron-withdrawing groups, such as cyano, trifluoromethyl, aldehydes, ketones and nitro, in the ortho and para positions on the aromatic ring decreases the electron density, which allows sufficient activation for the nucleophilic substitution [15]. It is also feasible to perform isotopic exchange, [19F]fluoride to [18F]fluoride, however, the specific activity will be low. Depending on the substrate, it is preferable to conduct direct fluorination on the aromatic ring. However, not all the precursors for the labeling can handle high temperature and basic conditions of the fluorination, they may decompose during the radiosynthesis. For example, in Scheme 4a the precursor for labeling contains nitro and trifluoromethyl groups on the aromatic ring, but direct fluorination cannot be done probably due to the electron withdrawing group that inductively polarizes the electron density towards the aromatic ring, which leads to instability of the molecules and decomposition. Hence, in order to label this molecule, several radiosynthesis steps were required (Scheme 4b) [69, 70].
Scheme 4.
Aromatic nucleophilic fluorination. a; attempt to synthesize [18F]-radiolabeled hydroxyflutamide derivative in a direct fluorination on the aromatic nitro group. b; Radiosynthesis of [18F]-radiolabeled hydroxyflutamide derivative with several radiosynthesis steps.
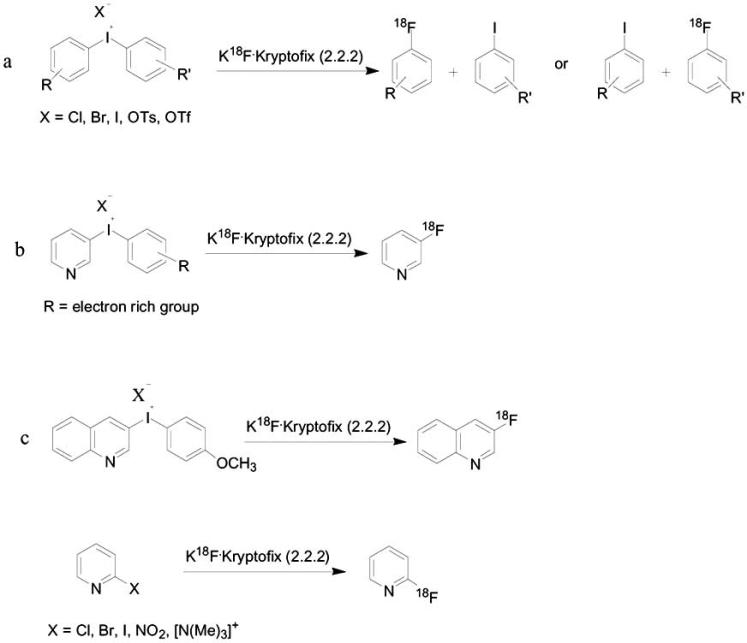
Aromatic nucleophilic substitution can also be done using diaryliodonium salts, and without the need of electron withdrawing groups, fluoride can be introduced into the arene. The introduction of [18F]fluoride into dihomoaryliodonium salts precursor gives [18F]-fluoroarenes and the corresponding iodoarenes (Scheme 5a) [71]. There are two factors that affect fluorination on diaryliodonium salts. First, if the aromatic ring is substituted on the ortho position, then the fluorination occurs on this ring. Secondly, the electronic effect results in a fluorination of the more electron-deficient ring. Coenen and co-workers showed that electron-rich aryl moieties, such as heteroaromatic, can allow a direct single-step nucleophilic 18F-introduction into the electron-rich aryl and control the regioselectivity of fluoride attack [71, 72] (Scheme 5b). Diaryliodonium salts derivatives of pyridines and quinolines were also subjected to F-18 introduction (Scheme 5c) [72]. The labeling can be done using DMF as a solvent under relatively high temperature of 130°C.
Scheme 5.
Aromatic nucleophilic fluorination on diaryliodonium salts. a; fluorination on dihomoaryliodonium. b; 18F-introduction into electron-rich aryl diaryliodonium salts. c; fluorination on pyridines and quinolines.
Fluoride ion can be used in nucleophilic heteroaromatic substitutions, displace groups such as nitro, trimethylammonuim salt, chloro, bromo and iodine at the ortho position (Scheme 5c) [9]. In contrast to nucleophilic substitution of fluoride on homoaromatic ring, in the case of substitution on hetroaromatic ring, there is no need for an additional strong electron-withdrawing group. The labeling is generally performed in DMSO using conventional heating at rather high temperatures (120-150°C) or in microwave irradiation for a short period of time (few minutes) and often leads to high radiochemical yields [9, 72].
4. F-18 LABELING OF BIOMOLECULES
Biomolecules such as peptides, proteins, affibodies, antibodies and oligonucleotides can be labeled with fluoride-18 and evaluated for their potential as diagnostic imaging agents. When imaging with fluoride-18, the timetable for the evaluation of the biological system is limited to 4 hours, (two half lives of the isotope). Therefore, to label biomolecule with fluoride-18, one needs to consider a fast clearance from the blood and high accumulation in target tissue. Out of the biomolecules mentioned above, peptides fit these demands best, with rapid clearance from the blood and high concentrations in target tissue. In addition, from the chemical point of view, the small size of peptides usually makes them relatively easy to synthesize “at home” with chemical modifications if needed. Moreover, peptides can often tolerate harsh chemical conditions for modification or radiolabeling. Another advantage of the small size is that peptides penetrate tumors fairly easily and may have low non-specific binding, leading to high accumulation in target tissue. Larger biomolecules, such as antibodies have slow pharmaco-kinetics (slow clearance from the blood), high non-specific binding and in terms of short imaging timetable, they usually have lower uptake in target tissue and sometimes dependent on protein concentrations [73].
Similar to the radiosynthesis of small molecules, radiosynthesis of biomolecules need to take under consideration several requirements. The specific activity of the tracer should be high enough to avoid saturation of binding sites. Otherwise, under saturating conditions, it would be impossible to measure changes in the concentration of available binding sites. Hence, generally speaking, nucleophilic non-carrier added fluoride ion will be preferable over the labeling with fluoro-electrophile.
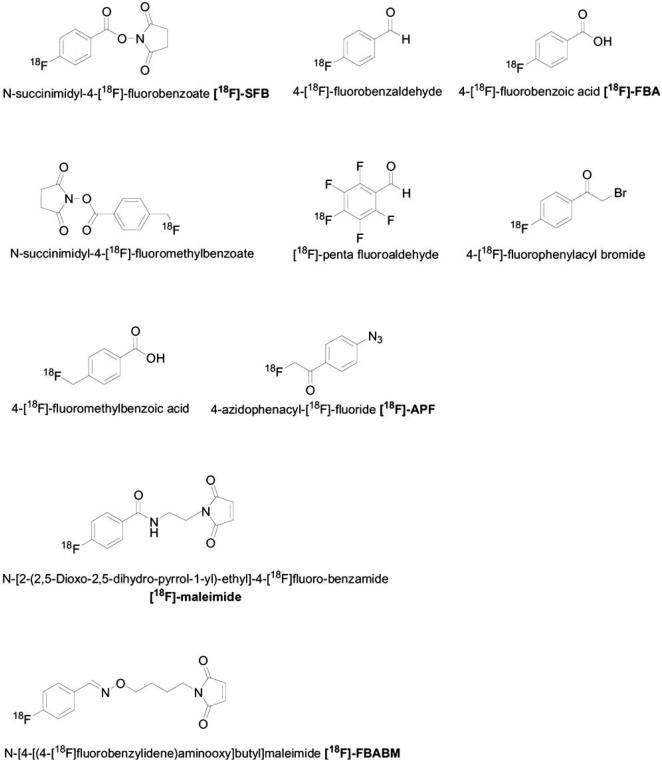
As discussed above, nucleophilic fluorination (aliphatic and aromatic) requires strong basic conditions and moderate to high temperatures. Most of the biomolecules have free amino or carboxylic groups, disulfide bonds and H-acidic moieties which are not stable under such harsh conditions. In addition, most peptides and proteins contain a variety of interfering nucleophilic groups that may lead to cross-linking reactions [74]. Therefore, it is hard to apply direct fluorination with non-carrier added fluoride on biomolecules. To overcome this limitation, labeling biomolecules with fluoride-18 ion is usually done in several radiosynthesis steps using prosthetic groups, which can be attached to the biomolecule, mostly through amino- or thiol-reactive groups via acylation, alkylation, amidation, imidation, oxime or hydrazone formations [75-78]. Another possibility for conjugation is the use of photochemical reactions [79]. The choice of prosthetic group for the labeling should take into account the complexity of the radiosynthesis and the yield of the labeling that need to be as high as possible. In addition, the prosthetic group has to be stable against in vivo defluoridation. Ideally, [18F]-prosthetic group is chemoselectively conjugated to a specifically functionalized, unprotected biomolecules. However, some biomolecules need to undergo some modifications before the conjugation. A large number of 18F-labeled prosthetic groups have been developed for labeling biomolecules with 18F (Scheme 6) [73, 79-84]. Some of the methods have limitations in labeling specific peptides and proteins [74, 79], insufficient specific activity of the labeled protein [80, 85], extensive HPLC purification post-labeling [86, 87] and low in vivo stability which results in defluoridation.
Scheme 6.
Examples of 18F-labelled prosthetic groups.
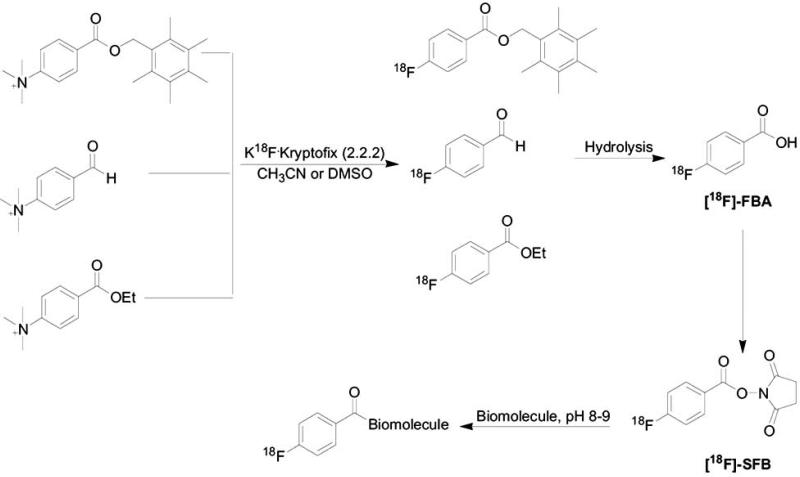
To date, the most common 18F- prosthetic group for labeling biomolecules through the reactive amine group of lysine is the N-succinimidyl-4-[18F]-fluorobenzoate ([18F]-SFB) [82, 88, 89] . [18F]-SFB can be synthesized in different routes, starting from different precursors. Various routes for synthesizing [18F]-SFB are described in Scheme 7. The radiosynthesis of [18F]-SFB involves another potential 18F-prosthetic group, [18F]-fluorobenzoic acid ([18F]-FBA). [18F]-FBA is converted into more activated form (such as [18F]-SFB) in order to form the amide bond with the biomolecules. Coupling of [18F]-SFB with peptides or proteins can be performed under mild pH and temperature conditions in aqueous media (pH 8-9) [82, 88]. Acylation with [18F]-SFB was shown to be a convenient labeling method in terms of in vivo stability and radiochemical yield. HPLC or size-exclusion chromatography purification is done to the final conjugation product.
Scheme 7.
Radiosynthesis of [18F]SFB using different precursors for the labeling, for biomolecules conjugation.
Another possible method involves labeling a thiol group (for example, in cysteine) using 18F-prosthetic group- maleimide (18F-maleimide) and its derivatives ([18F]-FBABM) (Scheme 6) [21, 75]. The radiosynthesis of 18F-maleimide and its derivatives can also be done through the formation of other 18F-prosthetic groups such as 4-[18F]-fluorobenzaldehyde or [18F]-FBA. These two major intermediates are further reacted with different malemide precursors to give various derivatives of 18F-maleimide prosthetic groups [21, 75]. One advantage of this method is that coupling of 18F-maleimides with biomolecules is done in buffer at pH 7 or less. At this pH the free amines in the biomolecules are protonated and are not labile for reactions, thus the conjugation will be selective to free thiol (SH). Another possibility for radiolabeling thiol group in the biomolecule is by coupling with an α-halo ketone 18F-prosthetic group such as 4-[18F]-fluorophenacyl bromide [73], to give thioethers. α-halo ketones can alkylate O, N and S atoms in a biomolecule and thus can potentially alkylate lysines, tyrosines, histidine, cysteins and methionines [73]. The coupling reaction of 4-[18F]-fluorophenacyl bromide and the biomolecule is performed at pH 7-8.
Another familiar 18F-prosthetic group for biomolecules conjugation is 4-[18F]-fluorobenzaldehyde which can form a chemoselective oxime bond with aminooxy group [90]. 4-[18F]-fluorobenzaldehyde is obtained in one step labeling using trimethylammonuim benzaldehyde triflate precursor with a reasonable radiochemical yield and time of reaction (Scheme 7). The formation of oxime bond is usually done in aqueous media, pH 2-4 at 60-100°C. The acidity and heat required in the formation of oxime bond are major limitations of this method, because large biomolecules usually don't tolerate such conditions. However, these conditions can sometimes be applied to labeli small biomolecules such as oligonucleotides and peptides. 4-[18F]-fluorobenzaldehyde is capable of forming bond with hydrazino-group in the biomolecule, to form hydrazone. The coupling is done with high concentration of biomolecules at 50-60°C [91] and the biomolecules need to undergo modifications before the coupling reaction. Recently it was reported on the use of [18F]-FDG as a prosthetic group by forming oxime bond with small peptides such as RGD [78]. [18F]-FDG can also form oxime bond with different maleimide derivatives to create a thiol-reactive prosthetic group [92].
Wester et al. [79] reported on the preparation of 4-azidophenacyl-[18F] fluoride ([18F]APF) from the corresponding bromo precursor with high radiochemical yield and reasonably short reaction time. [18F]APF contains electrophilic groups such as arylnitrene, that can be used for fluorination by UV radiation in the presence of biomolecules to yield the conjugated product (Scheme 6).
Meldal et al. [93] and Sharpless et al. [94] reported in 2002 a high chemoselective and efficient reaction between 1,3-dipolar cycloaddition of terminal alkynes and azides in the presence of Cu(I) as a catalyst, to give the corresponding triazole. They found that under mild reaction conditions they dramatically enhanced yields and reduced reaction times from days to hours. Cycloaddition can theoretically be performed in the presence of water and oxygen and is orthogonal to any functional group, hence it can be performed without the protection of other functional groups [95]. Due to the high efficiency and selectivity, the new variant of this cycloaddition can be suitable for bioconjugation of F-18 derivatives with biomolecules.
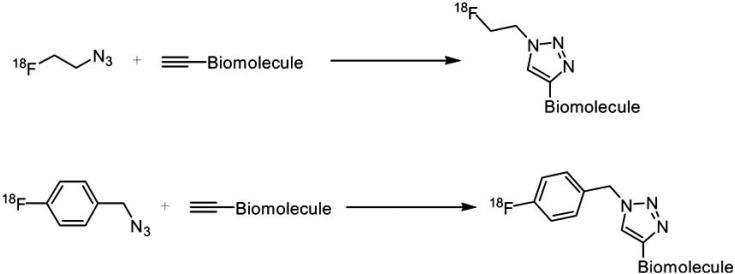
The reaction of 1,3-dipolar cyloaddition and flexible 18F-labeling chemistry dipolar known as “click chemistry” and its use in radiochemistry was reported by Marik and Sutcliffe [96] in 2006, who used 18F-labeled alkynes for a click reaction with azide-functionalized peptides [96]. Since then, 18F-click chemistry has been used in several studies for labeling peptides [95, 97] with high efficiency (Scheme 8).
Scheme 8.
18F-Labeling “click chemistry” reactions, using 2-[18F]fluoroethylazide and 1-(azidomethyl)-4-[18F]-fluorobenzene.
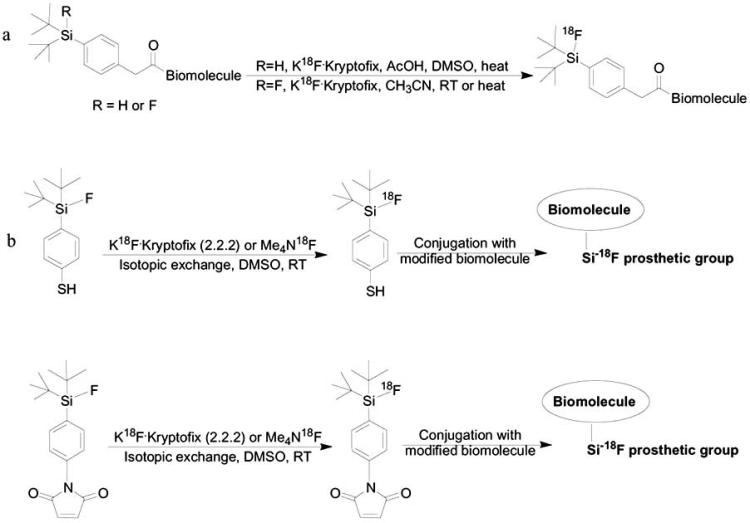
Fluoride-18 can also be introduced into biomolecules containing silicon, by the formation of high energy bond Si-18F [98, 99] (Scheme 9a). Schirrmacher and co-workers reported on the direct 19F-18F exchange of modified peptides which contains di-tert-butylphenyl-fluorosilane group with high radiochemical yield [100]. The isotopic exchange can also be done at room temperature which is very useful for labeling a variety of biomolecules which are usually not stable with heat. Si-18F bond formation can also be applied for non-carrier added fluoride-18. Hohne et al. reported on the direct fluorination of bombesin analogues by nucleophilic displacement of fluoride ion on alkoxy, hydroxy or hydride leaving groups using modified di-tert-butylsilyl based peptides [99, 101, 102] (Scheme 9a). This fluorination yielded with high specific activity but required heating the modified peptides for fluoride-18 displacement. In addition, several Si-18F prosthetic groups were developed for labeling biomolecules in two radioactive steps (Scheme 9b). Usually, the fluorination of organofluorosilane derivatives, based on the formation of Si-18F bond derive from isotopic exchange of fluoride-19 with fluoride-18, under mild conditions and relatively high radiochemical yield [103-105].
Scheme 9.
18F-Labeling of silicon based biomolecules. a; direct fluorination on silica-modifies biomolecules. b; labeling of Si-18F-prosthetic groups for further conjugation with biomolecules.
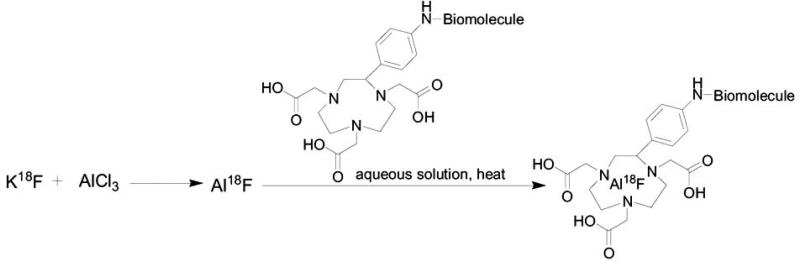
A new method for fluoride-18 labeling was published recently by McBride et al. [106]. They reported on the direct labeling of chelate-attached-peptide with aluminum-fluoride (Al18F) [106]. Initially, fluoride-18 is attached to aluminuim to form Al18F, which is further reacted with peptides that contain chelator group such as 1,4,7-triazacyclononane-1,4,7-triaceticacid (NOTA), to form a stable complex of Al18F-NOTA-peptide (Scheme 10). Another advantage of this method is that it doesn't require anhydrous environment conditions, therefore there is no need to prepare a reactive fluoride ion by azeotropic removal of the water, which also saves time in the radiosynthesis [106]. This method may be applicable to other chelate-conjugated biomolecules and will allow a suitable route for a direct labeling with fluoride-18.
Scheme 10.
Al18F-complexation with NOTA-conjugated biomolecule.
5. CONCLUSIONS
Flouride-18 is the most frequently used radioisotope because of the optimal properties described above. The versatile chemistry for labeling with fluoride-18 that has been developed over the last decades allows the incorporation of the isotope into various molecules. Multiple markers targeting various receptors in the fields of oncology, neurology, inflammatory conditions, artery diseases and other pathologies will enable in the future personalized treatment of patients, depending on the properties of their condition, and will facilitate and assist the evaluation of treatment.
REFERENCES
- 1.Bonasera TA, Ortu G, Rozen Y, Krais R, Freedman NM, Chisin R, Gazit A, Levitzki A, Mishani E. Potential (18)F-labeled biomarkers for epidermal growth factor receptor tyrosine kinase. Nucl. Med. Biol. 2001;28:359–374. doi: 10.1016/s0969-8051(01)00200-1. [DOI] [PubMed] [Google Scholar]
- 2.Jhanwar YS, Straus DJ. The role of PET in lymphoma. J. Nucl. Med. 2006;47:1326–1334. [PubMed] [Google Scholar]
- 3.Gofrit ON, Mishani E, Orevi M, Klein M, Freedman N, Pode D, Shapiro A, Katz R, Libson E, Chisin R. Contribution of 11C-choline positron emission tomography/computerized tomography to preoperative staging of advanced transitional cell carcinoma. J. Urol. 2006;176:940–944. doi: 10.1016/j.juro.2006.04.018. discussion 944. [DOI] [PubMed] [Google Scholar]
- 4.Niemeyer MG, Kuijper AF, Gerhards LJ, D'Haene EG, van der Wall EE. Nitrogen-13 ammonia perfusion imaging: relation to metabolic imaging. Am. Heart J. 1993;125:848–854. doi: 10.1016/0002-8703(93)90180-h. [DOI] [PubMed] [Google Scholar]
- 5.Ter-Pogossian MM, Herscovitch P. Radioactive oxygen-15 in the study of cerebral blood flow, blood volume, and oxygen metabolism. Semin. Nucl. Med. 1985;15:377–394. doi: 10.1016/s0001-2998(85)80015-5. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: A [11C]raclopride PET study. Synapse. 2007;61:637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y, Kawai K, Magata Y, Takeda R, Hashiguchi H, Abe H, Mukai T, Saji H. Changes in dopamine D2 receptors and 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine uptake in the brain of 6-hydroxydopamine-lesioned rats. Neurodegener. Dis. 2004;1:109–112. doi: 10.1159/000080051. [DOI] [PubMed] [Google Scholar]
- 8.Saleem A, Charnley N, Price P. Clinical molecular imaging with positron emission tomography. Eur. J. Cancer. 2006;42:1720–1727. doi: 10.1016/j.ejca.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Dolle F. Fluorine-18-labelled fluoropyridines: advances in radiopharmaceutical design. Curr. Pharm. Des. 2005;11:3221–3235. doi: 10.2174/138161205774424645. [DOI] [PubMed] [Google Scholar]
- 10.Qiam SM. Recent Developments in the Production of 18F, 75/76/77Br and 123I. Int. J. Radiat. Appl. Instrum. Part A. 1986;37:803–810. [Google Scholar]
- 11.Nickles RJ, Gatley SJ, Votaw JR, Kornguth ML. Production of reactive fluorine-18. Int. J. Rad. Appl. Instrum. A. 1986;37:649–661. doi: 10.1016/0883-2889(86)90258-3. [DOI] [PubMed] [Google Scholar]
- 12.McQuade P, Rowland DJ, Lewis JS, Welch MJ. Positron-emitting isotopes produced on biomedical cyclotrons. Curr. Med. Chem. 2005;12:807–818. doi: 10.2174/0929867053507397. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MR, Suzuki K. [18F]Fluoroalkyl agents: synthesis, reactivity and application for development of PET ligands in molecular imaging. Curr. Top. Med. Chem. 2007;7:1817–1828. doi: 10.2174/156802607782507448. [DOI] [PubMed] [Google Scholar]
- 14.Shiue CY, Welch MJ. Update on PET radiopharmaceuticals: life beyond fluorodeoxyglucose. Radiol. Clin. North Am. 2004;42:1033–1053. doi: 10.1016/j.rcl.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MS, Tewson TJ. Chemistry of fluorine-18 radiopharmaceuticals. Int. J. Rad. Appl. Instrum. A. 1986;37:685–693. doi: 10.1016/0883-2889(86)90262-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee BC, Dence CS, Zhou H, Parent EE, Welch MJ, Katzenellenbogen JA. Fluorine-18 labeling and biodistribution studies on peroxisome proliferator-activated receptor-gamma ligands: potential positron emission tomography imaging agents. Nucl. Med. Biol. 2009;36:147–153. doi: 10.1016/j.nucmedbio.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, Li F, Chen X. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J. Nucl. Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parent EE, Dence CS, Jenks C, Sharp TL, Welch MJ, Katzenellenbogen JA. Synthesis and biological evaluation of [18F]bicalutamide, 4-[76Br]bromobicalutamide, and 4-[76Br] bromo-thiobicalutamide as non-steroidal androgens for prostate cancer imaging. J. Med. Chem. 2007;50:1028–1040. doi: 10.1021/jm060847r. [DOI] [PubMed] [Google Scholar]
- 19.Ilovich O, Jacobson O, Aviv Y, Litchi A, Chisin R, Mishani E. Formation of fluorine-18 labeled diaryl ureas--labeled VEGFR-2/PDGFR dual inhibitors as molecular imaging agents for angiogenesis. Bioorg. Med. Chem. 2008;16:4242–4251. doi: 10.1016/j.bmc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 20.Dissoki S, Aviv Y, Laky D, Abourbeh G, Levitzki A, Mishani E. The effect of the [18F]-PEG group on tracer qualification of [4-(phenylamino)-quinazoline-6-YL]-amide moiety--an EGFR putative irreversible inhibitor. Appl. Radiat. Isot. 2007;65:1140–1151. doi: 10.1016/j.apradiso.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Kiesewetter DO, Kramer-Marek G, Ma Y, Capala J. Radiolabeling of HER2 specific Affibody(R) molecule with F-18. J. Fluor. Chem. 2008;129:799–805. doi: 10.1016/j.jfluchem.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiesewetter DO, Eckelman WC, Cohen RM, Finn RD, Larson SM. Syntheses and D2 receptor affinities of derivatives of spiperone containing aliphatic halogens. Int. J. Rad. Appl. Instrum. A. 1986;37:1181–1188. doi: 10.1016/0883-2889(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 23.Nickles RJ, Daube ME, Ruth TJ. An 18O2 target for the production of [18F]F2. Int. J. Appl. Radiat. Isot. 1984;35:117–122. [Google Scholar]
- 24.Helus F, Maier-Borst W, Sahm U, Wiebe LI. F-18 cyclotron production methods. Radiochem. Radioanal. Lett. 1979;38:395–410. [Google Scholar]
- 25.Schlyer DJ. PET tracers and radiochemistry. Ann. Acad. Med. Singapore. 2004;33:146–154. [PubMed] [Google Scholar]
- 26.Casella V, Ido T, Wolf AP, Fowler JS, MacGregor RR, Ruth TJ. Anhydrous F-18 labeled eslemental flurine for radiopharmaceutical preparation. J. Nucl. Med. 1980;21:750–757. [PubMed] [Google Scholar]
- 27.Palmer AJ, Clark JC, Goulding RW. The Preparation of Fluorine- 18 Labelled Radiopharmaceuticals. Int. J. Appl. Radiat. Isot. 1977;28:53–65. doi: 10.1016/0020-708x(77)90160-0. [DOI] [PubMed] [Google Scholar]
- 28.Furuya T, Kuttruff CA, Ritter T. Carbon-fluorine bond formation. Curr. Opin. Drug Discov. Dev. 2008;11:803–819. [PubMed] [Google Scholar]
- 29.Adachi K, O. Y, Tomizawa G, Ishihara S, Oishi S. Electrophilic fluorination with N,N’-difluoro-2,2′-bipyridinium salt and elemental fluorine. J. Fluorine Chem. 2003;120:173–183. [Google Scholar]
- 30.Oberdorfer F, Hofmann E, Maier-Borst W. Preparation of a new 18F-labelled precursor: 1-[18F]fluoro-2-pyridone. Int. J. Radiat. Appl. Instrum. Part A. 1988;39:685–688. [Google Scholar]
- 31.Ogawa M, Hatano K, Oishi S, Kawasumi Y, Fujii N, Kawaguchi M, Doi R, Imamura M, Yamamoto M, Ajito K, Mukai T, Saji H, Ito K. Direct electrophilic radiofluorination of a cyclic RGD peptide for in vivo alpha(v)beta3 integrin related tumor imaging. Nucl. Med. Biol. 2003;30:1–9. doi: 10.1016/s0969-8051(02)00387-6. [DOI] [PubMed] [Google Scholar]
- 32.Diksic M, Farrokhzad S, Yamamoto YL, Feindel W. A simple synthesis of 18F-labelled 5-fluorouracil using acetylhypofluorite. Int. J. Nucl. Med. Biol. 1984;11:141–142. doi: 10.1016/0047-0740(84)90049-4. [DOI] [PubMed] [Google Scholar]
- 33.Murali D, Flores LG, Roberts AD, Nickles RJ, DeJesus OT. Aromatic L-amino acid decarboxylase (AAAD) inhibitors as carcinoid tumor-imaging agents: synthesis of 18F-labeled alpha-fluoromethyl-6-fluoro-m-tyrosine (FM-6-FmT). Appl. Radiat. Isot. 2003;59:237–243. doi: 10.1016/s0969-8043(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 34.Teare H, Robins EG, Arstad E, Luthra SK, Gouverneur V. Synthesis and reactivity of [18F]-N-fluorobenzenesulfonimide. Chem. Commun. (Camb) 2007;23:2330–2332. doi: 10.1039/b701177f. [DOI] [PubMed] [Google Scholar]
- 35.Oberdorfer F, Hofmann E, Maier-Borst W. Preparation of 18F-labelled N-fluoropyridinium triflate. J. Labelled Comp. Radiopharm. 1988;25:999–1005. [Google Scholar]
- 36.Groves AM, Win T, Haim SB, Ell PJ. Non-[18F]FDG PET in clinical oncology. Lancet Oncol. 2007;8:822–830. doi: 10.1016/S1470-2045(07)70274-7. [DOI] [PubMed] [Google Scholar]
- 37.Seibyl JP, Chen W, Silverman DH. 3,4-dihydroxy-6-[18f]-fluoro-L-phenylalanine positron emission tomography in patients with central motor disorders and in evaluation of brain and other tumors. Semin. Nucl. Med. 2007;37:440–450. doi: 10.1053/j.semnuclmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J. Nucl. Med. 2008;49:573–586. doi: 10.2967/jnumed.107.045708. [DOI] [PubMed] [Google Scholar]
- 39.Namavari M, Bishop A, Satyamurthy N, Bida G, Barrio JR. Regioselective radiofluorodestannylation with [18F]F2 and [18F]CH3COOF: a high yield synthesis of 6-[18F]Fluoro-L-dopa. Int. J. Rad. Appl. Instrum. A. 1992;43:989–996. doi: 10.1016/0883-2889(92)90217-3. [DOI] [PubMed] [Google Scholar]
- 40.Dolle F, Demphe S, Hinnen F, Fournier D, Vaufrey F, Crouzel C. 6-[18F]Fluoro-L-DOPA by radiofluorodestannylation: a short and simple synthesis of a new labelling precursor. J. Labelled. Comp. Radiopharm. 1998;41:105–114. [Google Scholar]
- 41.Szajek LP, Channing MA, Eckelman WC. Automated synthesis of 6-[l8F]-fluoro-L-DOPA using polystyrene supports with 6-mercuric DOPA precursors. Appl. Radiat. Isot. 1998;49:795–804. [Google Scholar]
- 42.de Vries EFJ, Luurtsema G, Brüssermann M, Elsinga PH, Vaalburg W. Fully automated synthesis module for the high yield one-pot preparation of 6-[18F]-Fluoro-L-DOPA. Appl. Radiat. Isot. 1999;51:389–394. [Google Scholar]
- 43.Dolbier WR, Jr., Li AR, Koch CJ, Shiue CY, Kachur AV. [18F]-EF5, a marker for PET detection of hypoxia: synthesis of precursor and a new fluorination procedure. Appl. Radiat. Isot. 2001;54:73–80. doi: 10.1016/s0969-8043(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 44.Guillaume M, Luxen A, Nebeling B, Argentini M, Clark JC, Pike VW. Recommendations for fluorine-18 production. Appl. Radiat. Isot. Instrum. A. 1991;42:74–762. [Google Scholar]
- 45.Kim DW, Jeong HJ, Lim ST, Sohn MH, Katzenellenbogen JA, Chi DY. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: significantly enhanced reactivity of alkali metal fluorides and improved selectivity. J. Org. Chem. 2008;73:957–962. doi: 10.1021/jo7021229. [DOI] [PubMed] [Google Scholar]
- 46.Schlyer DJ, Bastos MA, Alexoff D, Wolf AP. Separation of [18F]fluoride from [18O]water using anion exchange resin. Int. J. Rad. Appl. Instrum. A. 1990;41:531–533. doi: 10.1016/0883-2889(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 47.Jewett DM, Toorongian SA, Bachelor MA, Kilbourn MR. Extraction of [18F]Fluoride from [18O]water by a fast fibrous anion exchange resin. Inr. J. Radial. Appl. Insfrum. Parr A. 1990;41:583–586. doi: 10.1016/0883-2889(90)90044-h. [DOI] [PubMed] [Google Scholar]
- 48.Romer J, Fuchtner F, Steinbach J, Johannsen B. Automated production of 16alpha-[18F]fluoroestradiol for breast cancer imaging. Nucl. Med. Biol. 1999;26:473–479. doi: 10.1016/s0969-8051(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 49.Chi DY, Kilbourn MR, Katzenellenbogen JA, Brodack JW, Welch MJ. Synthesis of no-carrier-added N-([18F] fluoroalkyl)spiperone derivatives. Int. J. Rad. Appl. Instrum. A. 1986;37:1173–1180. doi: 10.1016/0883-2889(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 50.Iwata R, Pascali C, Bogni A, Furumoto S, Terasaki K, Yanai K. [18F]fluoromethyl triflate, a novel and reactive [18F]fluoromethylating agent: preparation and application to the on-column preparation of [18F]fluorocholine. Appl. Radiat. Isot. 2002;57:347–352. doi: 10.1016/s0969-8043(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 51.Chi DY, Kilbourn MR, Katzenellenbogen JA, Welch MJ. A rapid and efficient method for the fluoroalkylation of amines and amides. Development of a method suitable for incorporation of the short-lived positron emitting radionuclide fluorine-18. J. Org. Chem. 1987;52:658–664. [Google Scholar]
- 52.Zhang MR, Tsuchiyama A, Haradahira T, Yoshida Y, Furutsuka K, Suzuki K. Development of an automated system for synthesizing 18F-labeled compounds using [18F]fluoroethyl bromide as a synthetic precursor. Appl. Radiat. Isot. 2002;57:335–342. doi: 10.1016/s0969-8043(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 53.Coenen HH, Colosimo M, Schüller M, Stöcklin G. Preparation of n.c.a. [18F]-CH2BrF via aminopolyether supported nucleophilic substitution. J. Labelled. Comp. Radiopharm. 1986;23:587–595. [Google Scholar]
- 54.Bergman J, Eskola O, Lehikoinen P, Solin O. Automated synthesis and purification of [18F]bromofluoromethane at high specific radioactivity. Appl. Radiat. Isot. 2001;54:927–933. doi: 10.1016/s0969-8043(00)00358-4. [DOI] [PubMed] [Google Scholar]
- 55.Vallabhajosula S. (18)F-labeled positron emission tomographic radiopharmaceuticals in oncology: an overview of radiochemistry and mechanisms of tumor localization. Semin. Nucl. Med. 2007;37:400–419. doi: 10.1053/j.semnuclmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Grierson AF, Shields AF. An improved radiosynthesis of [18F]-FLT. J. Labelled. Comp. Radiopharm. 1999;42:S525–S526. [Google Scholar]
- 57.Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[(18)F]fluorothymidine: [(18)F]FLT for imaging of cellular proliferation in vivo. Nucl. Med. Biol. 2000;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 58.Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch MJ. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. J. Nucl. Med. 1984;25:1212–1221. [PubMed] [Google Scholar]
- 59.Grierson JR, Link JM, Mathis CA, Rasey JS, Krohn KA. A radiosynthesis of fluorine-18 fluoromisonidazole. J. Nucl. Med. 1989;30:343–350. [PubMed] [Google Scholar]
- 60.Lim JL, Berridge MS. An efficient radiosynthesis of [18F]fluoromisonidazole. Appl. Radiat. Isot. 1993;44:1085–1091. doi: 10.1016/0969-8043(93)90110-v. [DOI] [PubMed] [Google Scholar]
- 61.Tang G, Wang M, Tang X, Gan M, Luo L. Fully automated one-pot synthesis of [18F]fluoromisonidazole. Nucl. Med. Biol. 2005;32:553–538. doi: 10.1016/j.nucmedbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, Krohn KA. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int. J. Radiat. Oncol. Biol. Phys. 1996;36:417–428. doi: 10.1016/s0360-3016(96)00325-2. [DOI] [PubMed] [Google Scholar]
- 63.Rasey JS, Grunbaum Z, Magee S, Nelson NJ, Olive PL, Durand RE, Krohn KA. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat. Res. 1987;111:292–304. [PubMed] [Google Scholar]
- 64.Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, Krohn KA, Griffin TW. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int. J. Radiat. Oncol. Biol. Phys. 1992;22:199–212. doi: 10.1016/0360-3016(92)91001-4. [DOI] [PubMed] [Google Scholar]
- 65.Tewson TJ. Synthesis of [18F]fluoroetanidazole: a potential new tracer for imaging hypoxia. Nucl. Med. Biol. 1997;24:755–760. doi: 10.1016/s0969-8051(97)00135-2. [DOI] [PubMed] [Google Scholar]
- 66.Kachur AV, Dolbier WR, Jr., Evans SM, Shiue CY, Shiue GG, Skov KA, Baird IR, James BR, Li AR, Roche A, Koch CJ. Synthesis of new hypoxia markers EF1 and [18F]-EF1. Appl. Radiat. Isot. 1999;51:643–650. doi: 10.1016/s0969-8043(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 67.Kim DW, Choe YS, Chi DY. A new nucleophilic fluorine-18 labeling method for aliphatic mesylates: reaction in ionic liquids shows tolerance for water. Nucl. Med. Biol. 2003;30:345–350. doi: 10.1016/s0969-8051(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 68.Kim DW, Ahn DS, Oh YH, Lee S, Kil HS, Oh SJ, Lee SJ, Kim JS, Ryu JS, Moon DH, Chi DY. A new class of SN2 reactions catalyzed by protic solvents: Facile fluorination for isotopic labeling of diagnostic molecules. J Am. Chem. Soc. 2006;128:16394–16397. doi: 10.1021/ja0646895. [DOI] [PubMed] [Google Scholar]
- 69.Jacobson O, Bechor Y, Icar A, Novak N, Birman A, Marom H, Fadeeva L, Golan E, Leibovitch I, Gutman M, Even-Sapir E, Chisin R, Gozin M, Mishani E. Prostate cancer PET bioprobes: synthesis of [18F]-radiolabeled hydroxyflutamide derivatives. Bioorg. Med. Chem. 2005;13:6195–6205. doi: 10.1016/j.bmc.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 70.Mishani E, Cristel ME, Dence CS, McCarthy TJ, Welch MJ. Application of a novel phenylpiperazine formation reaction to the radiosynthesis of a model fluorine-18-labeled radiopharmaceutical (18FTFMPP). Nucl. Med. Biol. 1997;24:269–273. doi: 10.1016/s0969-8051(97)00063-2. [DOI] [PubMed] [Google Scholar]
- 71.Ross TL, Ermert J, Hocke C, Coenen HH. Nucleophilic 18F-fluorination of heteroaromatic iodonium salts with no-carrier-added [18F]fluoride. J. Am. Chem. Soc. 2007;129:8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 72.Carroll MA, Nairne J, Woodcraft JL. Diaryliodonium salts: a solution to 3-[18F]-fluoropyridine. J. Labelled Comp. Radiopharm. 2007;50:452–454. [Google Scholar]
- 73.Kilbourn MR, Dence CS, Welch MJ, Mathias CJ. Fluorine-18 labeling of proteins. J. Nucl .Med. 1987;28:462–470. [PubMed] [Google Scholar]
- 74.Okarvi SM. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nucl. Med. 2001;28:929–938. doi: 10.1007/s002590100508. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Link JM, Stekhova S, Yagle KJ, Smith C, Krohn KA, Tait JF. Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconjug. Chem. 2008;19:1684–1688. doi: 10.1021/bc800164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Z, Li ZB, Cai W, He L, Chin FT, Li F, Chen X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of alphavbeta3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1823–1831. doi: 10.1007/s00259-007-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl. Med. Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Namavari M, Cheng Z, Zhang R, De A, Levi J, Hoerner JK, Yaghoubi SS, Syud FA, Gambhir SS. A Novel Method for Direct Site-Specific Radiolabeling of Peptides Using [(18)F]FDG. Bioconjug. Chem. 2009;20(3):432–436. doi: 10.1021/bc800422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wester HJ, Hamacher K, Stocklin G. A comparative study of N.C.A. fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl. Med. Biol. 1996;23:365–372. doi: 10.1016/0969-8051(96)00017-0. [DOI] [PubMed] [Google Scholar]
- 80.Shai Y, Kirk KL, Channing MA, Dunn BB, Lesniak MA, Eastman RC, Finn RD, Roth J, Jacobson KA. 18F-labeled insulin: a prosthetic group methodology for incorporation of a positron emitter into peptides and proteins. Biochemistry. 1989;28:4801–4806. doi: 10.1021/bi00437a042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jakobson K, Furlano DC, Kirk KL. A prosthetic group for the rapid introduction of fluorine into peptides and functionalized drugs. J. Fluor. Chem. 1988;39:339–347. doi: 10.1016/S0022-1139(00)81606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaidyanathan G, Zalutsky MR. Improved synthesis of N-succinimidyl 4-[18F]fluorobenzoate and its application to the labeling of a monoclonal antibody fragment. Bioconjug. Chem. 1994;5:352–356. doi: 10.1021/bc00028a012. [DOI] [PubMed] [Google Scholar]
- 83.Wilchek M, Knudsen KL, Miron T. Improved method for preparing N-hydroxysuccinimide ester-containing polymers for affinity chromatography. Bioconjug. Chem. 1994;5:491–492. doi: 10.1021/bc00029a018. [DOI] [PubMed] [Google Scholar]
- 84.Hostetler ED, Edwards WB, Anderson CJ, Welch MJ. Synthesis of 4-[18F]fluorobenzoyl octreotide and biodistribution in tumour-bearing Lewis rats. J. Labelled. Comp. Radiopharm. 1999;42:S720–722. [Google Scholar]
- 85.Herman LW, Fischman AJ, Tompkins RG, Hanson RN, Byon C, Strauss HW, Elmaleh DR. The use of pentafluorophenyl derivatives for the 18F labelling of proteins. Nucl. Med. Biol. 1994;21:1005–1010. doi: 10.1016/0969-8051(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 86.Lang L, Eckelman WC. One-step synthesis of 18F labeled [18F]-N-succinimidyl 4-(fluoromethyl)benzoate for protein labeling. Appl. Radiat. Isot. 1994;45:1155–1163. doi: 10.1016/0969-8043(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 87.Lang L, Eckelman WC. Labeling proteins at high specific activity using N-succinimidyl 4-[18F](fluoromethyl) benzoate. Appl. Radiat. Isot. 1997;48:169–173. doi: 10.1016/s0969-8043(96)00151-0. [DOI] [PubMed] [Google Scholar]
- 88.Vaidyanathan G, Zalutsky MR. Labeling proteins with fluorine-18 using N-succinimidyl 4-[18F]fluorobenzoate. Int. J. Rad. Appl. Instrum. B. 1992;19:275–281. doi: 10.1016/0883-2897(92)90111-b. [DOI] [PubMed] [Google Scholar]
- 89.Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat. Protoc. 2006;1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
- 90.Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N. Synthesis of a new heterobifunctional linker, N-[4-(aminooxy)butyl]maleimide, for facile access to a thiol-reactive 18F-labeling agent. Bioconjug. Chem. 2003;14:1253–1259. doi: 10.1021/bc034107y. [DOI] [PubMed] [Google Scholar]
- 91.Chang YS, Jeong JM, Lee YS, Kim HW, Rai GB, Lee SJ, Lee DS, Chung JK, Lee MC. Preparation of 18F-human serum albumin: a simple and efficient protein labeling method with 18F using a hydrazone-formation method. Bioconjug. Chem. 2005;16:1329–1333. doi: 10.1021/bc050086r. [DOI] [PubMed] [Google Scholar]
- 92.Berndt M, Pietzsch J, Bergmann R, Wuest F. A novel role for [18F]FDG: Synthesis and application of a [18F]FDG-based prosthetic group for peptides and protein labeling. J. Nuc. Med. 2006;47:S29. doi: 10.1021/bc8000112. [DOI] [PubMed] [Google Scholar]
- 93.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 94.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 95.Thonon D, Kech C, Paris J, Lemaire C, Luxen A. New strategy for the preparation of clickable peptides and labeling with 1-(azidomethyl)-4-[(18)F]-fluorobenzene for PET. Bioconjug. Chem. 2009;20:817–823. doi: 10.1021/bc800544p. [DOI] [PubMed] [Google Scholar]
- 96.Marik J, Sutcliffe J. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–6684. [Google Scholar]
- 97.Ross TL, Honer M, Lam PY, Mindt TL, Groehn V, Schibli R, Schubiger PA, Ametamey SM. Fluorine-18 click radiosynthesis and preclinical evaluation of a new 18F-labeled folic acid derivative. Bioconjug. Chem. 2008;19:2462–2470. doi: 10.1021/bc800356r. [DOI] [PubMed] [Google Scholar]
- 98.Rosenthal MS, Bosch AL, Nickles RJ, Gatley SJ. Synthesis and some characteristics of no-carrier added [18F]fluorotrimethylsilane. Int. J. Appl. Radiat. Isot. 1985;36:318–319. doi: 10.1016/0020-708x(85)90094-8. [DOI] [PubMed] [Google Scholar]
- 99.Mu L, Hohne A, Schubiger PA, Ametamey SM, Graham K, Cyr JE, Dinkelborg L, Stellfeld T, Srinivasan A, Voigtmann U, Klar U. Silicon-based building blocks for one-step 18F-radiolabeling of peptides for PET imaging. Angew. Chem. Int. Ed. Engl. 2008;47:4922–4925. doi: 10.1002/anie.200705854. [DOI] [PubMed] [Google Scholar]
- 100.Schirrmacher R, Bradtmoller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wangler B, Niemeyer CM, Jurkschat K. 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem. Int. Ed. Engl. 2006;45:6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- 101.Hohne A, Mu L, Honer M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Borkowski S, Berndorff D, Klar U, Voigtmann U, Cyr JE, Friebe M, Dinkelborg L, Srinivasan A. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug. Chem. 2008;19:1871–1879. doi: 10.1021/bc800157h. [DOI] [PubMed] [Google Scholar]
- 102.Hohne A, Yu L, Mu L, Reiher M, Voigtmann U, Klar U, Graham K, Schubiger PA, Ametamey SM. Organofluorosilanes as model compounds for 18F-labeled silicon-based PET tracers and their hydrolytic stability: experimental data and theoretical calculations (PET = positron emission tomography). Chemistry. 2009;15:3736–3743. doi: 10.1002/chem.200802437. [DOI] [PubMed] [Google Scholar]
- 103.Rosa-Neto P, Wangler B, Iovkova L, Boening G, Reader A, Jurkschat K, Schirrmacher E. [18F]SiFA-isothiocyanate: a new highly effective radioactive labeling agent for lysine-containing proteins. ChemBioChem. 2009;10:1321–1324. doi: 10.1002/cbic.200900132. [DOI] [PubMed] [Google Scholar]
- 104.Iovkova L, Wangler B, Schirrmacher E, Schirrmacher R, Quandt G, Boening G, Schurmann M, Jurkschat K. para-Functionalized aryl-di-tert-butylfluorosilanes as potential labeling synthons for (18)F radiopharmaceuticals. Chemistry. 2009;15:2140–2147. doi: 10.1002/chem.200802266. [DOI] [PubMed] [Google Scholar]
- 105.Wangler B, Quandt G, Iovkova L, Schirrmacher E, Wangler C, Boening G, Hacker M, Schmoeckel M, Jurkschat K, Bartenstein P, Schirrmacher R. Kit-like 18F-labeling of proteins: synthesis of 4-(di-tert-butyl[18F]fluorosilyl)benzenethiol (Si[18F] FA-SH) labeled rat serum albumin for blood pool imaging with PET. Bioconjug. Chem. 2009;20:317–321. doi: 10.1021/bc800413g. [DOI] [PubMed] [Google Scholar]
- 106.McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, Chang CH, Boerman OC, Goldenberg DM. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]