Abstract
New blood vessel formation (angiogenesis) is fundamental to tumor growth, invasion, and metastatic dissemination. The vascular endothelial growth factor (VEGF) signaling pathway plays pivotal roles in regulating tumor angiogenesis. VEGF as a therapeutic target has been validated in various types of human cancers. Different agents including antibodies, aptamers, peptides, and small molecules have been extensively investigated to block VEGF and its pro-angiogenic functions. Some of these agents have been approved by FDA and some are currently in clinical trials. Combination therapies are also being pursued for better tumor control. By providing comprehensive real-time information, molecular imaging of VEGF pathway may accelerate the drug development process. Moreover, the imaging will be of great help for patient stratification and therapeutic effect monitoring, which will promote effective personalized molecular cancer therapy. This review summarizes the current status of tumor therapeutic agents targeting to VEGF and the applications of VEGF related molecular imaging.
Keywords: Vascular endothelial growth factor, VEGF, targeted therapy, molecular imaging
Tumor Angiogenesis
Angiogenesis is the process of new blood vessel development, which is critical in both physiological development and pathological processes such as tumor progression, wound healing, cardiovascular, inflammatory, ischemic, and infectious diseases (1–3). It has been found several decades ago that tumors implanted into isolated perfused organs failed to grow beyond a few millimeters in diameter (4) as diffusion of nutrients and oxygen from nearby capillaries is inadequate to go beyond 100–200 μm in order to sustain cell function (5–7). For multicellular tumor clones to grow beyond this size, they must recruit new blood vessels by angiogenesis and vasculogenesis. It is now widely accepted that both mutations of oncogenes and tumor suppressor genes lead to the switch into an angiogenic tumor, i.e., the endogenous balance between pro-angiogenic and anti-angiogenic molecules is tipped in favor of angiogenesis (8–10). Tumor angiogenesis also involves an intricate interplay between the tumor and surrounding or supportive cells, including vascular endothelial cells, pericytes, smooth muscle cells, fibroblasts and tumor-associated macrophages (11).
Tumor vessels can grow by several different patterns including sprouting, intussusception or incorporation of bone marrow-derived endothelial precursors. In addition, tumor cells can co-opt existing vessels (12). Sprouting angiogenesis is the most important mechanism for tumor vascularization, which involves several steps from the growth of endothelial sprouts from preexisting post-capillary venules to the growth and remodeling process of the primitive network into a complex network (2, 13–15). In response to hypoxia, tumor tissues produce and release angiogenic growth factors such as vasculoendothelial growth factor (VEGF), the acidic and basic fibroblast growth factors (aFGF, bFGF), and the platelet-derived endothelial cell growth factor (PD-ECGF) (16). When these angiogenic growth factors bind to their corresponding specific receptors located on the endothelial cells of pre-existing blood vessels, various signal transduction pathways are activated to promote the activation of endothelial cells (17, 18). Subsequently, the original vessels undergo characteristic morphological changes, including enlargement of the diameter, basement membrane degradation, a thinned endothelial cell lining, increased endothelial number, decreased number of pericytes and detachment of pericytes (19). At the sprouting tips of growing vessels, endothelial cells secrete matrix metalloproteinases (MMPs) to facilitate the degradation of extracellular matrix and cell invasion (20). Cell surface adhesion molecules such as integrins also play an important role in endothelial cell migration and in contact with the extracellular tumor matrix, facilitating cell survival (21, 22). Next, a lumen within an endothelial cell tubule has to be formed, which requires interactions between the extracellular matrix and cell-associated surface proteins, among them are galectin-2, PECAM-1, and VE-cadherin (23). Finally, newly formed vessels are stabilized through the recruitment of smooth muscle cells and pericytes.
Unlike blood vessels in healthy tissues, the tumor vasculatures appear as disorganized tubular structures, which are often interconnected, tortuous, highly leaky, resembling premature sinusoidal vasculatures (24, 25). These abnormal vessels usually lack a clear separation between arterioles and venules and the recruitment of pericytes and vascular smooth muscle cells (26). A fast growing tumor almost always creates a hypoxic environment due to several interconnected reasons including unsynchronized growth rates of tumor cells and endothelial cells, disorganized vascular architecture, sluggish blood flow and high interstitial fluid pressure (IFP) (27, 28). Hypoxia leads to increased levels of hypoxia inducible factor-1alpha (HIF-1α) by inhibiting proline hydroxylase activity and stabilizing HIF-1α , which increases VEGF expression by activating its promoter (29). High level of VEGF could further increase vascular disorganization, permeability, and IFP, leading to severe hypoxia in turn (30). The high leakage and poor perfusion of tumor blood vessels facilitate cancer cells spreading throughout the body to develop metastasis (22, 31). Moreover, tumor-associated endothelial cells can acquire cytogenetic abnormalities while in the tumor microenvironment. This genetic instability may allow endothelial cells to survive modifications to the intratumor ecosystem, and become resistant to anti-angiogenic agents (32).
Vascular Endothelial Growth Factor (VEGF)
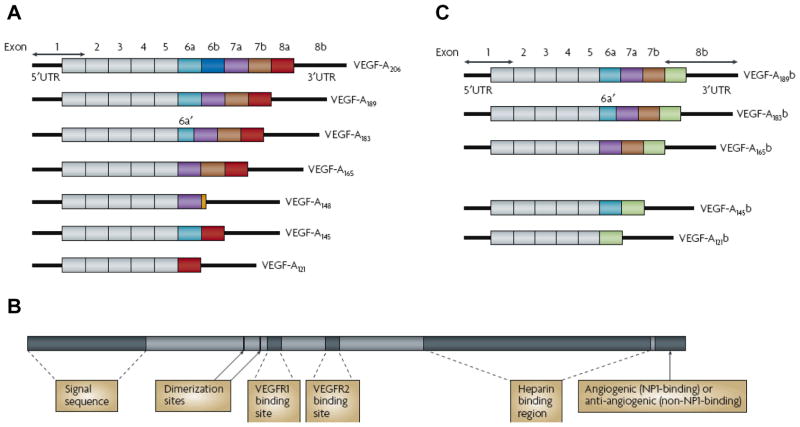
VEGF is a key tumor-derived angiogenic factor that exerts multiple functions including stimulation of angiogenesis, vasculogenesis, inflammation and vascular permeability (33, 34). The VEGF family of growth factors and its receptors constitute the most important signaling pathways in tumor angiogenesis (35–37). Initially, VEGF-A was identified as a vascular permeability factor (VPF) in 1983 and later characterized as an endothelial-specific mitogen (38). Subsequently, the whole VEGF family has been identified to comprise eight members with a common VEGF homology domain: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placenta growth factor (PIGF) -1 and -2 (36). VEGF-A is a dimeric, disulfide-bound glycoprotein existing in at least seven homodimeric isoforms due to alternative exon splicing of exons 6 and 7, which consist of 121, 145, 148, 165, 183, 189, or 206 amino acids (Figure 1A). Structurally VEGF belongs to the VEGF-PDGF (platelet-derived growth factor) super-gene family (Figure 1B). Among these gene products, 8 cysteine residues are conserved at the same positions, the these products function as a dimeric form since 2 out of 8 cysteines generate intermolecular cross-linking disulfide bonds. The other 6 cysteines make 3 intermolecular disulfide bonds to form 3 loop structures (39). Besides the difference in molecular weight, these isoforms also differ in their biological properties such as the ability to bind to cell surface heparin sulfate proteoglycans (36). For example, the shortest form, VEGF121, is a freely diffusible protein; VEGF165 is also secreted, although a significant fraction remains bound to the cell surface and the extracellular matrix.VEGF189, by contrast, is almost completely sequestered in the extracellular matrix (40). Among them, VEGF165 is the predominant isoform and is commonly overexpressed in a variety of human solid tumors (41).
Figure 1. Protein and mRNA products of human vascular endothelial growth factor A (VEGF-A).
A The pro-angiogenic isoforms (VEGF-Axxx, left) are generated by proximal splice site (PSS) selection in exon 8. B Protein structure of VEGF-A containing the dimerization sites and binding sites for heparin, VEGF-A receptor 1 (VEGFR1; encoded by exon 3) and VEGFR2 (encoded by exon 4), which are present in all isoforms. C The anti-angiogenic family (VEGF-Axxxb, right) from exon 8 distal splice site (DSS) choice. UTR, untranslated region. Reprinted with the permission of reference (64).
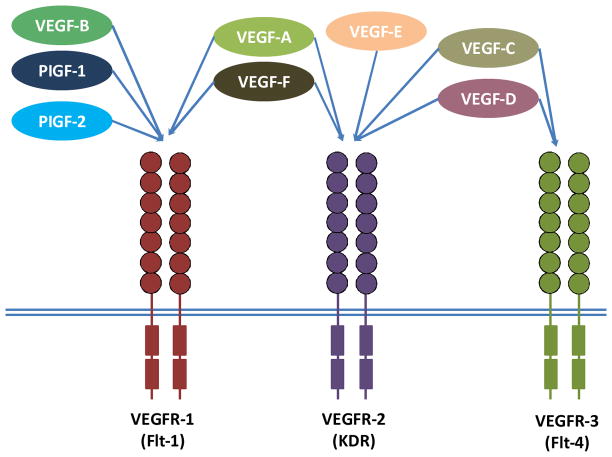
As shown in Figure 2, VEGFs signal through three tyrosine kinase receptors, known as Flt-1 (VEGFR-1), Flk-1/KDR (VEGFR-2) and VEGFR-3 predominantly expressed by endothelial cells (37, 42). A degree of specificity has been shown for growth factor-receptor binding, VEGF-B and PlGF-1 and -2 bind to VEGFR-1, whereas VEGF-A interacts with both VEGFR-1 and -2. Vascular endothelial growth factor-C and -D specifically bind to VEGFR-3 (43). Both VEGF-E and VEGF-F are exogenous subtypes. VEGF-E, encoded by a gene found in the NZ7 Orf viral genome, has approximate 25% of amino acid identity with VEGF-A and 17% with PDGF (44). VEGF-E tightly binds and stimulates VEGFR-2 similarly to VEGF-A, but not VEGFR-1 or VEGFR-3 (45). VEGF-F is a family of snake venom-derived VEGFs (46). Among them, vammin and VR-1 fromthe venoms of Vipera a. ammodytes and Daboia r. russelli bind only KDR with high affinity similar to VEGF-A but not to other VEGF receptors (47). Tf-svVEGF and Pm-VEGF from the venoms of Trimeresurus flavoviridis and Protobothrops mucrosquamatus have been shown to bind Flt-1 in preference to KDR, unlike vammin and VR-1 (48, 49).
Figure 2.
Binding specificity of various vascular endothelial growth factor (VEGF) family members and their receptors. VEGF-E and VEGF-F are exogenous subtypes.
Both VEGFR-1 and -2 can promote angiogenesis and VEGFR-3 stimulation leads to lymphangiogenesis (50). Binding with VEGFs leads to the dimerization of VEGFRs and activation of downstream signaling cascades. Activation of the VEGF/VEGFR pathway promotes endothelial cell growth, migration and survival. This pathway also mediates vessel permeability and mobilizes endothelial progenitor cells. There is a general consensus that VEGFR-2 is the dominant receptor in mediating the pro-angiogenic functions of VEGF-A and this pathway has been prioritized for the development of antiangiogenic therapies. Though VEGFR-1 has a 10-fold higher binding affinity for VEGF-A, its activation has less impact on the activation of intracellular signaling intermediates than VEGFR-2 (51). It has been reported that VEGFR-1 is critical for physiologic and developmental angiogenesis and its function varies with the stages of development, the states of physiologic and pathologic conditions, and the cell types in which it is expressed (36, 52). Apart from VEGFRs, Neuropilin-1 and -2 are cell surface proteins that bind to the most common isoform of VEGF-A, VEGF165, and may act as co-receptors to enhance VEGF signaling through VEGFR-1 (53).
VEGF promotes tumor angiogenesis through several mechanisms, including enhanced endothelial cell proliferation and survival; increased migration and invasion of endothelial cells; increased permeability of existing vessels, forming a lattice network for endothelial cell migration; and enhanced chemotaxis and homing of bone marrow derived vascular precursor cells (54, 55). In addition to having proangiogenic effects, VEGF has several important functions that are independent of vascular processes, including autocrine effects on tumor cell function (survival, migration, invasion), immune suppression, and homing of bone marrow progenitors to ‘prepare’ an organ for subsequent metastasis (56). Higher angiogenesis and VEGF expression have been detected in various human cancers including colorectal cancer (57), breast cancer (58), non small cell lung cancer (59), renal cell cancer (60), glioblastoma multiforme (61) and other tumors than corresponding nonmalignant normal tissue. Among patients with the highest levels of VEGF expression, survival was significantly worse than in patients with negative or lower levels of VEGF expression (62). VEGF levels were predictive of future metastases independently of nodal status and adjuvant chemotherapy, with a positive predictive value of 73% (63).
Recently, it has been found that VEGF-A mRNA splicing generates two families of proteins by exon 8 distal splice site (DSS) selection that differ by their C′ terminal six amino acids (Figure 1C), and these are termed VEGF-Axxx and VEGF-Axxxb, where xxx denotes the amino acid number of the mature protein (64–66). Basal expression is dominated by VEGF-Axxxb isoforms in many tissues such as in human vitreous fluid, circulating plasma, urine, renal cortex, colonic epithelium, bladder smooth muscle, lung and pancreatic islets (66, 67). However, in melanoma, colorectal carcinoma and bladder cancer cells as well as proliferating dedifferentiated podocytes, VEGF-Axxx isoforms comprise the majority of VEGF-A (68). Although both VEGF-A165 and VEGF-A165b bind VEGFR-2 with equal affinity (68), VEGF-A165b inhibits several VEGF-A165-mediated processes including endothelial cell migration in vitro, proliferation in vitro and vasodilatation ex vivo (65). In tumors, overexpression of transfected VEGF-A165b delays the growth of melanoma, kidney, prostate and Ewing sarcoma tumors (69). Tumors treated with VEGF-A165b are paler, less haemorrhagic and visibly less vascularized, with reduced microvascular density and increased necrosis (70).
VEGF as a Therapeutic Target
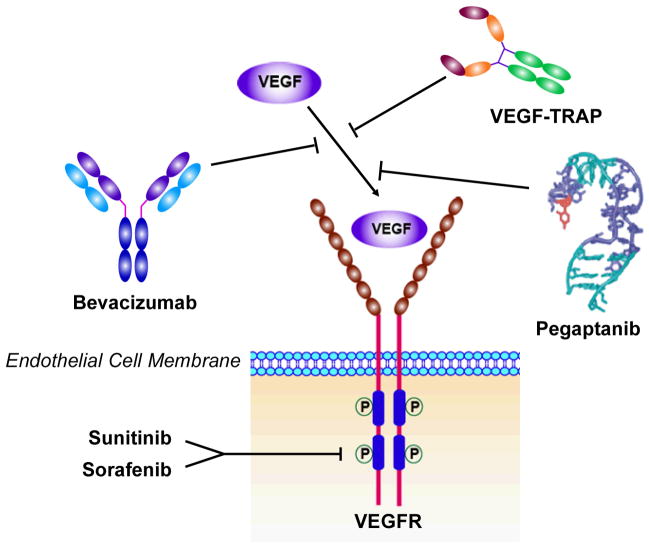
In as early as 1971, Folkman proposed that anti-angiogenesis might be an effective anticancer strategy (7) based on the observation that tumor growth was associated with marked vascularity (35). Recognition of the VEGF pathway as a key regulator of angiogenesis has led to the development of several VEGF-targeted agents, including agents that prevent VEGF-A binding to its receptors (71), antibodies that directly block VEGFR-2 (72, 73), and small molecules that inhibit the kinase activity of VEGFR-2 thereby block growth factor signaling (74–76). Some of them were approved by FDA to clinical applications (Figure 3).
Figure 3.
VEGF targeted and anti-VEGF drugs.
Mechanisms of VEGF-targeted therapy
To block VEGF, several anti-angiogenic effects are expected (77). Firstly, it will inhibit new vessel growth, perhaps accompanied by vessel regression and subsequent tumor cell death. VEGF is a survival factor for endothelial cells (78) and VEGF withdrawal can induce tumor endothelial cell death as well as prevent further angiogenesis (79, 80). Results from clinical trials suggested a cytostatic effect of VEGF-targeted therapy, that is, tumor growth was delayed, but there was little evidence of tumor shrinkage (81), providing indirect evidence that VEGF-targeted therapy is cytostatic for blood vessel growth, as was first hypothesized (7).
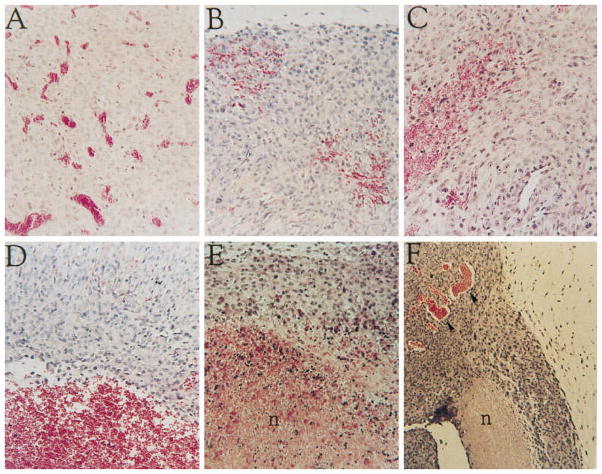
Secondly, blocking VEGF will induce endothelial cell apoptosis. VEGF mediates numerous pro-survival pathways in endothelial cells including induction or activation of BCL2, Akt, survivin and inhibitor of apoptosis proteins (IAPs) (82, 83). As VEGF mediates endothelial cell survival functions, loss of VEGF signaling has been proposed to lead to endothelial cell apoptosis. Using an inducible VEGF expression system in glioma cells, Benjamin and Keshet (84) demonstrated that shutting off VEGF production leads to the detachment of endothelial cells from the walls of preformed vessels and their subsequent death by apoptosis, indicating that VEGF was required for endothelial cell survival in tumor xenografts (Figure 4). Many studies using VEGF-targeted therapies in murine models demonstrated that inhibition of VEGF signaling could lead to tumor endothelial cell apoptosis, although rarely did such therapies lead to regression of established tumors (85, 86).
Figure 4.
Vessel regression and tumor necrosis following shut off of VEGF expression. Hematoxylin and eosin staining of sections from C6 tumors grown in Nude mice for 2 weeks in the absence of tetracycline and resected 0 h (A), 24 h (B), 48 h (C), 72 h (D), 4 days (E), and 5 days (F) after administration of tetracycline to shut off expression of VEGF from the transfected VEGF165. n, Necrosis. Reprinted with permission of reference (84)
In addition, anti-VEGF therapy will cause blockade of incorporation of haematopoietic and endothelial progenitor cells. VEGF can stimulate vasculogenesis in tumors by recruiting bone marrow-derived haematopoietic progenitor cells (HPCs) and endothelial progenitor cells (EPCs) (55, 87). In preclinical models the rate of incorporation of EPCs is typically 5–50% for most studies (77). While in humans the percentage of tumor vessels derived from bone marrow precursors is much lower (88). Although the incorporation rate of EPCs into tumor vasculature may be low, these cells provide a crucial function in sustaining angiogenesis in growing tumors. Thus, blockade of VEGFR-1+ HPCs and VEGFR-2+ EPC-mediated vasculogenesis in tumors may be an important mechanism of VEGF-targeted therapy in selected tumor types. Indeed, selective inhibition of VEGFR-1+ HPCs eliminates the pre-metastatic niche, reducing the formation of micrometastases. Selective targeting of VEGFR-2+ EPCs results in the formation of micrometastases without vascularization (56). The chemotaxis of EPCs is largely under the influence of VEGF (89). Preclinical studies, as well as early-phase clinical studies, have shown that VEGF-targeted therapy can inhibit the mobilization of EPCs and their presumed incorporation into the tumor vasculature (90). However, there exists controversial report that BM-derived, VEGFR-2 positive cells or other endothelial cell precursors do not contribute to the angiogenic tumor vasculature (91).
VEGF can increase the expression of nitric oxide (NO), prostacyclins and other soluble mediators that lead to vasodilation (92). Therefore, VEGF targeted therapy will cause vascular constriction. In clinical trials, shortly after the administration of VEGF-targeted therapy (within 48 h), a decrease in tumor perfusion has been observed with functional CT scan (93), which suggested that this is not due to vessel destruction, but more probably effects on the integrity (permeability) and function (vessel perfusion) of the vascular bed. The acute effects on blood flow are probably due to decreases in production of the vasodilators NO and prostacyclin. Owing to the fact that many of the abnormalities of the tumor vascular network are secondary to VEGF, Jain has hypothesized that VEGF-targeted therapy can ‘normalize’ the vasculature of tumors (24, 94). This window of normalization in mice is relatively short (days) and occurs soon after the initiation of VEGF-targeted therapy. Expression of all VEGFRs, as well as NP1 and NP2, has been detected on tumor cells (95), which have been shown to promote survival, proliferation and metastasis via autocrine mechanisms (96, 97). Thus, VEGF-targeted therapy might have a direct inhibitory effect on tumor cells in addition to its effect on the vasculature (98, 99). Immune modulation may also be involved in anti-VEGF therapy. Accumulating evidences suggest that VEGF has an important role in establishing immune privilege of tumors by blocking dendritic cell (DC) differentiation (100). Treatment of tumor-bearing mice with neutralizing VEGF antibodies resulted in an increased number of spleen and lymph node DCs and improved DC differentiation (101). Treatment with VEGF-targeted antibodies was also shown to improve anti-tumor peptide cytotoxic T-lymphocyte responses and efficacy of tumor immunotherapy in mouse models (102). Thus, VEGF is a strong negative modulator of DC function in the tumor microenvironment, which contributes to immune privilege of tumors in the host. It is also now clear that VEGF receptors can be expressed and functional on cancer cells, indicating that anti-VEGF treatment strategies may have direct antitumor effects (103).
VEGF Targeted and anti-VEGF Drugs
Bevacizumab
At present, antibodies are indisputably the best established class of binding molecules for tumor diagnosis and therapy (104, 105). Bevacizumab is a humanized monoclonal antibody composed of the consensus human IgG1 framework and antigen-binding regions (93%) and compliment-determining regions from the murine mAb A.4.6.1 (7%) (106). Bevacizumab neutralizes all isoforms of human VEGF and inhibits VEGF-induced proliferation of endothelial cells in vitro with an ED50 of around 50 ng/ml. Distribution analyzed in a rabbit model demonstrated that the majority of bevacizumab remained in the plasma, with a large quantity being distributed to the heart, testes, bladder and kidney as compared to other organs (107) with a circulating half-life of ~20 days (108). Bevacizumab was tested in combination with several chemotherapeutic drugs such as doxorubicin, topotecan, paclitaxel and docetaxel, showing an additive antitumor effect (109, 110). The combination of bevacizumab with paclitaxel resulted in marked tumor growth suppression in both the CWR22R androgen-independent xenograft model of prostate cancer or in the OVCAR3 ovarian tumor model (111, 112). The combination regimen significantly reduced tumor growth as compared to bevacizumab or paclitaxel alone. It has also been reported that bevacizumab could reverse the protection effect of endothelial cells by the high levels of VEGF produced by the tumor from the antiangiogenic effects of docetaxel (113). Bevacizumab is currently approved by the FDA for patients with metastatic colorectal cancer, non-small cell lung cancer, recurrent glioblastoma multiforme and metastatic breast cancer in combination with chemotherapy (114, 115) (116) and renal cell carcinoma in combination with interferon alpha (117).
Pegaptanib, the VEGF-A aptamer
Aptamers are RNA or DNA oligonucleotides that have been selected for their ability to bind proteins with both high affinity and high specificity. The selection of aptamers with specificity for virtually any protein target has become straightforward following the advent of systematic evolution of ligands by exponential enrichment (SELEX) (118). It is believed that aptamers are essentially non-immunogenic even when administered in excess of therapeutic doses (119, 120). Unmodified nucleic acids are very susceptible to nuclease attack and an unmodified antisense oligonucleotide has a serum half-life less than a minute (121). Therefore, F- and NH2-substituted ribonucleotides were subsequently used in the development of resistant aptamers (122, 123).
Pegaptanib is an anti-VEGF RNA aptamer, selected by having chosen VEGF165 as the target with SELEX methodology using F-substituted nucleotides (122–124). For detailed screening process, please see the review (125). Pegaptanib inhibited binding of 125I-labeled VEGF165 to human umbilical vein endothelial cells (HUVECs) and also to human dermal microvascular endothelial cells, with IC50 values in the range of 0.75–1.4 nM. Pegaptanib also inhibited VEGF165-mediated phosphorylation of VEGFR-2 and phospholipase Cγ and inhibited VEGF165- induced calcium mobilization in HUVECs (126). The binding of pegaptanib to VEGF involves close contact with cysteine-137 of VEGF165 so pretreatment of the cells with pegaptanib inhibited proliferative responses only to VEGF165, but not to VEGF121 (123). This residue is contained within the 55-amino-acid heparin-binding domain of VEGF, which is not present in VEGF121 (Figure 4) (127). In 2004, the US FDA approved pegaptanib sodium (MacugenR) for the treatment of all types of neovascular age-related macular degeneration (AMD) (128).
Certain guanosine-rich phosphodiester oligodeoxynucleotides, termed G-rich oligonucleotides (GRO), have antiproliferative activity against a variety of cancer cell lines (129, 130). An active GRO (named GRO29A) can cause cell cycle arrest and induction of cell death in human cancer cell lines but not in nonmalignant human cells (130). Several phase I clinical trials showed that AS1411, the truncated version of GRO29A was well tolerated and had promising clinical activity and now it is in phase II clinical trial to treat patients with relapsed or refractory acute myeloid leukemia (AML) and renal cell carcinoma (RCC) (131). It has been reported that AS1411 binds with nucleolin and nuclear factor-κB (NF-KB) essential modulator (NEMO) to inhibit NFκB pathway most probably(132). Other VEGF specific aptamers have also been developed and showed potential clinical applications in cancer detection and therapy (133).
VEGF Trap
VEGF Trap is a soluble receptor to VEGF developed by Regeneron, Inc. VEGF Trap is a high-affinity anti-VEGF compound, engineered by combining domains from VEGFR-2 and VEGFR-1. Initial animal data revealed that soluble VEGFR had poor bioavailability when administered subcutaneously (s.c.). These characteristics were hypothesized to be related to a high positive charge of the protein, which led to the deposition of Trap molecules at the s.c. injection site due to nonspecific adhesion to components of the extracellular matrix. To overcome this issue, investigators removed the third Ig domain of VEGFR-1 (with significant basic charge), replaced it with the third Ig domain from VEGFR-2, and added a dimerization domain from the Fc region of human IgG (134). These changes decreased the isoelectric point of VEGF Trap and increased bioavailability after s.c. administration, with an increase in Cmax and area under the curve of VEGF Trap. The structural changes introduced into VEGF Trap dramatically improved the affinity of the VEGF Trap molecule for VEGF-A, VEGF-B, and placental growth factor. In equilibrium binding assays, VEGF Trap, with a molecular weight of 115 kDa, had an affinity for VEGF of about 1 pM, significantly improved from the 5 pM binding affinity seen with a Trap molecule made solely from VEGFR-1 domains (134). In addition, VEGF Trap binds placental growth factor with an affinity of 45 pM, compared with the VEGF affinity of bevacizumab of 500 pM. With higher affinity, the goal of depleting tissue and plasma VEGF is hypothesized to be better accomplished.
Several in vitro experiments have shown the potential antitumor efficacy of VEGF Trap (134, 135). When given s.c. twice weekly, VEGF Trap led to significant decrease in tumor size for the xenografts tested when compared with control treated animals. Immunohistochemical staining showed a dramatic, dose-dependent reduction in tumor vasculature in the VEGF-treated mice (134). VEGF Trap treatment also led to the suppression of growth in pancreatic cell lines and was accompanied by a significant reduction of tumor microvessel density (MVD) (135). In a neuroblastoma xenograft modal, treatment with VEGF Trap biweekly led to 79% decrease in mean tumor weight after 36 days of treatment (136). VEGF Trap also has been used in combination with conventional cytotoxic chemotherapeutic agents. The combination of VEGF Trap and paclitaxel led to a 98% reduction in tumor volume with associated reduction in ascites (137). Correlative work showed significant decrease in tumor vasculature in the tumors treated with VEGF Trap and paclitaxel. Treatment with VEGF Trap alone or paclitaxel alone resulted in only modest rates of apoptosis (10% and 40%, respectively), whereas the combination of VEGF Trap and paclitaxel led to apoptosis in >90% of tumor cells. In a phase I clinical trial, 47 patients with refractory solid tumors or non-Hodgkin's lymphoma received VEGF Trap at doses ranging from 0.3 to 7.0 mg/kg IV every 2 weeks. The results showed that i.v. VEGF Trap was well tolerated at the dose levels tested. In addition, changes in volume transfer constant measured by DCE-MRI at baseline and at 24 hours after administration indicate a possible dose-related change in this pharmacodynamic marker (138).
VEGFR tyrosine kinase inhibitors sunitinib and sorafenib
Tyrosine kinase inhibitors currently approved for use in patients with solid tumors include imatinib, erlotinib, gefitinib, sorafenib and sunitinib. These agents compete with ATP for binding within the intracellular domain of various wild-type and/or mutated receptor tyrosine kinases, which represent a new paradigm in anticancer therapy (139). Unlike Bevacizumab, VEGF Trap and Pegaptanib, which target extracellular VEGF, tyrosine kinase inhibitors target the intracellular signaling pathways of VEGF receptors. Tyrosine kinase inhibitors target not only VEGF receptors and but also a variety of receptors that rely on a tyrosine kinase component to function properly, including PDGF receptor, FMS-like tyrosine kinase 3 (FLT3), RAF, and c-KIT receptors (140) .
Sunitinib malate, marketed by Pfizer as Sutent, has been shown to be a potent inhibitor of VEGF receptors, FLT3, c-KIT, and PDGF receptors in vitro (141). These targets give sunitinib direct antitumor and antiangiogenic properties (140).Certain types of cancer, including RCC, demonstrate a hypervascular histology, which is thought to be due to overexpression of VEGF and PDGF (142). Inhibition of VEGF and PDGF by sunitinib prevents further growth of new vessels. The direct antitumor effects of sunitinib are a result of its actions on the FLT3 and c-KIT receptors. Currently, sunitinib is approved by FDA as a second-line treatment of mRCC in patients who have not responded to or who are not eligible to receive IL-2 and as a monotherapy for treatment of advanced renal cell carcinoma (RCC) (143).
Similar to sunitinib, sorafenib inhibits VEGF receptors, PDGF receptors, FLT3, RAF-1, and BRAF in vitro (144). RAF-1 and BRAF belong to RAS family and are involved in normal cell proliferation, differentiation, and transformation. This signaling pathway is also present in tumor cell lines and plays a role in the pathogenesis of various cancers. VEGF requires a signaling cascade through the RAF–MEK–ERK pathway to take into effects. This allows sorafenib to counteract the activity of VEGF by two mechanisms: inhibiting the VEGF receptor and blocking the downstream activity of VEGF (144). In preclinical trials, sorafenib was shown to have broad-spectrum antitumor activity in mouse models and was found to prevent the growth of tumors but not to reduce tumor size (144). Sorafenib was approved by FDA for patients with metastatic renal cell carcinoma and hepatocellular carcinoma (145). Sunitinib and sorafenib share a similar mechanism of action and primarily target tumor angiogenesis by inhibiting a variety of tyrosine kinases. However, their efficacy appears to be differerent. Sorafenib does not result in tumor shrinkage, but sunitinib significantly reduces tumor size (146).
Pazopanib (GW786034) is an oral, second-generation multi-targeted tyrosine kinase inhibitor targeting VEGF-1, -2, and -3 receptors, PDGF-α and-β receptors, and c-kit. Pazopanib exhibited good potency against all of the human VEGFRs and closely related tyrosine receptor kinases in vitro, and demonstrated antitumor activity in several human tumor xenografts, including renal cell carcinoma (RCC), and breast and lung cancer (147). A phase II clinical trial with 225 patients with metastatic RCC showed that overall RR was 35% and median duration of response was 68 weeks, supporting the further development of pazopanib in advanced RCC (148). Another phase II single-arm study in patients with recurrent glioblastoma demonstrated that pazopanib was reasonably well tolerated with a spectrum of toxicities similar to other anti-VEGF/VEGFR agents. Single-agent pazopanib did not prolong PFS in this patient population but showed in situ biological activity as demonstrated by radiographic responses (149).
Besides TKIs, antibodies blocking VEGFR2 also has been developed. Ramucirumab (IMC-1121B; ImClone Systems, New York, NY) is a fully human immunoglobulin G1 monoclonal antibody (MAb) that binds with high affinity (approximately 50 pM) to the extracellular VEGF-binding domain of VEGFR-2 (150). Ramucirumab binds to a VEGFR-2 epitope involved in ligand binding and block VEGF ligands from binding this site and activating the receptor (151). Inhibition of VEGF-stimulated VEGFR-2 activationby ramucirumab conferred significant antitumor activity in a range of malignancies in animal models as single agents and in combination with other therapeutics (152). In a phase I clinical trial, patients with advanced solid malignancies were treated onceweekly with escalating doses of ramucirumab. The results showed that tumor perfusion and vascularity decreased in 69% of evaluable patients (153).
Combination of anti-VEGF drugs with either chemotherapy or radiation therapy
Anti-angiogenetic drugs are either used either as a single agent or adjunctive to chemotherapy. It is a sound deduction that a treatment aimed at reducing the blood supply of a tumor would also reduce the delivery of any other therapy such as chemotherapy and reduce the oxygen supply necessary for a response to radiotherapy (154). Indeed, a phase II trial tested the combination of 5-fluorouracil and leucovorin with two different doses of bevacizumab for treating metastatic colorectal cancer. The higher-dose group resulted in lower response rate than the lower-dose group, indicating that while bevacizumab might be antagonistic with chemotherapy, especially in excessive doses (155). Another example is that addition of bevacizumab to capecitabine treatment of metastatic breast cancer patients did not increase TTP or OS, although RR increased from 9.1% to 19.8 (115). However, synergism of anti-angiogenetics and chemotherapeutics has been observed in patients with colon cancers (114), non-small cell lung cancers (156). One explanation is that blockage of VEGF signaling, bevacizumab induces normalization of newly formed vessels, and thus reduces the interstitial tissue pressure (ITP) within tumors, allowing enhanced delivery of chemotherapy to the tumors (90). Moreover, the combination of anti-VEGF and chemotherapy might enhance killing of endothelial cells in the tumor blood vessels (79). Multiple preclinical studies suggest that combining radiation and angiogenesis inhibitors enhances the therapeutic ratio of radiation therapy (157, 158), though there is still concern that a reduction of tumor oxygenation resulting from inhibition of angiogenesis with destruction of the tumor vasculature could render the tumor hypoxic and thereby more radioresistant. The rationale came from the fact that endothelial cell apoptosis determines tumor response to RT (159) and RT induces VEGF expression and secretion by tumor cells, thereby inducing a paracrine endothelial protective stress response (160). However, the optimal timing and duration of antiangiogenic therapies to maximize radiation’s therapeutic ratio need be further determined (161).
Toxicity and Resistance with VEGF Inhibitors
Treatments that interfere with VEGF function are generally well tolerated although a specific side-effect profile is associated with this treatment modality. The most common toxicities are hypertension (grade 3 in approximately 10% patients), proteinuria (usually grade 1–2), hemorrhage, arterial and venous thrombotic events (104), impaired wound healing and, of particular concern in the initial phase II bevacizumab trials in ovarian cancer, gastrointestinal perforation (162). Although the role of VEGF in normal endothelial homoeostasis is not well understood, the rapid regression of capillaries in several different tissues such as pancreatic islets, thyroid, adrenal cortex, choroid plexus and small intestinal villi is seen within a few days of starting treatment with VEGF inhibitors in animal models (163). Loss of fenestration of renal glomerular capillaries also occurs, which might contribute to hypertension and proteinuria (164). VEGF also promotes endothelial nitric oxide production, and the removal of this stimulus may lead to vasoconstriction and hypertension (163, 165). VEGFR TKIs have also been associated with clinical hypothyroidism, which could be caused by the inhibition of iodine uptake in the thyroid (166).
One of the most common features after anti-VEGF therapy was the occurrence of a rapid decrease in density in central areas of the tumor in contrast to a sustained rim of well-vascularized tumor tissue in the surrounding area, forming a pseudo-capsule at the interface between the tumor and normal tissue. After various durations of exposure, some tumors eventually re-grew from peripheral areas, suggesting that the tumor rim is the primary place of evasion from anti-angiogenic VEGFR-targeting agents (167). It has been proposed that tumor collapse causes central regions of hypoxia that may help circumvent VEGFR-inhibited signaling pathways and promote evasion from VEGFR inhibitors in peripheral areas (168). Mutation of VEGFR/PDGFR or altered receptors or polymorphisms may also have a role in the resistance to anti-VEGF/VEGFR therapy. The resistance of this peripheral rim of viable tumor cells may be overcome by combination TKIs with targeted agents directed against kinases such as mammalian target of rapamycin (mTOR), mitogen-activated protein kinases (MAPKs) and protein kinase C (PKC) or the addition of cytotoxic drugs to destroy subclones evading multitargeted agents (139). In addition to morphological differences, tumor endothelial cells have distinct gene expression profiles (169) compared with normal endothelium and can also be cytogenetically abnormal (32), which may also contribute to the resistance to antiangiogenic treatment strategies.
A decrease in the expression level of soluble VEGFR has been consistently reported in patients receiving sunitinib treatment. Conversely, an increased level of VEGF seems to occur in many patients receiving treatment with sunitinib and may have a role in the flare-up of tumor growth that may occur after sunitinib discontinuation (170). Activation of alternative signaling pathways may overcome VEGFR inhibition. In this latter phase of circumvention, which produces phenotypic resistance to VEGFR-2 blockade, tumor cells upregulate the expression of other pro-angiogenic factors including PDGF/PDGFR and FGFs that reactivate angiogenesis in a VEGFR-independent manner (171). Exposure to VEGFR inhibitors upregulates ephrin B2 and ephrin B4, which are primarily expressed by endothelial cells and have an important role in regulating the assembly of vascular cells into stable networks by mediating endothelial–mesenchymal cell interactions (172). As mentioned above, VEGF-A165b contains binding domains for the vast majority of anti-VEGF-A antibodies, including therapeutic antibodies such as bevacizumab. The dose of Bevacizumab required to prevent tumor growth in VEGF-A165-expressing tumors had absolutely no effect on VEGF-A165b-expressing tumors (68), which suggests that treatment of patients with tumors expressing significant levels of VEGF-Axxxb with bevaciumab may not be effective, because VEGF-A165b will inhibit the effect of this anti-VEGF-A antibody.
Evaluation of Anti-angiogenesis Therapy
Imaging blood flow and vascular volume
The most commonly used end-point for assessing anti-angiogenic treatment in clinical studies is microvessel density (MVD), measured from biopsies taken before and at one or more times after treatment, using a variety of immunohistochemical vascular markers such as CD34, CD31, CD105 and von Willebrand factor (vWF) to identify the vessels (90). However, measurement of MVD is problematic for assessing the vascular efficacy of antiangiogenic agents (82) since blocking angiogenesis may be accompanied by a proportional reduction in tumor growth that would not result in a net change in MVD. Besides, vessel counts and/or density may remain unchanged even in the face of effective therapy (173). Therefore, although a reduction in MVD following treatment is indicative of an antiangiogenic effect, it does not mean that no change in MVD is indicative of no antiangiogenic effect, as is commonly assumed. In fact, recent studies with anti-DLL4 therapy (targeting the Notch pathway on endothelial cells) have indicated that vascular functions but not numbers are essential for promoting tumor growth (174, 175). Notch ligands and their receptors are involved in arteriogenesis, vascular remodeling, and maturation (176). Paradoxically, inhibition of Notch signaling evokes suppression of tumor growth accompanied by increased levels of tumor neovascularization (177). These new findings challenge the classical thinking of tumor angiogenesis and tumor growth where more tumor blood vessels would make a tumor growing faster (7).
Non-invasive imaging methods for measuring functional vascular volume are available and can provide a noninvasive means of detecting angiogenesis within and about the perimeter of the whole tumor and give functional information. For instance, PET studies with 15O-oxygen and related tracers can offer direct physiological measurement of circulatory parameters of regional blood flow and vascular volume (178). Ultrasound (particularly microbubble contrast enhanced ultrasound) is also a valuable imaging modality to determine the tumor microvascular blood volume and blood velocity (179). Especially, dynamic contrast-enhanced ultrasonography (DCE-US) allows repeated examinations and provides both morphologic and functional analyses. Several quantitative parameters considered as indicators of tumor flow such as the peak intensity (PI) or time-to-PI can be extracted from the time-intensity curves of contrast uptake (180). Using DCE-US, the antitumor efficacy of AVE8062, a tumor vasculature disruptive agent, has been assessed in melanoma-bearing nude mice (181). In a human melanoma xenograft model, CEU measures of tumor neovascularity was compared with the expression of molecular markers of angiogenesis (182). After power Doppler and intermittent pulse-inversion harmonic imaging (PI-HI), the tumor tissues were surgically removed and sectioned in the same planes as the ultrasound images and immunohistochemical staining for VEGF were carried out. Although there is a trend of correlation between percent area stained with VEGF and intermittent PI-HI results, no statistical significance was achieved. In a follow-up study using similar approach in two melanoma models, linear regression analysis indicated statistically significant correlations between percent area stained with VEGF and power Doppler and intermittent PI-HI measures of tumor neovascularity (183). Power Doppler ultrasonography has been used to demonstrate the presence of blood flow in small vessels and it was also found that the vascular signal correlates with histopathological quantification of the vascular density of synovial tissue (144).
Dynamic contrast-enhanced MRI (DCE-MRI) has also been well established to investigate angiogenesis within tumors, and in particular the response to antiangiogenic therapy. The leakage of MR contrast agent through tumor vessels results in a fast “wash-in” of contrast coupled with the rapid “wash-out,” and allows a functional analysis of the tumor microcirculation (184). DCE-MRI has been the most utilized pharmacodynamic imaging modality in early phase clinical trials of angiogenic inhibitors. This functional imaging technique is non-invasive and can be used to serially assess tumor vasculature in vivo (185). DCE-MRI can be performed with low-molecular-weight contrast media (LMCM) such as Gd-diethylenetriamine pentaacetic acid (Gd-DTPA) or macromolecular contrast media (MMCM) such as Gd conjugated human serum albumin (Gd-HSA) (186). DCE-MRI can be used to demonstrate the antiangiogenic effects of drugs early after their administration, and can predict traditional treatment response parameters such as changes in tumor size. The ability to accurately monitor angiogenesis response to therapy means that drug efficacy can be established at a very early stage of treatment so that non-responding patients may be detected and management plans altered on a timely basis (187). It has been shown that DCE-MRI can detect responses to PTK/ZK (a VEGF receptor tyrosine kinase inhibitor) therapy as early as two days after therapy with significant reductions in area under gadolinium-contrast-medium curve (AUGC) (30) or permeability parameters (188), which also predict subsequent response. LMCM DCE-MRI has also shown significant reductions in permeability values in patients treated with the antivascular agents AG-013736 (an inhibitor of the VEGF, PDGF, c-Kit receptor tyrosine kinases) and SU5416 (a selective inhibitor of VEGFR-2 tyrosine kinase) activity (189). Although consensus is still lacking on the exact kinetic model to be used in analyzing DCE-MRI data, the differences among the various methods are often marginal. Therefore, DCE-MRI is rapidly emerging as the imaging technique of choice for monitoring clinical response in trials of new antiangiogenic and antivascular therapies.
However, like MVD measurements, a negative effect on vascular volume indicated by non-invasive imaging cannot be interpreted as absence of antiangiogenic effect, either (190). Indeed, a study in a xenografted model of human breast cancer showed a poor correlation between MVD and fractional blood volume estimates as measured by functional MRI and macromolecular contrast agents (86). Tumor blood flow rate is an also accessible end-point for clinical studies. A decrease in tumor blood flow rate would be expected if MVD decreased and its measurement would provide additional functional information linked to oxygen availability and tumor growth. However, some pre-clinical studies have demonstrated an increase in tumor blood flow rate following antiangiogenic therapy. For example, Teicher et al. (191) showed that tumor blood flow and oxygenation significantly increased in the first weeks of treatment with TNP-470, a synthetic analogue of fumagillon. Following antiangiogenic therapy, blood flow rate within individual vessels may be improved, which has been termed as ‘‘normalizing tumor vasculature’’ (192). The mechanisms may lie in that the most immature and inefficient tumor blood vessels was ‘‘pruned’’ from the tumor vascular network by antiangiogenic therapy, leaving a more efficient system (192). In addition, many pro-angiogenic growth factors are associated with high vascular permeability and their withdrawal can reverse this effect (193). It is possible that a decrease in vascular permeability to macromolecules could improve blood flow rate by reducing tumor interstitial fluid pressure. Thus, measurement of vascular permeability or interstitial fluid pressure could provide alternative end-points for assessing tumor vascular effects of antiangiogenic agents (190).
Biomarkers and molecular imaging
A biological marker (biomarker) is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (194). For example, in response to sunitinib exposure, genes involved in cell survival and proliferation (p85PI3K); adhesion (cadherin 11 (CDH11) and ephrin receptor B4 (EPHB4)); and transcription (VHL) were overexpressed, and may be investigated as potential biomarkers for clinical studies (195). In addition, a Phase II study in patients with HCC who were treated with sorafenib suggested that phosphorylated extracellular signal regulated kinase (ERK) may represent a useful marker, as higher baseline levels of phosphorylated ERK in tumors correlated with slower tumor progression (196). However, transcription profiling usually requires quality-controlled fresh tumor tissues that may not be readily available in clinical trials and clinical practice. Thus, surrogate molecular markers that were more easily accessible for pre-treatment and post-treatment monitoring in clinical studies are in great need. Other potentially promising biomarkers for anti-angiogenesis therapy monitoring include circulating proteins related to angiogenesis such as soluble VEGFR-2 (sVEGFR-2), sVEGFR-3 and sKIT, circulating endothelial cells and/or their stem-cell progenitors (197). Biomarkers have great value in early efficacy and safety evaluations, disease diagnosis/staging, indicating disease prognosis, and prediction/monitoring of clinical response to an intervention. Imaging techniques can also be used as biomarkers to provide valuable information at the structural/functional and/or molecular level.
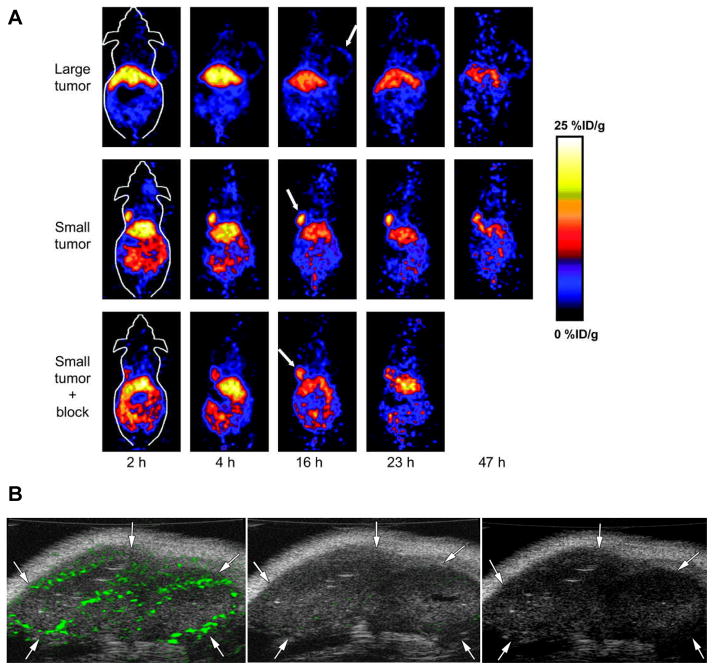
In view of the critical role of VEGF/VEGFR in cancer progression, development of VEGF- or VEGFR-targeted molecular imaging probes could serve as a new paradigm for the assessment of anti-angiogenic therapeutics, and for better understanding the role and expression profile of VEGF/VEGFR in many angiogenesis-related diseases. Due to the soluble and more dynamic nature of VEGF, imaging VEGF expression and explanation of the imaging results are very difficult, although single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging of VEGF has been performed with radioisotopes labeled anti-VEGF antibody (198). The more rational design is to use radiolabeled VEGF isoforms for SPECT or PET imaging of VEGFR expression. With SPECT imaging, recombinant human VEGF121 was labeled with 111In for the identification of ischemic tissue in a rabbit model, where unilateral hind-limb ischemia was created by femoral artery excision (15). VEGF121 has also been labeled with 99mTc through an “Adapter/Docking” strategy and the tracer was tested in a murine mammary carcinoma with tumor uptake of about 3 %ID/g (199). Cai et al. have labeled VEGF121 with 64Cu for PET imaging of tumor angiogenesis and VEGFR expression (200). MicroPET imaging revealed the dynamic nature of VEGFR expression during tumor progression in that even for the same tumor model, VEGFR expression level can be dramatically different at different stages (Figure 5A). It is well known that all VEGF-A isoforms bind to both VEGFR-1 and VEGFR-2 (201). A VEGFR-2-specific PET tracer has been developed using the D63AE64AE67A mutant of VEGF121 (VEGFDEE) generated by recombinant DNA technology. The renal uptake of 64Cu-DOTA-VEGFDEE was significantly lower than that of 64Cu-DOTA-VEGF121 as rodent kidneys expressed high levels of VEGFR-1 based on immunofluorescence staining (202). With the development of new tracers with better targeting efficacy and desirable pharmacokinetics, clinical translation will be critical for the maximum benefit of VEGF-based imaging agents. Peptidic VEGFR antagonists can be labeled with short-lived isotopes such as 18F and they may allow for higher throughput than antibody- or protein-based radiotracers, as one hour post-injection is usually sufficient for a peptide-based tracer to clear from the non-targeted organs and give high contrast images (203).
Figure 5.
Molecular imaging of tumor angiogenesis. A MicroPET of 64Cu-DOTA-VEGF121 in U87MG tumor-bearing mice. Serial small animal PET scans of large and small U87MG tumor-bearing mice injected intravenously with 5–10 MBq of 64Cu-DOTA-VEGF121 ( 2–4 μg of VEGF121). Mice injected with 64Cu-DOTA-VEGF121 30 min after injection of 100 μg VEGF121 are also shown (denoted as “Small tumor + block”). Tumors are indicated by arrows. Reprinted with the permission of reference (200). B Transverse color-coded US images of subcutaneous 9-mm malignant glioma tumor (arrows) in same nude mouse. Images obtained 4 minutes after intravenous administration of anti-VEGFR2 monoclonalantibody conjugated microbubbles (MBV, left), IgG conjugated microbubbles (MBC, middle) or non-targeted microbubbles (MBN, right). Differences in video intensity from subtraction of pre- and postdestruction images (green) on gray scale images were higher with MBV than with MBC. No signal was detected after MBN application. Reprinted with the permission of reference (208).
Ultrasonography (US) is by far one of the most commonly used clinical imaging modalities because it is safe and cost effective. Ultrasonic contrast agents such as microbubbles have been the subject of active research, especially in recent years, with added interest in developing site-directed ultrasonic contrast agents (204). With at least several micrometers in diameter, microbubbles are too large to extravasate so only the tumor endothelium can be targeted (205). Moreover, acoustic destruction of “payload-bearing” microbubbles can be used to deliver drugs or to augment gene transfection (206). Angiogenesis-targeted microbubbles may also have applications in site-specific therapy for ischemic tissues or tumors. Thus, VEGFR-2 is an excellent candidate for targeted untrasound imaging since it is almost exclusively expressed on activated endothelial cells (207). In a mouse model of pancreatic adenocarcinoma, anti-VEGFR2 or anti VEGF-VEGFR complex antibodies conjugated microbubbles were used to image and quantify vascular effects of two different anti-tumor therapies in both subcutaneous and orthotopic pancreatic tumors (205). Significant signal enhancement of tumor vasculature was observed when compared with untargeted or control IgG-targeted microbubbles. Video intensity from targeted microbubbles also correlated with the expression level of the target (VEGFR-2 or the VEGF-VEGFR complex) and with MVD in tumors under therapy. In another report, Willmann et al. have imaged VEGFR-2 expression in two murine tumor models using anti-VEGFR2 monoclonal antibody conjugated microbubbles (208, 209). Contrast-enhanced ultrasound imaging using targeted microbubbles showed significantly higher average video intensity compared with control microbubbles in both tumor models (Figure 5B). These studies support that targeted microbubbles can be used for non-invasive, vascularture-targeted molecular imaging of tumor angiogenesis and for in vivo monitoring of vascular effects after therapy.
Although optical imaging may not be widely used in clinical settings, near infrared (NIR) (700–900 nm) approaches provide opportunities for rapid and cost-effective preclinical evaluation in small animal models. Optical imaging has been used to study gene expression (210), tumor angiogenesis, physiological function of tumors, and tumor metastasis (211). In a transgenic mouse model where a VEGF promoter was chosen to drive a GFP reporter gene, VEGF expression during wound healing and possible impairment of wound healing due to collateral tissue damage was imaged in vivo (210). Human VEGF has also been conjugated to a self-assembled “dock and lock” system and retained its functional activities (212). After incorporating an additional cysteine residue for site-specific modification, a NIR fluorescent dye Cy5.5 (maximum emission 696 nm) was conjugated and the resulting Cy5.5-VEGF was used for in vivo imaging. Although tumor contrast was observed after administration of the probe, no information was reported about the whole body distribution of Cy5.5-VEGF (212, 213). Another component of optical imaging is bioluminescence imaging (BLI), which can be used to detect very low levels of signal because the emitted light is virtually background free (214). Non-invasive indirect imaging of VEGF expression with BLI in living transgenic mice has also been reported, where a two-step transcriptional amplification approach was used to augment the transcriptional activity of the relatively weak VEGF promoter (215).
Conclusions and perspectives
As a key regulator of tumor angiogenesis, VEGF is definitely an effective target for prevention and control of malignant diseases especially solid tumors, which has been substantiated by emerging evidences both preclinically and clinically. However, the complexity of the interplaying pathways during the angiogenesis process is still not fully unveiled. Sometimes controversial conclusions have been drawn from different experiments and clinical trials with different tumor types and patients subgroups. How to develop new agents to better block VEGF/VEGFR pathway and combine currently available agents to realize better tumor control are two major issues.
One obstacle in anti-VEGF/VEGFR therapy lies in that VEGF is not the only angiogenic factor generated by tumor tissues. For example, fibroblast growth factor-2 (FGF-2), platelet-derived growth factors (PDGFs), angiopoietins (Ang), hepatocyte growth factor (HGF), and insulin-like growth factors (IGFs) are all frequently produced by malignant cells (216). Moreover, tumor-produced angiogenic factors not only individually induce angiogenesis or vasculogenesis via their own receptors but also cross-communicate with each other to synergistically induce tumor neovascularization (217). In addition to a positive interplay between various angiogenic factors, some of them are negatively interacted. One example is the interaction between PlGF and VEGF-A .When PlGF and VEGF-A are expressed in different cell populations, the homodimeric form of PlGF can compete with VEGF-A for binding to VEGFR-1, allowing more VEGF-A to interact with its functional receptor, VEGFR-2. However, when PlGF and VEGF are synthesized in the same cell population, PlGF and VEGF can preferentially form biologically inactive heterodimers, which makes less VEGF-A homodimers available (218, 219). Thus, PlGF both positively and negatively modulate VEGF-A function depending upon its temporal and spatial relation to VEGF-A expression. The multitargeted approach with tyrosine kinase inhibitors or combination of specific single-targeted agents seems to have advantages in synergistic therapeutic effect and to overcome drug resistance, especially for anti-angiogenesis related therapy. A disadvantage of using multitargeted agents is that it might be difficult to determine which particular kinase inhibition(s) results in an antitumor effect.
Another hurdle is that preclinical models have limitations and thus we may not be able to appreciate the full extent of mechanisms associated with inhibiting VEGF in these types of studies. Typically, preclinical models of tumor growth are quite rapid and are supported by vascular structures that likewise grow rapidly. Thus, tumor vessels in murine models tend to be more plastic and responsive to anti-angiogenic therapy. Although functional changes in the vasculature of humans receiving anti-VEGF therapy have been clearly observed with sophisticated imaging techniques, the tumor response (if any) is much less dramatic than observed in mice (220, 221). Therefore, preclinical findings should be extrapolated to clinical situations with caution.
The effectiveness of anti-angiogenesis drugs does not always translate into changes in the diameter of the tumor, making the radiological evaluation of efficacy using standard RECIST and/or WHO criteria inappropriate (167). Although related biomarkers such as RNA profiling from biopsy samples, soluble receptors and progenitor cells from serum are being investigated as correlating indicators, non-invasive imaging modalities are under intensive investigation to reflect both functional and molecular changes with therapeutic interventions. However, several challenges still exist to impede its clinical translation. Firstly, the imaging based quantification and explanation still need be refined to reflect target expression or activity more accurately. For example, the imaging probes accumulation on certain regions reflected by images relates mainly to targets expression and probe-target interaction. However, other factors such as blood flow, extravascularization of the probes, interstitial pressure also need to be taken into account (222). Thus, a more thorough decipher of the images acquired by multiple molecular imaging modalities is critical to effectively replace the conventional sampling methods for pharmacokinetic (PK) parameters and pharmacodynamic (PD) endpoints evaluation. To achieve this goal, probes with optimal specificity and affinity to target molecules must be developed. In addition, further improvements in sensitivity and spatial/temporal resolution of the imaging techniques are still needed. In addition, advanced quantification algorithm and models may also be required.
Table 1.
Summarization of Anti-VEGF drugs
| Drug name | Mechanism | Indications | Status | References |
|---|---|---|---|---|
| Bevacizumab (Avastin) | A humanized anti-VEGF antibody | Metastatic colorectal cancer, non-small cell lung cancer, recurrent glioblastoma multiforme and metastatic breast cancer in combination with chemotherapy Renal cell carcinoma in combination with interferon alpha | FDA approved | (114, 115) 116) (117) |
| Pegaptanib | An anti-VEGF RNA aptamer | All types of neovascular age-related macular degeneration (AMD) | FDA approved | (128) |
| VEGF Trap | A soluble VEGF receptor | Refractory solid tumors or non-Hodgkin's lymphoma | Phase I | (138) |
| Sunitinib malate | A inhibitor of VEGFRs, FLT3, c-KIT, and PDGFRs | Gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate Advanced renal cell carcinoma |
FDA approved | (143) |
| Sorafenib | A inhibitor of VEGFRs, PDGFRs, FLT3, RAF-1, and BRAF | Metastatic renal cell carcinoma and hepatocellular carcinoma | FDA approved | (145) |
| Pazopanib | A inhibitor of VEGFRs, PDGFRs, and c-kit | Metastatic RCC Recurrent glioblastoma |
Phase II | (148) (149) |
| Ramucirumab | A fully humanized MAb targeting to the extracellular VEGF-binding domain of VEGFR-2 | Advanced solid malignancies | Phase I | (153) |
Acknowledgments
This project was supported by National Cancer Institute (NCI) R01 CA128908 and a DOD Prostate Postdoctoral Fellowship from Department of Defense (to G . Niu). Dr. Niu currently is an Imaging Sciences Training Fellowship jointly supported by the Radiology and Imaging Sciences Department, NIH Clinical Center and the Intramural Research Program, NIBIB, NIH.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003 Jun;3(6):401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005 Dec 15;438(7070):932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–7. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 6.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999 Jun 18;284(5422):1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996 Aug 9;86(3):353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 9.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):861–6. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–4. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000 Sep 14;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 13.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007 Dec;26(3–4):489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auguste P, Lemiere S, Larrieu-Lahargue F, Bikfalvi A. Molecular mechanisms of tumor vascularization. Crit Rev Oncol Hematol. 2005 Apr;54(1):53–61. doi: 10.1016/j.critrevonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003 Jun;3(6):422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M. Angiogenic factors as tumor markers. Invest New Drugs. 1997;15(1):29–37. doi: 10.1023/a:1005766511385. [DOI] [PubMed] [Google Scholar]
- 17.Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene. 1998 Jan 22;16(3):359–67. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 18.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999 Feb;154(2):375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paku S, Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991 Sep;65(3):334–46. [PubMed] [Google Scholar]
- 20.Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998 Sep;8(3):171–7. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995 Dec 1;270(5241):1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 23.Gamble J, Meyer G, Noack L, Furze J, Matthias L, Kovach N, et al. B1 integrin activation inhibits in vitro tube formation: effects on cell migration, vacuole coalescence and lumen formation. Endothelium. 1999;7(1):23–34. doi: 10.3109/10623329909165309. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005 Jan 7;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 25.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007 Dec 1;67(23):11244–53. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996 Feb;2(2):167–8. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 27.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004 Oct;4(10):806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci. 2009;14:3962–73. doi: 10.2741/3504. [DOI] [PubMed] [Google Scholar]
- 29.Folkman J. Looking for a good endothelial address. Cancer Cell. 2002 Mar;1(2):113–5. doi: 10.1016/s1535-6108(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 30.Yang AD, Bauer TW, Camp ER, Somcio R, Liu W, Fan F, et al. Improving delivery of antineoplastic agents with anti-vascular endothelial growth factor therapy. Cancer. 2005 Apr 15;103(8):1561–70. doi: 10.1002/cncr.20942. [DOI] [PubMed] [Google Scholar]
- 31.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–36. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 32.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004 Nov 15;64(22):8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF. VPF/VEGF and the angiogenic response. Semin Perinatol. 2000 Feb;24(1):75–8. doi: 10.1016/s0146-0005(00)80061-0. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. Exs. 2005;(94):209–31. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002 Oct;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 37.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005 Feb 10;23(5):1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 38.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–8. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 39.Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997 Nov 28;91(5):695–704. doi: 10.1016/s0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 40.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993 Dec;4(12):1317–26. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 42.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, et al. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000 Apr 20;19(17):2138–46. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 43.Kumaran GC, Jayson GC, Clamp AR. Antiangiogenic drugs in ovarian cancer. Br J Cancer. 2009 Jan 13;100(1):1–7. doi: 10.1038/sj.bjc.6604767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyttle DJ, Fraser KM, Fleming SB, Mercer AA, Robinson AJ. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol. 1994 Jan;68(1):84–92. doi: 10.1128/jvi.68.1.84-92.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998 Nov 20;273(47):31273–82. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006 Nov;10(4):515–27. doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki Y, Takani K, Atoda H, Morita T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2) J Biol Chem. 2003 Dec 26;278(52):51985–8. doi: 10.1074/jbc.C300454200. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem. 2004 Oct 29;279(44):46304–14. doi: 10.1074/jbc.M403687200. [DOI] [PubMed] [Google Scholar]
- 49.Chen YL, Tsai IH, Hong TM, Tsai SH. Crotalid venom vascular endothelial growth factors has preferential affinity for VEGFR-1. Characterization of Protobothrops mucrosquamatus venom VEGF. Thromb Haemost. 2005 Feb;93(2):331–8. doi: 10.1160/TH04-09-0568. [DOI] [PubMed] [Google Scholar]
- 50.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007 Feb 26;96(4):541–5. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994 Oct 28;269(43):26988–95. [PubMed] [Google Scholar]
- 52.Broumas AR, Pollard RE, Bloch SH, Wisner ER, Griffey S, Ferrara KW. Contrast-enhanced computed tomography and ultrasound for the evaluation of tumor blood flow. Invest Radiol. 2005 Mar;40(3):134–47. doi: 10.1097/01.rli.0000152833.35744.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007 Jul;212(3):237–48. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 54.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973 Jan;50(1):219–28. doi: 10.1093/jnci/50.1.219. [DOI] [PubMed] [Google Scholar]
- 55.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002 Nov;2(11):826–35. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005 Dec 8;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000 Apr;36(6):748–53. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 58.Hlatky L, Tsionou C, Hahnfeldt P, Coleman CN. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994 Dec 1;54(23):6083–6. [PubMed] [Google Scholar]
- 59.Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001 Jan 15;19(2):432–41. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 60.Nicol D, Hii SI, Walsh M, Teh B, Thompson L, Kennett C, et al. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol. 1997 Apr;157(4):1482–6. [PubMed] [Google Scholar]
- 61.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007 Aug;8(8):610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995 Sep 15;55(18):3964–8. [PubMed] [Google Scholar]
- 63.Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003 Jun;30(3 Suppl 6):39–50. doi: 10.1016/s0093-7754(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 64.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008 Nov;8(11):880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002 Jul 15;62(14):4123–31. [PubMed] [Google Scholar]
- 66.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005 Nov;48(11):2422–7. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 67.Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. Faseb J. 2008 Apr;22(4):1104–12. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 68.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008 Apr 22;98(8):1366–79. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008 Apr 8;98(7):1250–7. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schuler Y, Kelly SP, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer. 2008 Sep;44(13):1883–94. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Wang DA, Jain RK, Carie A, Paquette S, Ennis E, et al. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene. 2005 Jul 7;24(29):4701–9. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe H, Mamelak AJ, Wang B, Howell BG, Freed I, Esche C, et al. Anti-vascular endothelial growth factor receptor-2 (Flk-1/KDR) antibody suppresses contact hypersensitivity. Exp Dermatol. 2004 Nov;13(11):671–81. doi: 10.1111/j.0906-6705.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 73.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999 Oct 15;59(20):5209–18. [PubMed] [Google Scholar]
- 74.Ciardiello F, Caputo R, Damiano V, Troiani T, Vitagliano D, Carlomagno F, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003 Apr;9(4):1546–56. [PubMed] [Google Scholar]
- 75.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Curwen JO, Hennequin LF, et al. ZD4190: an orally active inhibitor of vascular endothelial growth factor signaling with broad-spectrum antitumor efficacy. Cancer Res. 2000 Feb 15;60(4):970–5. [PubMed] [Google Scholar]
- 76.Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Muller M, et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000 Sep 1;60(17):4819–24. [PubMed] [Google Scholar]
- 77.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008 Aug;8(8):579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 78.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004 Jun;4(6):423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 79.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000 Apr 1;60(7):1878–86. [PubMed] [Google Scholar]
- 80.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000 Apr;105(8):R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 82.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999 Jun 4;274(23):16349–54. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998 Nov 13;273(46):30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 84.Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8761–6. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000 Aug 1;89(3):488–99. [PubMed] [Google Scholar]
- 86.Laird AD, Christensen JG, Li G, Carver J, Smith K, Xin X, et al. SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid apoptosis of tumor vasculature and tumor regression in mice. Faseb J. 2002 May;16(7):681–90. doi: 10.1096/fj.01-0700com. [DOI] [PubMed] [Google Scholar]
- 87.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006 Nov;6(11):835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 88.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005 Mar;11(3):261–2. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 89.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004 Dec 1;104(12):3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 90.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004 Feb;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004 Aug 15;64(16):5535–8. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 93.Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003 Nov 1;21(21):3955–64. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 94.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001 Sep;7(9):987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 95.Dallas NA, Fan F, Gray MJ, Van Buren G, 2nd, Lim SJ, Xia L, et al. Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 2007 Dec;26(3–4):433–41. doi: 10.1007/s10555-007-9070-2. [DOI] [PubMed] [Google Scholar]
- 96.Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, et al. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10857–62. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood. 2006 Feb 15;107(4):1608–16. doi: 10.1182/blood-2005-06-2530. [DOI] [PubMed] [Google Scholar]
- 98.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001 Aug 1;61(15):5736–40. [PubMed] [Google Scholar]
- 99.Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, Yang AD, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008 Jan 16;100(2):109–20. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 100.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003 Jun 15;101(12):4878–86. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 101.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999 Oct;5(10):2963–70. [PubMed] [Google Scholar]
- 102.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007 Aug 15;13(16):4840–8. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 103.Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006 Feb 10;24(5):769–77. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 104.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. Jama. 2008 Nov 19;300(19):2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 105.Bernier J. Drug Insight: cetuximab in the treatment of recurrent and metastatic squamous cell carcinoma of the head and neck. Nat Clin Pract Oncol. 2008 Sep 30; doi: 10.1038/ncponc1228. [DOI] [PubMed] [Google Scholar]
- 106.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997 Oct 15;57(20):4593–9. [PubMed] [Google Scholar]
- 107.Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999 Jan;288(1):371–8. [PubMed] [Google Scholar]
- 108.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005 Feb 1;65(3):671–80. [PubMed] [Google Scholar]
- 109.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 110.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003 Jun 16;88(12):1979–86. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fox WD, Higgins B, Maiese KM, Drobnjak M, Cordon-Cardo C, Scher HI, et al. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res. 2002 Oct;8(10):3226–31. [PubMed] [Google Scholar]
- 112.Hu L, Hofmann J, Zaloudek C, Ferrara N, Hamilton T, Jaffe RB. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol. 2002 Nov;161(5):1917–24. doi: 10.1016/S0002-9440(10)64467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001 Apr 15;61(8):3369–72. [PubMed] [Google Scholar]
- 114.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 115.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007 Dec 27;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 116.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009 Nov;14(11):1131–8. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 117.Summers J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus interferon for advanced renal cell carcinoma. Oncologist. 15(1):104–11. doi: 10.1634/theoncologist.2009-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eaton BE. The joys of in vitro selection: chemically dressing oligonucleotides to satiate protein targets. Curr Opin Chem Biol. 1997 Jun;1(1):10–6. doi: 10.1016/s1367-5931(97)80103-2. [DOI] [PubMed] [Google Scholar]
- 119.White RR, Sullenger BA, Rusconi CP. Developing aptamers into therapeutics. J Clin Invest. 2000 Oct;106(8):929–34. doi: 10.1172/JCI11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wlotzka B, Leva S, Eschgfaller B, Burmeister J, Kleinjung F, Kaduk C, et al. In vivo properties of an anti-GnRH Spiegelmer: an example of an oligonucleotide-based therapeutic substance class. Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8898–902. doi: 10.1073/pnas.132067399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Smidt PC, Le Doan T, de Falco S, van Berkel TJ. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991 Sep 11;19(17):4695–700. doi: 10.1093/nar/19.17.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Green LS, Jellinek D, Bell C, Beebe LA, Feistner BD, Gill SC, et al. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem Biol. 1995 Oct;2(10):683–95. doi: 10.1016/1074-5521(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 123.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, et al. 2'-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998 Aug 7;273(32):20556–67. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 124.Jellinek D, Green LS, Bell C, Janjic N. Inhibition of receptor binding by high-affinity RNA ligands to vascular endothelial growth factor. Biochemistry. 1994 Aug 30;33(34):10450–6. doi: 10.1021/bi00200a028. [DOI] [PubMed] [Google Scholar]
- 125.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006 Feb;5(2):123–32. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 126.Bell C, Lynam E, Landfair DJ, Janjic N, Wiles ME. Oligonucleotide NX1838 inhibits VEGF165-mediated cellular responses in vitro. In Vitro Cell Dev Biol Anim. 1999 Oct;35(9):533–42. doi: 10.1007/s11626-999-0064-y. [DOI] [PubMed] [Google Scholar]
- 127.Lee JH, Canny MD, De Erkenez A, Krilleke D, Ng YS, Shima DT, et al. A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):18902–7. doi: 10.1073/pnas.0509069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou B, Wang B. Pegaptanib for the treatment of age-related macular degeneration. Exp Eye Res. 2006 Sep;83(3):615–9. doi: 10.1016/j.exer.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 129.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J Biol Chem. 1999 Sep 10;274(37):26369–77. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- 130.Xu X, Hamhouyia F, Thomas SD, Burke TJ, Girvan AC, McGregor WG, et al. Inhibition of DNA replication and induction of S phase cell cycle arrest by G-rich oligonucleotides. J Biol Chem. 2001 Nov 16;276(46):43221–30. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- 131.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009 Jun;86(3):151–64. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Girvan AC, Teng Y, Casson LK, Thomas SD, Juliger S, Ball MW, et al. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol Cancer Ther. 2006 Jul;5(7):1790–9. doi: 10.1158/1535-7163.MCT-05-0361. [DOI] [PubMed] [Google Scholar]
- 133.Hasegawa H, Sode K, Ikebukuro K. Selection of DNA aptamers against VEGF165 using a protein competitor and the aptamer blotting method. Biotechnol Lett. 2008 May;30(5):829–34. doi: 10.1007/s10529-007-9629-6. [DOI] [PubMed] [Google Scholar]
- 134.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004 May 15;10(10):3327–32. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 136.Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7785–90. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6966–71. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 138.Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010 Jan 10;28(2):207–14. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006 Aug;33(4):407–20. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, et al. The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther. 2006 Oct;5(10):2522–30. doi: 10.1158/1535-7163.MCT-06-0071. [DOI] [PubMed] [Google Scholar]
- 141.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006 Jan 1;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 142.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996 Sep 19;335(12):865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 143.Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007 Jan;12(1):107–13. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 144.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 145.Hasskarl J. Sorafenib. Recent Results Cancer Res. 2010;184:61–70. doi: 10.1007/978-3-642-01222-8_5. [DOI] [PubMed] [Google Scholar]
- 146.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005 Feb 10;23(5):965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 147.Sloan B, Scheinfeld NS. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs. 2008 Dec;9(12):1324–35. [PubMed] [Google Scholar]
- 148.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010 Jan 20;28(3):475–80. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 149.Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06–02) Neuro Oncol. 2010 Mar 3; doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002 Jan 20;97(3):393–9. doi: 10.1002/ijc.1634. [DOI] [PubMed] [Google Scholar]
- 151.Tessler S, Rockwell P, Hicklin D, Cohen T, Levi BZ, Witte L, et al. Heparin modulates the interaction of VEGF165 with soluble and cell associated flk-1 receptors. J Biol Chem. 1994 Apr 29;269(17):12456–61. [PubMed] [Google Scholar]
- 152.Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem. 2003 Oct 31;278(44):43496–507. doi: 10.1074/jbc.M307742200. [DOI] [PubMed] [Google Scholar]
- 153.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010 Feb 10;28(5):780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bar J, Onn A. Combined anti-proliferative and anti-angiogenic strategies for cancer. Expert Opin Pharmacother. 2008 Apr;9(5):701–15. doi: 10.1517/14656566.9.5.701. [DOI] [PubMed] [Google Scholar]
- 155.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003 Jan 1;21(1):60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 156.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 157.Schueneman AJ, Himmelfarb E, Geng L, Tan J, Donnelly E, Mendel D, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 2003 Jul 15;63(14):4009–16. [PubMed] [Google Scholar]
- 158.Zwolak P, Jasinski P, Terai K, Gallus NJ, Ericson ME, Clohisy DR, et al. Addition of receptor tyrosine kinase inhibitor to radiation increases tumour control in an orthotopic murine model of breast cancer metastasis in bone. Eur J Cancer. 2008 Nov;44(16):2506–17. doi: 10.1016/j.ejca.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 159.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz- Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003 May 16;300(5622):1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 160.Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001 Mar 15;61(6):2413–9. [PubMed] [Google Scholar]
- 161.Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol Biosyst. 2009 Nov;5(11):1262–70. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer. 2004 Oct;4( Suppl 2):S62–8. doi: 10.3816/ccc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 163.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H560–76. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 164.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007 Aug;50(2):203–18. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 165.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H547–59. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 166.Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007 Sep;92(9):3531–4. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 167.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007 Sep;6(9):734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 168.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003 Jan;9(1):327–37. [PubMed] [Google Scholar]
- 169.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007 Jun;11(6):539–54. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005 Oct;8(4):299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Huang J, Soffer SZ, Kim ES, McCrudden KW, Huang J, New T, et al. Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res. 2004 Jan;2(1):36–42. [PubMed] [Google Scholar]
- 173.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002 Jun 19;94(12):883–93. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 174.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006 Dec 21;444(7122):1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 175.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006 Dec 21;444(7122):1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 176.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007 Feb 15;445(7129):781–4. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 177.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007 May;7(5):327–31. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 178.Miller KD, Soule SE, Calley C, Emerson RE, Hutchins GD, Kopecky K, et al. Randomized phase II trial of the anti-angiogenic potential of doxorubicin and docetaxel; primary chemotherapy as Biomarker Discovery Laboratory. Breast Cancer Res Treat. 2005 Jan;89(2):187–97. doi: 10.1007/s10549-004-2044-y. [DOI] [PubMed] [Google Scholar]
- 179.Hughes MS, Marsh JN, Zhang H, Woodson AK, Allen JS, Lacy EK, et al. Characterization of digital waveforms using thermodynamic analogs: detection of contrast-targeted tissue in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2006 Sep;53(9):1609–16. doi: 10.1109/tuffc.2006.1678189. [DOI] [PubMed] [Google Scholar]
- 180.Li PC, Yang MJ. Transfer function analysis of ultrasonic time-intensity measurements. Ultrasound Med Biol. 2003 Oct;29(10):1493–500. doi: 10.1016/s0301-5629(03)00968-2. [DOI] [PubMed] [Google Scholar]
- 181.Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, et al. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008 Feb;43(2):100–11. doi: 10.1097/RLI.0b013e3181577cfc. [DOI] [PubMed] [Google Scholar]
- 182.Forsberg F, Dicker AP, Thakur ML, Rawool NM, Liu JB, Shi WT, et al. Comparing contrast-enhanced ultrasound to immunohistochemical markers of angiogenesis in a human melanoma xenograft model: preliminary results. Ultrasound Med Biol. 2002 Apr;28(4):445–51. doi: 10.1016/s0301-5629(02)00482-9. [DOI] [PubMed] [Google Scholar]
- 183.Forsberg F, Ro RJ, Potoczek M, Liu JB, Merritt CR, James KM, et al. Assessment of angiogenesis: implications for ultrasound imaging. Ultrasonics. 2004 Apr;42(1–9):325–30. doi: 10.1016/j.ultras.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 184.Padhani AR. MRI for assessing antivascular cancer treatments. Br J Radiol. 2003;76(Spec No 1):S60–80. doi: 10.1259/bjr/15334380. [DOI] [PubMed] [Google Scholar]
- 185.O'Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007 Jan 29;96(2):189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007 Feb 15;67(4):1555–62. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 187.Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007 Aug;26(2):235–49. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 188.Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005 Jun 20;23(18):4162–71. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 189.Medved M, Karczmar G, Yang C, Dignam J, Gajewski TF, Kindler H, et al. Semiquantitative analysis of dynamic contrast enhanced MRI in cancer patients: Variability and changes in tumor tissue over time. J Magn Reson Imaging. 2004 Jul;20(1):122–8. doi: 10.1002/jmri.20061. [DOI] [PubMed] [Google Scholar]
- 190.Tozer GM. Measuring tumour vascular response to antivascular and antiangiogenic drugs. Br J Radiol. 2003;76(Spec No 1):S23–35. doi: 10.1259/bjr/30165281. [DOI] [PubMed] [Google Scholar]
- 191.Teicher BA, Emi Y, Kakeji Y, Northey D. TNP-470/minocycline/cytotoxic therapy: a systems approach to cancer therapy. Eur J Cancer. 1996 Dec;32A(14):2461–6. doi: 10.1016/s0959-8049(96)00380-2. [DOI] [PubMed] [Google Scholar]
- 192.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004 Nov 15;22(22):4442–5. doi: 10.1200/JCO.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 193.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14765–70. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001 Mar;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 195.Morimoto AM, Tan N, West K, McArthur G, Toner GC, Manning WC, et al. Gene expression profiling of human colon xenograft tumors following treatment with SU11248, a multitargeted tyrosine kinase inhibitor. Oncogene. 2004 Feb 26;23(8):1618–26. doi: 10.1038/sj.onc.1207268. [DOI] [PubMed] [Google Scholar]
- 196.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006 Sep 10;24(26):4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 197.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006 Aug;6(8):626–35. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 198.Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med. 2007 Aug;48(8):1313–9. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- 199.Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007 Apr;13(4):504–9. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 200.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006 Dec;47(12):2048–56. [PubMed] [Google Scholar]
- 201.Dayton PA, Pearson D, Clark J, Simon S, Schumann PA, Zutshi R, et al. Ultrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of alphavbeta3-expressing cells. Mol Imaging. 2004 Apr;3(2):125–34. doi: 10.1162/1535350041464883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang H, Cai W, Chen K, Li ZB, Kashefi A, He L, et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007 Dec;34(12):2001–10. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 203.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin αvβ3 antagonism. Anti-Cancer Agents Med Chem. 2006;6:407–28. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 204.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003 Mar 1;17(5):545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 205.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007 Jan 1;13(1):323–30. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 206.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, et al. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000 Jun 6;101(22):2554–6. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 207.Underiner TL, Ruggeri B, Gingrich DE. Development of vascular endothelial growth factor receptor (VEGFR) kinase inhibitors as anti-angiogenic agents in cancer therapy. Curr Med Chem. 2004 Mar;11(6):731–45. doi: 10.2174/0929867043455756. [DOI] [PubMed] [Google Scholar]
- 208.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008 Feb;246(2):508–18. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Willmann JK, Cheng Z, Davis C, Lutz AM, Schipper ML, Nielsen CH, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008 Oct;249(1):212–9. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Izzo AD, Mackanos MA, Beckham JT, Jansen ED. In vivo optical imaging of expression of vascular endothelial growth factor following laser incision in skin. Lasers Surg Med. 2001;29(4):343–50. doi: 10.1002/lsm.1127. [DOI] [PubMed] [Google Scholar]
- 211.Lin PC. Optical imaging and tumor angiogenesis. J Cell Biochem. 2003 Oct 15;90(3):484–91. doi: 10.1002/jcb.10630. [DOI] [PubMed] [Google Scholar]
- 212.Backer MV, Patel V, Jehning BT, Backer JM. Self-assembled “dock and lock” system for linking payloads to targeting proteins. Bioconjug Chem. 2006 Jul-Aug;17(4):912–9. doi: 10.1021/bc060037u. [DOI] [PubMed] [Google Scholar]
- 213.Backer MV, Gaynutdinov TI, Patel V, Bandyopadhyaya AK, Thirumamagal BT, Tjarks W, et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005 Sep;4(9):1423–9. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 214.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006 Jun;6(6):484–90. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 215.Wang Y, Iyer M, Annala A, Wu L, Carey M, Gambhir SS. Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol Genomics. 2006 Jan 12;24(2):173–80. doi: 10.1152/physiolgenomics.00308.2004. [DOI] [PubMed] [Google Scholar]
- 216.Cao Y, Liu Q. Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv Cancer Res. 2007;97:203–24. doi: 10.1016/S0065-230X(06)97009-2. [DOI] [PubMed] [Google Scholar]
- 217.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007 Oct;117(10):2766–77. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schomber T, Kopfstein L, Djonov V, Albrecht I, Baeriswyl V, Strittmatter K, et al. Placental growth factor-1 attenuates vascular endothelial growth factor-A-dependent tumor angiogenesis during beta cell carcinogenesis. Cancer Res. 2007 Nov 15;67(22):10840–8. doi: 10.1158/0008-5472.CAN-07-1034. [DOI] [PubMed] [Google Scholar]
- 219.Xu L, Cochran DM, Tong RT, Winkler F, Kashiwagi S, Jain RK, et al. Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res. 2006 Apr 15;66(8):3971–7. doi: 10.1158/0008-5472.CAN-04-3085. [DOI] [PubMed] [Google Scholar]
- 220.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007 Jan;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004 Jul;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Niu G, Li Z, Xie J, Le QT, Chen X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med. 2009 Jul;50(7):1116–23. doi: 10.2967/jnumed.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]