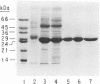
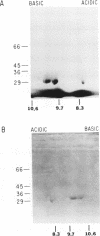
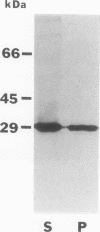
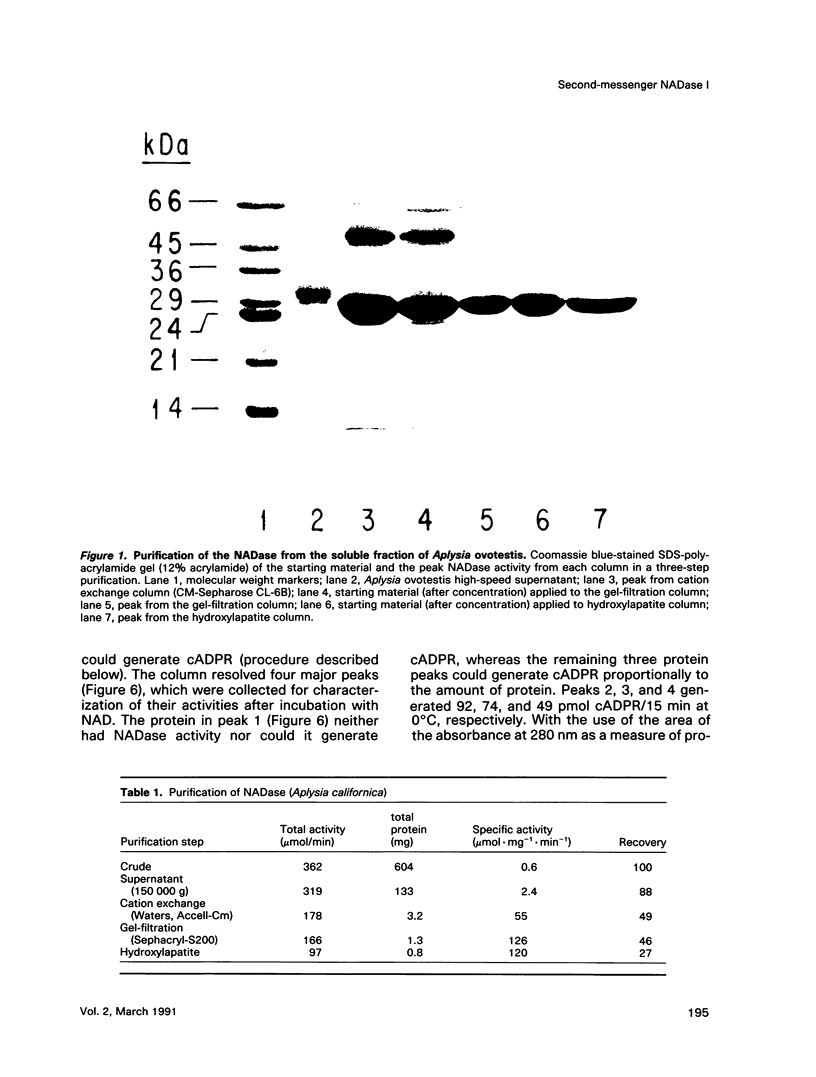
Abstract
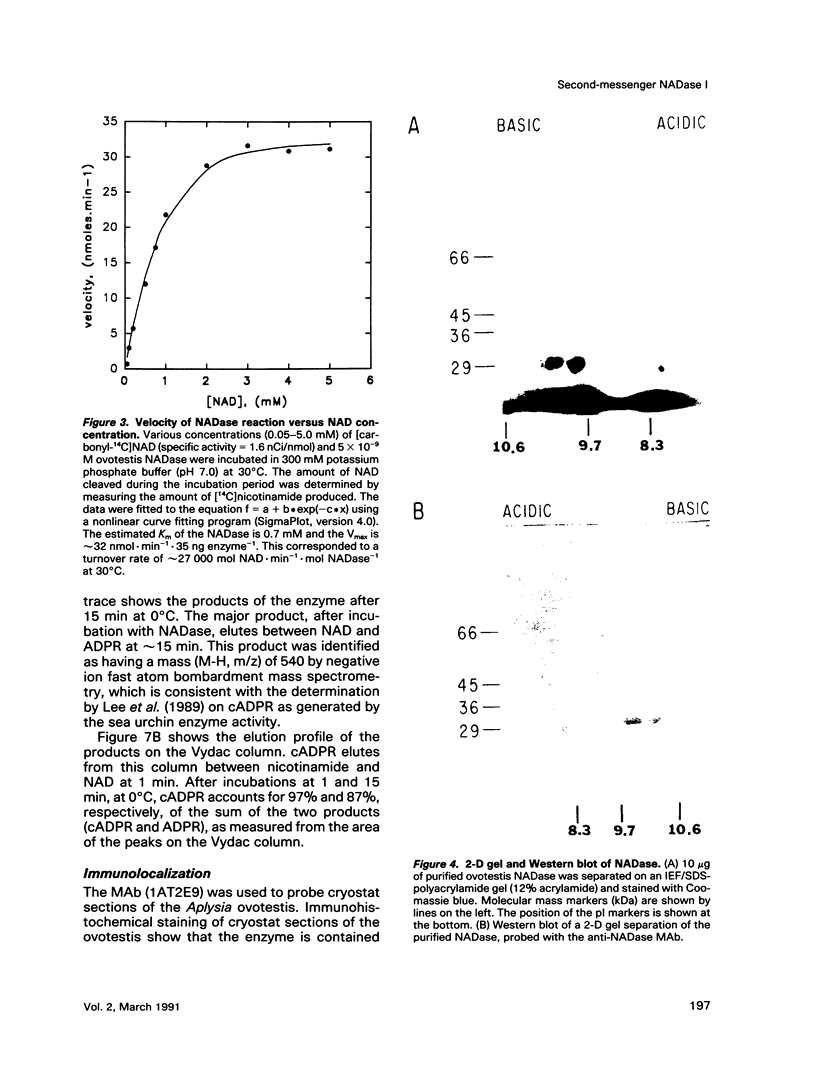
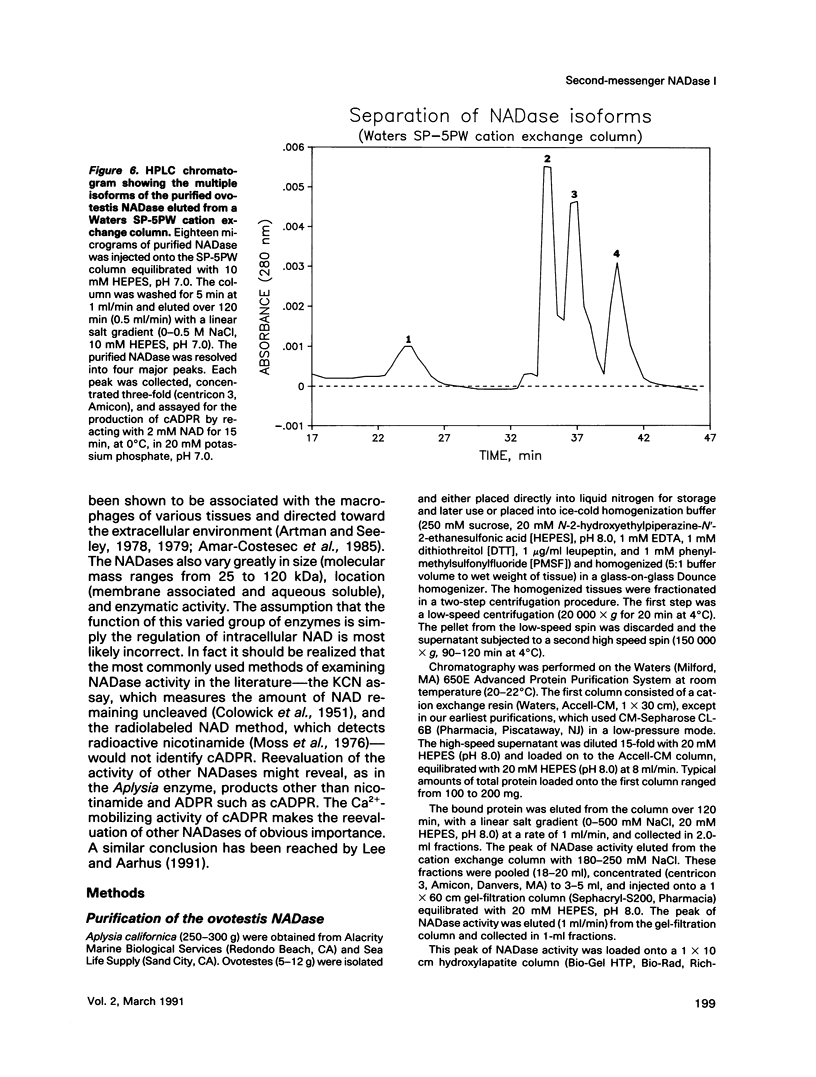
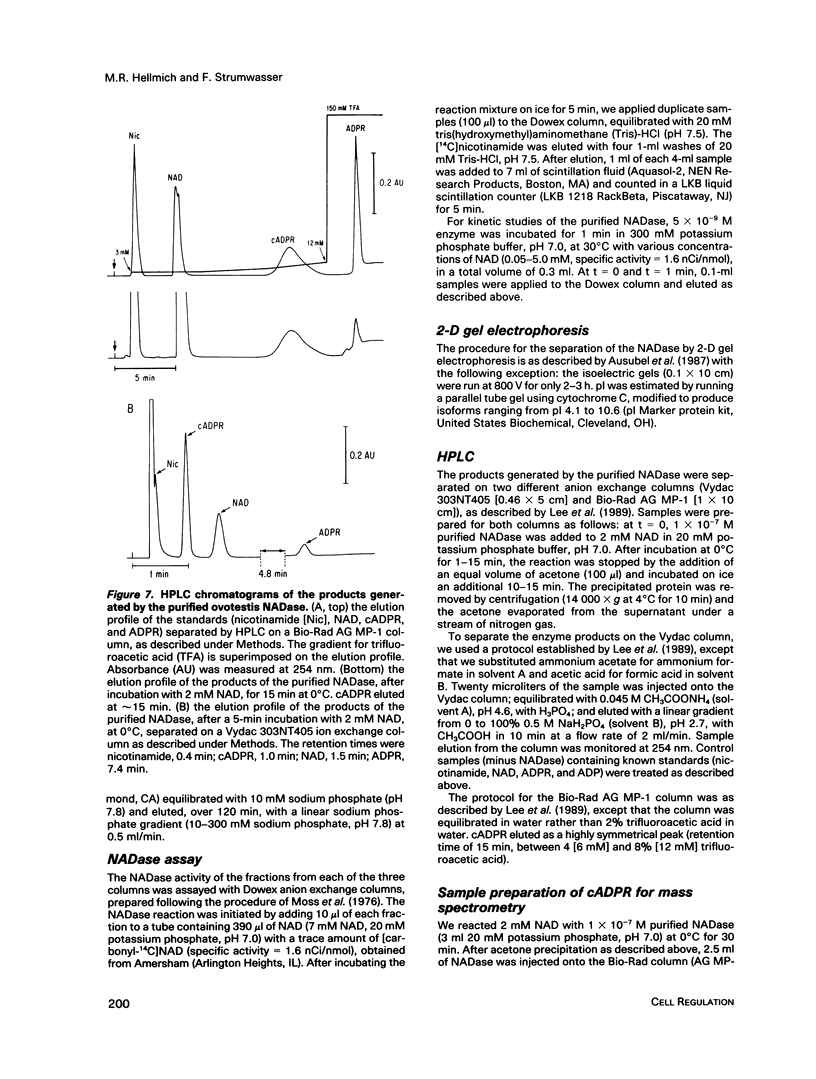
An egg-specific NADase has been purified to homogeneity from the ovotestis of the opisthobranch mollusk Aplysia californica. Unlike other NADases, the Aplysia enzyme generates primarily cyclic-ADP-ribose (cADPR) rather than ADP-ribose from NAD. cADPR has been shown to stimulate the release of Ca2+ from microsomes prepared from sea urchin egg and, when injected into intact eggs, to activate the cortical reaction, multiple nuclear cycles, and DNA synthesis. The Aplysia enzyme was initially identified as an inhibitor of cholera and pertussis toxin-catalyzed ADP-ribosylation. By the use of an NADase assay, it was purified from the aqueous-soluble fraction of ovotestis by sequential column chromatography. The enzyme has an apparent molecular mass of 29 kDa, a Km for NAD of 0.7 mM, and a turnover rate of approximately 27,000 mol NAD.min-1.mol enzyme-1 at 30 degrees C. Monoclonal antibodies were generated to the NADase. Immunoblots of two-dimensional gels revealed multiple isoforms of the enzyme, with pls ranging from 8.1 to 9.8. The multiple isoforms were resolved with a cation exchange high-pressure liquid chromatography column and shown to generate cADPR. Immunohistochemical analysis of cryostat sections of Aplysia ovotestis shows that the enzyme is specific to the eggs and restricted to large 5- to 10-microns granules or vesicles. To date the cADPR-generating enzyme activity has been identified in various organisms, including mammals. The Aplysia enzyme is the first example in which the enzyme that generates cADPR has been purified. All of the available evidence indicates that this NADase is a second-messenger enzyme, implying that other NADases may serve a similar function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Prado-Figueroa M., Beaufay H., Nagelkerke J. F., van Berkel T. J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IX. Nicotinamide adenine dinucleotide glycohydrolase: a plasma membrane enzyme prominently found in Kupffer cells. J Cell Biol. 1985 Jan;100(1):189–197. doi: 10.1083/jcb.100.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a characteristic of the mouse macrophage. Science. 1978 Dec 22;202(4374):1293–1295. doi: 10.1126/science.214853. [DOI] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a plasma membrane protein of murine macrophages. Arch Biochem Biophys. 1979 Jun;195(1):121–127. doi: 10.1016/0003-9861(79)90333-3. [DOI] [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987 Jul 15;262(20):9561–9568. [PubMed] [Google Scholar]
- Dargie P. J., Agre M. C., Lee H. C. Comparison of Ca2+ mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regul. 1990 Feb;1(3):279–290. doi: 10.1091/mbc.1.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D. L., Hellmich M. R., Beushausen S., Tempst P., Bayley H., Strumwasser F. Primary structure of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991 Mar;2(3):211–218. doi: 10.1091/mbc.2.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NASON A. Neurospora diphosphopyridine nucleotidase. J Biol Chem. 1951 Aug;191(2):473–483. [PubMed] [Google Scholar]
- Kim U. H., Rockwood S. F., Kim H. R., Daynes R. A. Membrane-associated NAD+ glycohydrolase from rabbit erythrocytes is solubilized by phosphatidylinositol-specific phospholipase C. Biochim Biophys Acta. 1988 Apr 14;965(1):76–81. doi: 10.1016/0304-4165(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991 Mar;2(3):203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jan 25;264(3):1608–1615. [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. M., Muller C. D., Schuber F. NAD+ glycohydrolase, an ecto-enzyme of calf spleen cells. Biochem J. 1983 May 15;212(2):459–464. doi: 10.1042/bj2120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinko N., Lee H. C. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jul 15;264(20):11725–11731. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]