Abstract
Penetrating keratoplasty is the most common type of tissue transplant in humans. Irreversible immune rejection leads to loss of vision and graft failure. This complex immune response further predisposes future corneal transplants to rejection and failure. A diverse armamentarium of surgical and pharmacologic tools is available to improve graft survival. In this review, we will discuss the various gene therapeutic strategies aimed at potentiating the anterior chamber-associated immune deviation to extend graft survival.
Keywords: Adenovirus, Corneal Transplant, Dendrimer, Gene Therapy, Immune Privilege, Neovascularization, Plasmid, Rejection
INTRODUCTION
Penetrating keratoplasty (PKP), a highly successful tissue transplant procedure, is performed worldwide for many indications, e.g. visual rehabilitation, improvement of tectonic support, or removal of infectious agent(s). Tissue shortage and the current transition to lamellar keratoplasties to preserve healthy tissue have modulated the numerical success of PKP.1–3 Its long-term success is limited by graft failures and allograft rejection accounts for up to 40% of all corneal transplant failures.4–10 A wide variety of premorbid risk factors, such as ocular inflammatory diseases, corneal neovascularization, prior graft failures, and glaucoma, increase the predisposition to allograft failure.3,9,11–14 A broad spectrum 15–20 of strategies has been proposed to prolong graft survival with protean success: tissue matching, reduction of antigenic factors, immunosuppression, lamellar transplantation, penetrating limbo-keratoplasty, human amniotic membrane transplantation, tissue engineering, and gene therapy.
Gene therapy is a potent modality that promises remedies to many inherited and acquired diseases. The unique features of the cornea and the eye present a splendid platform for gene therapies, particularly modulation of allograft rejection. These properties include stable ex vivo storage of donor tissue, ex vivo and in vivo accessibility, transparency of the cornea, and immune privilege of the eye. This review, however, will focus only on gene therapy to prolong PKP graft survival. We will describe the immune privilege of the eye, strategies to maintain this immune privilege, and the relevant literature.
IMMUNOLOGY OF CORNEAL GRAFT REJECTION
PKP enjoys a higher success rate than other tissue and organ transplantations, despite the routine human leucocyte antigen (HLA-) matching. Indeed, the Collaborative Corneal Transplantation Studies Research Group concluded that neither HLA-A, -B, nor -DR antigen matching substantially reduces the likelihood of graft failure.15 This remarkable property is achieved by maintenance of the integrity of the ocular immune privilege. Notwithstanding, the long-term survival rate remains unsatisfactory, especially in high-risk recipients.1,13 Irreversible allograft rejection precipitates 16-30% of all corneal graft failures.5,19 The prognosis worsens substantially with subsequent grafts, and the survival rates for third and fourth regrafts diminish to 25% and 0%, respectively.8,12 Allograft rejection is an labyrinthine, multifactorial process that requires the recognition of a foreign tissue by the host, initiation of the immune response cascade, and subsequent destruction of the foreign tissue. The literature substantiates a cell-mediated immune response as the primary mechanism involved in corneal allograft rejection; however, current evidence also suggests that alloantibodies play a role as well. Both direct and indirect antigen presentation appear to be involved in the sensitization phase, followed by activation against both major and minor histocompatibility antigens that are resident on the corneal graft. The three-legged stool analogy of the immune privilege is depicted in Figure 1. Figure 1 also outlines potential targets for gene therapy.
Figure 1.
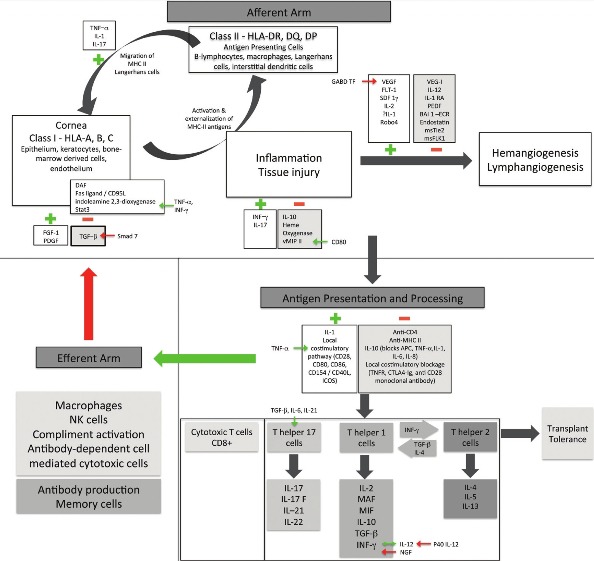
Gene therapeutic strategy for corneal allograft rejection
MAF: Macrophage activation factor; MIF: migration inhibition factor, APCs: Antigen-presenting cells, NK: Natural killer cells, vMIP II: viral macrophage inflammatory protein, NGF: Neuronal growth factor, PDGF: Platelet-derived growth factor, FGF: Fibroblast growth factor, INF: Interferon, TNF: tumor necrosis factor, SDF: Stromal-derived factor, PEDF: Pigment epithelium-derived factor, msTie2: murine-soluble Tie2, msFLK1: murine-soluble vascular endothelial growth factor receptor-2, IDO: Indoleamine 2,3 dioxygenase, IL: Interleukin, TGF: Transforming growth factor, TNFR: Tumor necrosis factor receptor, DAF: Decay accelerating factor, ICOS: Inducible Co-stimulator, Stat 3: Signal transducers and activators of transcription
Note: Green arrows and (+) signs denote positive interaction / upregulation. Red arrows and (-) denote negative interaction / downregulation.
Afferent arm
Similar to the human brain and testes, the cornea is an immunologically privileged site that is protected by multiple mechanisms. The avascular cornea maintains its immune privilege by the reduced expression of the major histocompatibility complex (MHC) class I and absent expression of MHC class II on antigen-presenting cells (APCs), by the lack of Langerhans cells in the epithelium, and by the expression of Fas ligand (FasL) by endothelial cells. FasL triggers apoptosis of Fas+ neutrophils and T cells that infiltrate the anterior chamber. Importantly, the anterior chamber allows the corneal allograft to induce deviation of systemic delayed-type hypersensitivity. This afferent blockage is violated by inflammation and neovascularization. Inflammatory stressors induce MHC II expression by keratocytes, bone marrow-derived stromal cells, and endothelium, thereby assisting antigen recognition by the host. The inflammatory cytokines also induce hemangiogenesis and lymphangiogenesis, further potentiating the rejection cascade.
Vascularization expedites the penetration of class II MHC+ Langerhans cells and centrifugal migration of allograft passenger leukocytes into the host tissue, the subsequent transformation into allostimulatory APCs, and the interaction with T-cells in the conjunctival associated lymphoid tissue and/or regional lymph nodes. This cascade vitiates the integrity of the anterior chamber immune privilege, thereby worsening the prognosis of subsequent transplant. Clinically, corneal neovascularization is a significant risk factor for graft rejection.3,9,11–14 One study12 reported that endothelial allograft reactions increased from 3.8% to 36.7% in patients with preoperative corneal vascularization; and 90% of allograft rejection reactions occurred within 1 year after transplant.
Consequently, neovascularization has generated profound interest, not only for corneal graft rejection, but also for tumorigenesis or organogenesis. Neovascularization has been associated with upregulation of growth factors. Angiogenesis suppression demonstrates its powerful utility in the arsenal against many ocular diseases, such as neovascular age-related macular degeneration, proliferative diabetic retinopathy, neovascular glaucoma, or retinopathy of prematurity. In addition to HLA-typing and inhibition of the inflammation cascade, pre-sensitization to activate the anterior chamber-associated immune deviation and anti-vascularization strategies such as anti-vascular endothelium growth factor receptor (anti-VEGFR), photodynamic therapy, and needle diathermy have all been proposed to improve allograft survival. We will discuss important advances in gene therapy for afferent blockage in the following sections.
Central processing stage
The central stage includes activation, differentiation, and proliferation of lymphocytes. The CD4+ T cells appear to be the principal conductors of the cell-mediated immune response. T helper 1 (Th1) cells orchestrate mononuclear cell infiltration and production of interferon (INF)-γ and tumor necrosis factor (TNF)-α, whereas Th2 cells are involved in the production of interleukin-4 (IL-4), IL-5, and IL-13 and transplant tolerance. Th17 cells are thought to be induced by transforming growth factor (TGF)-β, IL-6, and IL-21 via the orphan nuclear receptors and the retinoid-related orphan receptor gamma tau. These cells disseminate cytokines such as IL-17, IL-17F, IL-21, and IL-22. Interleukin-17 is a proinflammatory cytokine that involves chemokine expression, leukocyte infiltration, and tissue inflammation. Its participation in allograft rejection in cardiac, lung, renal, and vascular transplantations has been documented. Accordingly, therapeutic targets include T helper (Th) cells and cytokine production cascades as well.
Inhibition of the central processing stage can be accomplished mainly by using monoclonal antibodies directed against antigen presentation or T-cell activation steps, anti-interleukin 2, calcineurin inhibitors, and antimetabolites. Immunosuppressive targets for gene therapy currently being explored include CD4, CD8, T-cell receptors, and costimulatory molecules such as CD80, CD86, CD154, and CD25. Nonetheless, these steps typically occur centrally, and local transgene products may be inadequate. A recent study designed to investigate the effects of cervical and thoracic lymphadenectomy on corneal graft rejection in inbred rats failed to demonstrate any difference.21 The authors suggest that sensitization and clonal expansion of corneal alloantigen-reactive cells may not be limited only to the regional lymph nodes (superficial cervical, facial, internal jugular, and posterior cervical) in rat. Thus, therapies may need to target both locally and systemically.
Efferent arm
The efferent arm is, effectively, the destruction of the allograft by the cellular and humoral effector mechanisms. The cornea exercises efferent blockage by multiple mechanisms. Graft membrane-bound and host anterior chamber-soluble molecules can neutralize immune effector elements at the host-graft junction and the anterior chamber. Two notable factors are the decay-accelerating factor (DAF, CD55) and Fas ligand (FasL, CD95). DAF expression has been detected in lacrimal gland acinar cells, ocular surface epithelium, corneal endothelium, trabecular meshwork, and retinal pigment epithelium. It is a membrane complement regulatory protein that protects cells from autologous complement activation, formation of the membrane attack complex, and cell lysis.22,23 Similarly, FasL preferentially induces apoptosis of neutrophils and effector T cells; corneal epithelium and endothelium demonstrate constitutive expression of Fas ligand (CD 95) as a self-defense mechanism against neutrophils and activated T cells.
During an acute rejection, elevated concentrations of many cytokines are detected in lacrimal secretion, the anterior chamber, and the cornea. One study24 reported a high concentration of IL-6 and IL-8 in the tears of patients with concomitant corneal graft rejection, whereas expression of IL-10, tumor necrosis factor (TNF)-α, and IL-12p70 abated. Elevation of IL-6 was observed in aqueous humor and IL-8 was detected in corneal tissue.25,26 Similarly in a rat model, increased levels of IL-1β, IL-6, IL-10, IL-12 p40, and the macrophage inflammatory protein (MIP)-II were documented in both syngeneic and allogeneic recipients during the initial phase of rejection. The cytokine levels regressed to the pretransplant levels by week 2 after the onset of allograft rejection in the syngeneic group, but remained elevated in the allogeneic group. Of note, T cell-derived cytokines IL-4, IL-13, and interferon (INF)-γ were detected only during the rejection phase in allogeneic recipients. Migration of Langerhans cells expressing class II MHC into the cornea is mediated by IL-1 and TNF-β. TNF-α is responsible for proinflammatory and immunoregulatory functions; it upregulates adhesion and costimulatory molecules and activates the NK-kB signal pathway. Interleukine-10 is a potent immunomodulatory cytokine that interacts with APCs and inhibits production of monokines such as IL-1, IL-6, IL-8 and TNF-α. Recent discoveries in gene therapy for efferent blockage are discussed later.
GENE THERAPY
Irreversible immunological rejection is the leading cause of corneal transplant failure. Multidisciplinary pharmacologic strategies have been advocated to reduce failure. Topical or systemic corticosteroids typically constitute the first line of defense. Corticosteroids provide broad-spectrum immunosuppression by suppression of APCs, T lymphocytes and other cells, as well as inhibition of cytokine production. Systemic immunosuppression regimens are more commonly reserved for high-risk grafts. Systemic calcineurin inhibitors, antimetabolites, or monoclonal antibodies have been employed, either as monotherapy or in combination with corticosteroids, with variable success. Systemic cyclosporine has shown inconsistent results in prolongation of survival in high-risk patients and, in fact, may induce severe adverse effects, such as systemic hypertension, renal deficiency, and malignancy. Use of a multi-agent regimen has been proposed to leverage the synergistic mechanisms to achieve maximal therapeutic effects and minimal adverse effects in these high-risk transplants.17
Gene delivery methods
Corneal grafts constitute an excellent platform for gene therapies. Donor corneas are readily available for ex vivo manipulation. Both corneal endothelium and epithelium are accessible for broad range of in vivo and in vitro transduction techniques. Ex vivo gene transfer presents tantalizing opportunities, as the excess vectors and culture media may be eliminated to minimize the risks of vector spread and xenobiotic reaction; quality control measures can be implemented. A diverse selection of in vivo modalities encompasses topical instillation, intracorneal injection, ballistic delivery, electroporation, ultrasound-assisted, etc. Transgenes can be administered with naked plasmid, viral vector, or nonviral vectors. Naked plasmid offers the advantages of less associated inflammation and less concern for xenobiotic reaction; but the disadvantages include short-term stability and gene expression and poor penetration through intact epithelium, and typically, they must be delivered by direct intracorneal injection. Viral vectors include adenovirus, adeno-associated virus, herpes virus, retrovirus, lentivirus, baculovirus, etc. And nonviral vectors encompass gold particles, liposomes, chitosan, biocompatible and degradable polymers, and dendrimer.
Ensuring effective local concentration is a fundamental challenge in pharmacology. Ocular gene delivery is facilitated by the ease of access; and instantaneous administration can be achieved by direct topical instillation, subconjunctival injection, and intracameral or intravitreal injection. For topical application, precorneal factors such as small load, poor conjunctival, corneal, or scleral penetration, or mechanical elimination by tears and blinking can produce suboptimal bioavailability and reduce transfection efficiency. Clearance via the lacrimal drainage system may also lead to systemic absorption and toxicity, especially if a viral vector is employed. Inherent risks exist with intracameral or intravitreal deliveries such as endophthalmitis, retinitis, or retinal tear or detachment.
Other modalities are being developed to improve delivery and reduce adverse effects. A helium-driven ballistic gene gun, used to deliver plasmid-coated gold microparticles, has been available for more than 10 years. Gene delivery efficacy and the extend of tissue injury depend on the size of the microparticles and the cornea-gene gun interface.27 Use of a pegylated liposome showed promising data for green fluorescent protein (GFP) cDNA delivery in rabbit and rat models.28,29 Liposome can also be utilized for endothelium regeneration; Luo et al.35 performed lipofectine-mediated delivery of plasmid pcDNA4 for platelet-derived growth factor (PDGF)-B in cultured cat corneal endothelial cells and detected increased expression of PDGF. Femtosecond laser-assisted delivery of HIV1-derived lentiviral vector has been reported to result in more efficient transduction of the porcine stromal keratocytes compared to direct intracorneal injection.30 Given the inflammatory response incited by femtosecond laser, it will be interesting to see whether this technique will actually influence long-term allograft survival. Chitosan-modified adenoviral vector has been used in primary cultures of bovine and rat corneal epithelial cells.31 More recently, Klausner et al.32 reported the successful transduction of rat keratocytes after intracorneal injection of chitosan nanoparticles to deliver a DNA sequence encoding a marker protein. Delivery of plasmids encoding eGFP or β-gal markers by electroporation showed good transfection efficiency, with low early endothelial cell death in preserved human cornea.33
Gene therapies
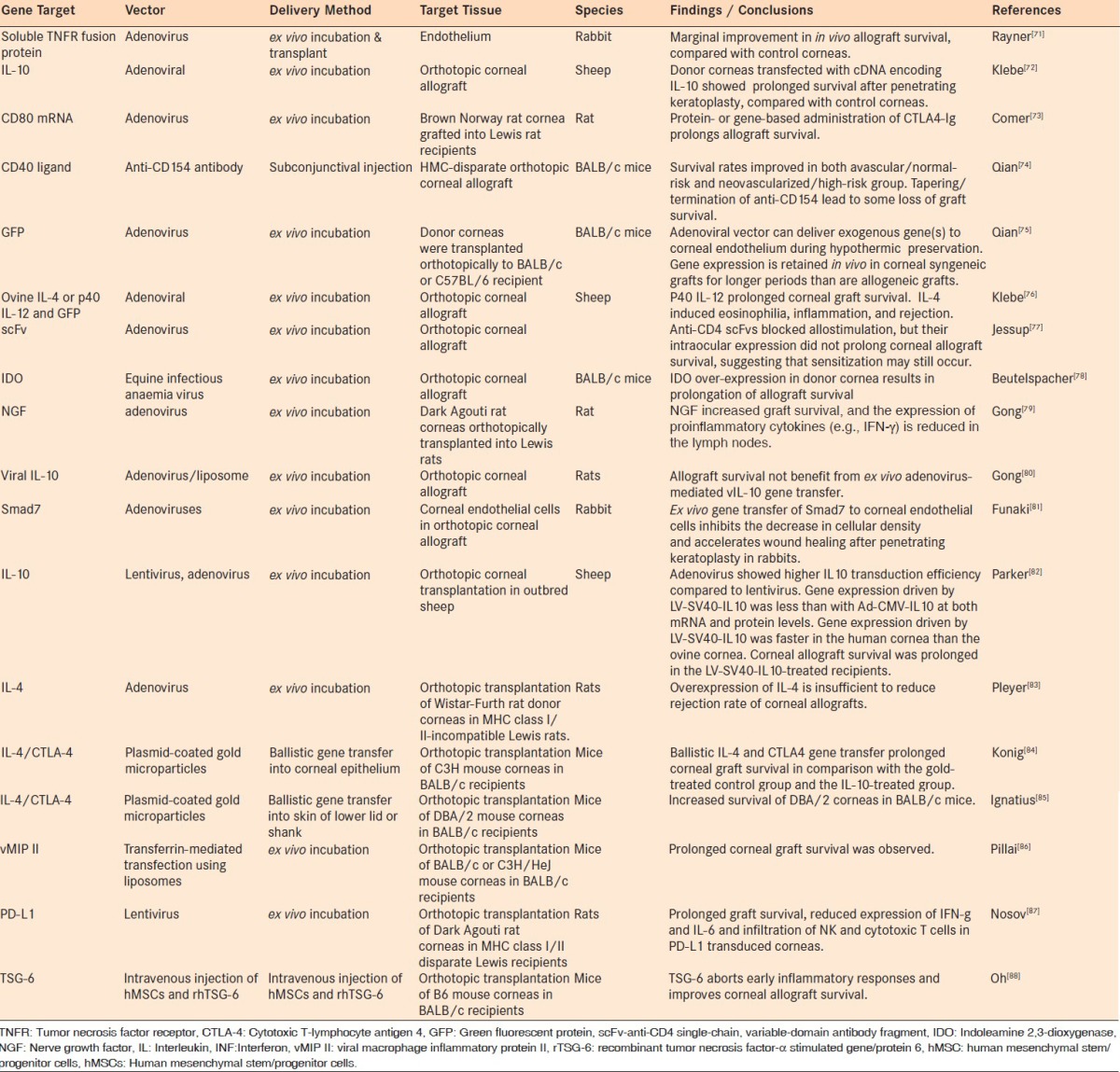
It has been reported that gene transduction and expression might be different in in vivo or ex vivo compared to in vitro experiments. Accordingly, although some in vitro tissue culture studies will also be discussed throughout this review and summarized in Tables 1 and 2, the bulk of the discussion will be focused on animal orthotopic keratoplasty models, as tabulated in Table 3.
Table 1.
Gene delivery using cultured tissues
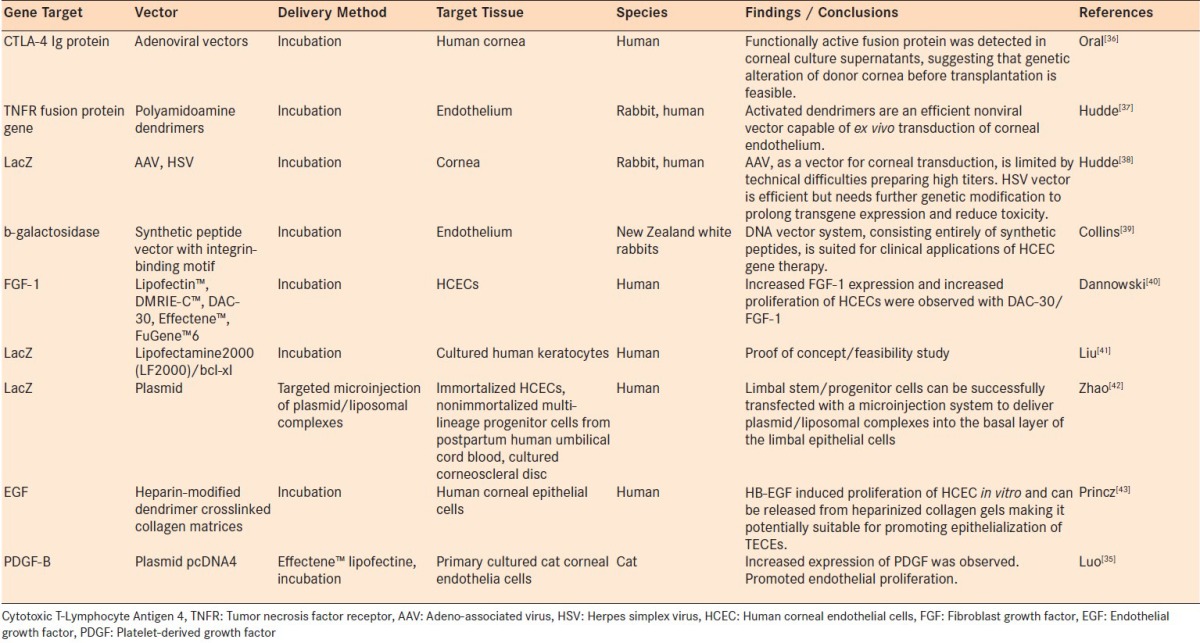
Table 2.
Gene delivery using ex vivo and in vivo models
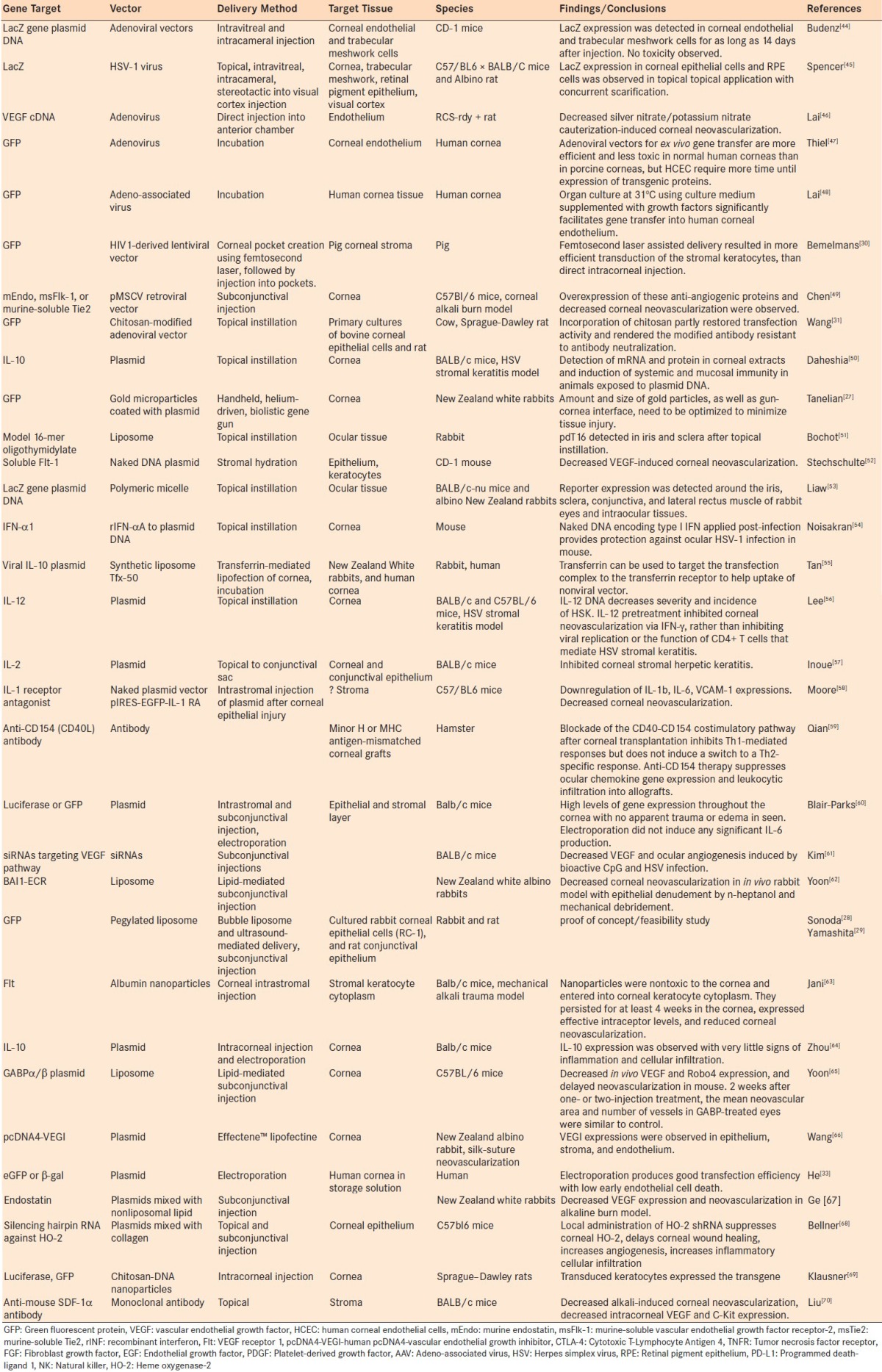
Table 3.
Gene delivery using orthotopic allograft animal models
Angiogenesis suppression
Suppression of angiogenesis is a well-documented strategy. Angiogenesis inhibitors that have been identified and explored for medical applications include prolactin, endostatin, angiostatin, somatostatin, cyclosporin A, the fumagillin analog TNP-470, steroids, nonsteroidal anti-inflammatory agents, thalidomide, methotrexate, and even cultured supernatant of human amniotic cells. Important advances in gene therapy have enabled us to evaluate angiosuppressive modalities for graft failure using animal models.
Diverse delivery methods have been tried to introduce an anti-angiogenic gene for corneal diseases. Direct instillation of anti-mouse SDF-1α antibody was found to decrease intracorneal VEGF and C-Kit expression and the resultant alkali-induced corneal neovascularization in a mouse model.34 In a RCS-rdy + rat model, direct injection of adenovirus carrying VEGF cDNA into the anterior chamber reduced corneal neovascularization induced by silver nitrate or potassium nitrate cauterization.46 Naked plasmid delivery of Flt-1, the vascular endothelial growth factor (VEGF) receptor 1, to the corneal stroma has been shown to decrease VEGF-induced corneal neovascularization in the CD-1 mouse.52 Subconjunctival injection of siRNAs targeting the VEGF pathway in BALB/c mice decreased VEGF production and ocular angiogenesis induced by CpG and herpes simplex virus (HSV) infection.61 More recently, in a mouse corneal alkali burn model, subconjunctival injection of retroviral vectors for delivery of murine endostatin (mEndo), murine-soluble vascular endothelial growth factor receptor-2 (msFlk-1), or murine-soluble Tie2 (msTie2) caused overexpression of these antiangiogenic proteins and decreased corneal neovascularization.49 Various non-viral vectors have been described as well.
Modeled after biological organelles, liposomal systems designed to encapsulate therapeutic agents for drug delivery have been found to have diverse ocular and systemic applications. In vivo delivery of BAI1-ECR, the extracellular region of brain-specific angiogenesis inhibitor gene, using liposome resulted in decreased corneal neovascularization in albino rabbits after epithelial denudement by n-heptanol and mechanical debridement.62 Yoon et al.65 investigated subconjunctival delivery of GABPα/β plasmid using a liposomal vehicle in C57BL/6 mice. The authors discovered an initial decrease in in vivo VEGF and Robo4 expression with delayed neovascularization; nonetheless, after 2 weeks they found no significant difference in the mean neovascular area and number of vessels in the eyes treated with GABP versus those treated with empty vectors. Another group used lipofectine-mediated delivery of human pcDNA4-vascular endothelial growth inhibitor (pcDNA4-VEGI). In this silk-suture-induced corneal neovascularization rabbit model, Wang et al.66 observed VEGI expression in the epithelium, stroma, and endothelium. More recently, plasmids encoding for endostatin mixed with non-liposomal lipid for subconjunctival injection were shown to attenuate VEGF expression and neovascularization in a rabbit alkaline burn model.67
Other encapsulating materials include albumin, collagen, chitosan, and biodegradable polymers. Commercially available albumin, a major protein constituent found in blood and secreted by the kidneys, can be synthesized into nanoparticles for sustained gene delivery. Jani et al.63 reported good results with intrastromal delivery of Flt-1, using albumin nanoparticles in a mechanical alkali trauma animal model. These authors found that the nontoxic albumin nanoparticles penetrated the corneal keratocyte cytoplasm; their presence was detected for at least 4 weeks and was correlated with reduced corneal neovascularization. Although synthesis of collagen nanoparticles has been described,89 Bellner et al.68 formed mixture of collagen and silencing hairpin RNA against heme oxygenase-2 (HO-2) instead. Subsequent to topical and subconjunctival deliveries, the authors noted delayed corneal wound healing, and increased angiogenesis and inflammatory cellular infiltration, identifying another target for anti-angiogenesis. Nanoparticles comprising of chitosan69 or biocompatible and degradable polymer poly(d, l-lactic-co-glycolic) acid90 have been reported as carriers for marker genes and antiangiogenic plasmid DNA pFlt23k.
Immunomodulatory gene therapy using orthotopic keratoplasty models
Recruitment of inflammatory cells is an intricate, multistep, coordination between cells of lymphocytic, granulocytic, and monocytic lineages, soluble mediators, and target cells. Suppression of the allogeneic rejection cascades often requires intervention at many levels. Multiple targets have been investigated and are discussed below.
IL-10 is an immunomodulatory cytokine that interacts with APCs and inhibits production of proinflammatory cytokines and TNF-α. Using a sheep model, Klebe et al.72 found prolonged survival of cornea transfected with cDNA encoding IL-10 after allogeneic transplantation. Another study64 applied electroporation technique to drive gene expression after intracorneal injection of IL-10 plasmid in mice and found IL-10 expression with minimal evidence of inflammation.
Viral vectors have been researched extensively for gene delivery using cornea models. Gong et al.80 investigating the role of viral IL-10 gene transfer using adenovirus and liposome, determined that neither mode of local ex vivo gene transfer led to prolonged allograft survival in a MHC class I/II mismatched rat model. Interestingly, the authors reported enhanced corneal allograft survival in host animals receiving systemic adenovirus IL-10 gene transfer and found increased mRNA expression in the draining lymph nodes but not in the corneal allografts, suggesting that IL-10 gene therapy modulation may occur more centrally. A study by Parker et al.82 observed that adenovirus has higher IL10 transduction efficiency compared to lentivirus. However, gene expression driven by lentivirus-SV40-IL10 in the human cornea was faster than in the ovine cornea and allograft survival was prolonged in the lentivirus-SV40-IL10-treated sheep recipients.
Interleukin-1 is a potent proinflammatory cytokine, associated with corneal neovascularization, chemotaxis of inflammatory cells, up-regulation of adhesion/costimulatory factors, and mediation of the acute phase response. Accordingly, the role of IL-1 receptor antagonist (IL-1 RA) in immunomodulation was investigated. It was found that IL-1 RA not only reduced the levels of cytokines, but it also decreased corneal neovascularization and improved graft survival in animal models. In a mouse model, transfection with IL-1 RA by direct injection of naked plasmid vector pIRES-EGFP-IL-1 RA resulted in downregulated expression of IL-1β and VCAM-1, as well as decreased corneal neovascularization and conjunctivalization.58 Topical application of IL-1-RA was found to decrease all cytokine and chemokine levels, reduce corneal damage and cell infiltration, and improve corneal transparency.91 One study in MHC-disparate corneal transplant mice showed decreased rejection rates in the treated group as well.92
Adenovirus delivered a gene encoding the soluble TNF-receptor fusion protein to rabbit corneal endothelium.71 Here, the authors detected good transgene expression in ex vivo corneal endothelial cells. However, only marginal improvement of graft survival was observed after orthotopic transplantation, compared to controls. Klebe et al.76 showed that adenovirus delivery of P40 IL-12 prolonged corneal graft survival in a sheep orthotopic transplant model, whereas IL-4 induced eosinophilia, inflammation, and allograft rejection. Smad 7 encodes a nuclear protein that appears to inhibit the fibrogenic responses of keratocytes to TGF-β. It interacts with TGF-β receptor type-1 (TGFBR1), leading to degradation of both the protein and TGFBR1. In a rabbit orthotopic corneal transplant model, ex vivo gene transfer of Smad7 to corneal endothelial cells inhibited cellular loss and accelerated wound healing after PKP.81
Viral macrophage inflammatory protein (vMIP) is a chemokine analogue encoded by Kaposi’s sarcoma herpes-associated virus (human herpesvirus-8) that demonstrates in vitro antagonism to the chemokine receptors that control cell migration. In a murine model of corneal allograft rejection, transferrin-mediated liposome transfection of vMIP II gene plasmid showed prolongation of graft survival.86 This was associated with decreased leukocyte infiltration of the corneal stroma.
A recent study exploited the roles of programmed death-ligand 1 (PD-L1) to inactivate lymphocytes and induce tolerance to corneal allografts.87 Here, Nosov and colleagues introduced rat PD-L1 using lentivirus vectors into rat transplant model and found that PD-L1 attenuates rejection by modulating the expression of INF-γ and IL-6. The reduced expression of these proinflammatory cytokines correlated with decreased severity of cellular infiltration, lower corneal opacity score, and improved graft survival.87
TNF-α stimulated gene/protein 6 (TSG-6), a multifunctional anti-inflammatory protein, has been reported to suppress a wide range of proinflammatory cytokines, such as INF-γ, IL-1β, IL-2, IL-6, IL-12, and myeloperoxidase (MPO). In this study, Oh et al.88 performed intravenous injection of both human mesenchymal stem/progenitor cells (hMSCs) and TSG-6 in mouse model of corneal allotransplantation. The authors reported that most of the hMSCs were trapped in the lungs and, subsequently, activated to produce TSG-6. Overexpression of TSG-6 modulated the proinflammatory cytokines, reducing early inflammation, and late rejection of the corneal allografts. Immunostaining suggested decreased number of cells expressing class II MHC in the allograft button. Intravenous injection of TSG-6 achieved similar results. Given that hMSCs had been reported to be safe in randomized, double-blind, placebo-controlled trials in patients after acute myocardial infarction93,94, we anticipate further data on the use and long-term safety profiles of intravenous hMSCs and TSG-6 for corneal transplantation.
Co-stimulatory pathway using orthotopic transplant models
Traditionally, the key drivers of allograft rejection are Th1 and Th2 cells. Th1 cells mediate allograft rejection by releasing inflammatory cytokines and upregulating monocellular infiltration. Th2 cells are involved in the induction of transplant tolerance and production of IL-4, IL-5, and IL-13. Generally, IL-4, IL-6, and IL-10 activate Th2 cells and B-lymphocytes, tilting the Th1/Th2 response, because these cells are mutually inhibitory via cytokine production. Researchers aim to influence the Th1/Th2 balance and block the costimulatory pathway to prolong allograft survival.
A previous study95 showed that anti-CD4 monoclonal antibodies delayed graft rejection in a rat model, whereas anti-CD8 monoclonal antibodies did not improve graft survival. However, Jessup et al.77 used adenovirus to deliver an anti-CD4 single-chain, variable-domain antibody fragment (anti-CD4 scFvs) and found that, although these antibody fragments blocked allostimulation, their intraocular expression did not prolong corneal allograft survival, suggesting the possibility of tachyphylaxis.
Nerve growth factor (NGF) was reported to modulate an immune response from predominantly Th1 type to a Th2 type with pleiotropic effects on corneal wound healing.96,97 NGF gene therapy has been investigated for a variety of diseases. In a corneal allograft transplant model, NGF transfection using adenovirus resulted in improved graft survival and reduced inflammatory markers.75 The authors also found that NGF and cytotoxic T-lymphocyte antigen 4 (CTLA-4)-Ig synergistically reduced apoptosis of corneal endothelium.
CTLA-4 is expressed on the surface of T helper cells, participating in the CD28–CD80–CD86 T-lymphocyte costimulatory pathway. CTLA4-Ig is a recombinant form of CTLA4 that acts as a competitive inhibitor of this CD28–CD80–CD86 pathway. Gene-based administration of CTLA4-Ig has shown promise in prolonging allograft survival in animal models.73,107–8 Comer and colleagues73 described suppression of CD80 and CD28 expression using adenovirus-mediated delivery of CD80 mRNA into Brown Norway rat corneas, which were subsequently grafted into Lewis rat recipients. CD80 gene therapy was found to prolong graft survival. As expected, systemic administration produced better allograft survival. Using a ballistic transfer technique with plasmid-coated gold microparticles, Konig et al.84 found that IL-4 and CTLA4 prolong corneal graft survival in comparison with the gold-treated control group and the IL-10-treated group. However, overexpression of IL-4 was not sufficient to reduce the rejection rate of corneal allografts in an experimental keratoplasty model of MHC class I/II-incompatible rats.83 Ignatius et al.85 reported increased survival of orthotopic corneal allograft in mice after ballistic delivery of IL-4/CTLA-4 plasmid-coated gold microparticles into the skin of the lower lid or shank.
Recent data suggest that Th17 and its products, e.g. IL-17, IL-21 and IL-22, may play a role in transplant immunity; however, this role is controversial. IL-17 is a proinflammatory cytokine that has been implicated in a variety of autoimmune diseases, e.g. multiple sclerosis, and allograft rejection of multiple tissues and organs in some studies. In vitro differentiation of the murine Th17 cell linage is controlled by intricate interactions between the cytokines and growth factors. CD28/B7-costimulation was found to downregulate Th17 development, whereas CTLA4-Ig, which interrupts the CD28/B7 pathway, favors Th17 differentiation. Antonysamy et al.99 reported that IL-17 promotes differentiation of dendritic cells and up-regulates costimulatory molecules; IL-17 blockage using recombinant murine IL-17R:Fc fusion protein inhibits in vitro T cell proliferation and prolongs cardiac allograft survival. However, Gorbacheva et al.100 reported that IL-17 promotes early allograft inflammation and tissue injury by facilitating T-cell recruitment into the graft. Recently, Chen et al.101 showed that neutralization of IL-17 using intraperitoneal injection of anti-IL-17 monoclonal antibody decreases angiogenesis and inflammatory cellular infiltration and improves allogeneic corneal graft survival. But, Yamada et al.102 used knockout mice (C57BL/6) to investigate the roles of IL-17 and INF-γ and concluded that neither INF-γ nor IL-17 played a critical role in the development of minor-specific allograft rejection in MHC disparate allograft transplantation. Another study reported that IL-17A regulates VEGF-A and VEGFR-1 expression and promotes corneal angiogenesis after HSV infection.103 More recently, Cunnusamy and colleagues104–6 found that IL-17A blocking, in mouse orthotopic keratoplasty model, actually increased the incidence of allograft rejection. The authors proposed that CD4+CD25+ regulatory T cells require IL-17A to mediate contact-dependent and time-dependent suppression.106 Further investigations to unravel the roles of Th17/IL-17 in graft rejection are underway.
Indoleamine 2,3-dioxygenase (IDO) is an intracellular enzyme that induces tryptophan depletion, arrests activated T lymphocytes in the G1 phase, and interferes T cell-mediated allograft rejection. It has also been proposed that IDO mediates CTLA4Ig-induced tolerance via reverse B7 signaling in dendritic cells, leading to T-cell suppression.109–10 Beutelspacher et al.78 presented data showing that transfection of IDO to mouse cornea using equine infectious anemia virus produces overexpression of IDO and prolongation of allograft survival. IDO overexpression has been reported to improve allograft survivals in other solid organ transplant models.111 Interestingly, cigarette smoke exposure appears to reduce long-term allograft survival by suppressing IDO expression.112–113
CONCLUSION
The current literature suggests that significant and exciting progress has been made in the field of gene therapy for ocular diseases and, specifically, in experimental models for treatment of allograft rejection. The unique properties of the anterior chamber and the cornea facilitate laboratory research to elucidate the intricate molecular and cellular mechanism of allograft rejection and to identify targets for gene therapy. The diversity of cornea models allows the formulation of critical and clinically relevant questions and subsequent hypothesis testing. Current lines of investigation raise the possibility of gene-based prevention and treatment of allograft rejection in the near future.
Footnotes
Source of Support: NEI core grant EY03040 and an unrestricted grant from Research to Prevent Blindness. Pho Nguyen is supported by the Heed Ophthalmic Foundation and the Fletcher Jones Foundation
Conflict of Interest: None declared.
REFERENCES
- 1.Waldock A, Cook SD. Corneal transplantation: How successful are we? Br J Ophthalmol. 2000;84:813–5. doi: 10.1136/bjo.84.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sit M, Weisbrod DJ, Naor J, Slomovic AR. Corneal graft outcome study. Cornea. 2001;20:129–133. doi: 10.1097/00003226-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RW, Jr, Price MO, Bowers PJ, Price FW., Jr Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 4.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99:599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 5.Williams KA, Muehlberg SM, Win SJ, Coster DJ. The Australian corneal graft registry. 1990-1992 report. Aust NZ J Ophthalmol. 1993;21:1–48. on behalf of all contributors. [PubMed] [Google Scholar]
- 6.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye (Lond) 1995;9:219–27. doi: 10.1038/eye.1995.43. [DOI] [PubMed] [Google Scholar]
- 7.Naacke HG, Borderie VM, Bourcier T, Touzeau O, Moldovan M, Laroche L. Outcome of corneal transplantation rejection. Cornea. 2001;20:350–3. doi: 10.1097/00003226-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bertelmann E, Jaroszewski J, Pleyer U. Corneal allograft rejection: current understanding. Ophthalmologica. 2002;216:2–12. doi: 10.1159/000048289. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Rank RM, Budde WM. Immunologic graft reactions after allogeneic penetrating keratoplasty. Am J Ophthalmol. 2002;133:437–43. doi: 10.1016/s0002-9394(01)01426-x. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen P, Yiu SC. Multi-agent pharmaceutical therapy for modulation of corneal allograft immunologic rejection. Curr Insights. 2007;18:509–14. [Google Scholar]
- 11.Hill JC. The relative importance of risk factors used to define high-risk keratoplasty. Ger J Ophthalmol. 1996;5:36–41. [PubMed] [Google Scholar]
- 12.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Conclusions of the corneal transplant follow up study. Collaborating Surgeons. Br J Ophthalmol. 1997;81:631–6. doi: 10.1136/bjo.81.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79:251–5. doi: 10.1034/j.1600-0420.2001.790308.x. [DOI] [PubMed] [Google Scholar]
- 14.Pleyer U, Dannowski H, Volk HD, Ritter T. Corneal allograft rejection: current understanding. 1. Immunobiology and basic mechanisms. Ophthalmologica. 2001;215:254–62. doi: 10.1159/000050870. [DOI] [PubMed] [Google Scholar]
- 15.The Collaborative Corneal Transplantation Studies Research Group. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–403. [PubMed] [Google Scholar]
- 16.Armitage WJ. HLA matching and corneal transplantation. Eye (Lond) 2004;18:231–2. doi: 10.1038/sj.eye.6700661. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen P, Barte F, Shinada S, Yiu SC. Management of corneal graft rejection – a case series report and review of the literature. J Clin Exp Ophthalmol. 2010:1. doi: 10.4172/2155-9570.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinhard T, Spelsberg H, Henke L, Kontopoulos T, Enczmann J, Wernet P, et al. Long-term results of allogeneic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology. 2004;111:775–82. doi: 10.1016/j.ophtha.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Eberwein P, Bohringer D, Schwartzkopff, Birnbaum F, Reinhard T. Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin c, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology. 2012;119:930–7. doi: 10.1016/j.ophtha.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:836–901. doi: 10.1097/01.tp.0000185197.37824.35. [DOI] [PubMed] [Google Scholar]
- 21.Brice SL, Kirk K, Brereton HM, Coster DJ, Williams KA. The influence of cervical and thoracic lymphadenectomy on corneal allograft rejection in inbred rats. Br J Ophthalmol. 2012;96:448–50. doi: 10.1136/bjophthalmol-2011-300934. [DOI] [PubMed] [Google Scholar]
- 22.Lass JH, Walter EI, Burris TE, Grossniklaus HE, Roat MI, Skelnik DL, et al. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Invest Ophthalmol Vis Sci. 1990;31:1136–48. [PubMed] [Google Scholar]
- 23.Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19:451–5. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]
- 24.Fodor M, Gogolák P, Rajnavölgyi E, Berta A, Kardos L, Módis L, et al. Long-term kinetics of cytokine responses in human tears after penetrating keratoplasty. J Interferon Cytokine Res. 2009;29:375–80. doi: 10.1089/jir.2008.0116. [DOI] [PubMed] [Google Scholar]
- 25.Funding M, Vorum H, Nexø E, Moestrup SK, Ehlers N, Møller HJ. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol Scand. 2005;83:234–9. doi: 10.1111/j.1600-0420.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 26.Shtein RM, Garcia DD, Musch DC, Elner VM. Herpes simplex virus keratitis: histopathologic inflammation and corneal allograft rejection. Ophthalmology. 2009;116:1301–5. doi: 10.1016/j.ophtha.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanelian DL, Barry MA, Johnston SA, Le T, Smith G. Controlled gene gun delivery and expression of DNA within the cornea. Biotechniques. 1997;23:484–8. doi: 10.2144/97233st06. [DOI] [PubMed] [Google Scholar]
- 28.Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, et al. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006;47:558–64. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita T, Sonoda S, Suzuki R, Arimura N, Tachibana K, Maruyama K, et al. A novel bubble liposome and ultrasound-mediated gene transfer to ocular surface: RC-1 cells in vivo and conjunctiva in vivo. Exp Eye Res. 2007;85:741–8. doi: 10.1016/j.exer.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Bemelmans AP, Arsenijevic Y, Majo F. Efficient lentiviral gene transfer into corneal stroma cells using a femtosecond laser. Gene Ther. 2009;16:933–8. doi: 10.1038/gt.2009.41. [DOI] [PubMed] [Google Scholar]
- 31.Wang IJ, Jhuang MC, Chen YH, Yeh LK, Liu CY, Young TH. Chitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cells. PLoS One. 2010;5:e12085. doi: 10.1371/journal.pone.0012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klausner EA, Zhang Z, Wong S, Chapman RL, Volin MV, Harbottle RP. Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J Gene Med. 2012;14:100–8. doi: 10.1002/jgm.1634. [DOI] [PubMed] [Google Scholar]
- 33.He Z, Pipparelli A, Manissolle C, Acquart S, Garraud O, Gain P, et al. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic Res. 2010;43:43–55. doi: 10.1159/000246577. [DOI] [PubMed] [Google Scholar]
- 34.Liu GQ, Lu PR, Li LB, Zhang XG. Inhibited experimental corneal neovascularization by neutralizing anti-SDF-1α antibody. Int J Ophthalmol. 2012;5:7–12. doi: 10.3980/j.issn.2222-3959.2012.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo WJ, Wang XJ, Xing XM, Wang CF. The study of human PDGF-B gene transferred to cat corneal endothelial cells. Int J Ophthalmol. 2012;5:18–22. doi: 10.3980/j.issn.2222-3959.2012.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oral HB, Larkin DF, Fehervari Z, Byrnes AP, Rankin AM, Haskard DO, et al. Ex vivo adenovirus-mediated gene transfer and immunomodulatory protein production in human cornea. Gene Ther. 1997;4:639–47. doi: 10.1038/sj.gt.3300443. [DOI] [PubMed] [Google Scholar]
- 37.Hudde T, Rayner SA, Comer RM, Weber M, Isaacs JD, Waldmann H, et al. Activated polyamidoamine dendrimers, a nonviral vector for gene transfer to the corneal endothelium. Gene Ther. 1999;6:939–43. doi: 10.1038/sj.gt.3300886. [DOI] [PubMed] [Google Scholar]
- 38.Hudde T, Rayner SA, De Alwis M, Thrasher AJ, Smith J, Coffin RS, et al. Adeno-associated and herpes simplex viruses as vectors for gene transfer to the corneal endothelium. Cornea. 2000;19:369–73. doi: 10.1097/00003226-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Collins L, Fabre JW. A synthetic peptide vector system for optimal gene delivery to corneal endothelium. J Gene Med. 2004;6:185–94. doi: 10.1002/jgm.482. [DOI] [PubMed] [Google Scholar]
- 40.Dannowski H, Bednarz J, Reszka R, Engelmann K, Pleyer U. Lipid-mediated gene transfer of acidic fibroblast growth factor into human corneal endothelial cells. Exp Eye Res. 2005;80:93–101. doi: 10.1016/j.exer.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Li X, Zhu X, Li G. Cationic liposome-mediated bcl-xl gene transfection into human keratocytes. J Huazhong Univ Sci Technolog Med Sci. 2005;25:365–7. doi: 10.1007/BF02828170. [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Allinson S, Ma A, Bentley AJ, Martin FL, Fullwood NJ. Targeted cornea limbal stem/progenitor cell transfection in an organ culture model. Invest Ophthalmol Vis Sci. 2008;49:3395–401. doi: 10.1167/iovs.07-1263. [DOI] [PubMed] [Google Scholar]
- 43.Princz MA, Sheardown H. Heparin-modified dendrimer crosslinked collagen matrices for the delivery of heparin-binding epidermal growth factor. J Biomed Mater Res Part A. 2012;100:1929–37. doi: 10.1002/jbm.a.34128. [DOI] [PubMed] [Google Scholar]
- 44.Budenz DL, Bennett J, Alonso L, Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36:2211–5. [PubMed] [Google Scholar]
- 45.Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus–mediated gene delivery to the rodent visual system. Invest Ophthalmol Vis Sci. 2000;41:1392–401. [PubMed] [Google Scholar]
- 46.Lai CM, Spilsbury K, Brankov M, Zaknich T, Rakoczy PE. Inhibition of corneal neovascularization by recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res. 2002;75:625–34. doi: 10.1006/exer.2002.2075. [DOI] [PubMed] [Google Scholar]
- 47.Thiel MA, Saydam C, Pavlovic J, Hemmi S. Effect of ex vivo gene transfer with an adenoviral vector on human eye bank corneas. Ophthalmic Res. 2005;37:67–71. doi: 10.1159/000084247. [DOI] [PubMed] [Google Scholar]
- 48.Lai L, Lin K, Foulks G, Ma L, Xiao X, Chen K. Highly efficient ex vivo gene delivery into human corneal endothelial cells by recombinant adeno-associated virus. Curr Eye Res. 2005;30:213–9. doi: 10.1080/02713680590927515. [DOI] [PubMed] [Google Scholar]
- 49.Chen P, Yin H, Wang Y, Mi J, He W, Xie L, et al. Multi-gene targeted antiangiogenic therapies for experimental corneal neovascularization. Mol Vis. 2010;16:310–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–52. [PubMed] [Google Scholar]
- 51.Bochot A, Mashhour B, Puisieux F, Couvreur P, Fattal E. Comparison of the ocular distribution of a model oligonucleotide after topical instillation in rabbits of conventional and new dosage forms. J Drug Target. 1998;6:309–13. doi: 10.3109/10611869808996838. [DOI] [PubMed] [Google Scholar]
- 52.Stechschulte SU, Joussen AM, von Recum HA, Poulaki V, Moromizato Y, Yuan J, et al. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci. 2001;42:1975–9. [PubMed] [Google Scholar]
- 53.Liaw J, Chang SF, Hsiao FC. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly (propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001;8:999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 54.Noisakran S, Carr DJJ. Topical application of the cornea post-infection with plasmid DNA encoding interferon-a1 but not recombinant interferon-aA reduces herpes simplex virus type 1-induced mortality in mice. J Neuroimmunol. 2001;121:49–58. doi: 10.1016/s0165-5728(01)00442-8. [DOI] [PubMed] [Google Scholar]
- 55.Tan P, King W, Chen D, Awad HM, Mackett M, Lechler RI, et al. Transferrin Receptor-mediated gene transfer to the corneal endothelium. Transplantation. 2001;71:552–560. doi: 10.1097/00007890-200102270-00011. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, Zheng M, Deshpande S, Eo SK, Hamilton TA, Rouse BT. IL-12 suppresses the expression of ocular immunoinflammatory lesions by effects on angiogenesis. J Leukoc Biol. 2002;71:469–76. [PubMed] [Google Scholar]
- 57.Inoue T, Inoue Y, Hayashi K, Yoshida A, Nishida K, Shimomura Y, et al. Topical administration of HSV gD-IL-2 DNA is highly protective against murine herpetic stromal keratitis. Cornea. 2002;21:106–10. doi: 10.1097/00003226-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 58.Moore JE, McMullen TC, Campbell IL, Rohan R, Kaji Y, Afshari NA, et al. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest Ophthalmol Vis Sci. 2002;43:2905–15. [PubMed] [Google Scholar]
- 59.Qian Y, Leong FL, Kazlauskas A, Dana MR. Ex vivo adenovirus-mediated gene transfer to corneal graft endothelial cells in mice. Invest Ophthalmol Vis Sci. 2004;45:2187–93. doi: 10.1167/iovs.03-0901. [DOI] [PubMed] [Google Scholar]
- 60.Blair-Parks K, Weston BC, Dean DA. High-level gene transfer to the cornea using electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–85. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW, Seo MS, et al. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther. 2005;12:617–24. doi: 10.1038/sj.gt.3302442. [DOI] [PubMed] [Google Scholar]
- 63.Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–6. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- 64.Zhou R, Dean D. Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp Biol Med (Maywood) 2007;232:362–9. [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon KC, Bae JA, Park HJ, Im SK, Oh HJ, Lin XH, et al. Subconjunctival gene delivery of the transcription factor GA-binding protein delays corneal neovascularization in a mouse model. Gene Ther. 2009;16:973–81. doi: 10.1038/gt.2009.50. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Wang B. Inhibition of corneal neovascularization by vascular endothelia growth inhibitor gene. Int J Ophthalmol. 2010;3:295–8. doi: 10.3980/j.issn.2222-3959.2010.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ge HY, Xiao N, Yin XL, Fu SB, Ge JY, Shi Y, et al. Comparison of the antiangiogenic activity of modified RGDRGD-endostatin to endostatin delivered by gene transfer in vivo rabbit neovascularization model. Mol Vis. 2011;17:1918–28. [PMC free article] [PubMed] [Google Scholar]
- 68.Bellner L, Patil KA, Castellano K, Halilovic A, Dunn MW, Schwartzman ML. Targeted suppression of HO-2 gene expression impairs the innate anti-inflammatory and repair responses of the cornea to injury. Mol Vis. 2011;17:1144–52. [PMC free article] [PubMed] [Google Scholar]
- 69.Klausner EA, Zhang Z, Wong SP, Chapman RL, Volin MV, Harbottle RP. Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J Gene Med. 2012;14:100–8. doi: 10.1002/jgm.1634. [DOI] [PubMed] [Google Scholar]
- 70.Liu GQ, Lu PR, Li LB, Zhang XG. Inhibited experimental corneal neovascularization by neutralizing anti-SDF-1α antibody. Int J Ophthalmol. 2012;5:7–12. doi: 10.3980/j.issn.2222-3959.2012.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rayner SA, Larkin DF, George AJ. TNF receptor secretion after ex vivo adenoviral gene transfer to cornea and effect on in vivo graft survival. Invest Ophthalmol Vis Sci. 2001;42:1568–73. [PubMed] [Google Scholar]
- 72.Klebe S, Sykes PJ, Coster DJ, Krishnan R, Williams KA. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation. 2001;71:1214–20. doi: 10.1097/00007890-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 73.Comer RM, King WJ, Ardjomand N, Theoharis S, George AJ, Larkin DF. Effect of administration of CTLA4-Ig as protein or cdna on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1095–103. [PubMed] [Google Scholar]
- 74.Qian Y, Dana MR. Effect of locally administered anti-CD154 (CD40 ligand) monoclonal antibody on survival of allogeneic corneal transplants. Cornea. 2002;21:592–7. doi: 10.1097/00003226-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 75.Qian Y, Leong FL, Kazlauskas A, Dana MR. Ex vivo adenovirus-mediated gene transfer to corneal graft endothelial cells in mice. Invest Ophthalmol Vis Sci. 2004;45:2187–93. doi: 10.1167/iovs.03-0901. [DOI] [PubMed] [Google Scholar]
- 76.Klebe S, Coster DJ, Sykes PJ, Swinburne S, Hallsworth P, Scheerlinck JP, et al. Prolongation of sheep corneal allograft survival by transfer of the gene encoding ovine IL-12-p40 but not IL-4 to donor corneal endothelium. J Immunol. 2005;175:2219–26. doi: 10.4049/jimmunol.175.4.2219. [DOI] [PubMed] [Google Scholar]
- 77.Jessup CF, Brereton HM, Sykes PJ, Thiel MA, Coster DJ, Williams KA. Local gene transfer to modulate rat corneal allograft rejection. Invest Ophthalmol Vis Sci. 2005;46:1675–81. doi: 10.1167/iovs.04-1140. [DOI] [PubMed] [Google Scholar]
- 78.Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, et al. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36:690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- 79.Gong N, Pleyer U, Vogt K, Anegon I, Flügel A, Volk HD, et al. Local overexpression of nerve growth factor in rat corneal transplants improves allograft survival. Invest Ophthalmol Vis Sci. 2007;48:1043–52. doi: 10.1167/iovs.06-1084. [DOI] [PubMed] [Google Scholar]
- 80.Gong N, Pleyer U, Volk HD, Ritter T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene Ther. 2007;14:484–90. doi: 10.1038/sj.gt.3302884. [DOI] [PubMed] [Google Scholar]
- 81.Funaki T, Ebihara N, Murakami A, Nakao A. Ex vivo transfer of Smad7 decreases damage to the corneal endothelium after penetrating keratoplasty. Jpn J Ophthalmol. 2008;52:204–10. doi: 10.1007/s10384-007-0526-2. [DOI] [PubMed] [Google Scholar]
- 82.Parker DG, Coster DJ, Brereton HM, Hart PH, Koldej R, Anson DS, et al. Lentivirus-mediated gene transfer of interleukin 10 to the ovine and human cornea. Clin Experiment Ophthalmol. 2010;38:405–13. doi: 10.1111/j.1442-9071.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- 83.Pleyer U, Bertelmann E, Rieck P, Hartmann C, Volk HD, Ritter T. Survival of corneal allografts following adenovirus-mediated gene transfer of interleukin-4. Graefes Arch Clin Exp Ophthalmol. 2000;238:531–6. doi: 10.1007/pl00007896. [DOI] [PubMed] [Google Scholar]
- 84.König Merediz SA, Zhang EP, Wittig B, Hoffmann F. Ballistic transfer of minimalistic immunologically defined expression constructs for IL4 and CTLA4 into the corneal epithelium in mice after orthotopic corneal allograft transplantation. Graefes Arch Clin Exp Ophthalmol. 2000;238:701–7. doi: 10.1007/s004170000144. [DOI] [PubMed] [Google Scholar]
- 85.Ignatius R, Zhang EP, Schulte F, Schroff M, Ruschendorf U, Muller A, et al. Transplantation of DBA/2 mouse corneas in BALB/c recipients significantly delays graft rejection compared with C3H grafts and facilitates studies on gene transfer. Open Transplant J. 2008;2:9–12. [Google Scholar]
- 86.Pillai RG, Beutelspacher SC, Larkin DF, George AJ. Expression of the chemokine antagonist vmip ii using a non-viral vector can prolong corneal allograft survival. Transplantation. 2008;85:1640–7. doi: 10.1097/TP.0b013e318172813f. [DOI] [PubMed] [Google Scholar]
- 87.Nosov M, Wilk M, Morcos M, Cregg M, O′Flynn L, Treacy O, et al. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am J Transplant. 2012;12:1313–22. doi: 10.1111/j.1600-6143.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 88.Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous Mesenchymal Stem Cells Prevented Rejection of Allogeneic Corneal Transplants by Aborting the Early Inflammatory Response. Mol Ther. 2012;20:2143–52. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papi M, Palmieri V, Maulucci G, Arcovito G, Grecio E, Quintiliani G, et al. Controlled self assembly of collagen nanoparticle. J Nanopart Res. 2011;11:6141–7. [Google Scholar]
- 90.Mayo AS, Ambati BK, Kompella UB. Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions. Int J Pharm. 2010;387:278–85. doi: 10.1016/j.ijpharm.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamada J, Dana MR, Sotozono C, Kinoshita S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res. 2003;76:161–7. doi: 10.1016/s0014-4835(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 92.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501–7. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 93.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansour S, Roy DC, Bouchard V, Nguyen BK, Stevens LM, Gobeil F, et al. COMPARE-AMI trial: comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction: study rationale and design. J Cardiovasc Transl Res. 2010;3:153–9. doi: 10.1007/s12265-009-9145-2. [DOI] [PubMed] [Google Scholar]
- 95.Ayliffe W, Alam Y, Bell EB, McLeod D, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol. 1992;76:602–6. doi: 10.1136/bjo.76.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, et al. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998;28:3240–51. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 97.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–80. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 98.Bouguermouh S, Fortin G, Baba N, Rubio M, Sarfati M. CD28 Co-Stimulation Down Regulates Th17 Development. PLoS ONE. 2009;4:e5087. doi: 10.1371/journal.pone.0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–84. [PubMed] [Google Scholar]
- 100.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177:1265–73. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X, Zhao S, Tang X, Ge H, Liu P. Neutralization of mouse interleukin-17 bioactivity inhibits corneal allograft rejection. Mol Vis. 2011;17:2148–56. [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada J, Hamuro J, Fukushima A, Ohteki T, Terai K, Iwakura Y, et al. Mhc-matched corneal allograft rejection in an ifn-gamma/il-17-independent manner in c57bl/6 mice. Invest Ophthalmol Vis Sci. 2009;50:2139–46. doi: 10.1167/iovs.08-2993. [DOI] [PubMed] [Google Scholar]
- 103.Suryawanshi A, Veiga-Parga T, Reddy PB, Rajasagi NK, Rouse BT. Il-17a differentially regulates corneal vascular endothelial growth factor (vegf)-a and soluble vegf receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J Immunol. 2012;188:3434–46. doi: 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cunnusamy K, Chen PW, Niederkorn JY. IL-17 Promotes immune privilege of corneal allografts. J Immunol. 2010;185:4651–8. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cunnusamy K, Chen PW, Niederkorn JY. Paradigm shifts in the role of cd4+ t cells in keratoplasty. Discov Med. 2010;10:452–61. [PMC free article] [PubMed] [Google Scholar]
- 106.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A–dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186:6737–45. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoffmann F, Zhang EP, Pohl T, Kunzendorf U, Wachtlin J, Bulfone-Paus S. Inhibition of corneal allograft reaction by ctla4-ig. Graefes Arch Clin Exp Ophthalmol. 1997;235:535–40. doi: 10.1007/BF00947013. [DOI] [PubMed] [Google Scholar]
- 108.Gebhardt BM, Hodkin M, Varnell ED, Kaufman HE. Protection of corneal allografts by CTLA-4 Ig. Cornea. 1999;18:314–20. doi: 10.1097/00003226-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 109.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. Ctla-4-ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 110.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, et al. Cutting edge: Induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–5. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 111.Li J, Meinhardt A, Roehrich ME, Golshayan D, Dudler J, Pagnotta M, et al. Indoleamine 2,3-dioxygenase gene transfer prolongs cardiac allograft survival. Am J Physiol Heart Circ Physiol. 2007;293:H3415–23. doi: 10.1152/ajpheart.00532.2007. [DOI] [PubMed] [Google Scholar]
- 112.Vavrincova-Yaghi D, Deelman LE, Goor H, Seelen M, Kema IP, Smit-van Oosten A, et al. Gene therapy with adenovirus-delivered indoleamine 2,3-dioxygenase improves renal function and morphology following allogeneic kidney transplantation in rat. J Gene Med. 2011;13:373–81. doi: 10.1002/jgm.1584. [DOI] [PubMed] [Google Scholar]
- 113.Wan F, Dai H, Zhang S, Moore Y, Wan N, Dai Z. Cigarette smoke exposure hinders long-term allograft survival by suppressing indoleamine 2, 3-dioxygenase expression. Am J Transplant. 2012;12:610–9. doi: 10.1111/j.1600-6143.2011.03820.x. [DOI] [PubMed] [Google Scholar]


