Abstract
The purpose of this study was to examine the influence of the lactam bridge cyclization on melanoma targeting and biodistribution properties of the radiolabeled conjugates. Two novel lactam bridge-cyclized α-MSH peptide analogues, DOTA-CycMSH (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-c[Lys-Nle-Glu-His-DPhe-Arg-Trp-Gly-Arg-Pro-Val-Asp]) and DOTA-GlyGlu-CycMSH (DOTA-Gly-Glu-c[Lys-Nle-Glu-His-DPhe-Arg-Trp-Gly-Arg-Pro-Val-Asp]), were synthesized and radiolabeled with 111In. The internalization and efflux of 111In-labeled CycMSH peptides were examined in B16/F1 melanoma cells. The melanoma targeting properties, pharmacokinetics and SPECT/CT imaging of 111In-labeled CycMSH peptides were determined in B16/F1 melanoma-bearing C57 mice. Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited fast internalization and extended retention in B16/F1 cells. The tumor uptake values of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were 9.53±1.41 %injected dose/gram (%ID/g) and 10.40±1.40 %ID/g at 2 h post-injection, respectively. Flank melanoma tumors were clearly visualized with 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH by SPECT/CT images at 2 h post-injection. Whole-body clearance of the peptides was fast, with greater than 90% of the radioactivities cleared through urinary system by 2 h post-injection. There was low radioactivity (<0.8 %ID/g) accumulated in blood and normal organs except kidneys at all time points investigated. Introduction of a negatively-charged linker (-Gly-Glu-) into the peptide sequence decreased the renal uptake by 44% without affecting the tumor uptake at 4 h post-injection. High receptor-mediated melanoma uptakes coupled with fast whole-body clearance in B16/F1 melanoma-bearing C57 mice demonstrated the feasibility of using 111In-labeled lactam bridge-cyclized α-MSH peptide analogues as a novel class of imaging probes for receptor-targeting melanoma imaging.
Keywords: Melanoma imaging, lactam bridge cyclization, alpha-MSH, melanocortin-1
Introduction
Skin cancer is the most commonly diagnosed cancer in the United States. Malignant melanoma is the most lethal form of skin cancer and the most commonly diagnosed malignancy among young adults with an increasing incidence. It is predicted that there will be 59,940 cases of malignant melanoma newly reported and 8,110 fatalities in 2007 (1). There is a great need to develop novel imaging probes and treatment approaches for melanoma detection and therapy since early diagnosis and prompt surgical removal are a patient’s best hope for a cure. Although 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) is commonly used for positron emission tomography (PET) diagnosis and staging of melanoma, [18F]FDG is not melanoma-specific and some melanoma cells are not detected by [18F]FDG since they use substrates other than glucose as energy sources (2, 3). Alternatively, the G protein-coupled melanocortin-1 (MC1) receptors have been used as targets for melanoma imaging peptides due to their over-expression on human and mouse melanoma cells (4-8). Radiolabeled α-melanocyte stimulating hormone (α-MSH) peptide analogues, derived from wild-type α-MSH, are very promising candidates for melanoma imaging and therapy due to their nanomolar MC1 receptor binding affinities and high receptor-mediated tumor uptakes in murine melanoma-bearing mice and human melanoma xenografts (9-13).
The strategy of peptide cyclization was used to improve binding affinity, in vivo stability and receptor selectivity of the α-MSH peptides (14-16). Compared to the linear peptides, the cyclic peptides possess less conformational freedom due to the stabilization of secondary structures such as beta turns, which results in greater receptor binding affinities. Furthermore, the constrained cyclic peptides confer higher stability against the proteolytic degradations in vivo (17). Peptide cyclization strategies can be generally classified into four types, namely, 1) backbone to backbone; 2) N-terminus to C-terminus; 3) one side chain to C-terminus or N-terminus; 4) two side chains via disulfide or lactam bridges. Over the past several years, peptide cyclization through metal coordination has been successfully employed in developing radiolabeled cyclic α-MSH peptide analogues for melanoma imaging and therapy. Metal cyclization made the α-MSH peptide analogues resistant to chemical and proteolytic degradation in vivo (18, 19). 111In-DOTA-Re[Cys3,4,10, d-Phe7]-α-MSH3-13} (111In-DOTA-ReCCMSH) exhibited 11.4±2.89 %injected dose/gram (%ID/g) tumor uptake 2 h post-injection in B16/F1 murine melanoma mouse model (19). A comparison of biodistribution data between 111In-labeled DOTA-conjugated metal-cyclized and disulfide bridge-cyclized α-MSH peptide analogues demonstrated that the metal cyclization resulted in more favorable pharmacokinetics of radiolabeled peptides, such as higher tumor uptake and lower renal uptake values (19), indicating that the different cyclization strategies might dramatically affect the biodistribution properties of peptides with comparable in vitro binding affinities.
In this study, Lys-Asp lactam bridge cyclization was employed to the α-MSH peptides to examine its effect on melanoma targeting and pharmacokinetics of the radiolabeled peptides. Two novel DOTA-conjugated lactam bridge-cyclized α-MSH peptide analogues, namely DOTA-CycMSH and DOTA-GlyGlu-CycMSH, were synthesized and characterized by liquid chromatography-mass spectrometry (LC-MS). A negatively-charged linker of -Gly-Glu- was introduced between DOTA and CycMSH sequences to determine its effect in reducing the renal uptake value of the radiolabeled peptide. The pharmacokinetics and SPECT/CT imaging of 111In-labeled lactam bridge-cyclized α-MSH peptides were determined in B16/F1 melanoma-bearing C57 mice to evaluate their potential as SPECT imaging probes for melanoma detection.
Experimental Procedures
Chemicals and Reagents
Amino acid and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). DOTA-tri-t-butyl ester was purchased from Macrocyclics Inc. (Richardson, TX). 111InCl3 was purchased from Mallinckrodt, Inc. (St. Louis, MO). All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Peptide Synthesis
Intermediate scaffold of H2N-Lys(Dde)-Nle-Glu(OtBu)-His(Trt)-dPhe-Arg(Pbf)-Trp(Boc)-Gly-Arg(Pbf)-Pro-Val was synthesized on Val-2-Chlorotrityl Chloride (Val-2ClTrt) resin using standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). A small aliquot of the scaffold material was cleaved and characterized by LC-MS. Gly-Glu(OtBu) and DOTA-tri-t-butyl ester were manually attached to the intermediate scaffold using standard Fmoc chemistry, respectively. Protected branched peptide was cleaved from the resin treating with 25% hexafluoroisopropanol (HFIP) and 5% triisopropylsilane (TIS) in dichloromethane (DCM) and characterized by LC-MS. Peptide cyclization between the acid moiety of Val and the amino group of Asp coupled to the Lys side chain was achieved by overnight reaction in DMF in presence of 1 mM 1-hydroxybenzotriazole (HOBT), 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetranethyluronium hexafluorophosphate (HBTU) and N,N-diisopropylethylamine (DIEA) mixture. The protecting groups were totally removed by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at room temperature (25°C). The peptides were precipitated and washed with ice-cold ether for four times. The final products were purified by RP-HPLC and characterized by LC-MS.
In vitro Competitive Binding Assay
The IC50 values of DOTA-CycMSH and DOTA-GlyGlu-CycMSH were determined by using methods described previously (8). B16/F1 cells were harvested and seeded into a 24-well cell culture plate (5×105/well) and incubated at 37°C overnight. After being washed once with binding media (MEM with 25 mM HEPES, pH 7.4, 0.2% BSA, 0.3 mM 1,10-phenathroline), the cells were incubated at room temperature (25°C) for 2 h with approximately 50,000 cpm of 125I-Tyr2-[Nle4, d-Phe7]-α-MSH {NDP-MSH} (GE HealthCare, Piscataway, NJ) in presence of increasing concentrations of DOTA-CycMSH or DOTA-GlyGlu-CycMSH in 0.3 ml of binding media. The reaction media were aspirated after incubation. Cells were rinsed with 0.5 ml of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS twice and lysed in 0.5 ml of 1 N NaOH for 5 minutes. The activities in cells were measured in a gamma counter. The IC50 values for the peptides were calculated by using the Grafit software (Erithacus Software Limited, UK).
Complexation of the Peptide with 111In
111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were prepared using a 0.5 M NH4OAc-buffered solution at pH 5.4. Briefly, 50 μl of 111InCl3 (18.5-37.0 MBq in 0.05 M HCl), 10 μl of 1 mg/ml DOTA-CycMSH or DOTA-GlyGlu-CycMSH aqueous solution and 400 μl of 0.5 M NH4OAc (pH 5.4) were added into a reaction vial and incubated at 75°C for 45 min. After the incubation, 20 μl of 0.5% EDTA aqueous solution was added into the reaction vial to scavenge potential unbound 111In. The radiolabeled complexes were purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vadyc C-18 reverse phase analytical column (Deerfield, IL) using a 20-minute gradient of 16-26% acetonitrile in 20 mM HCl aqueous solution with a flowrate of 1 ml/min. Purified peptide samples were purged with N2 gas for 20 minutes to remove the acetonitrile. The pH of final solution was adjusted to 7.4 with 0.1 N NaOH and normal saline for animal studies. The stability of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH was determined by incubation in mouse serum at 37°C according to the published procedure (20) for various time periods, and monitored for degradation by RP-HPLC.
Cellular Internalization and Efflux of 111In-labeled Peptides
Cellular internalization and efflux of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were evaluated in B16/F1 cells as described by Miao et al (21). After being washed once with binding media (MEM with 25 mM HEPES, pH 7.4, 0.2% BSA, 0.3 mM 1,10-phenathroline), B16/F1 cells in cell culture plates were incubated at 25°C for 20, 40, 60, 90 and 120 min (n=4) in presence of approximately 200,000 counts per minute (cpm) of HPLC purified 111In-DOTA-CycMSH (0.019 pmol) or 111In-DOTA-GlyGlu-CycMSH (0.019 pmol). After incubation, the reaction media were aspirated and cells were rinsed with 2×0.5 ml of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS. Cellular internalization of radiolabeled peptides was assessed by washing the cells with acidic buffer [40 mM sodium acetate (pH 4.5) containing 0.9% NaCl and 0.2% BSA] to remove the membrane-bound radioactivity. The remaining internalized radioactivity was obtained by lysing the cells with 0.5 ml of 1N NaOH for 5 min. Membrane-bound and internalized 111In activities were counted in a gamma counter. Cellular efflux of radiolabeled peptides was determined by incubating B16/F1 cells with 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH for 2 h at 25°C, removing non-specific-bound activity with 2×0.5 ml of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS rinse, and monitoring radioactivity released into cell culture media. At time points of 20, 40, 60, 90 and 120 min, the radioactivities in media, on cell surface and in cells were separately collected and counted in a gamma counter.
Biodistribution Studies
All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The pharmacokinetics of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were determined in B16/F1 murine melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). C57 mice were inoculated subcutaneously with 1×106 B16/F1 murine melanoma cells in the right flank. After 10 days, when the weight of tumors reached approximately 0.2 g, 0.037 MBq of 111In-DOTA-CycMSH or 111In-DOTA-GlyGlu-CycMSH was injected into each mouse through the tail vein. Groups of 5 mice were sacrificed at 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The tumor uptake specificity of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH was determined by blocking tumor uptake with the co-injection of 10 μg (6.07 nmol) of unlabeled NDP-MSH, a linear α-MSH peptide analogue with picomolar affinity for the MC1 receptors present on murine melanoma cells. Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significance between radiolabeled compounds, with p<0.05 being significantly different.
Imaging Melanoma with 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH
Two B16/F1 melanoma-bearing C57 mice were injected with 13.7 MBq of 111In-DOTA-CycMSH or 111In-DOTA-GlyGlu-CycMSH via the tail vein, respectively. The mice were euthanized for micro-CT imaging immediately followed by micro-SPECT imaging at 2 h post-injection. Micro-SPECT scans of 60 frames for each animal were acquired and total counts acquisitions of 524,672 and 278,242 counts (2 h acquisition) were achieved for both SPECT scans, respectively. The micro-SPECT images were obtained using the Micro-CAT II CT/SPECT (Siemens) unit equipped with high resolution 2 mm pinhole collimators (22). Reconstructed data from SPECT and CT were visualized and co-registered using Amira 3.1 (Ascent Media System & Technology Service, Northvale, NJ).
Urinary Metabolites of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH
One hundred microliter of HPLC purified 111In-DOTA-CycMSH (0.74-1.11 MBq, 0.82-1.12 ng) or 111In-DOTA-GlyGlu-CycMSH (0.74-1.11 MBq, 0.90-1.35 ng) was injected into each B16/F1 murine melanoma-bearing C57 mouse through the tail vein. At 2 h after dose administration, mice were sacrificed and the urine was collected. The radioactive metabolites in the urine were analyzed by injecting aliquots of urine into HPLC. A 20-minute gradient of 16-26% acetonitrile / 20 mM HCl was used for the urine analysis.
Results
DOTA-CycMSH and DOTA-GlyGlu-CycMSH were synthesized, purified by RP-HPLC and the identities of peptides were confirmed by electrospray ionization mass spectrometry. The synthetic schemes are presented in Fig. 1. The competitive binding curves of DOTA-CycMSH and DOTA-GlyGlu-CycMSH are shown in Fig. 2. The IC50 values of DOTA-CycMSH and DOTA-GlyGlu-CycMSH were 1.75 nM and 0.90 nM in B16/F1 cells. The peptides were labeled with 111In using a 0.5 M NH4OAc-buffered solution at pH 5.4. The radiolabeling yields were greater than 95% for both peptides. 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were completely separated from their excess non-labeled peptides by RP-HPLC. The retention times of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were 15.4 and 18.0 min, respectively. The specific activities of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were 9.03×108 and 8.23×108 MBq/g, respectively. 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were stable in mouse serum at 37°C. Only the 111In-labeled peptides were detected by RP-HPLC after 24 h of incubation (Fig. 3).
Figure 1.
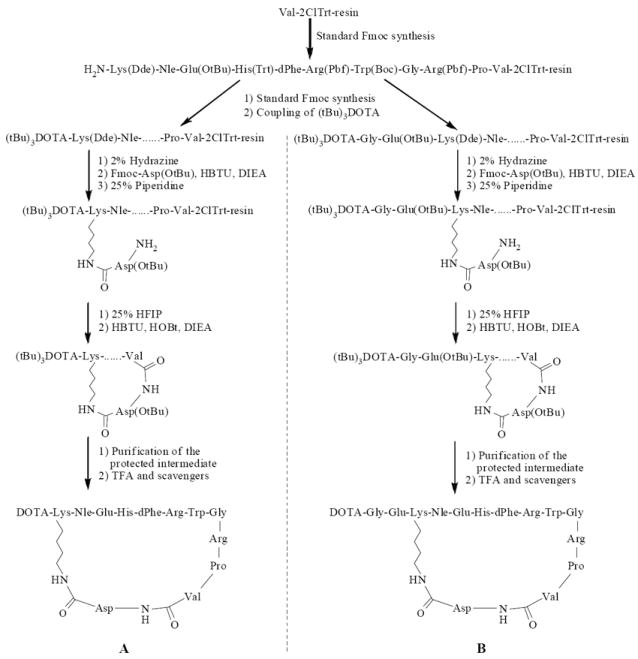
Synthetic schemes of DOTA-CycMSH (A) and DOTA-GlyGlu-CycMSH (B).
Figure 2.
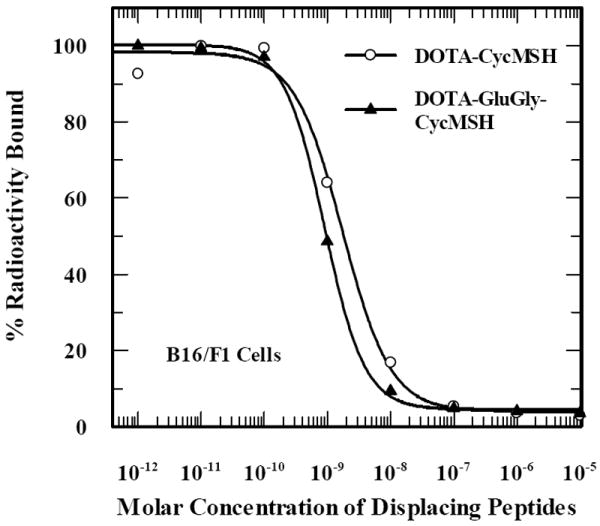
The competitive binding curves of DOTA-CycMSH and DOTA-GlyGlu-CycMSH in B16/F1 murine melanoma cells. The IC50 values of DOTA-CycMSH and DOTA-GlyGlu-CycMSH were 1.75 nM and 0.90 nM.
Figure 3.
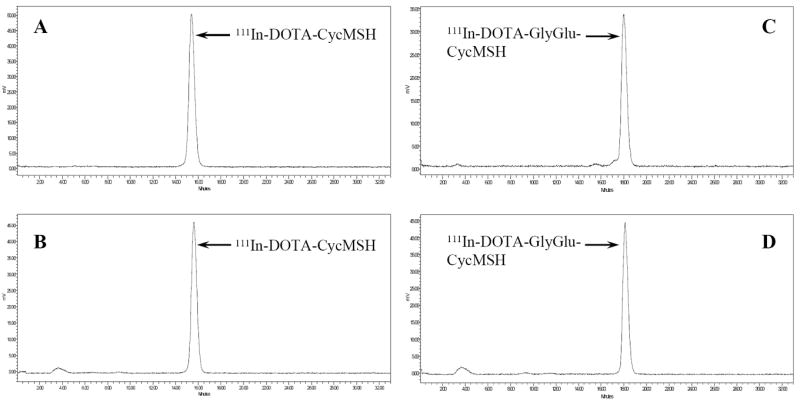
HPLC profiles of radioactive 111In-DOTA-CycMSH (A), 111In-DOTA-GlyGlu-CycMSH (C) and mouse serum stability of 111In-DOTA-CycMSH (B), 111In-DOTA-GlyGlu-CycMSH (D) after 24 h incubation at 37°C, respectively. The retention times of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were 15.4 and 18.0 min, respectively.
Cellular internalization and efflux of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were evaluated in B16/F1 cells at 25°C. Figure 4 illustrates cellular internalization and efflux of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH. Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited rapid cellular internalization and extended cellular retention. There were 73.71±1.71% of the 111In-DOTA-CycMSH and 66.19±2.12% of the 111In-DOTA-GlyGlu-CycMSH activity internalized in the B16/F1 cells 40 min post incubation. There were 81.98±2.02% of the 111In-DOTA-CycMSH and 78.93±0.64% of the 111In-DOTA-GlyGlu-CycMSH activity internalized in the cells after 2 h incubation. Cellular efflux experiments demonstrated that 90.26±1.71% of the 111In-DOTA-CycMSH activity and 90.00±1.10% of the 111In-DOTA-GlyGlu-CycMSH activity remained inside the cells 2 h after incubating cells in culture media at 25°C.
Figure 4.
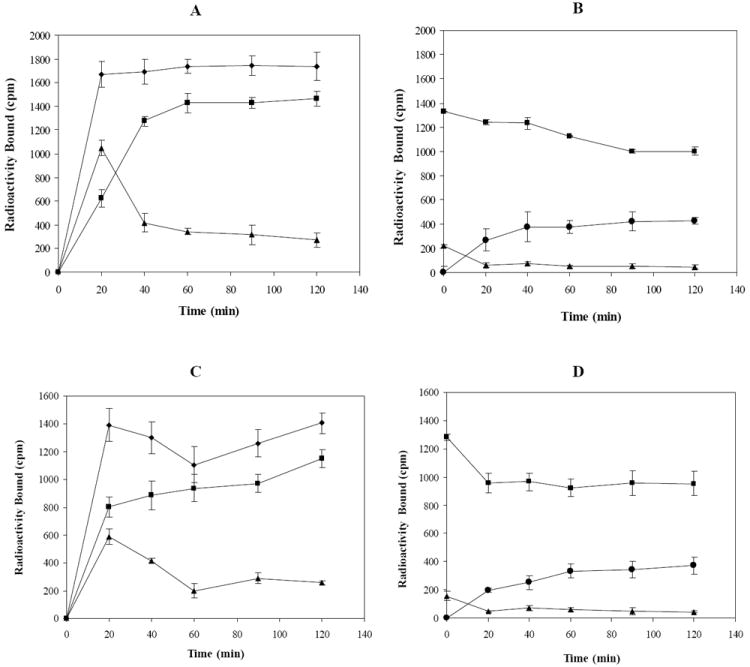
Cellular internalization and efflux of 111In-DOTA-CycMSH (A and B) and 111In-DOTA-GlyGlu-CycMSH (C and D) in B16/F1 murine melanoma cells at 25°C. Total bound radioactivity (◆), internalized activity (■), cell membrane activity (▲) and cell culture media activity (●) were presented as counts per minute (cpm).
The pharmacokinetics and tumor targeting properties of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were determined in B16/F1 murine melanoma-bearing C57 mice. The biodistribution of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH are shown in Table 1. Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited very rapid and high tumor uptakes. At 2 h post-injection, 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH reached their peak tumor uptake values of 9.53±1.41 and 10.40±1.40 %ID/g, respectively. There were 7.54±0.70 %ID/g of the 111In-DOTA-CycMSH and 7.40±0.43 %ID/g of the 111In-DOTA-GlyGlu-CycMSH activities remained in the tumors 4 h post-injection. The tumor uptake values of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH decreased to 2.22±0.51 and 2.37±0.28 %ID/g at 24 h post-injection. In competition studies, the tumor uptakes of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH with 10 μg (6.07 nmol) of non-radiolabeled NDP co-injection were only 3.0% and 2.6% of the tumor uptake without NDP co-injection at 2 h after dose administration (P<0.01), demonstrating that the tumor uptakes were specific and receptor-mediated. Whole-body clearance of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH was very rapid, with approximately 90% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 1). Normal organ uptakes of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were generally very low (<0.8 %ID/g) except for the kidneys at all time points investigated in this study. High tumor/blood and tumor/normal organ uptake ratios were demonstrated 2 h post-injection (Table 1). Although there was no statistical difference in tumor uptake between 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH, the renal uptake of 111In-DOTA-GlyGlu-CycMSH was significantly lower (p<0.05, Table 1) than that of 111In-DOTA-CycMSH at 2, 4 and 24 h post-injection. 111In-DOTA-GlyGlu-CycMSH displayed 44% less renal uptake value than 111In-DOTA-CycMSH at 4 h post-injection. At 24 h after dose administration, the kidney uptake values of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH decreased to 16.00±2.30 and 9.06±1.82 %ID/g, respectively. Bone uptake values of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were low (<0.2 %ID/g) at all time points investigated in this study.
Table 1.
Biodistribution of 111In-DOTA-Cyc(Arg11)CCMSH and 111In-DOTA-GlyGlu-Cyc(Arg11)CCMSH in B16/F1 murine melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (Mean±SD, n=5).
| Tissues | Percent injected dose/gram (%ID/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 111In-DOTA-CycMSH | 111In-DOTA-GlyGlu-CycMSH | |||||||
| 2 h | 2 h blockade | 4 h | 24 h | 2 h | 2 h blockade | 4 h | 24 h | |
| Tumor | 9.53±1.41 | 0.29±0.14 | 7.54±0.70 | 2.22±0.51 | 10.40±1.40 | 0.27±0.10 | 7.40±0.43 | 2.37±0.28 |
| Brain | 0.06±0.02 | 0.05±0.04 | 0.05±0.04 | 0.02±0.03 | 0.10±0.07 | 0.01±0.02 | 0.02±0.03 | 0.05±0.04 |
| Blood | 0.09±0.14 | 0.11±0.11 | 0.01±0.00 | 0.05±0.06 | 0.14±0.05 | 0.12±0.11 | 0.02±0.04 | 0.09±0.14 |
| Heart | 0.20±0.18 | 0.17±0.15 | 0.06±0.05 | 0.07±0.13 | 0.16±0.18 | 0.04±0.06 | 0.00±0.00 | 0.09±0.17 |
| Lung | 0.22±0.18 | 0.10±0.10 | 0.11±0.10 | 0.03±0.06 | 0.04±0.03 | 0.12±0.14 | 0.01±0.00 | 0.06±0.05 |
| Liver | 0.17±0.03* | 0.12±0.00 | 0.09±0.07* | 0.11±0.01* | 0.27±0.03 | 0.25±0.05 | 0.21±0.02 | 0.24±0.08 |
| Spleen | 0.39±0.41 | 0.42±0.37 | 0.80±0.39 | 0.22±0.39 | 0.16±0.33 | 0.15±0.14 | 0.27±0.40 | 0.15±0.25 |
| Stomach | 0.10±0.06 | 0.08±0.10 | 0.13±0.15 | 0.02±0.04 | 0.06±0.07 | 0.03±0.05 | 0.02±0.03 | 0.04±0.07 |
| Kidneys | 16.16±1.86* | 16.21±2.33 | 21.69±0.34* | 16.00±2.30* | 13.07±2.49 | 8.61±1.34 | 12.13±1.17 | 9.06±1.82 |
| Muscle | 0.14±0.10 | 0.28±0.32 | 0.01±0.01 | 0.03±0.04 | 0.06±0.12 | 0.13±0.13 | 0.05±0.06 | 0.05±0.06 |
| Pancreas | 0.07±0.06 | 0.13±0.04 | 0.03±0.05 | 0.07±0.06 | 0.07±0.10 | 0.09±0.15 | 0.05±0.08 | 0.02±0.02 |
| Bone | 0.20±0.16 | 0.16±0.14 | 0.04±0.05 | 0.18±0.13 | 0.14±0.07 | 0.42±0.37 | 0.00±0.00 | 0.06±0.05 |
|
| ||||||||
| Percent injected dose (%ID) | ||||||||
| Intestines | 0.34±0.11 | 0.23±0.06 | 0.25±0.08 | 0.05±0.02 | 0.27±0.15 | 0.27±0.06 | 0.15±0.01 | 0.07±0.02 |
| Urine | 91.70±0.62 | 94.53±0.65 | 92.34±0.80 | 94.69±0.49 | 90.32±1.64 | 96.52±0.20 | 92.36±0.74 | 95.04±0.84 |
|
| ||||||||
| Uptake ratio of tumor/normal tissue | ||||||||
| Tumor/Blood | 105.89 | 2.64 | 754.00 | 44.40 | 74.29 | 2.25 | 370.00 | 26.33 |
| Tumor/Kidneys | 0.59 | 0.02 | 0.35 | 0.14 | 0.80 | 0.0.3 | 0.61 | 0.26 |
| Tumor/Lung | 43.32 | 2.90 | 68.55 | 74.00 | 260.00 | 2.25 | 740.00 | 39.50 |
| Tumor/Liver | 56.06 | 2.42 | 83.78 | 20.18 | 38.52 | 1.08 | 35.24 | 9.88 |
| Tumor/Muscle | 68.07 | 1.04 | 754.00 | 74.00 | 173.33 | 2.08 | 148.00 | 47.40 |
P<0.05, significance comparison between 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH at 2, 4 and 24 h post-injection.
Two B16/F1 murine melanoma-bearing C57 mice were separately injected with 111In-DOTA-CycMSH (13.7 MBq, 15.2 ng) and 111In-DOTA-GlyGlu-CycMSH (13.7 MBq, 16.6 ng) through the tail vein to visualize the tumors at 2 h after dose administration. The whole-body SPECT images of the mice were fused with CT images, respectively. The transaxial tumor images and the whole-body images are presented in Fig. 5. Flank melanoma tumors were visualized clearly by both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH at 2 h post-injection. Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited high tumor to normal organ uptake ratios except for the kidney, which was coincident with the trend observed in the biodistribution results. In view of the substantial renal uptake values of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH in the biodistribution results, the urinary metabolites of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were analyzed by RP-HPLC at 2 h post-injection. The HPLC elution profiles of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH are shown in Fig. 6. All of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were transformed to two more polar metabolites at 2 h post-injection, respectively.
Figure 5.
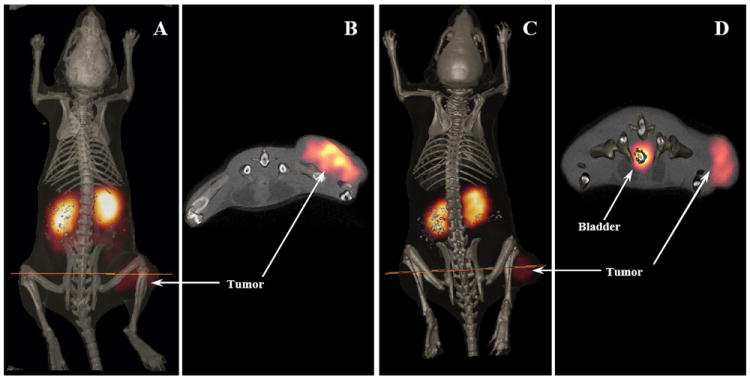
Whole-body and transaxial images of 111In-DOTA-CycMSH (A, B) and 111In-DOTA-GlyGlu-CycMSH (C, D) in B16/F1 flank melanoma-bearing C57 mice at 2 h post-injection.
Figure 6.
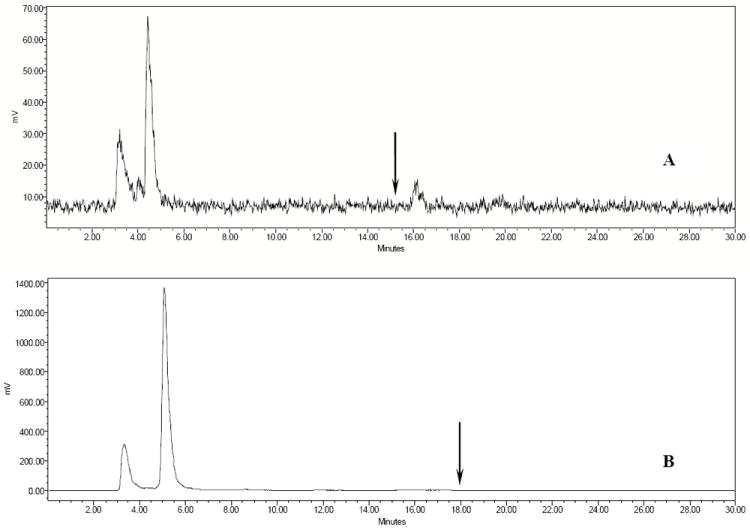
HPLC profiles of radioactive urine samples of B16/F1 murine melanoma-bearing C57 mice at 2 h post-injection of 111In-DOTA-CycMSH (A) and 111In-DOTA-GlyGlu-CycMSH (B). Arrows indicate retention time of the original compound of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH prior to the tail vein injection.
Discussion
Previous publications have demonstrated that the cyclization of α-MSH peptide analogues was able to improve the binding affinities and in vivo stability of peptides (14-16). The cyclic peptides possess less conformational freedom and higher stability than the linear peptides due to the stabilization of secondary structures, such as beta turns. The stabilization of secondary structures makes the cyclic peptides better fit receptor binding pocket, enhancing the binding affinities of the cyclic peptides. Peptide cyclization can be achieved through disulfide bridges, lactam bridges, covalent bonds such as N-N and N-C bonds or metal coordination. Site-specific bond formation or metal coordination allows one to control the ring size of the cyclic peptide, which is critical for optimal receptor binding. Over the past several years, we have successfully employed radiometal to cyclize CCMSH peptides through radiolabeling processes (6, 7, 18). Labeling the CCMSH peptides with 99mTc or 188Re simultaneously completed the peptide cyclization and the coupling of radionuclides to the peptides for melanoma imaging or therapy. The metal cyclization made α-MSH peptide analogues resistant to chemical and proteolytic degradation in vivo (18, 19). Furthermore, the metal cyclization resulted in greater tumor uptake and lower renal uptake values of the radiolabeled peptides compared to the disulfide bridge cyclization (19), demonstrating that the different cyclization strategies affect the biodistribution properties of radiolabeled peptides with similar in vitro receptor binding affinities.
Two novel lactam bridge-cyclized α-MSH peptide analogues were synthesized to examine the effect of lactam bridge cyclization on the tumor targeting and pharmacokinetics of the radiolabeled peptides. DOTA-CycMSH and DOTA-GlyGlu-CycMSH displayed IC50 values of 1.75 and 0.9 nM, that were comparable to metal- and disulfide-cyclized α-MSH peptide analogues previously reported (19). The lactacm bridge cyclization remained the nanomolar receptor binding affinities of the peptides, demonstrating its suitability and feasibility as a strategy to cyclize the peptides. Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited rapid cellular internalization and extended cellular retention in B16/F1 cells, with approximately 70% of the activity internalized in the cells 40 min post incubation and 90% of internalized activity remained in the cells after 2 h incubation in culture media. Efficient cellular internalization coupled with extended retention made the lactam bridge-cyclized α-MSH peptide analogues suitable for melanoma imaging and therapy (6, 23).
Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited high receptor-mediated tumor uptakes of 9.53±1.41 and 10.40±1.40 %ID/g at 2 h post-injection, respectively. The tumor uptakes of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH (lactam bridge-cyclized α-MSH peptides) were comparable to that of 111In-DOTA-ReCCMSH (a metal-cyclized α-MSH peptide), but slightly higher than the tumor uptake of 111In-DOTA-[Cys4,10, d-Phe7]-α-MSH4-13 (111In-DOTA-CMSH, a disulfide bridge-cyclized α-MSH peptide) (19). Two novel 111In-labeled DOTA-conjugated linear α-MSH peptide analogues, [Nle4, Asp5, d-Phe7, Lys11(DOTA)-111In]-α-MSH4-11 (111In-DOTA-NAPamide) and 111In-DOTA-[β-Ala3, Nle4, Asp5, d-Phe7, Lys10]-α-MSH3-10 (111In-DOTA-MSHoct), were reported for melanoma imaging (11, 12). 111In-DOTA-NAPamide displayed greater tumor uptake values and better pharmacokinetics than 111In-DOTA-MSHoct. The tumor uptake values of 111In-DOTA-NAPamide were 7.56±0.51 %ID/g at 4 h and 2.32±0.28 %ID/g at 24 h post-injection in the B16/F1 melanoma mouse model (12). Both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH exhibited comparable tumor uptake values with 111In-DOTA-NAPamide at 4 and 24 h post-injection. Flank melanoma tumors were clearly visualized with 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH at 2 h post-injection by SPECT/CT images (Fig. 5). The SPECT imaging of tumors accurately matched the anatomical information from CT images. 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH displayed high tumor to normal organ uptake ratios except for the kidney, which was coincident with the trend observed in biodistribution results. The high receptor-mediated tumor uptake and tumor to normal organ uptake ratios highlighted the potential of radiolabeled lactam bridge-cyclized α-MSH peptides as a novel class of peptide radiopharmaceuticals for melanoma imaging.
Both biodistribution results and SPECT/CT images demonstrated that the kidney was primary excretion pathway of the radiolabeled lactam bridge-cyclized α-MSH peptides. The strategy of infusing basic amino acids such as lysine or arginine has been employed to decrease the renal uptakes of radiolabeled peptides by shielding the electrostatic interaction between positively-charged peptides and negatively-charged surface of tubule cells (7, 24, 25). Recently, it has been reported that different pathways may play roles in the mechanism of the renal uptake of radiolabeled peptides besides the electrostatic interaction between the positively-charged peptides and negatively-charged tubule cells (26, 27). For instance, the use of colchicine, which prevents endocytosis in tubular cells, has successfully decreased the renal uptake up to 25% in a rat model (27). Moreover, transmembrane glycoproteins such as megalin, have been reported to be involved in the renal uptakes of radiolabeled somatostatin analogues (28). Since the extracellular domains of megalin can accommodate a variety of ligands, megalin may be involved in the renal uptakes of other radiolabeled peptides. Glutamic acid has been introduced into the peptide sequences to reduce the renal uptakes of radiolabeled metal-cyclized α-MSH peptides (29), as well as a PKM linker to modify the biodistribution of radiolabeld RGD peptides (30-35). In this study, the structural modification of increasing the overall negative charge of the peptide (introducing a glutamic acid) was investigated to demonstrate whether the structural modification could reduce the renal uptake values of the radiolabeled peptides. As a matter of fact, the introduction of a negatively-charged glutamic acid as a linker significantly (p<0.05) decreased the renal uptake of 111In-DOTA-GlyGlu-CycMSH by 44% compared to 111In-DOTA-CycMSH (Table 1) 4 h post-injection, suggesting that the electrostatic interaction played an important role in the renal uptakes of 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH. The urine analysis showed that both 111In-DOTA-CycMSH and 111In-DOTA-GlyGlu-CycMSH were metabolized into two moieties that might be responsible for the activity retention in kidneys. The kidney uptake value of 111In-DOTA-GlyGlu-CycMSH was 29% higher than 111In-DOTA-ReCCMSH at 4 h post-injection. However, the renal uptake value of 111In-DOTA-GlyGlu-CycMSH was only 34% of that of 111In-DOTA-CMSH at 4 h post-injection, demonstrating that the lactam cyclization was better than the disulfide cyclization in terms of less renal uptake value. Introduction of one more negatively-charged glutamic acid or lysine co-injection might further decrease the renal uptake of 111In-DOTA-GlyGlu-CycMSH. Another potential strategy to further reduce the kidney uptake would be to use shorter lactam bridge-cyclized α-MSH peptide since lower molecular weight 111In-DOTA-NAPamide exhibited less kidney uptake than 111In-DOTA-GlyGlu-CycMSH. Further reduction of the renal uptakes will facilitate the clinical evaluations of this novel class of radiolabeled α-MSH peptides as melanoma imaging probes, as well as promote the potential use of this novel class of radiolabeled α-MSH peptides as therapeutic agents for peptide-targeted radionuclide therapy of melanoma.
In conclusion, 111In-labeled DOTA-conjugated lactam bridge-cyclized α-MSH peptides exhibited high receptor-mediated tumor uptake coupled with fast whole-body clearance in B16/F1 murine melanoma model. Introduction of a negatively-charged linker (-Gly-Glu-) into the peptide sequence decreased the renal uptakes by 44% without affecting the tumor uptakes 4 h post-injection. 111In-labeled lactam bridge-cyclized α-MSH peptide analogues appear to be a novel class of very promising peptide radiopharmaceuticals for receptor-targeting melanoma imaging.
Acknowledgments
The authors express their gratitude to Drs. Wynn A. Volkert and Susan L. Deutscher for their helpful discussions. The authors thank Drs. Said D. Figueroa, Timothy J. Hoffman and Ms. Tiffani Shelton, Katherine Benwell for their assistance.
Grant support: UNM-LANL MOU on Research and Education Grant 2R76T (Y.M), American Foundation for Pharmaceutical Education Grant 3R48E (Y.M) and American Cancer Society Institutional Research Grant IRG-92-024 (Y.M) and National Cancer Institute P50 Imaging Center Grant P50-CA-103130 (T.P.Q)
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Nabi HA, Zubeldia JM. Clinical application of 18F-FDG in oncology. J Nucl Med Technol. 2002;30:3–9. [PubMed] [Google Scholar]
- 3.Dimitrakopoulou-Strauss A, Strauss LG, Burger C. Quantitative PET studies in pretreated melanoma patients: A comparison of 6-[18F]fluoro-L-DOPA with 18F-FDG and 15O-water using compartment and non-compartment analysis. J Nucl Med. 2001;42:248–256. [PubMed] [Google Scholar]
- 4.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 5.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 6.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mTechnetium-labeled cyclic α-melanocyte-stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- 7.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 8.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled alpha-Melanocyte stimulating hormone peptide analogues. Bioconjug Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 9.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re labeled α-melanocyte stimulating hormone peptide analogue in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–129. [PubMed] [Google Scholar]
- 10.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman TJ, Quinn TP. Melanoma therapy via peptide-targeted α-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 11.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-α-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 12.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A Gallium-labeled DOTA-α-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 13.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. J Nucl Med. 2005;46:887–895. [PubMed] [Google Scholar]
- 14.Sawyer TK, Hruby VJ, Darman PS, Hadley ME. [half-Cys4,half-Cys10]-α-melanocyte-stimulating hormone: a cyclic α-melanotropin exhibiting superagonist biological activity. Proc Natl Acad Sci U S A. 1982;79:1751–1755. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. Design of a new class of superpotent cyclic a-melanotropins based on quenched dynamic simulations. J Am Chem Soc. 1989;111:3413–3416. [Google Scholar]
- 16.Al-Obeidi F, de L Castrucci AM, Hadley ME, Hruby VJ. Potent and prolonged-acting cyclic lactam analogs of a-melanotropin: design based on molecular dynamics. J Med Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 17.Fung S, Hruby VJ. Design of cyclic and other templates for potent and selective peptide α-MSH analogues. Current Opinion in Chem Biol. 2005;9:352–358. doi: 10.1016/j.cbpa.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giblin MF, Wang NN, Hoffman TJ, Jurisson SS, Quinn TP. Design and charaterization of α-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proc Natl Acad Sci U S A. 1998;95:12814–12818. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- 20.Aloj L, Panico M, Caraco C, Del Vecchio S, Arra C, Affuso A, Accardo A, Mansi R, Tesauro D, De Luca S, Pedone C, Visentin R, Mazzi U, Morelli G, Salvatore M. In vitro and in vivo characterization of Indium-111 and Technetium-99m labeled CCK-8 derivatives for CCK-B receptor imaging. Cancer Biotherapy Radiopharm. 2004;19:93–98. doi: 10.1089/108497804773391739. [DOI] [PubMed] [Google Scholar]
- 21.Miao Y, Hoffman TJ, Quinn TP. Tumor-targeting properties of 90Y- and 177Lu-labeled α-melanocyte stimulating hormone peptide analogues in a murine melanoma model. Nucl Med Biol. 2005;32:485–493. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Miao Y, Benwell K, Quinn TP. 99mTc and 111In labeled alpha-melanocyte stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 23.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem Rev. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 24.Behr TM, Sharkey RM, Juweid ME, Blumenthal RD, Dunn RM, Bair HJ, Wolf FG, Becker WS, Goldenberg DM. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res. 1995;55:3825–3834. [PubMed] [Google Scholar]
- 25.Behr TM, Becker WS, Sharkey RM, Juweid ME, Dunn RM, Bair HJ, Wolf FG, Goldenberg DM. Reduction of renal uptake of monoclonal antibody fragments by amino acid infusion. J Nucl Med. 1996;37:829–833. [PubMed] [Google Scholar]
- 26.Béhé M, Kluge G, Becker W, Gotthardt M, Behr TM. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J Nucl Med. 2005;46:1012–1015. [PubMed] [Google Scholar]
- 27.Rolleman EJ, Krenning EP, Van Gameren A, Bernard BF, De Jong M. Uptake of [111In-DTPA0]octreotide in the rat kidney is inhibited by colchicine and not by fructose. J Nucl Med. 2004;45:709–713. [PubMed] [Google Scholar]
- 28.De Jong M, Barone R, Krenning EP, Bernard BF, Melis M, Vissor T, Gekle M, Willnow TE, Walrand S, Jamar F, Pauwels S. Megalin is essential for renal proximal tubule reabsorption of 111In-DTPA-Octreotide. J Nucl Med. 2005;46:1696–1700. [PubMed] [Google Scholar]
- 29.Miao Y, Fisher DR, Quinn TP. Reducing renal uptake of 90Y and 177Lu labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl Med Biol. 2006;33:723–733. doi: 10.1016/j.nucmedbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, He Z, Hsieh WY, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconjug Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijkgraaf I, Liu S, Kruijtzer JA, Soede AC, Oyen WJ, Liskamp RM, Corstens FH, Boerman OC. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconjug Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 34.Li ZB, Cai W, Cao Q, Chen K, Wu Z, He L, Chen X. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J Nucl Med. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- 35.Dijkgraaf I, Kruijtzer JA, Liu S, Soede AC, Oyen WJ, Corstens FH, Liskamp RM, Boerman OC. Improved targeting of the alpha(v)beta(3) integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34:267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]


