Abstract
Inflammatory mediators and drugs which affect inflammation can influence the healing of injured tissues. Leukotrienes are potent inflammatory mediators, and similar to prostaglandins, are metabolites of arachidonic acid which can have positive or negative effects on bone and cartilage tissues. Here we tested the hypothesis that blocking the negative regulation of leukotrienes, would lead to enhanced endochondral bone formation during fracture repair. A closed femoral fracture was created in mice. Animals were divided into three groups for treatment with either montelukast sodium, a cysteinyl leukotriene type 1 receptor antagonist (trade name Singulair), zileuton, a 5-lipoxygenase enzyme inhibitor (trade name Zyflo), or carrier alone. The fractures were analyzed using radiographs, quantitative gene expression, histology and histomorphometry, and immunohistochemistry. Both the montelukast sodium group and the zileuton group exhibited enhanced fracture repair when compared with controls. Both treatment groups exhibited increased callous size and earlier bone formation when compared to controls as early as day 7. Gene expression analysis of treatment groups showed increased markers of chondrocyte proliferation and differentiation, and increased early bone formation markers when compared with controls. Treatment with montelukast sodium directly targeted the cysteinyl leukotriene type 1 receptor, leading to increased chondrocyte proliferation at early time points. These novel findings suggests a potential mechanism by which the cysteinyl leukotriene type 1 receptor acts as a negative regulator of chondrocyte proliferation, with important and previously unrecognized implications for both fracture repair, and in a broader context, systemic chondrocyte growth and differentiation.
The immediate physiologic response to skeletal injury is a local and systemic inflammatory reaction. This first stage of fracture repair is followed by subsequent stages which culminate in skeletal regeneration. This cascade of events results in mesenchymal stem cell (MSC) recruitment to the zone of injury and terminal stem cell differentiation. Chondrocyte proliferation and differentiation predominate early in the process, giving way to matrix resorption, osteoblastic differentiation, and osseous tissue formation. In this sense, the skeletal repair process is recognized as highly sequential, progressing in a step wise fashion through well recognized stages (Schindeler et al., 2008). The early stages of repair are critical for successful healing, and inflammatory mediators which drive the initial cellular response set in motion a complex interplay of chondrogenesis, osteogenesis, and neovascularization (Einhorn, 2005).
The importance of the early inflammatory response is widely recognized, and many reports over several decades have examined the effects of commonly used non-steroidal anti-inflammatory drugs (NSAIDs) on fracture repair. These drugs, which modulate the initial inflammatory response, can have long-term effects on much later stages of fracture repair (Anon., 1978; Sudmann et al., 1979; Keller et al., 1989; Engesaeter et al., 1992; Hogevold et al., 1992; Beck et al., 2003; Bergenstock et al., 2005; Murnaghan et al., 2006; Gerstenfeld et al., 2007; Simon and O’Connor, 2007). Taken collectively, these studies demonstrated that arachidonic acid metabolism to prostaglandins is important for healing, and that inhibiting prostaglandin synthesis by administration of cyclo-oxygenase inhibitors generally results in delayed skeletal repair.
Arachidonic acid is formed from cell membrane bound phospholipids in response to many biological signals including injury. Collectively, the biologically relevant metabolites of arachidonic acid are known as eicosanoids, and comprise the families of prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs). The eicosanoids provide a wide range of inflammatory effects, variously affecting mucous membranes, airway constriction, gastric acid secretion, pain and fever modulation, and affect initiation of labor, among others (Boyce, 2008). Currently, there is limited knowledge concerning a potential role for leukotrienes and their cognate receptors in modulating fracture repair, particularly in early chondrogenic phases. One preliminary report using 5-lipoxygenase (5-LO) knockout mice demonstrated larger callous size and enhanced mechanical properties after fracture, although the mechanism for this effect remained unclear (Manigrasso and O’Connor, 2006).
5-LO functions for the leukotriene family in a manner analogous to cyclo-oxygenase in the prostaglandin family. This enzyme, acting in concert with 5-lipoxygenase activating protein (FLAP), catalyzes the conversion of arachidonic acid first to a series of intermediary metabolites, with the end result formation of two major groups of leukotrienes. These two groups consist of LTB4, and what a collectively known as the cysteinyl leukotrienes, LTC4, LTD4, LTE4, and LTF4.
The cysteinyl leukotrienes as a group have previously been implicated as negative regulators of MSC differentiation (Akino et al., 2006). In vitro studies of human MSCs cultured in the presence of pranlukast, a specific cysteinyl leukotriene type 1 (CysLT1) receptor antagonist, showed enhanced cellular differentiation based on morphology, highlighting the potential inhibitory effect of the CysLT1 pathway on MSC differentiation. Thus it is possible that MSC differentiation could be promoted during fracture repair via CysLT1 receptor inhibition. While it has long been recognized that local tissue mechanical trauma causes an up regulation of leukotriene production (Denzlinger et al., 1985), the effect of these potent inflammatory mediators on fracture repairs remains unknown.
Given the evidence that the CysLT1 receptor acts as a negative regulator of MSC differentiation (Akino et al., 2006), a process critical for fracture repair, along with previous murine fracture studies indicating larger callous size and enhanced mechanical properties of healing fractures in a 5-LO knockout model (Manigrasso and O’Connor, 2006), we hypothesized that oral medications which affect leukotriene synthesis could be used to enhance fracture repair. In the present study, we sought to determine potential mechanisms by which oral inhibition of the CysLT1 receptor alters the fracture repair response. Our studies provide evidence for a novel application using oral leukotriene inhibitors to promote fracture repair at early chondrogenic stages, which results in accelerated and enhanced endochondral bone formation. We demonstrate for the first time that chondrocytes express the CysLT1 receptor, suggesting a direct mechanism for the observed effect.
Materials and Methods
Animals
C57B/6 mice were purchased (Charles River, Inc., Wilmington, MA) and housed in the animal facility at the University of Massachusetts Medical School under IACUC approved protocol. Eight- to 9-week-old animals were used in the study.
Fracture technique
Institutional approval was obtained and all procedures were undertaken in accordance with approved IACUC methods. Animals were administered general anesthesia using IP injections of ketamine and xylazine. A midline skin incision over the knee joint was utilized and a median parapatellar arthrotomy was performed to expose the trochlear groove. A pilot hole was made using a 25 gauge needle to gain access to the femoral canal. The central cannula from a 22 gauge spinal needle was inserted into the canal and passed to the proximal femur in retrograde fashion. The wire was backed out slightly, cut, and reinserted. Wounds were closed, and the femur was held in a fixed position while a drop weight from a standard height was used to deliver a fixed traumatic injury to the mid portion of the femur, generating a fracture via three point bending, and ensuring that fractures were generated using a traumatic method with reproducible energy of injury (Marturano et al., 2008).
Medications
Study medications included montelukast sodium (trade name Singulair), provided by the manufacturer (Merck, Inc., Rahway, NJ) and zileuton (trade name Zyflo), purchased commercially (Sequoia, Inc., Pangbourne, UK). Medications were suspended in 1% methylcellulose (Sigma, Inc., St. Louis, MO) and delivered by direct intragastric delivery. Montelukast sodium was delivered at a dose of 1.5 mg/kg/day in a single dose, and zileuton was administered at 45 mg/kg/day in divided doses. As the study medications are both currently FDA approved for the treatment of reactive airway disease, our dosing frequency was based on current prescription guidelines. Montelukast sodium was administered once daily by oral gavage. At the time the study began, zileuton was only approved for four times per day dosing; as the drug needed to be administered by oral gavage and to maintain the normal sleep/awake cycle of the mouse colonies in the animal facilities, three doses of zileuton were dosed at 6-h intervals, with a 12-h break from 8 pm to 8 am. Control animals received 1% methylcellulose carrier only by a single daily oral gavage. The first dose of all medications was administered on post-fracture day 1.
Radiography
All animals were examined pre- and post-fracture using live fluoroscopy with an inverted Xiscan 1000 fluoroscope. Pre-fracture imaging was used to confirm correct positioning of the stabilizing wire, and post-fracture imaging was used to confirm correct fracture location and configuration. Additionally, standard radiographs were obtained in all animals immediately post-fracture with a high resolution MX-20 Faxitron on mammography film. Animals were anesthetized for additional X-rays to document fracture repair at 7, 14, 21, and 28 days post-fracture. Animals were sacrificed immediately post-fracture if the fracture was not diaphyseal and transverse.
Histology and immunohistochemistry
Fracture specimens were harvested at various times (7, 10, 14, and 21 days) and fixed in a solution of 4% paraformaldehyde, 0.1% CPC in PBS for 16 h at room temperature, and then embedded in paraffin after decalcification. Embedded tissues were sectioned into 6-μm slices, mounted on silane-coated glass slides (Fisher, Pittsburgh, PA), de-paraffinized, and re-hydrated. Safranin-O: Slides were stained sequentially with Weigert’s iron hematoxylin, fast green (FCF), and Safranin-O, then dehydrated sequentially in 95% ethyl alcohol, absolute ethyl alcohol, xylene and cover-slipped. Immunohistochemistry: Slides were washed in PBS. Non-specific tissue binding sites were blocked for 1 h at room temperature in 5% normal goat serum (NGS) (Santa Cruz, Santa Cruz, CA) and incubated in a humidified chamber overnight at 48°C in 150 μl of diluted primary antibody per individual tissue section. Primary antibodies to CysLT1 and 5-LO were diluted (1:100 for all) in blocking solution. Following primary antibody incubation, sections were washed with PBS (3 × 3 min each) and visualized using an ABC biotin/avidin (Dako, Inc., Carpinteria, CA) amplification/reporter method using DAB as chromogen (brown = positive identification). Slides were dried for 1 h at 37°C and cover-slipped using Pro-Texx (Learner Labs, Inc., Pittsburgh, PA) mounting medium.
Quantitative real-time PCR
Mice were sacrificed on days 7, 10, 14, and 21 post-fracture. The fractured limb was carefully dissected free and all overlying tissue was carefully removed to expose the fracture callous. Callous tissue alone was then placed in TRIzol reagent, avoiding the underlying cortical bone. The tissue was ground using a Polytron homogenizer and total RNA was isolated as per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Any potential DNA contamination was removed by RNase-free DNase treatment. The reverse transcription reaction was performed on 1 μg of total RNA using the First Strand Synthesis Kit and random hexamer primers (Invitrogen). Relative transcript levels were measured by real-time PCR in a 25 μl reaction volume on 96-well plate using ABI PRISM 7000 FAST sequence detection system (Applied Biosystems, Foster City, CA), following the recommended protocol for SYBR-Green (Applied Biosystems). Transcript levels were normalized with 18S ribosomal RNA levels using primers from Applied Biosystems and SYBR-Green master mix (Applied Biosystems). The primers used for amplification are described in Table 1.
TABLE 1.
List of primers used for quantitative real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| Runx2 | 5′-CGG CCC TCC CTG AAC TCT-3′ | 5′-TGC CTG CCT GGG ATC TGT A-3′ |
| Osteicalcin | 5′-CTG ACA AAG CCT TCA TGT CCA A-3′ | 5′-GCG CCG GAG TCT GTT CAC TA-3′ |
| Col 10a1 | 5′-CCC AAG GAA AAG AAG CAC GTC-3′ | 5′-AGG TCA GCT GGA TAG CGA CAT C-3′ |
| Collagen II | 5′-CTG GAA TGT CCT CTG CGA-3′ | 5′-TGA GGC AGT CTG GGT CTT CAC-3′ |
| Collagen X | 5′-CCT GCA GCA AAG GAA AAC TC-3′ | 5′-TGT GGT AGT GGT GGA GGA CA-3′ |
| Sox9 | 5′-GAG GCC ACG GAA CAG ACT CA-3′ | 5′-CAG CGC CTT GAA GAT AGC ATT-3′ |
| CDK2 | 5′-ACA GCC GTG GAT ATC TGG AG-3′ | 5′-TTA GCA TGG TGC TGG GTA CA-3′ |
| 5-LO | 5′-CCA TCA AGA GCA GGG AGA AG-3′ | 5′-ACC AGT CAT ACT GGC CGA AG-3′ |
| CystLT1 | 5′-CAT CTT CCT GCT TTG GCT TC-3′ | 5′-ATT GCC AAA GAA ACC CAC AA-3′ |
| Histone | 5′-CCAGCTGGTGTTTCAGATTACA-3′ | 5′-ACCCTTGCCTAGACCCTTTC-3′ |
Histomorphometry
Sectioning and histomorphometric measurements were performed in accordance with published methodologies (Gerstenfeld et al., 2005). Sagittal sections were reviewed and the most representative sections from the central portion of each specimen were chosen for analysis. The total callus area and cartilage area were measured in sections and quantified using imaging software.
Statistical methods
Student’s t-test was performed to analyze the significance of the data for gene expression and histomorphometric analysis.
Results
Fracture healing is enhanced in the montelukast sodium and zileuton groups
Given the potent inflammatory effects of leukotrienes and their role as negative regulators of MSC differentiation, we addressed whether inhibiting the effect of the cysteinyl leukotrienes would enhance fracture healing. Groups of mice were treated with either montelukast sodium, zileuton, or carrier and were sacrificed at days 7, 10, 14, and 21.
Characteristic radiographs following fracture are shown in Figure 1. Animals were fractured and underwent post-fracture X-ray while under anesthesia at day 0. Subsequent X-rays were taken at days 7, 10, 14, and 21 for all animals until the time of sacrifice. By day 21, all animals in all treatment groups had bridging bone across the ends of the fracture. Non-unions, delayed unions, or acute infections were entirely absent.
Fig. 1.
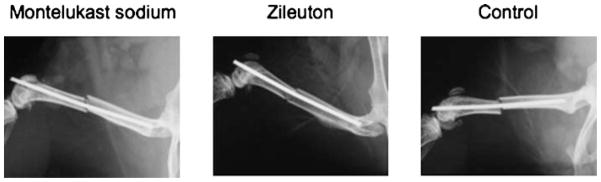
Representative X-rays, day 0. Animals underwent surgical stabilization and fracture of the right femur. Radiographic analysis was conducted on all animals at days 0, 7, 10, 14, and 21.
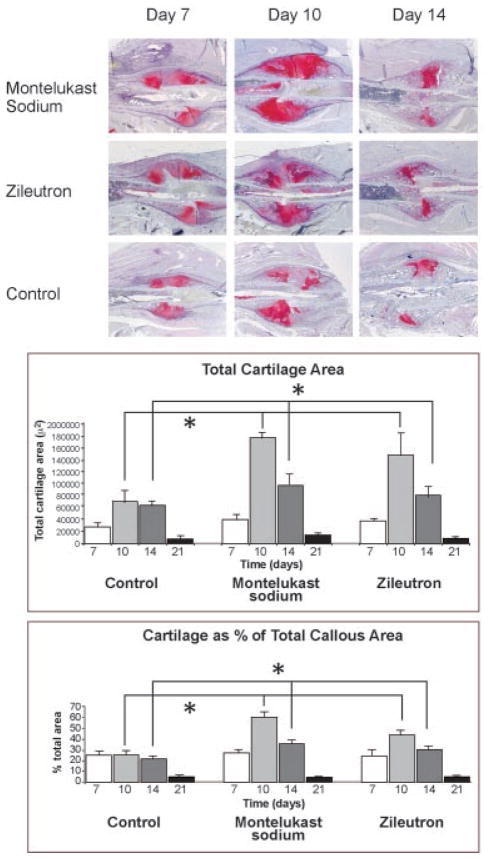
Animals in groups of n = 4 for each treatment group at each time point underwent hisotologic sectioning. Representative sections are shown in Figure 2 for days 7, 10, and 14 as the differences in the treated and control groups were most readily identifiable at early time points. As early as day 7, changes were readily visible between the two treatment groups versus control. Specifically, animals in both the montelukast sodium and the zileuton groups exhibited dramatically larger callous size with markedly increased amounts of unmineralized chondroid matrix at both days 7 and 10. This effect was restricted to the chondroid phase, and by day 14 the endochondral process had progressed in all groups. In treatment groups, the larger initial callous resulted in a net increase of bone relative to control. Quantification of total callous size, amount of chondroid, and percent chondroid were all significantly greater in both the montelukast sodium group and the zileuton group when compared to control, although changes were not significant when treatment with montelukast sodium was compared with zileuton as shown in Figure 2.
Fig. 2.
Treatment with either montelukast sodium or zileuton enhances early stages of fracture repair. Animals were treated with either montelukast sodium (1.5 mg/kg/day), zileuton (45 mg/kg/day), or control. Animals were sacrificed at days 7, 10, and 14. Histologic specimens were stained with Safranin-O to detect cartilage. Histology demonstrates that in treatments groups, enhanced chondroid formation was present as early as day 7 and was sustained to day 14, indicating rapid MSC differentiation and sustained chondrocyte proliferation. Histomorphometry data demonstrate enhanced cartilage formation in both treatment groups when compared with controls. Both total cartilage area and cartilage area as a percent of total callous area are enhanced (photographed at 40×).
At days 7 and 10 new bone formation was quantified using histomorphometry and was found to be significantly greater in the two treatment groups when compared with controls. Given the inherent limitations of histomorphometry and the difficulty in accurately separating new bone formation from underlying cortical bone at later time points, analysis was conducted only at days 7 and 10 when newly laid down bone was most readily discernible. By day 21 (data not shown), animals in all treatment groups had bridged the ends of the fracture and remodeled the callous to woven bone.
Interestingly, this data set reveals that enhanced early chondrogenesis is likely responsible for the larger callous size. The onset of bone formation, however, is not delayed, and new bone formation is clearly identifiable. This suggests that the endochondral process as a whole is enhanced, and that the increased chondroid phase does not occur at the expense of a delay in osteogenesis.
Taken together, these findings suggest that both treatment groups exhibit a similar effect of enhancing chondrogenesis after the initial inflammatory response. Montelukast sodium is a specific CysLT1 receptor antagonist, while zileuton broadly blocks leukotriene synthesis. The similarities in the two treatment groups suggest that the common effect of cysteinyl leukotriene inhibition is responsible for the early changes in callous formation, contributing to early fracture stabilization.
The CysLT1 receptor, and 5-LO, are expressed by chondrocytes
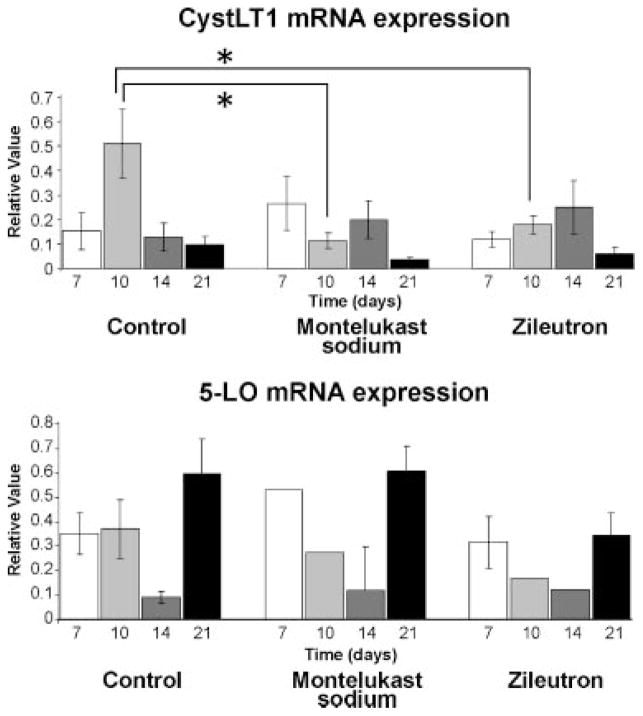
Given the potent effect of inhibiting leukotriene synthesis by treatment with zileuton and the similar effect observed with specific blockade of the CysLT1 receptor by montelukast sodium, we examined levels of the 5-LO enzyme and the CysLT1 receptor during fracture repair. Gene expression analysis indicated that both 5-LO and the CysLT1 receptor were expressed in fracture callous (Fig. 3). In control animals, peak expression of the CysLT1 receptor occurred at day 10, and was significantly higher than either the montelukast sodium or the zileuton treated groups. These gene expression data are consistent with our histology, particularly at day 10, demonstrating an inverse relationship between callous size and CysLT1 expression. Specifically, low levels of the receptor are seen in treatment groups corresponding to large callous size, implicating the cysteinyl leukotrienes as negative regulators of chondrocyte activity.
Fig. 3.
The CysLT1 receptor is expressed in early stages of fracture repair, and coincides with the expression of 5-lipoxygenase. Arachidonic acid conversion to the leukotriene pathway is catalyzed by the 5-LO enzyme, which is highly expressed in fracture repair. The CysLT1 receptor has peak expression at day 10 in controls, coinciding with the peak of chondrocyte proliferation, and is decreased at this time point in both treatment groups.
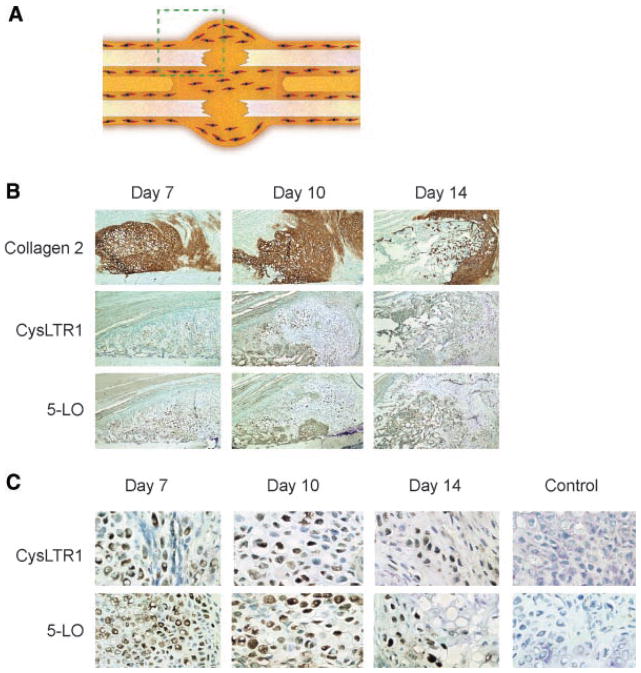
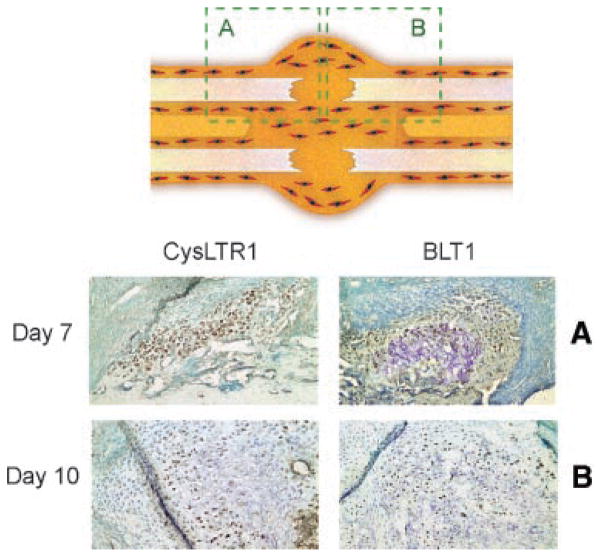
To elucidate the cells of origin which expressed the CysLT1 receptor, immunohistochemical staining on control sections was conducted. Control sections were chosen because peak expression was higher, and this also avoided potential regulatory effects of receptor expression in the presence of treatment drugs. Staining at day 7 was sparse, with clear staining at day 10 showing expression in pre-hypertrophic chondrocytes (Fig. 4). Interestingly, neither immature chondrocytes nor hypertrophic chondrocytes expressed the CysLT1 receptor in stained sections.
Fig. 4.
The CysLT1 receptor and 5-lipoxygenase are expressed by chondrocytes early in the fracture repair process. Histologic sections were analyzed and show evidence of CysLT1 and 5-LO expression in chondrocytes. A: The schematic diagram shows the region of fracture callous demonstrated. B: Abundant collagen 2 staining confirms the presence of chondroid matrix at days 7, 10, and 14 (photographed at 100×). Expression of CysLT1 and 5-LO are present in this chondroid tissue. C: At higher magnification expression of CysLT1 receptor is clearly seen in a restricted chondrocyte population, with no staining infibroblast appearing cells. A similar expression pattern in seen with 5-LO. Negative controls confirm specificity of staining (photographed at 200×).
Cysteinyl leukotrienes are negative regulators of chondrocyte proliferation
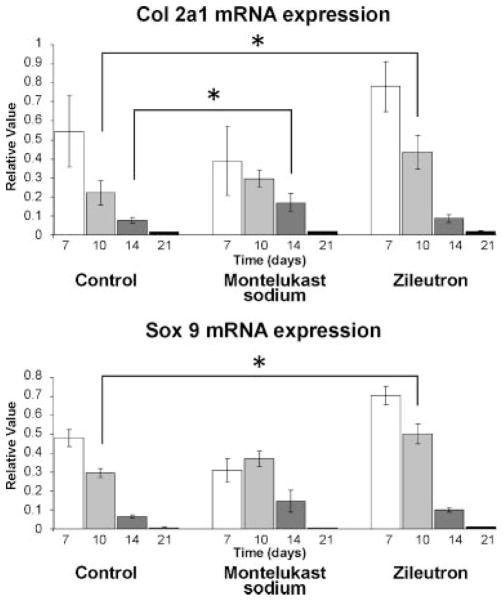
Given the novel finding of CysLT1 expression in fracture callous and restricted expression in pre-hypertrophic chondrocytes, we sought to identify the activity of study drugs during the endochondral process. Fracture callous was harvested from animals in the three treatment groups after sacrifice at days 7, 10, 14, and 21 with n = 4 animals per treatment group at each time point for expression of chondrogenic markers by qPCR (Fig. 5). Sox9 and Col 2a indicate a commitment to the chondrocyte phenotype. mRNA expression of these genes in controls exhibits peak levels at day 7, when proliferating chondrocyte committed populations begin to expand. In the zileuton group, expression levels of both genes are increased significantly at day 10. This is not seen in the montelukast sodium group; however, the Col 2a expression is elevated at day 14 and Sox9 levels peak later than controls at day 10. This suggests that the chondroid phase persists in the montelukast sodium group consistent with our histology (Fig. 2).
Fig. 5.
Cysteinyl leukotrienes are negative regulators of chondrocyte proliferation. Col 2a1 and SOX9 mRNA expression analyzed by qPCR of fracture callous tissue at days 7, 10, 14, and 21. Peak levels of chondrocyte markers seen in all groups at day 7. These markers showed prolonged expression in treatment animals compared with controls. In control animals, expression drops markedly after day 10. In the montelukast sodium group, Col 2a1 expression is elevated at day 14 (P < 0.05) and in the zileuton group both SOX9 and Col 2a1 are elevated at day 10 (P < 0.05).
Blocking the effect of leukotrienes leads to enhanced chondrocyte hypertrophy and early bone formation evidenced by changes in gene expression
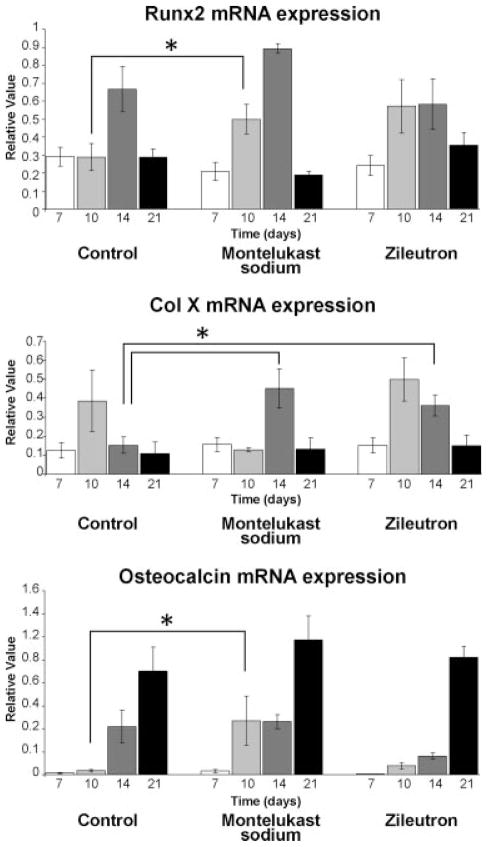
Given the sequential nature of endochondral ossification and the early and sustained chondrogenesis seen in the treatment groups, we undertook to examine if transition to osteogenesis was similarly affected. As part of our analysis, Runx2 and Col 10a1 gene expression were also analyzed as markers of hypertrophic chondrocyte formation. Col 1 and osteocalcin levels were analyzed as markers of bone formation. These data are summarized in Figure 6.
Fig. 6.
Blocking the effect of leukotrienes leads to enhanced chondrocyte hypertrophy and early bone formation. Runx2 and Col 10a1 gene expression were analyzed as markers of hypertrophic chondrocyte formation. In the montelukast sodium group, Runx2 levels are higher at day 10 (P < 0.05) and Col 10a1 levels are higher at day 14 (P < 0.01). In the zileuton group, Runx2 expression levels were increased at day 10 and approached significance (P = 0.06), while Col 10a1 expression was significantly higher (P < 0.01) at day 14. Early osteocalcin levels were elevated in the montelukast sodium group (P < 0.05) at day 10. In the zileuton group levels were also elevated at day 10, approaching significance (P = 0.07).
In the montelukast sodium group, Runx2 levels are higher at day 10 (P <0.05) and Col 10a1 levels are higher at day 14 (P <0.01). Similarly, in the zileuton group Runx2 expression levels were also increased at day 10 and approached significance (P = 0.06), while Col 10a1 expression was significantly higher (P <0.01) at day 14. The finding that Col 10a1 is elevated at day 14 in treatment groups likely reflects higher numbers of mature chondrocytes, a result of the sustained chondrocyte proliferation seen at earlier time points.
Interestingly, Col 1 levels are decreased at day 7 in both the treatment groups compared with controls, which suggests that some of the increase in chondrogenesis might occur at the expense of osteogenesis (data not shown). By day 10, though, no change is seen and the overall expression pattern and peaks of Col 1 is the same in all treatment groups at subsequent points. However, the increased expression of Runx2 in both montelukast sodium and zileuton treatment groups at day 10 suggests an early transition to hypertrophic chondrocytes, suggesting that the callous is maturing more rapidly in the montelukast sodium and zileuton groups. Our findings of significantly elevated osteocalcin expression at day 10 in the montelukast sodium group, and osteocalcin levels approaching significance in the zileuton group (P = 0.07) compared with controls, would also indicate that transition to osteogenesis occurs earlier in both treatment groups.
Differences between montelukast sodium treatment and zileuton treatment suggest a differential role for the BLT1 receptor
The overall effect of treatment is similar in animals treated with either montelukast sodium or zileuton, suggesting that since both drugs interfere with cysteinyl leukotriene signaling that the CysLT1 receptor plays a critical mechanistic role. In addition to its effect on cysteinyl leukotrienes, zileuton blocks formation of the non-cysteinyl leukotrienes, most relevantly LTB4. To determine if LTB4 played a potential role in regulating this process, we performed immunohistochemistry to localize the BLT1 receptor, the main target for LTB4, in fracture callous. In Figure 7, we demonstrate strong chondrocyte-specific BLT1 expression. Furthermore, histone gene expression by qRT-PCR is higher in the zileuton treated group (data not shown), suggesting a potential role for BLT1 in regulating cell proliferation.
Fig. 7.
Chondrocytes in fracture callous express LTB4. Immunohistochemical analysis of fracture callous taken from control animals at day 10 shows mature chondroid tissue and early osseous response. Staining for the BLT1 receptor is restricted to chondrocytes, although the expression pattern and timing differs from CysLT1 expression, and changes in gene expression between the two treatment groups suggest a differential role for the BLT1 receptor. day 7 staining is primarily cytoplasmic, and by day 10 staining is both cytoplasmic and nuclear.
Discussion
Fracture repair initiates from a strong inflammatory response, progressing in step wise fashion through a process of endochondral ossification. Mediators which influence leukotriene synthesis and activity may have potent effects on this inflammatory process. We undertook to study these effects in an in vivo model of fracture repair to determine the outcome of treatment with montelukast sodium and zileuton on events leading to skeletal regeneration. We are able to demonstrate that both zileuton, a 5-LO inhibitor which broadly blocks leukotriene synthesis, and montelukast sodium, a specific CysLT1 receptor antagonist, can be used to promote fracture healing, and that treatment with these drugs results in an enhanced chondroid phase and faster transition to bone formation. Furthermore, our data support a mechanism for this effect which results in a larger callous size seen at early time points and a faster transition to bone formation. Secondly, we find that CysLT1 receptor is expressed in pre-hypertrophic chondrocytes, suggesting a target cell which mediates the observed changes in the endochondral process. Pharmacologic blockade of this receptor by montelukast sodium, or alternatively by inhibition of cysteinyl leukotriene production, results in enhanced fracture healing. These findings, taken collectively, highlight the role of the CysLT1 receptor as a direct and potent negative regulator of chondrocyte proliferation, and may affect chondrocyte matrix production, providing new and novel insights into the direct regulatory effects of the cysteinyl leukotrienes on other biologic processes which may involve chondroid proliferation and differentiation.
The medications tested in this study are currently FDA approved for use in reactive airway disease. Montelukast sodium, whose trade name is Singulair, is approved for use in adults and children to control symptoms of asthma and for relief of symptoms of indoor and outdoor allergies. Zileuton, whose trade name is Zyflo, is approved for the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older. It has long been known that the eicosanoids, including both cysteinyl and non-cysteinyl leukotrienes, exert a potent effect on the airways and contribute to eosinophil and neutrophil migration. We have in these studies identified a novel role for the CysLT1 receptor in regulating either the proliferation and/or the formation of chondrocytes from MSC cells recruited to fracture repair sites.
Other investigators have previously reported a larger callous size with enhanced mechanical strength in a fracture study using 5-LO knockout mice (Manigrasso and O’Connor, 2006). It stands to reason that the larger callous size seen in this model originated with an enhanced chondroid phase. The initial phase of endochondral ossification is formation of a chondroid anlage; a large callous would thus require enhanced chondroid matrix formation. This is consistent with our findings in which inhibition of leukotrienes either by inhibition of formation (zileuton) or blockade of the CysLT1 receptor (montelukast sodium) resulted in enhanced cartilage tissue formation in early stages of the repair. The similar results of treatment with montelukast sodium and zileuton would further suggest that the driving mechanism behind this effect is primarily a function of the cysteinyl leukotrienes, since blockade of the CysLT1 receptor had comparable effect to broad inhibition of leukotriene synthesis. Montelukast sodium, a CysLT1 receptor antagonist, should only affect activity of cysteinyl leukotrienes, thus highlighting their role as negative regulators of chondrocyte activity.
Given the potent effect of inhibiting leukotriene synthesis by treatment with zileuton and the similar effect seen with specific blockade of the CysLT1 receptor by montelukast sodium, we performed immunohistochemistry to elucidate which cells of origin would be affected by treatment. Gene expression analysis indicated that both 5-LO and the CysLT1 receptor were expressed in fracture callous, and peak expression generally coincided with the peak of the chondroid phase. In control animals, peak expression of the CysLT1 receptor was dramatically higher in control than in either the montelukast sodium or the zileuton treated groups. This significant decrease in receptor expression following either inhibition of cysteinyl leukotriene formation (zileuton) or receptor blockade (montelukast sodium) may suggest a potential feedback mechanism in which transcription of the receptor if down regulated in response to decreased intracellular signaling. It is noteworthy that we find robust receptor levels by immunohistochemistry in pre-hypertrophic chondrocytes but not in other cell populations, specifically in immature chondroid tissue or bone tissue. This may be an issue of detection levels. However, our gene expression studies suggest an early response to the drugs in chondroid tissue as well as in transition to bone formation, with elevated markers of hypertrophic chondrocytes seen in the treatment groups.
We initiated these studies in part based on previous data which demonstrated that the LTB4 receptor is expressed on osteoblasts, and that osteoblastic activity in vitro is significantly decreased in the presence of LTB4, while the cysteinyl leukotrienes do not affect osteoblastic activity (Traianedes et al., 1998). Thus we had anticipated that zileuton, which blocks LTB4 production, would have a greater effect than montelukast sodium, which only affects cysteinyl leukotriene activity, and we further anticipated that the observed changes should occur after the onset of osteogenesis. Our findings of striking effects on early chondrocyte activity occur at a much earlier stage in fracture repair, and our observed finding that both drugs stimulated the chondrogenic phase and resulted in earlier transition to bone formation suggests that the CysLT1 receptor plays a mechanistic role in this process.
Recent evidence has expanded the potential effects of the leukotrienes in a variety of physiologic processes including arthritis. The cysteinyl leukotrienes in particular have been implicated as negative regulators of MSC differentiation (Akino et al., 2006). These findings are particularly interesting given the critical role of MSC differentiation to chondrocytes and osteoblasts required for successful fracture repair. The effects we see in treated animals are quantifiable at our earliest time point. For changes in enhancing chondrogenesis to be evident at day seven suggests an early and potent effect in promoting MSC differentiation, which is a consequence of inhibiting the effects of cysteinyl leukotrienes by either montelukast sodium or zileuton. Thus our findings during the fracture repair process are consistent with the in vitro data reported by Akino et al. (2006). In the treatment animals, our data indicates an early and sustained increase in chondrocyte formation observed at both 7 and 10 days, suggesting part of the effect of these drugs may promote MSC differentiation to chondroid and osteoblast lineage cells that is further supported by our gene expression studies.
While the overall effect of treatment with either montelukast sodium or zileuton is similar and our findings implicate the CysLT1 receptor as a negative regulator of chondrocyte activity, subtle differences in the treatment groups do exist and leave open the question of a complementary role of LTB4 during endochondral ossification. As our findings have identified novel expression of the CysLT1 receptor in chondrocytes, the focus of our studies has been to characterize the effect of the receptor on early stages of fracture healing. Zileuton broadly blocks synthesis of both cysteinyl and non-cysteinyl leukotrienes while montelukast sodium, as a CysLT1 receptor antagonist, only affects cysteinyl leukotriene activity. However, our data does show that both Sox9 and Col 2A1 levels were generally higher in the zileuton treated group (Fig. 3). Histone gene expression is similarly elevated in the zileuton treated group at early time points and not in the montelukast sodium group, indicating a potential role of LTB4 as a negative regulator of chondrocyte proliferation. Lastly, our immunohistochemistry shows that the BLT1 receptor is also expressed in chondrocytes, suggesting a potential secondary role for LTB4 which deserves further investigation. Our findings are summarized schematically in Figure 8.
Fig. 8.
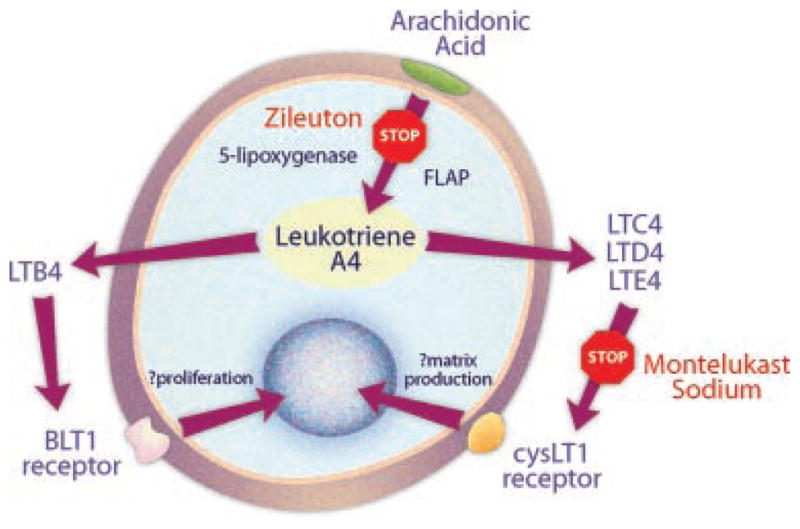
The CysLT1 receptor acts as a negative regulator of chondrocyte activity. Blockade of 5-lipoxygenase by zileuton results in broad inhibition of leukotriene synthesis. The effect of montelukast sodium is restricted to the cysteinyl leukotrienes. The similar effect of treatment with either montelukast sodium or zileuton highlights the mechanistic role of the CysLT1 receptor as a negative regulator of chondrocyte activity. The BLT1 receptor is unaffected by montelukast sodium, and differences between the treatment groups suggests a complementary role for BLT1.
Although these findings may have important clinical implications, translating these findings into human use will require further study. While the dose of montelukast sodium used in this study is higher than the human dose, zileuton dosing was kept at levels consistent with human dosing to limit potential hepatotoxicity, a known side effect of zileuton. Thus our conclusions that montelukast sodium and zileuton have similar effect in this study may or may not be translatable to current FDA dosing guidelines in humans.
Our findings that these drugs promote fracture healing are encouraging, and highlight the role of the CysLT1 receptor as a negative regulator of chondrocyte activity. While broadly applicable to many fractures, those clinical situations in which endochondral ossification is disordered, such as diabetic fractures or atrophic non-unions, may stand to benefit from an approach which enhances early chondrocyte activity and faster transition to bone. The current use of these medications for the treatment of asthma has shown them to be safe, and their FDA status should simplify efforts to investigate their usefulness in humans as part of a larger translational effort aimed at enhancing fracture healing by inhibition of the cysteinyl leukotriene pathway during early stages of fracture repair.
Acknowledgments
Contract grant sponsor: Investigator-Initiated Studies Program of Merck and Co., Inc..
This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck and Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck and Co., Inc.
Literature Cited
- Anonymous. International nomenclature of constitutional diseases of bone. Revision, May, 1977. Am J Roentgenol. 1978;131:352–354. doi: 10.2214/ajr.131.2.352. [DOI] [PubMed] [Google Scholar]
- Akino K, Mineda T, Mori N, Hirano A, Imaizumi T, Akita S. Attenuation of cysteinyl leukotrienes induces human mesenchymal stem cell differentiation. Wound Repair Regen. 2006;14:343–349. doi: 10.1111/j.1743-6109.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Beck A, Krischak G, Sorg T, Augat P, Farker K, Merkel U, Kinzl L, Claes L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg. 2003;123:327–332. doi: 10.1007/s00402-003-0537-5. [DOI] [PubMed] [Google Scholar]
- Bergenstock M, Min W, Simon AM, Sabatino C, O’Connor JP. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–723. doi: 10.1097/01.bot.0000184144.98071.5d. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- Denzlinger C, Rapp S, Hagmann W, Keppler D. Leukotrienes as mediators in tissue trauma. Science. 1985;230:330–332. doi: 10.1126/science.4048937. [DOI] [PubMed] [Google Scholar]
- Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–S6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- Engesaeter LB, Sudmann B, Sudmann E. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop Scand. 1992;63:330–333. doi: 10.3109/17453679209154794. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20:1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–125. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. A mechanical study of osteotomies in rats. Acta Orthop Scand. 1992;63:607–611. doi: 10.1080/17453679209169718. [DOI] [PubMed] [Google Scholar]
- Keller J, Bayer-Kristensen I, Bak B, Bunger C, Kjaersgaard-Andersen P, Lucht U, Melsen F. Indomethacin and bone remodeling. Effect on cortical bone after osteotomy in rabbits. Acta Orthop Scand. 1989;60:119–121. doi: 10.3109/17453678909150109. [DOI] [PubMed] [Google Scholar]
- Manigrasso MB, O’Connor JP. Accelerating fracture healing by manipulating arachidonic acid metabolism. ORS. 2006;31:0070. [Google Scholar]
- Marturano JE, Cleveland BC, Byrne MA, O’Connell SL, Wixted JJ, Billiar KL. An improved murine femur fracture device for bone healing studies. J Biomech. 2008;41:1222–1228. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Murnaghan M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: An inhibition of angiogenesis? J Bone Joint Surg Am. 2006;88:140–147. doi: 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89:500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- Sudmann E, Dregelid E, Bessesen A, Morland J. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest. 1979;9:333–339. doi: 10.1111/j.1365-2362.1979.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Traianedes K, Dallas MR, Garrett IR, Mundy GR, Bonewald LF. 5-Lipoxygenase metabolites inhibit bone formation in vitro. Endocrinology. 1998;139:3178–3184. doi: 10.1210/endo.139.7.6115. [DOI] [PubMed] [Google Scholar]