Abstract
Connexins (Cxs) form hemichannels and gap junction channels. Each gap junction channel is composed of two hemichannels, also termed connexons, one from each of the coupled cells. Hemichannels are hexamers assembled in the ER, the Golgi, or a post Golgi compartment. They are transported to the cell surface in vesicles and inserted by vesicle fusion, and then dock with a hemichannel in an apposed membrane to form a cell–cell channel. It was thought that hemichannels should remain closed until docking with another hemichannel because of the leak they would provide if their permeability and conductance were like those of their corresponding cell–cell channels. Now it is clear that hemichannels formed by a number of different connexins can open in at least some cells with a finite if low probability, and that their opening can be modulated under various physiological and pathological conditions. Hemichannels open in different kinds of cells in culture with conductance and permeability properties predictable from those of the corresponding gap junction channels. Cx43 hemichannels are preferentially closed in cultured cells under resting conditions, but their open probability can be increased by the application of positive voltages and by changes in protein phosphorylation and/or redox state. In addition, increased activity can result from the recruitment of hemichannels to the plasma membrane as seen in metabolically inhibited astrocytes. Mutations of connexins that increase hemichannel open probability may explain cellular degeneration in several hereditary diseases. Taken together, the data indicate that hemichannels are gated by multiple mechanisms that independently or cooperatively affect their open probability under physiological as well as pathological conditions.
Keywords: Connexon, Redox potential, Protein phosphorylation
1. Introduction
A connexin based gap junction channel is made of two hemichannels or connexons, one in each of the apposed membranes. Each hemichannel is composed of six protein subunits termed connexins. Mammalian connexins are encoded by a gene family of close to 20 members. We and others have published reviews on connexin hemichannels which give the primary references [1–7]. Recently, three homologs of the protostome gap junction forming proteins, pannexins or innexins, were found in the mouse and human genomes; two are expressed in the central nervous system and they will form gap junctions between Xenopus oocytes, but their function is not yet determined [8]. Here we summarize data on mechanisms of hemichannel gating. We do not address the possible functions of hemichannels that are not related to gating or insertion into the surface membrane [6].
Diverse actions are attributed to hemichannel opening (Table 1) including uptake and release of small molecules and passage of current (Table 2). These actions are inhibited by hemichannel and gap junction blockers (Table 1) and are absent or greatly reduced in cells lines that do not express connexins (e.g., HeLa and C6 cells) or cells from connexin knock out animals (e.g., astrocytes from Cx43 knockout mice). These actions are induced in “communication incompetent” cell lines by transfection with connexins [3]. Although numerous studies have focused on Cx43 hemichannels, other connexin types also form hemichannels with a non-zero open probability (Table 2). Hemichannels of commonly studied connexins have been detected on the cell surface by means of immunological, biochemical and morphological approaches as well as gene tagging with fluorescent proteins or a tetracysteine motif (Table 2). In HeLa cells transfected with Cx43-EGFP the rate of dye uptake is directly correlated with the expression level of Cx43-EGFP [9]. Furthermore, only cells expressing Cx43 or Cx43-EGFP show single channel events with conductance of about 220 pS, which is twice that of single cell–cell channels (Table 3). The ratio of hemichannel to cell–cell channel conductance follows from the series arrangement of two hemichannels in the cell–cell channel. (A deviation would be expected in the simplest model, because hemichannels and cell–cell channels would have the same access resistances at the two ends; thus, the unitary resistance is the sum of the channel lumen resistance and the two access resistances. The access resistances may be negligible with respect to the channel lumen resistances, or the access resistances differ in hemichannels and cell–cell channels.) The unitary conductance of hemichannels composed of several other connexin types is also about twice that of the cell–cell channels (Table 3).
Table 1.
Involvement of hemichannels in diverse actions and blocker(s) of hemichannels that prevent these actions
| Connexin | Function | Cell type | Blockers | |
|---|---|---|---|---|
| Cx43 | ATP release | Astrocytes and C6 glioma | FFA and Gd3+ | [17] |
| Cx43 | ATP release | Rat brain endothelial cell line and HeLa cells | FFA, NPPB, niflumic acid, α-GA connexin mimetic peptide, Gd3+ and La3+ | [67] |
| Cx43 | Glutamate release | Astrocytes | carbenoxolone, octanol, heptanol, FFA, α-GA Ca2+, Ba2+, Sr2+, Mg2+ and La3+ | [40] |
| Cx43 | NAD+ release | 3T3 fibroblast | β-GA, La3+, octanol and oleamide | [70] |
| Cx43 | Calcium waves propagation | HOBIT | α-GA | [68] |
| Calcium waves propagation | Radial glia | carbenoxolone | [69] | |
| Cx43 | Cell volume regulation | N2A | β-GA and oleamide | [39] |
| Cx43 | Transduction of survival signals | MLO-Y4, HeLa cells | α-GA, carbenoxolone and oleamide | [71] |
| Cx43 | Metabolic stress | HEK293 | halothane and La3+ | [20] |
| Cx43 | Metabolic stress | Myocytes | La3+, halothane and heptanol | [21,72] |
| Cx43 | Cell death under metabolic stress | Renal tubule cells | Gd3+ | [23] |
| Cx43 | Cell death under metabolic stress | Astrocytes | β-GA and octanol | [22] |
| Cx26 | Increase Shigella invasion | Caco-2/TC7 and HeLa cells | α-GA and carbenoxolone | [73] |
| Cx26 | Ephaptic inhibition retina | Retinal horizontal cells | carbenoxolone | [74,75] |
While all membrane permeant blockers affect both hemichannels and gap junction channels, La3+ and Gd3+, which are membrane impermeant, preferentially block hemichannels. C6: glial cell line from a rat astrocytoma; HOBIT: transformed human osteoblast-like cells; 3T3: mouse fibroblast cell line; N2A: mouse neuroblastoma cell line; MLO-Y4: osteocyte-like cell line; HeLa: human cervix epithelial adenocarcinoma cell line; HEK293: human embryo renal cortical cell line; FFA: flufenamic acid; α-GA: 18α-glycyrrhetinic acid; β-GA: 18β-glycyrrhetinic acid; NPPB: 5-nitro-2-(3-phenylpropylamino) benzoic acid; Caco-2/ TC7: human colorectal adenocarcinoma cell lines.
Table 2.
Summary of experimental techniques used to identify hemichannels in different cell types
| Connexins hemichannels |
Immunolabeling | Biotinylation | Tetracysteine tag |
Freeze-fracture | Released molecule (cell) |
Uptake assay (cell) |
Macroscopic current recording |
Single channel recording |
|---|---|---|---|---|---|---|---|---|
| Cx26 | Horizontal cells [74,75] | Ascorbic acid (liposomes) [26] | Oocytesa [25] | |||||
| ATP (HeLa) [73] | Ly (HeLa) [73] | |||||||
| Cx30 | ATP (HeLa) [54] | HeLa [54] | HeLa [76] | |||||
| Cx32 | Oocytesa [53] | ATP (C6) [90] | Sucrose, LY (liposomes) [93] | Oocytesa [49] | Planar lipid bilayer [93] | |||
| Cx35 | Oocytesa [16,64] | Oocytesa [36] | ||||||
| Cx38 | Spermidine (oocytesa) [91] | Oocytesa [18,91,95] | ||||||
| Cx43 | Cardiomyocytes [87] | MLO-Y4 [71] | HeLa [89] | NAD+(fibroblasts) [70] | EtdBr, LY (astrocytes) [9,22] | Myocytes [20,21] | HeLa [9] | |
| Astrocytes [88] | NRK [12] | LY (Novikoff) [56,92] | Carboxyfluorescein (Novikoff) [56] | |||||
| ATP (astrocytes) [17] | Coumarin based tracer (NRK) [55] | |||||||
| Glutamate (astrocytes) [40] | Fura-2 (HeLa) [55] Calcein, LY, PI (myocytes) [20,21,72] | |||||||
| Mn2+, IP3, LY (HOBIT) [68] Sucrose, LY (liposomes) [61,62] | ||||||||
| Cx45 | LY, PI (HeLa) [13] | HeLa [13] Oocytesa [33, 98] HeLa [76] | ||||||
| Cx46 | LY, sugars, PEG (oocytesa) [10,24] | Oocytesa [24,78,96] | ||||||
| Cx48.5 | Oocytesa [97] | |||||||
| Cx50 | Oocytesa [34] | Oocytesa [34] | Oocytesa [98] HeLa [76] | |||||
| Cx52.6 | N2A [99] | |||||||
| Cx56 | Oocytesa [96] | Oocytesa [77] | ||||||
| ND | Carboxyfluorescein (horizontal cells) [15] | Horizontal cells [31] | ||||||
| Carboxyfluorescein (epithelial cells) [94] | Epithelial cells [94] | |||||||
| Carboxyfluorescein (proximal tubule) [23] |
EtdBr: ethidium bromide.
PI: propidium iodide.
LY: Lucifer yellow.
PEG: polyethylene glycol.
ND: not determined.
Xenopus laevis.
Table 3.
Comparison of unitary conductance of hemichannels and the corresponding cell–cell channels for different connexins
| Type of connexin |
Hemichannel conductance (pS) |
Reference | Gap junction channel conductance (pS) |
Reference |
|---|---|---|---|---|
| Cx30 | 283 | [76] | 146 | [79] |
| Cx32 | 17–18 | [38,48] | 50–53 | [80] |
| Cx35 | 48 | [36] | ||
| Cx36 | ~10–15 | [81,82] | ||
| Cx38 | 250–320 | [36] | ||
| Cx43 | 223 | [9] | 110 | [83] |
| Cx43EGFP | 220 | [9] | 110 | [83] |
| Cx45 | 62 | [13] | 32 | [84] |
| Cx45.6 | 300 | [77] | 202 | [85] |
| Cx46 | 250–300 | [76,78] | 140 | [46] |
| Cx50 | 352 | [76] | 220 | [86] |
| Cx56 | 300 | [77] |
A recent study using excised patches of oocyte membrane containing Cx46 hemichannels demonstrated their accessibility to several uncharged molecules (molecular weights of 180–666 Da and 5.8 to 12 Å in diameter) [10]. The added molecules decreased single-channel conductance and open probability. The lifetimes of the apparent occupancy states were much greater than the transit times calculated from simple diffusion. An earlier report using the same approach of measuring single channel conductance in the presence of uncharged molecules of different sizes indicated that a particular Cx32 mutation associated with X-linked Charcot-Marie-Tooth disease decreased the diameter of the cell–cell channel [11]. Rigorous analyses of permeation including size and charge selectivity are still to be obtained. This information is likely to be necessary for full comprehension of the functional roles of hemichannels.
In most hemichannels so far studied, opening is enhanced at positive membrane potentials and low extracellular [Ca2+] [1–3]. These conditions allowed a convincing demonstration of functional hemichannels on the surface of HeLa cell transfectants. They also allowed the characterization of voltage-dependent gating and pharmacological properties of hemichannels in different cell types. However, they are not the most likely mechanisms that control hemichannel activity under physiological conditions (see below).
1.1. Might openings of Cx43 hemichannels be much more frequent at the resting potential than so far observed?
Unitary events measured under resting conditions are rarely recorded in HeLa Cx43 transfectants [9], which is consistent with the prediction that many open hemichannels would kill cells. However, dye uptake suggests that there are openings, and brief openings might be missed in whole cell patch recording. Given the uncertainty as to the actual rate of dye permeation, there is no clear discrepancy between dye uptake and the upper limit of channel opening inferred from the electrical measurements [9]. Moreover, dialysis of the cells against the pipette solution might reduce hemichannel activity.
Under conditions of resting membrane potential and millimolar extracellular [Ca2+], the rate of ethidium bromide uptake is very low but closely proportional to the total amount of Cx43-EGFP expressed (r=0.8) [9]. The correlation might be limited in part by variability in the relative sizes of connexin pools in other cell domains, such as endoplasmic reticulum, cytoplasmic transport vesicles going to and from the surface membrane, and gap junctions, as well as by local differences in the state of surface hemichannels. Further studies of cells cultured at low density to reduce the incidence of gap junctions might reveal an r value closer to one. Also, the incidence of gap junctions in confluent cultures might be reduced by treatment with casein kinase I inhibitors, because the phosphorylation of Cx43 by casein kinase I enhances the recruitment of hemichannels to gap junctions [12]. Under control conditions, the opening of most hemichannels studied is infrequent, and the proportionality of the hemichannel open probability to the rate of dye uptake and level of hemichannel expression is still not established. However, Cx45 hemichannels have a relatively large open probability at the resting potential, and dye uptake (of Lucifer yellow) is proportional to hemichannel conductance [13]. The quantitation of the surface expression of hemichannels (e.g., Western blot analyses of biotinylated cell surface proteins) and application of agents to alter their open probability should extend these findings to other connexins. We find that about 10% of the total Cx43 expressed by cortical astrocytes can be biotinylated [14] and that the application of agents to reduce the intracellular redox potential greatly enhances hemichannel opening under normal conditions of culture (see below).
Spontaneous or induced hemichannel opening has been blocked with diverse agents (octanol, heptanol, halothane, carbenoxolone, 18-α- and 18-β-glycyrrhetinic acid (α-, β- GA), oleamide, flufenamic acid (FFA), 5-nitro-2-(3-phenyl-propylamino)benzoic acid (NPPB), La3+ and Gd3+) (Table 1). Conversely, macroscopic currents ascribed to hemichannels composed of several connexin types are increased by quinine or quinidine [15–18], suggesting that their open probability is increased. It is not clear whether these agents increase open probability uniformly for all hemichannels, increase the open probability of a subpopulation of hemichannels that already have a small open probability, or transfer hemichannels from an inactive subpopulation into a subpopulation with a non-zero open probability.
Cloroquines including quinine have long been used as anti-malarial drugs. One can conclude that the degree of hemichannel opening caused by therapeutic quinine concentrations is not very deleterious. Treating astrocytes with quinine induces the opening of Cx43 hemichannels but does not cause an obvious reduction in the number of astrocytes in culture [17], indicating that normal cells can tolerate some degree of enhanced hemichannel opening. The transient opening of hemichannels in normal cells may even be beneficial, for instance by enhancing paracrine intercellular communication. During cell proliferation most cells lose their gap junction channels [19]; although the effect on hemichannels has not been determined and surface expression is almost certainly reduced, paracrine intercellular communication mediated by hemichannels might compensate for the loss of junctional communication.
Hemichannel opening is induced in cells under conditions that mimic ischemic episodes [20–22]. These conditions are stressful, and enhanced hemichannel activity can accelerate cell death [22,23].
Overall, the data demonstrate that there are mechanisms to either increase or decrease hemichannel opening.
1.2. Do hemichannels formed of different connexin types open under physiological conditions?
The first indication of hemichannel opening was obtained from the expression of Cx46 in Xenopus oocytes, which resulted in cell swelling and death unless the cells were bathed in high Ca2+ solution [24]; these observations confirmed the idea that hemichannel opening is deleterious. However, oocytes expressing human Cx26 were recently shown to exhibit large membrane conductances with little voltage dependence, suggesting that these hemichannels have a relatively high open probability in basal conditions and that they are less sensitive to block by extracellular [Ca2+] [25]. The level of presumptive hemichannel opening did not promote cell swelling and death, suggesting that their permeability properties are more compatible with cell life than those formed by some other connexins. Since reconstituted Cx26 hemichannels in liposomes are permeable to ascorbic acid [26], protocols designed to study ascorbic acid transport across the plasma membrane of cells expressing Cx26 should dissect the contribution of ascorbic acid transporters and Cx26 hemichannels. The same argument can be extended to other connexins and molecules to which their hemichannels are permeable.
2. Gating mechanisms of hemichannels
Stimuli that cause hemichannels to open or close include change in transmembrane voltage and application of chemical agents to either or both faces of the hemichannel. The nature of some of these stimuli suggests that hemichannels can participate in signal transduction systems.
Like some other ion channels, Cx46 hemichannels exhibit a degree of mechanical sensitivity [27]. Single channel and whole cell electrophysiological studies show that suction on the recording pipette or bathing in hyposmotic solution, which causes swelling, opens the hemichannels at negative potentials and closes them at positive potentials. Hemichannels formed by other connexins may share this property, although the expression of Cx43 and Cx38 does not induce mechanical sensitivity in oocytes [27]. Mechanical stimulation of astrocytes is known to cause brief conductance increases and to promote propidium iodide uptake and ATP release [17,28]. Mechanical stimuli trigger calcium waves in several cell types including astrocytes which express Cx43 [17] and hepatocytes which express Cx26 and/or Cx32 [29]. Calcium waves and ATP release are potentiated by quinine [17], an activator of several hemichannel types. The mechanosensitivity of hemichannels may be important in tissues frequently subjected to mechanical stress and may mediate responses to tissue distortion. Whether a similar mechanism explains the hyperosmolarity-induced opening of Cx43 hemichannels [4] remains unknown. Hyperosmolarity would shrink cells, presumably reducing tension overall, but might cause local increases in tension.
The open probability of most hemichannels studied is greatly reduced at negative membrane potentials or when the extracellular [Ca2+] ([Ca2+]o) is above 1 mM [18,30–37]. The Ca2+ sensitive domain for Cx32 hemichannels appears to be a ring of 12 Asp residues within the external vestibule of the pore [38]. Since physiological [Ca2+]o is between 1 and 2 mM, most hemichannels should have a low open probability, as generally observed. However, opening of Cx43 hemichannels has been demonstrated during cell volume regulation in response to small changes in [Ca2+]o (between 1.6 and 1.8 mM) under isosmotic conditions [39]. Zero [Ca2+]o causes dye uptake and ATP and glutamate release from astrocytes in culture, evidently released through hemichannels [17,40]. Glutamate release is measurable at [Ca2+]o=~0.2 mM, which can occur in the brain during ischemia or seizures [41].
Hemichannels formed by various connexins are sensitive to intracellular acidification [18,13,37,42,43]. The application of low pH solutions to the intracellular face of Cx46 hemichannels rapidly closes them, and they remain closed throughout the application and reopen at normal pH if the application is not too long lasting [42]. The application of acid solution to the extracellular face of an open hemichannel rapidly closes it, but it does not remain closed; rather it opens and closes irregularly. An analysis of closed times with acid applications to either side of the hemichannels indicates that the H+ binding site is on the cytoplasmic side of the pH gate. With acid application to the intracellular side, the pH remains low and the gate remains shut. With acid application to the extracellular side, the pH on the intracellular side of the gate rapidly returns to the cytoplasmic level after closure and the gate reopens. (The opening rate is the same as when pH is rapidly restored on the intracellular side after the application of low pH solutions on that side.) The application of weak, membrane permeable and strong, relatively membrane impermeable acids to HeLa cells expressing Cx43-EGFP indicates that a similar mechanism is operating [37]. Weak, membrane permeant acids prevent hemichannel opening in response to subsequent application of positive voltages. Strong acids have little immediate effect on the latency of first opening in response to positive voltages, but greatly shorten the openings, suggesting that H+ ions do not get to their binding site until the channels open. Similarly, gap junction channels formed by Cx43 with its C-terminal truncated are less sensitive to intracellular acidification than normal Cx43 is (pKa=6.1 and 6.6, respectively [43].
The use of the substituted cysteine accessibility method (SCAM) at the single channel level demonstrated that fixed negative charges in the first extracellular loop domain (E1) strongly influence charge selectivity, conductance, and rectification of hemichannels formed of Cx46 [44]. Moreover, the Vj sensor of Cx32 and other Group 1 connexins includes a segment of the N-terminus [45,46]. The addition of a single negatively charged amino acid residue at several of the first 10 positions reverses the polarity of Vj gating, and channel closure may involve the movement of this region [47]. Analysis utilizing the one-dimensional Poisson– Nernst–Plank model to determine the voltage profile of simple model channels containing fixed charges suggests how local variations in the electric field influence polarity and sensitivity of Vj-gating [48].
Abnormal hemichannel gating may contribute to genetic disease. Some mutations of Cx32 identified in X-linked Charcot-Marie-Tooth disease (CMTX) and Cx46 and −50 identified in congenital cataract prevent the formation of functional hemichannels as well as cell–cell channels, and it is unclear how the two effects contribute to the pathogenesis [49–51]. The loss of hemichannel opening can be associated with failure to reach the surface membrane, which perforce prevents the formation of cell–cell channels. Some disease causing mutations prevent the formation of hemichannels but not cell–cell channels, indicating a requirement for hemichannel function [51]. In the lens, hemichannel opening may facilitate the formation of cell–cell junctions as well as regulate cell volume [52]. Other disease associated mutations in Cx32 [38,53] and Cx30 [54] enhance hemichannel opening with little effect on cell–cell channels, suggesting that, for these mutations and tissues, hemichannel opening is deleterious. Moreover, a CMTX mutation (D178Y) in the external vestibule of the pore abolishes the Ca2+ regulation of Cx32 hemichannels, supporting that this excessive hemichannel opening can be involved in the pathogenesis [38].
2.1. Do covalent modifications affect the gating mechanisms of Cx43 hemichannels?
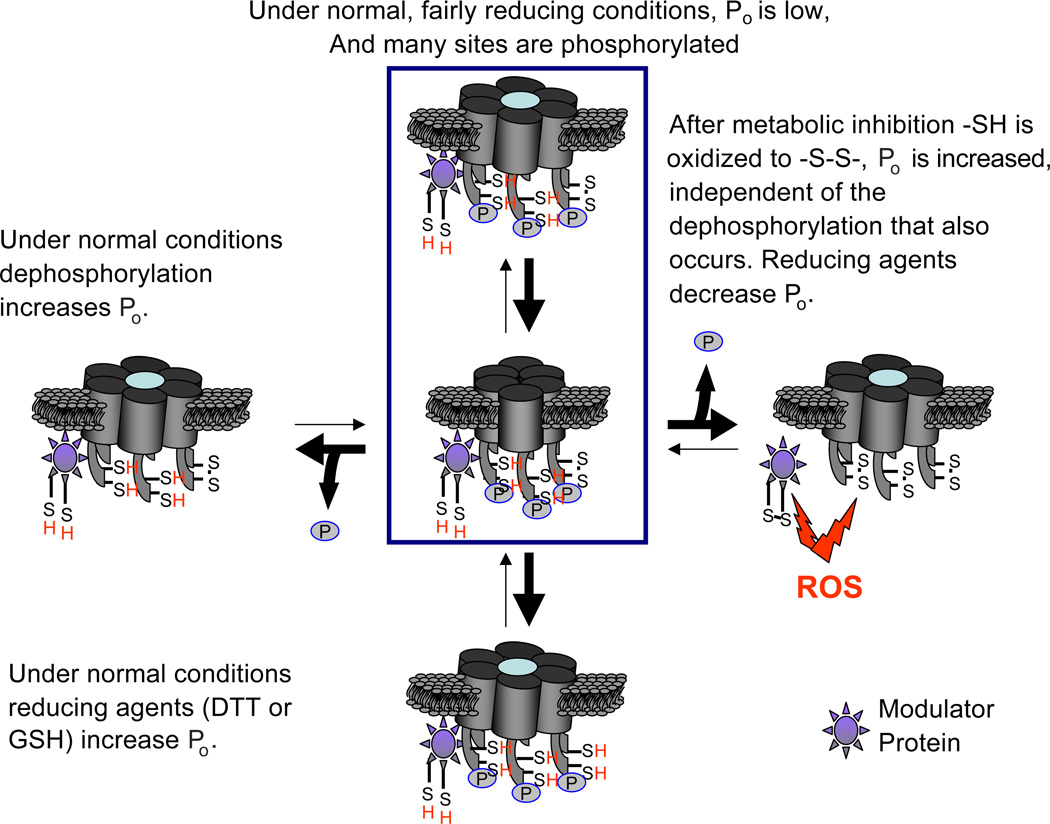
Although Cx26 and Cx36 are not phosphorylated [58,63], several other connexins are phosphoproteins [58], and changes in their phosphorylation state may be involved in regulating their hemichannel open probability. In mammalian cell lines, the opening of Cx43 hemichannels induced by low extracellular [Ca2+] is blocked by the activation of protein kinase C (PKC) [55,56]. Similarly, the opening of Cx46 hemichannels expressed in Xenopus oocytes is greatly reduced by the activation of PKC [57], and the direct phosphorylation of these connexins may control the open probability of their hemichannels. The expression of Cx43 with Ser368, a site of PKC phosphorylation [56], mutated to Ala produces hemichannels that remain preferentially open and cause cell swelling [59]. Cx43 hemichannels are downregulated by MAP kinase, and Cx43 is a substrate [60]; the permeability of Cx43 hemichannels reconstituted into liposomes is reduced by phosphorylation with MAP kinase, and the permeability of Cx43 hemichannels is increased by phosphatase treatment [61]. Similarly, when reconstituted into liposomes, Cx43(S368A), which forms hemichannels insensitive to PKC, induces permeability to small molecules including Lucifer yellow and sucrose [62]. The dephosphorylation at MAP kinase and/or PKC sites of phosphorylation may explain the hemichannel opening induced by hyperosmolarity, which induces the dephosphorylation of Cx43 [4] (Fig. 1).
Fig. 1.
Diagram of hemichannels depicting two putative covalent modifications, disulfide bond formation and phosphorylation, that the regulate open probability ( Po) of Cx43 hemichannels. Under normal conditions, most cysteines are in their reduced form, most potential sites are phosphorylated, and hemichannel Po is low. Dephosphorylation under normal conditions increases Po (left hemichannel). Metabolic inhibition generates reactive oxygen species, e.g., nitric oxide, that oxidize cystines to cysteines and increase Po. Dephosphorylation induced by metabolic inhibition appears not to be necessary for this opening (right hemichannel). Under normal conditions, reducing agents, DTT and GSH, increase Po, perhaps by reducing disulfide bonds formed under basal conditions (lowest hemichannel). Phosphorylation state may account for different effects of reducing agents in normal conditions and under metabolic inhibition. Reducing agents may produce different covalent changes depending on condition. Modulator proteins may participate in these actions. P: phosphate group on Cx43. –S–S–: disulfide bond. ROS: reactive oxygen species.
Perch Cx35 has a consensus site for protein kinase A (PKA) phosphorylation, and its hemichannels are closed by the activation of PKA [64]. This PKA consensus site is absent in skate Cx35, but a single residue mutation at the homologous site to generate a PKA consensus site confers the cAMP sensitivity of the hemichannel opening. The regulation of Cx35 hemichannels by PKA-dependent phosphorylation is species specific owing to this single amino acid difference.
Cx43 lacking the extracellular loop cysteines, known to be essential for gap junction channel formation [65], appears to form hemichannels as indicated by the uptake of carboxyfluorescein that is modulated by protein kinase C [66].
In astrocytes subjected to metabolic inhibition, the opening of Cx43 hemichannels in the cell surface accelerates cell death, and the dephosphorylation and/or oxidation of hemichannels were suggested as possible triggers of opening [22]. In order to examine whether metabolic inhibition affects the phosphorylation state of hemichannels on the surface of cortical astrocytes, proteins were biotinylated and Cx43 in hemichannels was quantified by Western blot analyses. Under basal conditions ~10% of total Cx43 was found on the surface and was mostly phosphorylated. After ~15 min of metabolic inhibition, the amount of surface Cx43 was doubled and most of this protein was not phosphorylated. Thereafter, levels of unphosphorylated surface Cx43 increased progressively. Cyclosporin A, a calcineurin inhibitor, significantly reduced the dephosphorylation of Cx43 induced by metabolic inhibition, measured in whole cell homogenates [7], but did not prevent an increased uptake of ethidium bromide (EtdBr). Moreover, trolox, a free radical scavenger, or dithiothreitol (DTT), a sulfhydril reducing reagent (applied 30 min before the end of a 60 min period of metabolic inhibition), did not prevent the metabolic inhibition-induced dephosphorylation of Cx43 [14] but greatly reduced EtdBr uptake [7,14]. In agreement with a role of reactive oxygen species (ROS), astrocytes loaded with CM-H2DCFDA show elevated generation of oxidants during metabolic inhibition. These studies suggest that the increase in Cx43 hemichannel opening observed in astrocytes during metabolic inhibition is associated with an oxidation reaction as well as increased insertion of Cx43 hemichannels into the cell surface and does not result from dephosphorylation [14].
A quite different picture emerged when we studied the effect of the reducing molecules, DTT and reduced glutathione (GSH), on hemichannel opening in normoxic conditions (M.A. Retamal, F.F. Bukauskas, M.V.L. Bennett, J.C. Sáez, unpublished observations). Single channel openings and dye uptake in HeLa cells expressing Cx43-EGFP were increased by bath applied DTT, which is membrane permeant. No effect was observed in parental cells or in cells expressing EGFP-Cx43 (EGFP bound to the N-terminal of Cx43, which does not form functional hemichannels). The DTT induced openings were blocked by the hemichannel blocker, La+3, or the gap junction and hemichannel blocker, flufenamic acid. When GSH, which is membrane impermeant, was applied in the bathing solution, there was no effect on the rate of dye uptake or on the open probability of Cx43-EGFP hemichannels. However, when GSH was present in the pipette solution, the frequency of open hemichannels was comparable to that observed at positive potentials in DTT solutions. These data suggest that a reducing cytosolic redox potential increases the open probability of Cx43 hemichannels under normoxic conditions.
To test whether intracellular cysteines might mediate the redox effect on Cx43 hemichannels, we made HeLa cells expressing Cx43 with the carboxy terminal truncated 12 residues after the fourth membrane spanning region at amino acid 258 (Cx43-Δ258); this mutant lacks cytoplasmic cysteines. In these cells DTT did not affect the rate of ethidium bromide uptake, suggesting that the intracellular cysteines are involved in the effects of changes in the cytoplasmic redox potential. The effect of DTT on Cx43-EGFP hemichannels was not associated with the changes in the phosphorylation state or cellular distribution of Cx43-EGFP, suggesting that opening of Cx43 hemichannels can be activated by simultaneous or independent changes in the redox and phosphorylation states of Cx43. This truncation would also cause loss of sites mediating interaction with binding partners, and actions on these proteins rather than Cx43 itself could mediate the DTT effect; replacement of individual cysteines should clarify this issue.
The above findings suggest that changes in redox state increase or decrease the open probability of Cx43 hemichannels depending on the conditions. Possible covalent modifications of sulfhydryl groups include oxidation to form disulfide bonds, nitrosylation, or glutathionation, all of which are sensitive to reduction by DTT. Future studies should identify the specific covalent modifications occurring in each condition as well as their differential effects on Cx43 hemichannels, phosphorylated or dephosphorylated at specific sites. Site directed mutagenesis will greatly facilitate the analysis of actions and interactions of the various covalent modifications. There is likely to be a wide range of effects given the divergence of connexin sequences in the cytoplasmic loop and C-terminal domain. Cysteine residues in the C-terminal vary in number and location (and are lacking in Cx45), and actions mediated by binding partners are also likely to contribute to the response repertoire.
Future research on hemichannels will examine the physiological relevance of opening and closing of hemichannels in vivo. Further analyses with biochemical and molecular techniques should elucidate the gating mechanisms of hemichannels and lead to strategies to control their activity as both an analytic tool and a therapeutic strategy.
Acknowledgements
This work was partially financed by a grant of Fondo Nacional para el Desarrollo de Ciencias y Tecnología (FONDECYT 1030945 to JCS), grants of the National Institute for Health (NS36706 to FFB and NS45837 to MVLB) and from the F.M. Kirby Foundation Program in Neuroprotection and Repair (to MVLB). Unpublished results on the phosphorylation and redox state on Cx43 hemichannels described here will be presented in partial fulfillment of the requirements to obtain the Ph.D. degree at the Pontificia Universidad Católica de Chile (M.A.R.).
References
- 1.Verselis VK, Trexler EB, Bukauskas FF. Connexin hemichannels and cell–cell channels: comparison of properties. Braz. J. Med. Biol. Res. 2000;33:379–389. doi: 10.1590/s0100-879x2000000400003. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;11:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sáez JC, Contreras JE, Bukauskas F, Retamal M, Bennett MVL. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol. Scand. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol. Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat. Rev., Mol. Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 6.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Contreras JE, Sánchez H, Véliz L, Bukauskas FF, Bennett MVL, Sáez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res. Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras JE, Sáez JC, Bukauskas FF, Bennett MVL. Gating and regulation of connexin-43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu Y, Dahl G. Accessibility of cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys. J. 2004;86:1502–1509. doi: 10.1016/S0006-3495(04)74218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S, Ri Y, Bennett MVL, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19:927–938. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J. Biol. Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 13.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retamal MA, Cortés CJ, Bukauskas FF, Bennett MVL, Sáez JC. Studies on putative gating mechanisms involved in opening Cx43 hemichannels in metabolically inhibited rat astrocytes. Mol. Biol. Cell. 2004:1718. (abstract). [Google Scholar]
- 15.Malchow RP, Qian H, Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J. Neurosci. Res. 1993;35:237–245. doi: 10.1002/jnr.490350303. [DOI] [PubMed] [Google Scholar]
- 16.White TW, Deans MR, O’Brien J, Al-Ubaidi MR, Goodenough DA, Ripps H, Bruzzone R. Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur. J. Neurosci. 1999;11:1883–1890. doi: 10.1046/j.1460-9568.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 17.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 18.Ripps H, Qian H, Zakevicius J. Pharmacological enhancement of hemi-gap-junctional currents in Xenopus oocytes. J. Neurosci. Methods. 2002;121:81–92. doi: 10.1016/s0165-0270(02)00243-1. [DOI] [PubMed] [Google Scholar]
- 19.Lampe PD, Kurata WE, Warn-Cramer BJ, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J. Cell. Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 20.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J. Biol. Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 21.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 22.Contreras JE, Sánchez HA, Eugenín EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MVL, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. U. S. A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergara L, Bao X, Cooper M, Bello-Reuss E, Reuss L. Gap-junctional hemichannels are activated by ATP depletion in human renal proximal tubule cells. J. Membr. Biol. 2003;196:173–184. doi: 10.1007/s00232-003-0636-9. [DOI] [PubMed] [Google Scholar]
- 24.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Good-enough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripps H, Qian H, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell. Mol. Neurobiol. 2004;24:647–665. doi: 10.1023/B:CEMN.0000036403.43484.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad S, Evans WH. Post-translational integration and oligomerization of connexin 26 in plasma membranes and evidence of formation of membrane pores: implications for the assembly of gap junctions. Biochem. J. 2002;365:693–699. doi: 10.1042/BJ20011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am. J. Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 28.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stout C, Charles A. Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia. 2003;43:265–273. doi: 10.1002/glia.10257. [DOI] [PubMed] [Google Scholar]
- 31.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch. 1999;437:345–353. doi: 10.1007/s004240050788. [DOI] [PubMed] [Google Scholar]
- 34.Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. J. Gen. Physiol. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedamzik B, Marten I, Ngezahayo A, Ernst A, Kolb HA. Regulation of lens rCx46-formed hemichannels by activation of protein kinase C, external Ca(2+) and protons. J. Membr. Biol. 2000;173:39–46. doi: 10.1007/s002320001005. [DOI] [PubMed] [Google Scholar]
- 36.Valiunas V, Mui R, McLachlan E, Valdimarsson G, Brink PR, White TW. Biophysical characterization of zebrafish connexin35 hemichannels. Am. J. Physiol. 2004;287:C1596–C1604. doi: 10.1152/ajpcell.00225.2004. [DOI] [PubMed] [Google Scholar]
- 37.Basilio D, Sáez JC, Bukauskas FF, Bennett MVL. pH gating of Cx43-GFP hemichannels. Mol. Biol. Cell. 2004:1708. (abstract). [Google Scholar]
- 38.Gómez-Hernaández JM, de Miguel M, Larrosa B, González D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. U. S. A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gapjunctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris RJ, Symon L. Extracellular pH, potassium, and calcium activities in progressive ischaemia of rat cortex. J. Cereb. Blood Flow Metab. 1984;4:178–186. doi: 10.1038/jcbfm.1984.26. [DOI] [PubMed] [Google Scholar]
- 42.Trexler EB, Bukauskas FF, Bennett MVL, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Taffet S, Stoner L, Delmar M, Vallano ML, Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: the carboxyl tail length. Biophys. J. 1993;64:1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J. Gen. Physiol. 2003;122:389–405. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 46.Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys. J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys. J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys. J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro C, Gómez-Hernández JM, Silander K, Barrio LC. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J. Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 2000;279:C596–C602. doi: 10.1152/ajpcell.2000.279.3.C596. [DOI] [PubMed] [Google Scholar]
- 51.Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys. J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beahm DL, Hall JE. Opening hemichannels in nonjunctional membrane stimulates gap junction formation. Biophys. J. 2004;86:781–796. doi: 10.1016/S0006-3495(04)74154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrams C, Bennett MVL, Verselis VK, Bargiello TA. Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu TF, Paulson AF, Li HY, Atkinson MM, Johnson RG. Inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate on dye leakage from single Novikoff cells and on dye transfer between reaggregated cell pairs. Methods Find. Exp. Clin. Pharmacol. 1997;19:573–577. [PubMed] [Google Scholar]
- 57.Ngezahayo A, Zeilinger C, Todt II, Marten II, Kolb H. Inactivation of expressed and conducting rCx46 hemichannels by phosphorylation. Pflügers Arch. 1998;436:627–629. doi: 10.1007/s004240050681. [DOI] [PubMed] [Google Scholar]
- 58.Sáez JC, Martínez AD, Brañes MC, González HE. Regulation of gap junctions by protein phosphorylation. Braz. J. Med. Biol. Res. 1998;31:593–600. doi: 10.1590/s0100-879x1998000500001. [DOI] [PubMed] [Google Scholar]
- 59.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am. J. Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 60.Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J. Biol. Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- 61.Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J. Biol. Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- 62.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 63.Sitaramayya A, Crabb JW, Matesic DF, Margulis A, Singh V, Pulukuri S, Dang L. Connexin 36 in bovine retina: lack of phosphorylation but evidence for association with phosphorylated proteins. Vis. Neurosci. 2003;20:385–395. doi: 10.1017/s0952523803204041. [DOI] [PubMed] [Google Scholar]
- 64.Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemichannels by cyclic AMP requires a protein kinase A phosphorylation site. J. Neurosci. Res. 2003;72:147–157. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- 65.Dahl G, Werner R, Levine E, Rabadan-Diehl C. Mutational analysis of gap junction formation. Biophys. J. 1992;62:172–180. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao X, Chen Y, Reuss L, Altenberg GA. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J. Biol. Chem. 2004;279:9689–9692. doi: 10.1074/jbc.M311438200. [DOI] [PubMed] [Google Scholar]
- 67.Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell. Physiol. 2003;197:205–213. doi: 10.1002/jcp.10365. [DOI] [PubMed] [Google Scholar]
- 68.Romanello M, D’Andrea P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: a role for gap-junctional hemichannels. J Bone Miner. Res. 2001;16:1465–1476. doi: 10.1359/jbmr.2001.16.8.1465. [DOI] [PubMed] [Google Scholar]
- 69.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin-43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 71.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 72.Li F, Sugishita K, Su Z, Ueda I, Barry WH. Activation of connexin-43 hemichannels can elevate [Ca2+]i and [Na+]i in rabbit ventricular myocytes during metabolic inhibition. J. Mol. Cell. Cardiol. 2001;33:2145–2155. doi: 10.1006/jmcc.2001.1477. [DOI] [PubMed] [Google Scholar]
- 73.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 74.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 75.Pottek M, Hoppenstedt W, Janssen-Bienhold U, Schultz K, Perlman I, Weiler R. Contribution of connexin26 to electrical feedback inhibition in the turtle retina. J. Comp. Neurol. 2003;466:468–477. doi: 10.1002/cne.10897. [DOI] [PubMed] [Google Scholar]
- 76.Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 77.Ebihara L, Xu X, Oberti C, Beyer EC, Berthoud VM. Co-expression of lens fiber connexins modifies hemi-gap-junctional channel behavior. Biophys. J. 1999;76:198–206. doi: 10.1016/S0006-3495(99)77189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valiunas V, Manthey D, Vogel R, Willecke K, Weingart R. Biophysical properties of mouse connexin30 gap junction channels studied in transfected human HeLa cells. J. Physiol. 1999;519:631–644. doi: 10.1111/j.1469-7793.1999.0631n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valiunas V, Niessen H, Willecke K, Weingart R. Electrophysiological properties of gap junction channels in hepatocytes isolated from connexin32-deficient and wild-type mice. Pflügers Arch. 1999;437:846–856. doi: 10.1007/s004240050854. [DOI] [PubMed] [Google Scholar]
- 81.Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J. Membr. Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 1999 Nov;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bukauskas FF, Bukauskiene A, Bennett MVL, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys. J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bukauskas FF, Buskauskiene A, Verselis VK, Bennett MVL. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tong JJ, Liu X, Dong L, Ebihara L. Exchange of gating properties between rat cx46 and chicken cx45.6. Biophys. J. 2004;87:2397–2406. doi: 10.1529/biophysj.104.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J. Physiol. 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.el Aoumari A, Dupont E, Fromaget C, Jarry T, Briand JP, Kreitman B, Gros D. Immunolocalization of an extracellular domain of connexin43 in rat heart gap junctions. Eur. J. Cell Biol. 1991;56:391–400. [PubMed] [Google Scholar]
- 88.Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 89.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 90.Cotrina ML, Lin JH, López-Garcí JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J. Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enkvetchakul D, Ebihara L, Nichols CG. Polyamine flux in Xenopus oocytes through hemi-gap junctional channels. J Physiol. 2003;553:95–100. doi: 10.1113/jphysiol.2003.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu TF, Li HY, Atkinson MM, Johnson RG. Intracellular lucifer yellow leakage from Novikoff cells in the presence of ATP or low extracellular Ca: evidence for hemi-gap junction channels. Methods Find. Exp. Clin. Pharmacol. 1995;17:23–28. [PubMed] [Google Scholar]
- 93.Rhee SK, Bevans CG, Harris AL. Channel-forming activity of immunoaffinity-purified connexin32 in single phospholipid membranes. Biochemistry. 1996;35:9212–9223. doi: 10.1021/bi960295m. [DOI] [PubMed] [Google Scholar]
- 94.Vanoye CG, Vergara LA, Reuss L. Isolated epithelial cells from amphibian urinary bladder express functional gap junctional hemichannels. Am. J. Physiol. 1999;276:279–284. doi: 10.1152/ajpcell.1999.276.1.C279. [DOI] [PubMed] [Google Scholar]
- 95.Ebihara L. Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys. J. 1996;71:742–748. doi: 10.1016/S0006-3495(96)79273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ebihara L, Berthoud VM, Beyer EC. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys. J. 1995;68:1796–1803. doi: 10.1016/S0006-3495(95)80356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng S, Shakespeare T, Mui R, White TW, Valdimarsson G. Connexin 48.5 is required for normal cardiovascular function and lens development in zebrafish embryos. J. Biol. Chem. 2004;279:36993–37003. doi: 10.1074/jbc.M401355200. [DOI] [PubMed] [Google Scholar]
- 98.Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 And Cx50 hemichannels and gap junction channels. Biophys. J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zoidl G, Bruzzone R, Weickert S, Kremer M, Zoidl C, Mitropoulou G, Srinivas M, Spray DC, Dermietzel R. Molecular cloning and functional expression of zfCx52.6: a novel connexin with hemichannel-forming properties expressed in horizontal cells of the zebrafish retina. J. Biol. Chem. 2004;279:2913–2921. doi: 10.1074/jbc.M304850200. [DOI] [PubMed] [Google Scholar]