Abstract
The purpose of this study was to investigate an engineered composite of multilayer acellular tendon slices seeded with bone marrow stromal cells (BMSCs) as a possible solution for tendon reconstruction. BMSCs were harvested from 15 rabbits and infraspinatus tendons were harvested from 17 dogs. The decellularized tendons were sectioned in longitudinal slices with a thickness of 50 μm. The BMSCs were seeded on the slices and then the slices were bundled into one composite. The composite was implanted into a rabbit patellar tendon defect. Tendon slices without BMSCs were implanted into the contralateral patellar tendon as a control. The composites were evaluated by histology and qRT–PCR. The viability of BMSCs was assessed using a fluorescent marker. Histology showed viable cells between the collagen fibres on the cell-seeded side. Analysis by qRT–PCR showed higher tenomodulin, collagen type III, MMP3 and MMP13 expressions and lower collagen type I expression in the cell-seeded composite than in the tendon slices without BMSCs. We conclude that BMSCs can survive in a multilayer composite, express a tendon phenotype and enhance the metabolism of tendon in vivo. This in vivo study suggests a potential utility of this composite in tendon reconstruction.
Keywords: bone marrow stromal cells, tendon, regeneration, gene expression, cell tracking, tissue engineering
1. Introduction
Cells, scaffolds and growth factors are the three key factors for tissue regeneration (Tabata, 2003). Various biosynthetic scaffolds, such as polyglycolic acid (PGA), polylactic acid (PLA) and polylactic polyglycolic acid (PLGA), have been used for tendon engineering (Liu et al., 2008). However, synthetic materials may alter tendon mechanical properties, decrease cell viability, lose strength and integrity over time, limit tendon ingrowth, increase the inflammatory response and cause scar hyperplasia around the repair site (van Wachem et al., 1985; Wan et al., 2003; Bostman and Pihlajamaki, 2000a, 2000b). Therefore, successful application of synthetic scaffolds is still a great challenge. Native acellular scaffolds made from animal tissues, such as small intestinal submucosa or human cadaver dermal matrix, have also been developed and used in both research and clinical studies (Barber et al., 2006; Wang et al., 2003; Le Visage et al., 2006; Adams et al., 2006). Although these materials can serve as a temporary scaffold and provide an environment for local cell infiltration, concerns regarding mechanical strength, delayed healing and adhesion formation have been reported (Butler et al., 2004; Cannon et al., 2005; le Roux 2005; Zhang et al., 2006; Fini et al., 2007).
The function of a tendon is to transmit the muscle force to the bone to move a joint. The structure of the tendon contains dense connective tissue, mainly type I collagen fibres. For tendon regeneration, the ideal scaffold would possess the basic structure of the tendon, in which the type I collagen fibres align densely in the same direction and unite biologically with the adjacent collagen fibres of uninjured tendon, for anatomical healing. The scaffold should provide an appropriate environment, such as interconnected cavities, for cells or growth factors and for the ingrowth of adjacent tissue.
A previous study reported a technique for tendon engineering, using stacked slices of acellular canine infraspinatus tendon tissue at a thickness of 50 μm (Omae et al., 2009). Each individual tendon slice was seeded with cultured bone marrow stromal cells (BMSCs), which have been shown to possess the ability of multipotential differentiation (Colter et al., 2001). The BMSCs could survive up to 2 weeks in vitro, organized along the collagen fibres on the tendon slices and expressed a marker of tendon phenotype, tenomodulin (Shukunami et al., 2006).
The purpose of this study was to investigate the viability and differentiation of BMSCs seeded on such tendon slices in a rabbit tendon repair model in vivo. We hypothesized that BMSCs could populate the tendon slice composite and remain viable after the composite was implanted in vivo. We also hypothesized that the seeded BMSCs would express genes related to the tendon phenotype after implantation in vivo.
2. Materials and methods
Acellular tendon slices were made from the infraspinatus tendon of a dog sacrificed in other studies. BMSCs were obtained from a rabbit, cultured and seeded on the tendon slices. The BMSCs–tendon slices composite was implanted into the defect created in the patellar tendon of the same rabbit. Two weeks after operation, the rabbits were sacrificed and the composite was examined an compared to the contralateral patellar tendon, with a defect repaired with similar but unseeded slices. This study was approved by our Institutional Animal Care and Use Committee (IACUC). NIHH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev, 1985) were observed.
2.1. Bone marrow stromal cells
We harvested bone marrow from both tibias of 15 adult rabbits. Six rabbits were used for a cell viability test, six rabbits for gene expression testing and three rabbits for histological assessment. The rabbits were anaesthetized with intravenous ketamine (35 mg/kg), acepromazine (1 mg/kg) and xylazine (5 mg/kg). A total of 6.0 ml of bone marrow was aspirated from the medial aspect of the proximal tibiae, using an 18 gauge needle and a 20 ml syringe (BD, Franklin Lakes, NJ, USA) containing 1.0 ml heparin solution (Heparin sodium injection; Baxter Healthcare Corporation, Deerfield, IL, USA). The heparinized bone marrow extract was added to 5.0 ml phosphate-buffered saline (PBS) and centrifuged at 1500 rpm (380 × g) for 5 min at room temperature. The bone marrow pellet was then resolubilized in 10 ml minimal essential medium (MEM) with Earle’s salts (Gibco, Grand Island, NY, USA), 10% fetal bovine serum (FBS; Gibco) and 5% antibiotics (Antibiotic–Antimycotic; Gibco). The cells from one rabbit were divided into four equal aliquots, placed in 100 mm culture dishes and incubated at 37 °C with 5% CO2 and 95% air at 100% humidity. After 5 days, the medium and any floating cells were removed and new medium was added to the remaining adherent cells. These adherent cells were defined as bone marrow stromal cells (BMSCs) (Prockop, 1997). The medium was then changed every other day until the adherent cells reached confluence. The cells were then released with trypsin–EDTA solution [0.25% trypsin, 0.1% EDTA in Hank’s balanced salt solution (HBSS); Mediatech Inc., Manassas, VA, USA] to produce a cell suspension and centrifuged at 1500 rpm for 5 min to remove the trypsin–EDTA solution. The concentrated cell suspension from each rabbit was then gathered into one tube. The cells were counted using a haemocytometer and the concentration of the cell suspension was adjusted to 5.0 × 106 cells/ml by adding additional medium.
For the analysis of cell viability, BMSCs from six rabbits were stained with the fluorescent marker PKH 26-GL (PKH 26 Red Fluorescent Cell Linker Kit for General Cell Membrane Labelling; Sigma, St. Louis, MO, USA), following the manufacturer’s instructions, before seeding on the tendon slices. This fluorescent marker was used for cell tracking in the study using bone marrow stromal cells (Urdzikova et al., 2006). The final concentration of labelled cell solution was also adjusted to 5.0 × 106 cells/ml. For the histological analysis in three rabbits and gene expression analysis in six rabbits, cells were not stained.
2.2. Tendon slices
We harvested 33 infraspinatus tendons from 17 mixed-breed dogs weighing 25–30 kg, which hadf been euthanized for other IACUC-approved studies. The dogs were euthanized by an overdose of pentobarbital. The infraspinatus tendon was exposed by removing the deltoid muscle and the tendinous portion between the insertion to the bone and the muscle–tendon junction was harvested. The size of harvested tendon was roughly 25 × 10 mm and rectangular in shape. The harvested tendons were frozen at −80 °C until processing. The infraspinatus tendons were harvested under sterile conditions.
Frozen infraspinatus tendons were prepared for use following thawing at room temperature. The infraspinatus tendons were trimmed into segments roughly 10 × 10 mm in size. The tendon segments were immersed in liquid nitrogen for 2 min and then thawed in saline solution at 37 °C for 10 min. This procedure was repeated five times to kill residual cells in the tendon (Arnoczky et al., 1992; Azuma et al., 2007). Following washing in PBS without ethylenediaminetetraacetic acid (EDTA) (3 × 30 min), the tendon segments were incubated in 20 ml nuclease solution (RNase from bovine pancreas, 1.5 U/ml; Roche Diagnostic, Indianapolis, IN, USA) for 12 h at 37 °C. Finally, the infraspinatus tendon segments were rinsed for 30 min in PBS (50 ml) at room temperature with gentle agitation. The rinsing was repeated three times. The tendon segments were then frozen to −80 °C and fixed to the cutting base plate of a cryostat (Leica CM 1850, Germany) with OCT compound (polyvinyl alcohol and polyethylene glycol; Tissue-Tek®, Sakura Finetek, Torrance, CA, USA). The excess OCT compound around the tendon was removed by a scalpel. The tendon segments were then sliced at a thickness of 50 μm and the slices were placed in a 100 mm culture dish. Fourteen slices were placed on each dish. The slices were thawed on the dish in an incubator at 37 °C with 5% CO2 and 95% air at 100% humidity for 10 min. The tendon slices were then washed three times with 10 ml PBS. At this point the sliced acellular tendon segments were ready to seed with BMSCs.
2.3. Composite of BMSCs and sliced tendon scaffold
The concentrated BMSCs solution (5.0 × 106 cells/ml, 10 ml/dish) was added to the sliced tendon scaffold dish and cultured at 37 °C with 5% CO2 and 95% air at 100% humidity for 1 day (Figure 1A). Just before the implantation, the slices were then carefully detached with forceps and 10 tendon slices were bundled together on a new dish. The ends of the bundled slices were fixed with 4-0 nylon suture (Ethicon, Piscataway, NJ, USA) (Figure 1B).
Figure 1.
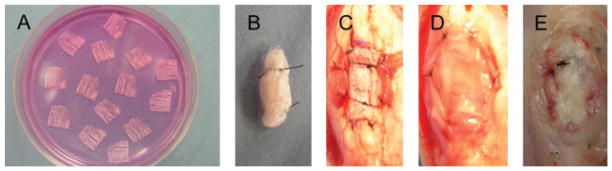
Preparation of composite and surgical procedures. (A) BMSCs solution was added to tendon slices (black arrow) on culture. (B) Tendon slices with BMSCs were bundled with sutures. (C) Bundled tendon slices with BMSCs were fixed into the patellar tendon defect (black arrows). (D) A membrane of subcutaneous bursa was placed around the implanted tendon slices with BMSCs (black arrows). (E) Two weeks after the operation, the implanted tendon slices with BMSCs were exposed (black arrows)
2.4. Surgical procedures
The rabbits were anaesthetized with intravenous ketamine (35 mg/kg), acepromazine (1 mg/kg) and xylazine (5 mg/kg). After standard sterile preparation and draping of the area over the patellar tendon, a longitudinal incision was made on the right knee. The subcutaneous tissue was divided and the patellar tendon was exposed. A 10 × 3 mm square defect (length × width) was made in the middle third of the tendon. The bundled tendon slices with seeded BMSCs were placed in the defect, orientated so that the slice fibres were aligned with the direction of the patellar tendon. The tendon slices was sutured to the host tendon at the four corners, using 4-0 nylon suture (Ethicon) (Figure 1C). A 10 × 10 mm section of the subcutaneous bursa around the patella was harvested, placed around the implanted tendon slices and sutured to the patellar tendon at the four corners using 4-0 nylon suture (Figure 1D). Following the patellar tendon defect repair, the wound was closed by 4-0 nylon suture. In nine rabbits (six rabbits for qRT–PCR analysis and three rabbits for histological assessment), as a control, a tendon defect was made in the contralateral patellar tendon and repaired with tendon slices without cells. The limbs were not immobilized after the operation. Two weeks after surgery, the rabbits were sacrificed by intravenous administration of a lethal dose of pentobarbital and the implanted composite was excised for analysis (Figure 1E). No adhesion formation was observed at the time of sacrifice. On gross observation, the excised tendon slices, with or without BMSCs, fused together and did not separate with gentle traction.
2.5. Assessment of cell viability
For the analysis of cell viability, BMSCs from six rabbits were stained with the fluorescent marker PKH 26-GL and seeded on the tendon slices. Two composites were made from one rabbit to compare the cell viability between day 0 (before implantation) and day 14 after implantation. A total of 12 composites (six composites with BMSCs at day 0 and six composites with BMSCs at day 14) from six rabbits were used for the assessment of cell viability. For day 0 analysis, 10 tendon slices with BMSCs were detached from the dish and sutured with 4-0 nylon suture at both ends and were immediately fixed in 2% paraformaldehyde. For day 14 analysis, immediately after sacrifice, the implanted composites with BMSCs were dissected from the patellar tendons and fixed in 2% paraformaldehyde. The samples were mounted using OCT compound on dry ice and sectioned longitudinally at a thickness of 50 μm using a cryostat (Leica CM 1850). A laser-scanning confocal microscope (LSM 310, Zeiss) was used to observe fluorescence. For the analysis, sections in the middle of the tendon slices were used. The number and distribution of the stained cells were compared between day 0 and day 14 composites.
2.6. Histological examination
A total of six composites (three with and three without BMSCs at day 14) from three rabbits were used for the histological analysis. Each composite was fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned longitudinally at a thickness of 5 μm. Haematoxylin and eosin (H&E) staining was performed. The morphology and distribution of the cells were examined.
2.7. Assessment of gene expression
A total of 12 composites (six with and six without BMSCs at day 14) from six rabbits were used for the assessment of gene expression. Immediately after sacrifice, the composite was dissected from the patellar tendon, put into liquid nitrogen and stored at −86 °C. A quantitative real-time reverse transcription–polymerase chain reaction (qRT–PCR) was performed to measure the gene expression levels of tenomodulin (a marker of tenocyte differentiation) (Colter et al., 2001), collagen type I, collagen type III, MMP2 (gelatinase), MMP3 (stromelysin) and MMP13 (collagenase) (Oshiro et al., 2003). RNA was extracted by TRIzol® reagent (monophasic solutions of phenol and guanidine isothiocyanate; Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using a First Strand cDNA Synthesis Kit (Roche Diagnostics) with random primers. Quantitative RT–PCR was performed on a LightCycler® (Roche Diagnostics). The sequences of the primers are shown in Table 1. The expression level was normalized to that of GAPDH. All mRNA expressions were confirmed by LightCycler® melting curve analysis.
Table 1.
Sequences of the primers used in assessment of gene expression
| Gene | Sequences of forward/reverse primers |
|---|---|
| GAPDH | 5′-CGAGCTGAACGGGAAAC-3′/5′-CCTGGTCCTCGGTGTAG-3′ |
| Collagen type I | 5′-GGAGAAGAAGCACGTGT-3′/5′-GCAGTGGTAGGTGATGTT-3′ |
| Collagen type III | 5′-TTATAAACCAACCTCTTCCT-3′/5′-TATTATAGCACCATTGAGAC-3′ |
| MMP2 | 5′-GACCAGAGCACCATCGAGA-3′/5′-AGGCATCATCCACTGTTT-3′ |
| MMP3 | 5′-TATGAAGTTATTAGCAGGGATACTGTT-3′/5′-TCCTTTCCTTATCAGAAATGGCA-3′ |
| MMP13 | 5′-AGAGAGCTACCTGAGATCATAC-3′/5′-AGCTAAGGTGTTATCATCAAGTT-3′ |
| Tenomodulin | 5′-GATCCCATGCTGGATGAG-3′/5′-TACAAGGCATGATGACACG-3′ |
2.8. Statistics
The results of the gene expressions were analysed using paired t-tests. p < 0.05 was considered significant.
3. Results
3.1. Cell tracking and histology
BMSCs labelled with PKH 26 fluoresced red under the confocal laser microscope. Stained BMSCs were observed in the composites at day 0 (before implantation; Figure 2A) and day 14 after implantation (Figure 2C; red = live BMSCs). In the composite at day 0 the red-stained BMSCs were aligned, while the BMSCs in the composite at day 14 were scattered. The number of red-stained BMSCs in the composite at day 14 was similar to that at day 0. A quantitative analysis of the cell number could not be done because the red-stained BMSCs at day 0 were overlapped, making it impossible to count cells accurately.
Figure 2.
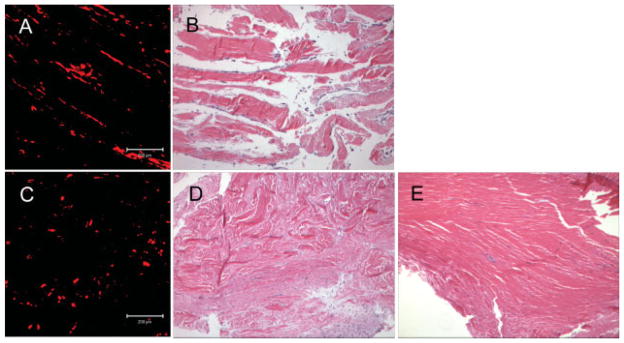
(A) BMSCs labelled with PKH 26 (red colour) were observed at day 0 under a confocal laser microscope (white arrows). (B) Cells were aligned on the tendon slices at day 0 (black arrows; H&E stain, original magnification ×100). (C) BMSCs labelled with PKH 26 (red colour) were scattered at day 14 when viewed under a confocal laser microscope (white arrows). (D) A large number of scattered cells were observed in the collagen fibres in the composite with BMSCs at day 14 (black arrows, H&E stain, original magnification ×100). (E) Collagen fibres with few cells were observed in the composite without BMSCs at day 14 (H&E stain, original magnification ×100)
Histological sections showed the cells aligned on the tendon slices at day 0 (before implantation; Figure 2B) and a large number of cells in the composite with BMSCs at day 14 (Figure 2D), but only a few cells in the tendon slices without BMSCs at day 14 (Figure 2E). Infiltration of inflammatory cells was not observed in either seeded or unseeded composites at day 14.
3.2. Gene expression
Significantly higher gene expression was detected in the composite with BMSCs than in the tendon slices without BMSCs for tenomodulin (p = 0.028), collagen type III (p = 0.028), MMP3 (p = 0.028) and MMP13 (p = 0.046). Collagen type I expression was lower in the composite with BMSCs than that in the tendon slices without BMSCs (p = 0.046). MMP2 expression showed no significant difference between with and without BMSCs (Figure 3).
Figure 3.
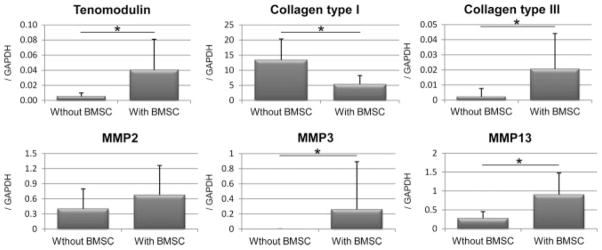
The results of gene expression levels of tenomodulin, collagen type I, collagen type III, MMP2, MMP3 and MMP13 by qRT–PCR. The expression level was normalized to that of GAPDH (n = 6)
4. Discussion
The repair of a large tendon defect has remained one of the most difficult surgical interventions in orthopaedics. The creation of a viable tendon substitute by tissue engineering might solve this problem.
Bone marrow stromal cells (BMSCs) were used as the cell source in this study because BMSCs have the potential to differentiate into a variety of tissue types (Colter et al., 2001). BMSCs are suitable for clinical application, because bone marrow is easily obtained with a minimally invasive procedure and the methods for isolation and culture of BMSCs are not complicated.
The ideal scaffold for tendon engineering is a material which is replaced by normal tendon tissue after implantation, can be combined biologically to the host tendon, possesses the same mechanical properties as the host tendon, has the space for holding cells and growth factors inside and for the migration of host cells and vessels, can be obtained and handled easily and is not expensive.
Multilayer acellular tendon slices were used as a scaffold in this study. This scaffold has aligned dense collagen fibres which can be biologically combined to the host tendon tissue. By slicing the donor tendon and bundling the resulting tendon slices into a multilayer composite, space is provided for the seeded cells inside the composite. The repeated deep freezing–thawing method makes the tissue acellular (Arnoczky et al., 1992; Azuma et al., 2007) and reduces antigenicity to a minimum (Minami et al., 1982). This method is easy and safe, compared with the other methods of tissue preparation using chemical agents.
In this study, BMSCs from rabbits were combined with acellular tendon slices from dogs and the composite was transplanted into rabbits. The rationale for using dog tendon slices instead of rabbit tendon was twofold. First, the larger dog tendon slices were technically easier to manipulate. Second, a xenograft concept may be useful to test for possible clinical applications, since animal tendon is far easier to obtain than human cadaver tissue. In addition, acellular collagen xenografts have been already manufactured commercially and used clinically (McCarron et al., 2008). In this study, the infiltration of inflammatory cells was not observed at day 14.
The terminology of MSCs is confusing. The ‘M’ may stand for either marrow or mesenchymal; the ‘S’ for either stromal or stem cells. In addition, the term ‘stem cell’ is itself controversial. The cells obtained by the procedure in our manuscript are plastic-adherent cells from bone marrow. These cells have many of the characteristics of stem cells, in that they can differentiate into other cell types, but they could also be defined as ‘mesenchymal’ or as marrow ‘stromal’ cells by some authors (MSCs; Prockop, 1997). In contrast, Horwitz et al. (2005) suggested that the plastic adherent cells isolated from bone marrow were ‘multipotent’ mesenchymal stromal cells, while the term mesenchymal ‘stem’ cells should be used only for cells that meet specified stem cell criteria. We used the term bone marrow ‘stromal’ cells in this paper to describe the plastic-adherent bone marrow cells.
There are many hurdles to clear for the clinical application of this composite as a method to reconstruct a tendon defect. First of all, the viability of BMSCs seeded on the tendon slices and the function of BMSCs in the composite must be examined in more detail. In vitro, the BMSCs survive and differentiate into tenocytes within 2 weeks (Omae et al., 2009). The current study assessed the viability and gene expression of BMSCs in vivo. The BMSCs in the composites which were implanted in patellar tendons were detected by a fluorescence tracking marker at 2 weeks after the operation. This showed that the seeded BMSCs could survive in the composite after in vivo implantation. The expression of the tenomodulin gene, a tenocyte differentiation marker, was higher in the composite with BMSCs than that in tendon slices without BMSCs. The expressions of the collagen type III, MMP3 and MMP13 genes were higher and the expression of the collagen type I gene was lower in the composite with BMSCs than that in tendon slices without BMSCs at 2 weeks after the operation. These results of collagen and MMP gene expressions might indicate that the composite with BMSCs was in a catabolic state at 2 weeks after the operation. The gene expression data for the BMSCs-seeded composite could not be isolated from the effect of host cells that might have infiltrated the composite during the 2 weeks of the study. Thus, while the BMSC-seeded constructs enhanced a tendon-like phenotype and metabolism, we could not determine whether the phenotype expression and metabolic changes arose exclusively within the BMSCs, the host cells or a combination of the two cell sources. It is also important to note that the expression of a particular gene does not mean that the associated protein is expressed. In this study we did not measure protein synthesis.
The main limitation of this study was its duration. Two weeks is too short to study full integration of the composite into the host tendon. The evaluations were performed at 2 weeks after the operation because the purpose of this study was to investigate the viability and differentiation of BMSCs seeded on the tendon slices in a rabbit tendon repair model in vivo, as a prelude to a longer study. Now that we have shown that the cells can survive in vivo, we will complete longer studies and compare the integration of autologous cells in both allograft and xenograft slice composites. In addition, the mechanical properties of the scaffold were not investigated; this was also due to the short-term nature of the present study.
5. Conclusion
We have developed a technique to create an engineered tendon substitute, using acellular tendon slices at a thickness of 50 μm and seeding the slices with cultured BMSCs. While xenogeneic tendon was used here, allogeneic sources could be used as well. We showed that BMSCs could survive in such a construct in vivo; the increased tenomodulin expression suggests that BMSCs might express or stimulate the expression of a tendon phenotype in vivo. The changes of collagen and MMP expressions suggest that the BMSCs also have an effect on collagen metabolism. We believe that this new composite might be useful for tendon tissue engineering.
Acknowledgments
This study was supported by a grant from the Mayo Foundation.
References
- Adams JE, Zobitz ME, Reach JS, Jr, et al. ; Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, DiCarlo EF, O’Brien SJ, et al. ; Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8:428–436. doi: 10.1016/0749-8063(92)90003-t. [DOI] [PubMed] [Google Scholar]
- Azuma C, Tohyama H, Nakamura H, et al. ; Antibody neutralization of TGF-β enhances the deterioration of collagen fascicles in a tissue-cultured tendon matrix with ex vivo fibroblast infiltration. J Biomech. 2007;40:2184–2190. doi: 10.1016/j.jbiomech.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Barber FA, Herbert MA, Coons DA. ; Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Bostman O, Pihlajamaki H. ; Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000a;21:2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Bostman OM, Pihlajamaki HK. ; Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000b;371:216–227. [PubMed] [Google Scholar]
- Butler DL, Shearn JT, Juncosa N, et al. ; Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;427(Suppl):S190–S199. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Sweeney DD, Conway DA, et al. ; A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005;96:664–669. doi: 10.1111/j.1464-410X.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. ; Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini M, Torricelli P, Giavaresi G, et al. ; In vitro study comparing two collageneous membranes in view of their clinical application for rotator cuff tendon regeneration. J Orthop Res. 2007;25:98–107. doi: 10.1002/jor.20295. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, et al. ; Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- le Roux PJ. ; Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: a pilot study. J Urol. 2005;173:140–143. doi: 10.1097/01.ju.0000146554.79487.7f. [DOI] [PubMed] [Google Scholar]
- Le Visage C, Yang SH, Kadakia L, et al. Small intestinal submucosa as a potential bioscaffold for intervertebral disc regeneration. Spine. 2006;31:2423–2430. doi: 10.1097/01.brs.0000238684.04792.eb. discussion, 2431. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ramanath HS, Wang DA. ; Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201–209. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- McCarron J, Derwin KA, Iannotti JP. Biologic augmentation of rotator cuff healing. In: Galatz LM, editor. Orthopaedic Knowledge Update: Shoulder and Elbow 3. 3. American Academy of Orthopedic Surgeons; Rosemont, IL, USA: 2008. pp. 211–219. [Google Scholar]
- Minami A, Ishii S, Ogino T, et al. ; Effect of the immunological antigenicity of the allogeneic tendons on tendon grafting. Hand. 1982;14:111–119. doi: 10.1016/s0072-968x(82)80001-6. [DOI] [PubMed] [Google Scholar]
- Omae H, Zhao C, Sun YL, et al. ; Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro W, Lou J, Xing X, et al. ; Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. ; Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, et al. ; Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9(suppl 1):S5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- Urdzikova L, Jendelova P, Glogarova K, et al. ; Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- van Wachem PB, Beugeling T, et al. ; Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials. 1985;6:403–408. doi: 10.1016/0142-9612(85)90101-2. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chen W, Yang J, et al. ; Biodegradable poly(L-lactide)-poly(ethylene glycol) multiblock copolymer: synthesis and evaluation of cell affinity. Biomaterials. 2003;24:2195–2203. doi: 10.1016/s0142-9612(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Watanabe Y, Toki A. ; Experimental assessment of small intestinal submucosa as a small bowel graft in a rat model. J Pediatr Surg. 2003;38:1596–1601. doi: 10.1016/s0022-3468(03)00567-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Frimberger D, Cheng EY, et al. ; Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98:1100–1105. doi: 10.1111/j.1464-410X.2006.06447.x. [DOI] [PubMed] [Google Scholar]


